Summary
OX40 (CD134) is a tumor necrosis factor (TNF) receptor expressed primarily on activated CD4+ and CD8+ T cells and transmits a potent costimulatory signal when engaged. OX40 is transiently expressed after T-cell receptor engagement and is upregulated on the most recently antigen-activated T cells within inflammatory lesions (e.g. sites of autoimmune destruction and on tumor-infiltrating lymphocytes). Hence, it is an attractive target to modulate immune responses: OX40 blocking agents to inhibit undesirable inflammation or OX40 agonists to enhance immune responses. In regards to this review, OX40 agonists enhance anti-tumor immunity, which leads to therapeutic effects in mouse tumor models. A team of laboratory and clinical scientists at the Providence Cancer Center has collaborated to bring the preclinical observations in cancer models from the bench to the bedside. This review describes the journey from in vitro experiments through preclinical mouse models to the successful translation of the first OX40 agonist to the clinic for the treatment of patients with cancer.
Keywords: immunotherapies, tumor immunity, T cells, costimulation
Preface
The events chronicled in this review relay how basic, preclinical, and clinical science were eventually brought together so that cancer patients could be treated with an OX40 agonist. There were several chance meetings and occurrences that influenced both the science and decisions to go forward with the clinical trial. Therefore, the review contains both scientific and personal events that directly influenced OX40 development and eventually achieving the goal of treating cancer patients. While it would have been nice to describe an exact ‘template’ for scientists to move forward with their discoveries into patients, this manuscript reviews the actual events that unfolded.
Introduction
Immunotherapy has been proposed as an effective cancer treatment for over 100 years, and there has been a focused effort to obtain approval of immune stimulatory drugs/techniques for cancer patients during the past 40 years (1–9). These immune stimulatory agents have included cancer vaccines, cytokine-based therapies, Toll-like receptor (TLR) agonists, adoptive T cell and natural killer (NK) cell therapies, antibody-based therapy directed against tumor antigens (Ags) and angiogenic factors, and antibodies to T-cell surface proteins that augment T-cell function (1–9). Clinical efforts in the 1980s focused on interleukin-2 (IL-2) and lymphokine activated killer cells (LAK) therapy with limited success; however, from this work, IL-2 monotherapy was approved for use in late stage melanoma and renal cancer patients (3, 4). Since the mid-1990s, several antibodies against tumor Ags [e.g. Her2/neu, CD20, and epidermal growth factor (EGF) receptors] were approved for use against different tumors, some of which showed greater efficacy when combined with chemotherapy (6, 10, 11). In the last 2 years, two immune-based therapies have been approved for use in cancer patients. One, ipilimumab, an antibody to the cytotoxic T-lymophocyte antigen-4 (CTLA-4) protein that blocks a negative T-cell signal and improves T-cell function, was approved for late stage melanoma (12). The other, sipuluecel-T, is a vaccine/adoptive therapy, which has been approved for hormone refractory late stage prostate cancer patients (13). Hence, there is renewed enthusiasm for immune-based/T-cell therapies for cancer patients. This review chronicles efforts to enhance T-cell stimulation with agonists to the tumor necrosis factor (TNF) receptor family member OX40 for use as a therapy in cancer patients.
A group at Oxford University in the1980s led by Alan Williams produced several monoclonal antibodies to activated rat T-lymphocyte cell surface proteins, one of which was named OX40 (14). In the early 1990s, the cDNA for the OX40 antigen was cloned, sequenced, and found to be a member of the TNF receptor super family (15, 16). The initial publication showed that an OX40 antibody (Ab) could increase T-cell proliferation, particularly during the later stages of in vitro T-cell activation (14). Around the same time that the initial OX40 Ab was produced, a group in Japan described an antibody that bound a protein on human T lymphocytes infected with human T-cell lymphoma/leukemia virus-1 (HTLV-1) (17), which was later confirmed to be the ligand for OX40 (OX40L) (18, 19). There is only one known ligand for OX40, and the crystal structure of OX40 binding to OX40 ligand was recently resolved (20). The crystal structure showed that the OX40 ligand, which is a TNF family member, forms a homotrimer and that OX40 interacts at several contact points within the groove between two OX40L subunits (20). It is inferred from the crystal structure that three OX40 monomers interact with the OX40L homo-trimer.
OX40 is mainly expressed on activated T cells and is preferentially expressed on CD4+ T cells although activated CD8+ T cells also express OX40, albeit at lower levels (21). In mice, OX40 is constitutively expressed on all T-regulatory (Treg) cells (22). However in humans, OX40 expression is low or absent on peripheral Tregs, but expression is higher on human Tregs isolated from sites of inflammation (e.g. tumors) (Weinberg laboratory, unpublished observations). OX40 has also been found on polymorphonuclear cells (PMNs) and dendritic cells and can have a biologic (proinflammatory) effect in hosts where T cells are absent (23, 24). OX40L is found mainly on activated antigen-presenting cells (e.g. dendritic cells, B cells, macrophages, and endothelial cells), but can also be expressed by activated T cells (25–30). In general, the OX40L is expressed at low levels throughout the body of normal individuals where there is little inflammation but is upregulated in individuals with autoimmune manifestations (31). In particular, the majority of OX40 ligand expression in hosts with autoimmunity appears to be confined to the lesions (32). Blocking OX40/OX40L interaction in vivo tempers the clinical signs of autoimmunity and overexpression of the OX40L in transgenic mice leads to increased signs of autoimmunity as they age (32–37). It is clear that engagement of OX40 by its ligand leads to potent biologic activity and restricted expression of the OX40L appears to limit the biologic potency of this TNF receptor/ligand pair (32, 33). Based on these findings, it was hypothesized that injecting an OX40 agonist in vivo might enhance biologic activity in otherwise dormant immune settings, such as hosts with tumors or chronic infections. This approach proved to be therapeutically effective in preclinical models, confirming the potential of the OX40 protein as a therapeutic target (38, 39). The rest of this article chronicles the scientific journey as well as the collaborative efforts at the Providence Cancer Center to produce an OX40 agonist that was eventually administered to cancer patients.
Initial exposure to OX40
Investigation into experimental autoimmune encephalomyelitis (EAE) in the Lewis rat model revealed a critical role for OX40 in immune function. In the late 1980’s most studies on cytokine-driven T-helper 1 (Th1) and Th2 differentiation (40–43) relied on in vitro cultures. Performing adoptive transfer studies in the EAE model allowed for in vivo observation of the biologic function of T helper cells (myelin-specific T cells), which in this case caused hind-limb paralysis within 4–6 days after transfer. Several groups had published that Ag-activated encephalitogenic T cells were able to penetrate the blood-brain barrier, invade central nervous system (CNS) tissue, recognize their cognate Ag in situ and produce cytokines, which ultimately led to paralysis (44–46). Our group characterized the phenotype and cytokine profile of auto-Ag specific CD4+ T cells isolated from the CNS of rats with clinical signs of EAE (47, 48).
Attending the 8th International Congress of Immunology in 1992, which was being held in Budapest, Hungary, led to a fortuitous encounter. This was a very interesting time in Eastern Europe as the Berlin wall had just come down and a lot of these countries including Hungary were transitioning from communism to a democracy. As far as the meeting was concerned, the weather was extremely hot and there was no air conditioning in the meeting halls, so I only attended sessions that were circled with major interest. At a poster presentation, a student from Allan William’s lab presented the cloning of mouse form of OX40, which was expressed on CD4+ T cells. This encounter prompted the initial examination of OX40 on autoantigen-specific CD4+ T cells that invaded the CNS of rats with EAE.
Expression of OX40 in EAE
In the early 1990s the only Ab known to bind OX40 was the original monoclonal that the William’s group had produced, which only recognizes rat OX40 (14). Hence, it was a perfect fit for the rat EAE studies that we were performing and in retrospect gave us a head start on studying OX40, as most immunologists were involved with mouse or human research. Initially, we assessed OX40 expression in an EAE study where autoantigen-specific T cells were adoptively transferred into congenic hosts (49). Therefore, the myelin-specific T cells could be detected via a congenic marker within the CNS of rats with EAE as well as in other lymphoid organs (spleen and lymph node). OX40 was highly expressed on the auto-antigen-specific CD4+ T cells isolated from the CNS, but the auto-antigen-specific T cells isolated from blood and spleen expressed low levels of OX40. The highest expression of OX40 on the auto-antigen-specific T cells within the CNS was found on the day prior to disease onset, as the disease peaked the amount of OX40 decreased, and was at its lowest levels as the disease resolved (5 days after disease onset) (49). The time course of OX40 expression suggested that an Ab to OX40 might be able to detect T cells that had recently engaged their cognate Ag in vivo (in this case within the CNS) and once upregulated the T cells internalized OX40, which in turn may confer biologic activity to the T cell.
The OX40 expression data from the initial studies in the adoptive transfer EAE model were intriguing, but there were still questions regarding the biologic role of this molecule. Does OX40 expression identify the most recently activated Ag-reactive T cells in situ? Does this cell surface protein confer specific biologic function upon cell surface engagement? Both questions were of critical importance because in the majority of autoimmune diseases the auto-antigen(s) being recognized by T cells are unknown. Hence, if a cell surface antibody could recognize the cells causing autoimmune damage, these cells could be selectively targeted even without knowing the antigen that caused an autoimmune event. Also, one could potentially dampen the destruction of normal tissue by autoreactive T cells by blocking the biologic activity of OX40. To directly address whether OX40 expression marked the auto-antigen T cells within autoimmune lesions, we performed experiments in the active immunization model of EAE (active EAE). In this model, the host is injected with auto-antigen (myelin basic protein) in complete Fruend’s adjuvant (CFA) and within 12–14 days clinical signs of autoimmunity develops (50). Hence, in this model, there is no prior knowledge of the auto-antigen-specific T cells that home to the CNS and causes the clinical signs of disease, as in the adoptive transfer studies. Close to the time these experiments were performed, it was shown by others that the T cells involved with inducing myelin basic protein (MBP)-specific Lewis rat EAE preferentially expressed the Vβ8.2 T-cell receptor. These autoreactive T-cell receptors were cloned and sequenced and three CDR3 MBP-binding motifs were identified as Asp-Ser, X-Ser, and Arg-Gly (51). Therefore, we isolated the Vβ8.2+ T cells from the CNS of rats during the initial clinical signs of active EAE. They were sorted directly ex vivo into OX40+ and OX40− fractions, and their T-cell receptors were sequenced. It was found that 16/17 of the sequences from the OX40+ fraction expressed one of these myelin-binding motifs, while only 5/17 TCRs from the OX40− fraction expressed these motifs (52). These data strongly suggested that the OX40+ T cells were being activated by their cognate Ag within the CNS and subsequently upregulated OX40. Therefore, it appeared that OX40 expression could be used as a surrogate marker to detect the auto-antigen-specific T cells from a site of inflammation. This finding led to experiments showing that selective depletion of OX40+ T cells in rats with EAE significantly decreased the number of auto-antigen-specific T cells within the CNS and diminished clinical signs of disease (53).
It was next ascertained whether the biologic activity of OX40 was important for eliciting the clinical signs of autoimmunity in EAE. First, OX40 ligand expression was assessed during ongoing signs of autoimmunity within the CNS. The OX40L was expressed on macrophages within the CNS as well as on the inflamed endothelial cells during clinical episodes of disease (25, 26). Similar to OX40 expression, the OX40L was selectively upregulated at the onset and peak of disease on CNS macrophages and downregulated as the disease resolved (25). CNS macrophages isolated during clinical signs of EAE had previously been used as potent Ag-presenting cells (54), and therefore, it was tested whether OX40L blockade could inhibit the activation of T cells by CNS isolated macrophages. Blocking OX40 and OX40L interaction within the CNS macrophage/T-cell cultures led to a significant decrease in Ag-induced T-cell proliferation (25). Therefore, in vivo OX40L blockade, using an OX40:Ig fusion protein, was tested in the EAE model. The OX40:Ig fusion was administered on the first day of disease onset and on two consecutive days thereafter. Blockade of OX40/OX40L in vivo dampened clinical signs of disease almost immediately (24 h after initial dose was administered) and appeared to have an anti-inflammatory effect for a 10 day period following the last injection (25). However, administration of the OX40:Ig fusion protein during the acute phase of disease had no impact on the development of subsequent relapses, suggesting that prevention of OX40 engagement did not tolerize an ongoing T-cell response but blocked their function while the OX40:Ig protein was in circulation. This form of OX40-specific inhibition was also effective if administered during relapses, but soon after the OX40:Ig injections were stopped, the autoimmune disease returned (25).
The EAE studies showed that the majority of the T cells expressing OX40 within the inflammatory lesions were auto-antigen-specific T cells and that OX40 was important for the biologic activity of these CD4+ T cells (55). Therefore therapeutic manipulation of the OX40/OX40L pathway could most likely be used to block autoimmunity without unwanted peripheral side effects. Studies performed in other autoimmune models (e.g. inflammatory bowel disease, rheumatoid arthritis, and asthma) confirmed these results (31, 36), and currently there is an ongoing clinical trial being performed to block OX40 signaling in patients with asthma. The general immune principles learned from the OX40-based autoimmune studies were always kept in mind as our research focus changed to experiments involving tumor immunology.
Similar to the autoimmunity studies, OX40 expression was confined to T cells isolated from the tumor microenvironment in cancer patient samples (56). The OX40+ T cells within these biopsies might represent recently activated tumor Ag-specific T cells, suggesting a new twist to the OX40 story that complemented the research mission at the Providence Cancer Center under the leadership of Dr. Walter Urba. Dr. Urba’s unique vision in forming this cancer center was that he strived for an academic environment in a community hospital where clinicians and scientists would work closely together to translate ideas from the ‘bench to the bedside’. Working on the EAE model allowed us to observe T cells inducing lesions and destroying CNS tissue. Therefore, our idea was to commandeer T cells to destroy cancerous tissue by reversing the principles used in reducing inflammation in EAE. Hence, the lessons learned from the OX40 studies in EAE were applied to the tumor immunology setting.
OX40 agonists augment tumor-specific T-cell responses
At the time I joined the Providence Cancer Center, there were two tumor immunology researchers with experience in the field. In particular, Dr. Bernard Fox had been trained in Dr. Steven Rosenberg’s lab at the Surgery Branch of the National Cancer Institute (NCI) and had multiple years of experience with tumor models. Dr. Fox helped our group to set up tumor models and trouble shoot problems, which ultimately contributed to translating this work into cancer patients.
There were some reports at meetings that agonist antibodies to OX40 were able to elicit potent costimulatory signals to T cells, and when injected in vivo they exacerbated clinical signs of autoimmunity (EAE). Hence, we started to assess the ability of OX40 agonists to stimulate and enhance tumor Ag-specific responses in cancer-bearing hosts. In particular, two different strategies were used: one that attempted to increase the Ag-presentation capacity of tumors through co-transfection of MHC class II and OX40L into tumors, and the other was to deliver soluble forms of OX40 agonists into tumor-bearing mice. Transfection of MHC class II and OX40L increased their expression in B16 melanoma cells, and these cells were used as irradiated vaccines prior to tumor challenge. It was hypothesized that dual expression of these two proteins in a cellular vaccine would increase the tumor’s capacity to elicit T-cell responses. This was indeed the case (Weinberg laboratory, unpublished observation); however, it was clear that this technique would be cumbersome to ultimately translate into cancer patients.
The use of a soluble OX40 agonist was an attractive approach because of the ease of administration and similar methods had met with success in mouse tumor models with anti-CTLA-4 and a 4-1BB agonist (57, 58). Leping Chen had recently published that a soluble 4-1BB agonist led to tumor regression (57). Based on discussions with Dr. Chen and emerging data from Jim Allison’s lab using anti-CTLA-4 (58, 59), a dose of 100 μg of OX40L:Ig fusion protein would be delivered 3 and 7 days after tumor inoculation. Dr Fox suggested starting with a moderately immunogenic sarcoma cell line, MCA 303, which was administered subcutaneously followed by the OX40 agonist therapy. We had previously only tested the OX40L:Ig fusion protein in a few in vitro assays. Hence, we were somewhat skeptical that addition of an OX40 agonist by itself would be effective to enhance anti-tumor immunity eventually leading to tumor destruction. The initial experiment evaluated three tumor-bearing mice receiving the OX40L:Ig fusion protein and three tumor-bearing mice receiving an equivalent amount of saline. Surprisingly, all of the mice receiving the OX40 agonist rejected the tumor, whereas all of the control mice succumbed to tumor growth within 40 days (39). A dose titration of the OX40L:Ig agent in tumor-bearing mice revealed that the 25 μg and 50 μg/dose had no affect on tumor growth; however, therapeutic efficacy was observed at 100 μg and plateaued at 250 μg (39). Mice cured by OX40 agonist treatment were resistant to rechallenge with the initial inoculating tumor (39). However, if the mice cured with the inoculating tumor were rechallenged with a tumor of different histologic origin they succumbed to the second tumor challenge. These data suggested that the OX40 agonist was augmenting a T-cell memory response that was specific for tumor Ags associated with the initial tumor inoculum, which is a hallmark of tumor-specific T-cell memory.
The preclinical data suggested that rejection of these tumors was T-cell mediated, and this hypothesis was tested by depletion of either CD4+ or CD8+ T cells prior to anti-OX40 treatment. Depletion of either T-cell subset in tumor-bearing mice completely abrogated the therapeutic efficacy (60). Also OX40+ T cells isolated from tumor-draining lymph nodes were enriched for tumor reactivity when compared to OX40– T cells (39), which was similar to what was observed in the EAE models described earlier. Finally, adoptive transfer of CD4+ T cells from mice cured with OX40 agonist treatment into naive mice were able to protect these hosts from tumor challenge (39). These data show that both CD4+ and CD8+ T cells are involved with the therapeutic efficacy of OX40 agonists, and there appeared to be a robust memory T-cell response to the tumor within mice that were cured with OX40 agonists.
To further understand the mechanisms involved with the OX40 agonist treatment, attention was focused to CD4+ and CD8+ T-cell immunization models, where in vivo Ag injections were followed by OX40 agonist stimulation. The T cells specific to the immunogen were then followed and/or isolated after Ag stimulation for in-depth analyses. Initially, studies were performed in the superantigen (SEA) T-cell stimulation model, which is notorious for quick activation and proliferation of superantigen-specific T cells followed by rapid deletion of the cells in vivo. Anti-OX40 administration enhanced proliferation and survival of the superantigen-stimulated T cells and the addition of LPS greatly augmented their survival (61, 62). Memory T-cell survival was next evaluated in a soluble Ag (ovalbumin) model, where the Ag was injected into mice harboring ova-specific TCR transgenic T cells (D011.10 model). This is another model where the Ag-specific CD4+ T cells initially proliferate and is then followed by rapid deletion. Addition of anti-OX40 greatly increased the proliferation, effector function, and survival of ova-specific T cells in vivo ultimately leading to increased long-lived memory. Again, it was found that the addition of the TLR agonist lipopolysaccharide (LPS) increased the number of surviving memory T cells when given in combination with anti-OX40 (62). Anti-OX40 stimulation was also compared to anti-CTLA-4 blockade in the D011 CD4+ T-cell model. While both antibodies greatly increased CD4+ T-cell proliferation in vivo, only anti-OX40 led to increased survival (63, 64). It is clear that both anti-CTLA-4 and anti-OX40 have potent immune enhancing properties that augment anti-tumor immunity (39, 58); however, these data suggest they have distinct modes of action and therefore may not necessarily have overlapping function. This was clearly the case in mouse models of cancer where anti-OX40 therapy was effective in some models where anti-CTLA-4 had little to no effect and vice versa (39, 58). Hence, as these immune-enhancing antibodies are evaluated in human clinical trials there will likely be several used to augment tumor immunity (e.g. anti-OX40, anti-CD40, anti-4-1BB, anti-CTLA-4, anti-PD-1, and anti-CD27) (7), and most likely combinations of these Abs will be tested in future clinical trials.
The main biologic function of OX40 was initially thought to involve CD4+ T-cell responses; however, OX40 stimulation clearly has direct and indirect effects on augmenting CD8+ T-cell function (65–73). Studies in the OT1 CD8+ T-cell model showed OX40 agonists could greatly augment proliferation, effector function, and survival of CD8+ T cells (65). Michael Croft’s group (66) crossed the OT1 TCR transgenic mice with OX40 knockout mice and found that the OX40-deficient CD8+ T cells did not function as well as their wildtype counterparts. Thus showing that OX40 expressed on CD8+ T cells was critical for their in vivo function. We also tested the ability of OT1/OX40 ko T cells to respond to OX40 agonists stimulation in both basic immunology and tumor immunology models. It was found that OX40 agonist augmentation of CD8+ T-cell function was both CD4 independent and dependent (71, 74, 75). Hence, there is clear evidence that OX40 agonists enhance both CD4+ and CD8+ T-cell function in both basic and tumor immunology models, and therefore within the OX40 agonist clinical, trial both CD4+ and CD8+ T-cell function were thoroughly investigated.
A patient advocate changes the course of OX40 research
Four articles described the initial effects of OX40 agonist stimulation in basic T-cell and tumor immunology models (39, 60, 62, 76), and we focused on the molecular mechanisms involved with the enhanced T-cell function. The initial work was published in 2000 and in the summer of the same year the Providence Cancer Center had a retreat at the Oregon Coast. A number of speakers were invited that were involved with the initial OX40 agonist work as well as a few prominent tumor immunologists, all of whom gave talks followed by round table discussions. Dr. Fox organized the meeting, and one of Dr. Urba’s cancer patients, Judy Hartman, who was a staunch supporter of research, donated her beach house for the occasion. Judy was a lawyer by training and had an insatiable curiosity. When she was diagnosed with cancer, she researched the disease and started to grasp the concept that research could make a difference in changing the outcome of the disease for many cancer patients. In particular, Judy sat on the Department of Defense Breast Cancer study section as a patient advocate, where she would read scientific grants and give scores based on the work’s ability to help patients with breast cancer. Therefore, Judy quickly learned of the investigations at the Providence Cancer Center and had become particularly interested in the OX40 agonist project. Judy was also a member of the Providence Cancer Center Foundation board and regularly joined in OX40 discussions with us in the hallway. During the OX40 agonist session, we discussed our recent findings showing that OX40 agonists were effective in several different preclinical mouse tumor models including sarcoma, breast carcinoma, melanoma, colon carcinoma, and renal cancer. The presentation concluded with future directions, none of which included translating these findings to patients. At the end of the talk, Judy raised her hand and asked why OX40-specific agents were not being developed for future clinical trials. There were several clinical colleagues in the audience including Dr. Urba and the group looked at each other and nodded their heads in approval at Judy’s suggestion. After Judy’s planting the seed, we discussed ways that could make this idea become a reality for cancer patient clinical trials.
Translating a scientific idea is easier said than done
There were numerous discussions at the Providence Cancer Center to develop a plan to proceed with clinical testing of an OX40 agonist. The group decided to produce a mouse monoclonal Ab to human OX40. At the time of this decision, human Ab libraries were not widely available to research scientists, and the first humanized Abs were just being approved for patient use. Therefore, the group at Providence Cancer Center pursued a ‘proof of concept’ clinical trial with a mouse anti-human OX40 Ab, and if the results were favorable, then a humanized version could be produced for future trials. While we had lots of experience treating mice and rats with monoclonal Abs, we had no experience in making a therapeutic agent suitable for human clinical trials. Nicholas Morris, a protein chemist, joined our group to address all the needed assays and issues with the US Food and Drug Administration (FDA). In retrospect, this was the single most important hire that allowed the group at the Providence Cancer Center to be successful in gaining approval from the FDA for the phase I trial with a murine anti-human OX40 Ab.
Initially, the production of several mouse monoclonal antibodies to human OX40 was contracted out and the four antibodies were selected for detailed analysis. Dr. Morris had perfected the in vitro OX40-specific human T-cell proliferation assay using the commercially available anti-OX40 Ab L106. The T-cell proliferation assay, an OX40-specific enzyme-linked immunosorbent assay, and a T-cell binding assay were all used to characterize this panel of Abs. The hybridoma clone 9B12 was picked based on the antibody’s ability to bind OX40, stimulate T-cell proliferation, and its high level of Ab production. At least $1–1.5 million was needed to complete the multitude of tasks just to start treating patients (Fig. 2), and the chances of the National Institutes of Health funding the project at this early stage without good manufacturing practice (GMP) material were slim. Hence, Dr. Urba and his team at the Providence Medical Foundation set out to raise the money via charitable donations through the Portland, Oregon community. This did seem like an arduous task at the time; however, other supporters of the Cancer Center realized just like Judy Hartman did that this project had real potential not just to advance science but also to benefit patients. This fund raising effort was quite a bit different than donations received to the general cancer center fund, and both Dr. Urba and Dr. Weinberg gave OX40-targeted presentations to interested donors. Within a 12-month period, the funds were raised and the goal now was to take the necessary steps to proceed towards cancer patient clinical trials.
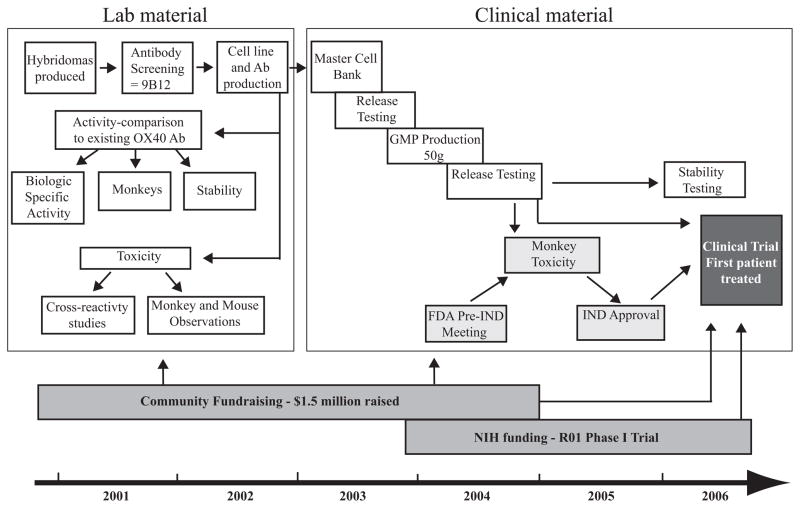
Fig. 2. Timeline for the development of a clinical grade OX40 agonist antibody.
The antibody development steps are depicted in white boxes, regulatory steps in light gray boxes, and funding in dark grey boxes. Characterization of the murine anti-human OX40 agonist monoclonal antibody 9B12 included three separate in vitro potency assays to measure specificity, specific activity, and stability. Initial toxicity and cross-reactivity studies with laboratory scale material indicated that there was little toxicity or off-target binding of the 9B12 Ab. After GMP master cell bank and antibody production had concluded, the FDA mandated that a GLP monkey toxicity study be performed prior to clinical testing. Once the FDA reviewed the monkey toxicity data, the IND was submitted, and the first patient was treated in March 2006. Community fundraising helped support all aspects of the project, and NIH funding supported the phase I clinical trial.
It is ambitious for any academic cancer center to take on the many regulatory and developmental tasks required to test a new therapeutic agent in patients with cancer. This work is generally performed by pharmaceutical companies working with clinicians at university hospitals that have an interest in cancer research. To perform drug development at a community hospital like Providence Medical Center was somewhat unprecedented and took a team effort and a bit of luck along the way. The group’s experience in producing clinical grade protein was extremely limited. Contract manufacturing companies for good laboratory practice (GLP) assays and GMP production of the Ab was used to supply expertise that was not available in house. During the production, process aliquots of Abs purified from qualifying runs were assessed in the OX40-specific assays described earlier and were assessed through the final GMP steps to ensure there was no loss in bioactivity or binding affinity to OX40. After the final purification steps, 52 grams of GMP grade antibody was vialed and ready for testing in patients with cancer.
After the GMP production was complete, the FDA was contacted for a pre-investigational new drug (IND) meeting to discuss the issues pertaining to moving forward with a clinical trial. Before the call, the group performed monkey studies with another anti-OX40 Ab (L106), and limited toxicity was observed. Therefore, the Providence Cancer Center group proposed to the FDA that they should be able to proceed directly with cancer patient clinical trials. However, after multiple discussions with the FDA toxicology group, it was clear that a toxicology study involving 32 monkeys would have to be performed using the OX40 Ab that was planned for patient use, prior to initiation of a phase I clinical study. The monkey toxicology study was performed with three doses of the GMP grade anti-OX40 Ab, 0.4 mg/kg, 2.0 mg/kg, and 10 mg/kg. Each dose was given three times during a weekly course (Monday, Wednesday, and Friday), and one group of monkeys was sacrificed seven days after the start and the other group was sacrificed on day 28 (77). There were no signs of overt toxicity in any of the monkeys treated. The analysis included measurements for body weight, electrocardiograms, urinalysis, coagulation, serum chemistries, and lymphocyte counts. At all doses, the peripheral blood lymphocyte counts decreased 8 days after anti-OX40 administration. The lymphocyte counts returned to baseline or greater by 15 days after anti-OX40 treatment and was no different than pretreatment levels by day 28 (77). Thirty-nine percent of the monkeys had enlarged spleens 8 days after anti-OX40 treatment, which mostly resolved when the 28 day time point was assessed. Several of the monkeys also showed increased lymph node size especially in the mesentery. Histologic evaluation of the enlarged spleen and lymph nodes within the anti-OX40 treated groups showed normal architecture with greatly increased numbers of B and T-cell blasts associated with mitotic figures. One surprising finding from this study was that the enlarged lymph nodes/spleens and the decrease in lymphocyte counts were observed at all dose levels of anti-OX40 treatment, which ultimately influenced the doses that were chosen for the phase I clinical trial.
Five years later cancer patients are treated
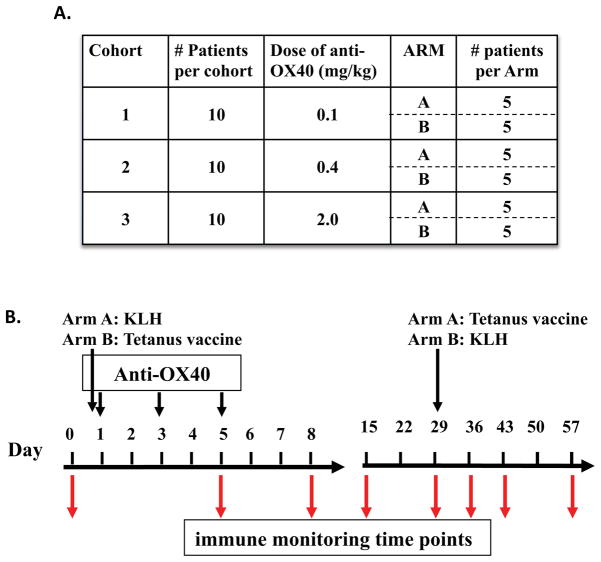
Five years had elapsed by the time the entire process, from hybridoma development to the final FDA discussions and IND submission was finished. While none of members of the OX40 team at the Providence Cancer Center knew exactly just how long it would take from bench to bedside, their determination had persevered through the long journey. The monkey studies indicated that every dose of anti-OX40 tested had biologic activity; hence, the FDA advised the group to start the clinical trial at a four-fold lower dose than the lowest dose administered to monkeys. The phase I trial was designed to test three different doses of anti-OX40: 0.1, 0.4, and 2.0 mg/kg. The optimal dose observed for tumor shrinkage in mice was between 1–2.0 mg/kg. While there is not always a direct correlation between an effective mouse and human dose, the preclinical data and the antibody supply suggested that these were appropriate doses to evaluate. Dr. Brendan Curti, who has extensive experience with immunotherapy clinical trials both at Providence and the NCI (78, 79), led this clinical study. The clinical objectives of the trial were to (i) determine the maximal tolerated dose in patients with advanced malignancy (all solid tumor types), (ii) measure pharmocokinetics of anti-OX40, (iii) determine the most immunologically active dose of anti-OX40, and (iv) monitor for tumor regression. A non-conventional phase I trial was designed where 10 patients per cohort were treated to produce more robust statistical analysis of OX40-specific immune modulation. The patients at each dose level were randomized to receive both tetanus or keyhole limpet hemocyanin (KLH) at the start of anti-OX40 treatment, and then 28 days later they would receive the opposite Ag (Fig. 3). Ab and T-cell responses to these surrogate Ags were followed to determine whether anti-OX40 could indeed enhance primary and secondary immune responses to well-defined Ags, as had been observed in animal models (63, 65). Serial blood draws were obtained from the patients before and on days 5, 8, 14, 28, 35, 42, and 56 after anti-OX40 treatment. Diagnostic imaging of metastatic lesions was performed prior to therapy and on days 28 and 56 post-treatment to determine whether tumor regression had occurred.
Fig. 3. OX40 agonist clinical trial scheme.
The trial design included 3 dose cohorts with 10 patients treated per dose. As shown in (A), equal numbers of patients within each cohort were randomized into Arm A or B. As shown in (B), patients in Arm A received the reporter antigen KLH on the same day as anti-OX40 administration and tetanus 29 days later, while patients in Arm B received tetanus on the same day as anti-OX40 administration and KLH 29 days later. Anti-OX40 was administered in three separate doses on days 1, 3, and 5, and peripheral blood lymphocytes were isolated at the indicated time points for immune monitoring.
Once the IND application was submitted to the FDA there was a 30 day period that the FDA had to respond or change the protocol. After the final IND application was submitted and just prior to treating the first patient in March 2006, the agonist anti-CD28 Ab developed by Tegenero was administered to six normal volunteers. The infusion was accompanied by a rapid onset of severe life-threatening toxicity requiring admission to the intensive care unit (80). The pathogenesis of this extreme toxicity was believed to be a ‘cytokine storm’ secondary to the activation of large numbers of T cells. Since CD28 and OX40 are both costimulatory molecules on T cells, the Providence group was concerned that the OX40 Ab could also cause a similar toxicity event within these patients. However, a major difference between CD28 and OX40 is that CD28 is expressed at high levels all the time on most T cells in humans, whereas OX40 has very limited expression. The majority of high expressing cells are typically found on recently activated T cells at sites of inflammation. The hope was that there would be far fewer OX40+ T cells to activate; hence despite the concern following the Tegenero experience, the first patient received anti-OX40 in late March, 2006. No acute toxicities were observed in any of the anti-OX40-treated patients, therefore the fear that anti-OX40 might cause events similar to the Tegenero clinical trial was unfounded, and the clinical work continued.
In total, 30 patients were treated in the phase I trial, and the last patient was enrolled in December of 2009. In general, the drug was well tolerated, and the trial did not reach a maximum tolerated dose. Another immune-enhancing Ab, anti-CTLA, which just gained FDA approval for use in advanced melanoma causes immune-mediated diarrhea, rash, and endocrinopathies. These severe toxicities were not observed with anti-OX40, and this may be due to the relatively short-half life of the mouse Ab that was administered. Recently, it has been shown that anti-OX40 stimulation within the gut can promote Treg expansion, which in turn suppressed colitis (81). Therefore the dual function of OX40 agonists to increase T-cell effector function as well as expanding Tregs in certain areas of the body (81) may also have limited the autoimmune side effects in patients treated with OX40 agonists.
The patients treated with the anti-OX40 Ab had failed conventional therapies; therefore, it was remarkable that some of them showed signs of tumor shrinkage (Curti et al., manuscript submitted), especially since only one round of treatment was administered. The early trials with anti-CTLA-4, anti-PD-1, and anti-4-1BB all showed similar signs of clinical activity, and these immune activating Abs have gone on to phase II trials and beyond. The results from this trial have spawned further clinical testing with OX40 agonists, which are described in greater detail later in the review.
Looking for increased immune activation after anti-OX40 treatment
Preclinical studies had showed that OX40 agonists increased T-cell proliferation and memory T-cell survival in vivo (61). Therefore, a major objective of the phase I trial was to determine whether T-cell responses to tetanus, KLH, or tumor Ags were increased after anti-OX40 administration, and whether there were increases in T-cell proliferation within the peripheral blood of patients. Fig. 3 shows the trial design where 5 patients in each cohort received KLH on day 0 and tetanus on day 28 (Arm A) or tetanus on day 0 and KLH on day 28 (Arm B) (Fig. 3A). The prediction was that the patients receiving KLH early with anti-OX40 would have higher Ab and T-cell responses to KLH than patients receiving KLH 28 days after anti-OX40 (same with tetanus). In general, this trend held true and the specific details have recently been submitted for publication (Curti et al., manuscript submitted).
Based on pre-clinical tumor models using OX40 agonists (75, 82), it was hypothesized that anti-OX40 would increase proliferation, activation and survival of T cells, some of which would be specific for the patient’s tumor cells. Ideally, tumor-specific immune assays would have been performed on all patients. However, tumor cell lines from three melanoma patients within the trial were generated, and therefore tumor-specific immune responses pre- and post-anti-OX40 administration could be assessed. In general, we observed increases in tumor-specific immune responses after patients were treated with anti-OX40 (Curti et al., manuscript submitted)
While the tumor-specific immune responses were upregulated in most individuals tested, only a few patients of the 30 treated could be assessed. T-cell activation after anti-OX40 administration was measured, so that all the patients’ samples could be examined. There had been a number of publications from Louis Picker’s lab (83–85) regarding T-cell proliferation that could be detected by Ki-67 expression (via flow cytometry) in the peripheral blood of simian immunodeficiency virus-infected and naive monkeys. The general premise underlying these studies was that T cells recognize cognate Ag within the lymphoid organs (e.g. LNs and spleen), and once activated, they are shunted to the peripheral blood where their proliferation can be detected (85). At steady state, this is occurring all the time within the body and in humans between 0.5 – 6.0% of peripheral blood CD4+ and CD8+ T cells are Ki-67+ (Weinberg laboratory, manuscript submitted). Therefore, it was tested whether anti-OX40 delivered to cancer patients would increase the levels of Ki-67 expression in peripheral blood T-cell subsets. A 10-color flow cytometry panel was developed, which included staining for CD3, CD4, CD8, CD25, FoxP3, CD28, CD95, CD127, CCR7, and Ki-67, to assess proliferation in T-cell subsets. Previous studies in monkeys and humans have shown that CD95+ (Fas) T cells represent the memory/activated population and that the CD95 negative population represents naive T cells. The Picker group showed that the majority of proliferating/Ki-67+ T cells are within the CD95+ subset (84); therefore, CD95+ T cells were gated on when assessing the OX40 patient samples. The FoxP3 marker was added to distinguish regulatory from effector CD4+ T cells. Ki-67 was assessed at several times points following anti-OX40 administration. There were increases in both CD4+ and CD8+ T-cell proliferation (Ki-67 expression) following anti-OX40 treatment (Curti et al., manuscript submitted). The Ki-67 assay has been used for more recent monkey studies and ongoing human trials to assess activity of OX40 agonists, as this technique is an efficient and cost effective way to assay OX40-specific function directly ex vivo.
Ongoing and future clinical trials
Having completed the phase I trial, the Providence group has a two-pronged strategy for future clinical trials: (i) produce humanized OX40 agonists to allow repeat dosing in cancer patients, and (ii) perform pilot studies to test combination therapies with the murine OX40 Ab in specific malignancies (e.g. prostate and breast cancer). This approach should allow a greater understanding of which combinations and malignancies may derive the most benefit, so that when the humanized OX40 agents are available for clinical testing, the knowledge gained from the murine OX40 Ab clinical trials will be used to proceed in a setting most likely to succeed.
Clinical trials to pursue the murine OX40 Ab in combination with chemotherapy and radiation have been recently funded by the Prostate Cancer Foundation and the Safeway Foundation. Both chemotherapy and radiation synergize with anti-OX40 in mouse models (86). It is postulated that tumor breakdown induced by chemotherapy and/or radiation leads to productive tumor Ag presentation and subsequent immune/T-cell activation. Hence, providing a bit of ‘self’ vaccination to one’s own tumor prior to enhancing T-cell function through OX40 stimulation should lead to increased anti-tumor efficacy. Alan Houghton and Jedd Wolchock’s group (82) have recently published that cyclophosphamide greatly enhanced anti-OX40 immunotherapy in several different mouse tumor models. This study was of particular interest, because cyclophosphamide treatment not only has a direct cytotoxic effect against tumors but also can ‘reset’ the immune system by deleting Treg cells, which can lead to enhanced immune function (87). Based on this preclinical data, a phase IB trial was designed to investigate combining anti-OX40 with increasing doses of cyclophosphamide and focal external beam radiation to bone metastatic sites in prostate cancer patients. Immune monitoring of this trial includes Ki-67/T-cell evaluation on all patients, similar to what was performed in the phase I trial. Patients will also be assessed for response by imaging, prostate-specific antigen test, and circulating tumor cells. Five patients at two different doses of cyclophosphamide have been treated thus far, and toxicity has been minimal with some intriguing T-cell proliferation data.
The breast cancer trial is spearheaded by a radiation oncologist and immunologist at Providence, Dr. Marka Crittenden, who along with Dr. Curti and other colleagues have shown that high dose fractional radiation (SBRT) in combination with IL-2 treatment has potent therapeutic synergy in melanoma patients (manuscript submitted). The SBRT/IL-2 trial was based on the premise that irradiation to one or two visceral metastases followed by high-dose IL-2 would improve a systemic response. Based on the therapeutic synergy observed with anti-OX40 and radiation in preclinical models and the fact the anti-OX40 appears to have less side effects than IL-2 treatment, we developed a clinical trial that mirrors the SBRT/IL-2 study in breast cancer with SBRT/anti-OX40. End points of the study will include safety, immunologic activation by measuring Ki-67 expression in T cells, examination of markers related to tumor inflammation, and tumor regression within the unirradiated lesions. The breast cancer trial should start accruing patients during the last quarter of 2011.
The clinical and immunologic activity observed in the phase I trial was sufficient to move forward with second-generation OX40 agonists. It is anticipated that multiple dosing regimens may increase the clinical efficacy thus there is a need for an OX40 agonist that can be given serially over time. Production has begun on humanized OX40 agonists that likely could be given multiple times without eliciting neutralizing Ab responses. We have produced a fully human OX40L:Ig fusion protein that binds to OX40 with greater affinity than the murine OX40 Ab and has a shorter half-life (88). The OX40L:Ig fusion protein has been tested in monkeys and has potent T-cell stimulatory properties (unpublished data). The OX40L:Ig fusion protein will be the next OX40 agonist tested in cancer patients, and the Providence group is in the final stages of cell line development prior to GMP protein production. The mouse anti-OX40 Ab that was used in the phase I clinical trial described earlier is in the process of being humanized and would most likely have a longer half-life than the OX40L:Ig protein. The reason this group has chosen to move forward with two different human OX40 agonists is that they can test a long-lived versus a short-lived OX40 agonist, which may have implication regarding toxicity and/or biologic and clinical activity. Ultimately, as has been learned from the phase I trial, taking a drug to human clinical trials can be somewhat empiric, hence it will not be known which approach is better until it is actually tested in patients.
Future directions – combined immunotherapies
There is a general consensus among tumor immunologists that achieving a Th1 cytokine pattern is the most efficient way to immunologically attack cancer (89). Future directions will attempt to make all the metastatic deposits within a cancer patient immunologically similar, preferentially to promote a Th1 immune response. It is clear from recent studies that OX40 agonists can drive all T-helper cell lineages, including Tregs (90). In particular, the interferon-γ (IFN-γ) to transforming growth factor-β (TGF-β) ratio is critical to predicting the T-helper cell lineage that is expanded upon OX40 agonist stimulation (90). If IFN-γ is higher than TGF-β, then Th1 cells emerge; however, if TGF-β is higher than IFN-γ then Tregs cells emerge (90). Therefore in preclinical tumor models where anti-OX40 alone shows little to no efficacy, we found that the addition of IL-12 (Th1 skewing cytokine) or blockade of TGF-β signaling synergizes with OX40 agonist therapy (91, authors’ unpublished observations). Ultimately, changing the cytokine balance in patients receiving OX40 agonists may help achieve the goal of increased therapeutic efficacy at all metastatic sites in stage IV cancer patients and these ideas will be pursued in future clinical trials.
There are a number of T-cell activating Abs and fusion proteins that are being pursued clinically. In particular, ipilimumab, which blocks negative signals to T cells via the CTLA-4 pathway, was recently approved for use in melanoma patients (12). Anti-programmed death-1 (anti-PD-1) antibodies also block negative T-cell signals and have shown therapeutic promise in both phase I and II clinical trials. There are also a number of T-cell activating Abs (agonist) to other TNF-receptor family members (CD27, 4-1BB, GITR) that have shown therapeutic promise in mouse tumor models, and an anti-4-1BB antibody has been translated to the clinic (7). Different combinations of the agonist and checkpoint blockade Abs have been tested in preclinical tumor and chronic viral models with added/synergistic results (67, 92–97). With several of these Abs already tested clinically, it is likely that combinations of these T-cell activators will elicit more potent anti-tumor activity in cancer patients and is probably the future direction for this new class of immune activators.
Conclusions
Some of the events that led this research to enter into clinical trials were driven by seemingly random events, but ultimately individuals made choices that allowed the research to be translated to patients. Our goals revolved around the basic immune parameters and performing properly controlled studies that lead to strong conclusions. In the last 20 years, autoimmune, tumor immunology, and basic immunology models have been used to understand T-cell function. The ultimate outcome of this research may benefit patients with cancer and/or autoimmunity. However, the experiments were not directly designed to cure disease but to have a strong understanding of the disease process so that the resulting data might be used to intervene with a particular disease. The research described herein will hopefully provide a productive pathway for future immunotherapy researchers to translate their ideas into clinical trials.
Once the research group at the Providence Cancer Center had made the commitment to take OX40 agonists to the clinic, they had to change from scientific discovery mode to managing a production process that conformed to FDA guidelines. The clinicians at this cancer center had experience with trial design and writing FDA protocols, hence the capabilities were in place and they were able to manage the production/manufacturing process. It took a long time (5 years) to perform the needed steps to get the OX40 Ab to patients, but once the trial had begun, the group was able to scientifically evaluate the clinical samples. Prior to this work, the Weinberg lab had performed very few scientific evaluations on human clinical trial samples. While mouse work allows for tightly controlled experimentation, human analyses are fraught with issues of running controlled experiments and how to best design studies to obtain properly powered results. Within the OX40 agonist clinical trial, the traditional phase I trial design was abandoned, and 10 patients were treated per cohort, which ultimately allowed for statistically significant results. Control samples were obtained from normal donors immunized with tetanus, which led to sound conclusions based on OX40 activity. The mindset for performing good science in humans is clearly different than mouse studies, and the group at the Providence Cancer Center is dedicated to a greater understanding of the results associated human trials so that their future immunotherapy trials will provide relevant information to the scientific community.
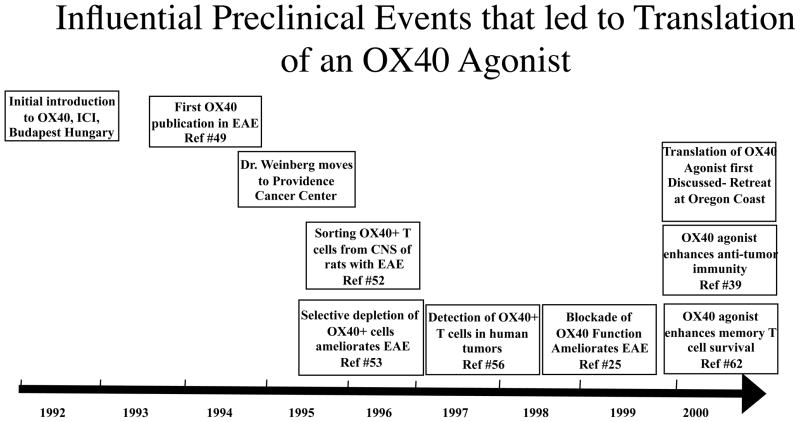
Fig. 1. Timeline of influential preclinical events that led to the translation of an OX40 agonist.
This figure chronicles the important preclinical events and publications that led to the translation of an OX40 agonist. The manuscripts highlighted within the boxes were the critical advances that allowed the project to advance towards clinical trials in cancer patients. These preclinical observations were made in autoimmune, basic, and tumor immunology models and ultimately led to the decision to move forward with the steps outlined in Figs 2 and 3.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med. 365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lotze MT, Matory YL, Rayner AA, Ettinghausen SE, Vetto JT, Seipp CA, Rosenberg SA. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer. 1986;58:2764–2772. doi: 10.1002/1097-0142(19861215)58:12<2764::aid-cncr2820581235>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Lotze MT, Chang AE, Seipp CA, Simpson C, Vetto JT, Rosenberg SA. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986;256:3117–3124. [PubMed] [Google Scholar]
- 5.Gulley JL, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol. 18:e150–157. doi: 10.3747/co.v18i3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuti M, Bellati F, Visconti V, Napoletano C, Domenici L, Caccetta J, Zizzari IG, Ruscito I, Rahimi H, Benedetti-Panici P, Rughetti A. Immune effects of trastuzumab. J Cancer. 2:317–323. doi: 10.7150/jca.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 8.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1:949–964. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner MK, Heslop HE. Adoptive T cell therapy of cancer. Curr Opin Immunol. 22:251–257. doi: 10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–527. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 11.Forero A, Lobuglio AF. History of antibody therapy for non-Hodgkin’s lymphoma. Semin Oncol. 2003;30:1–5. doi: 10.1053/j.seminoncol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia JA. Sipuleucel-T in patients with metastatic castration-resistant prostate cancer: an insight for oncologists. Ther Adv Med Oncol. 3:101–108. doi: 10.1177/1758834010397692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 15.Mallett S, Barclay AN. A new superfamily of cell surface proteins related to the nerve growth factor receptor. Immunol Today. 1991;12:220–223. doi: 10.1016/0167-5699(91)90033-P. [DOI] [PubMed] [Google Scholar]
- 16.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. Embo J. 1990;9:1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka Y, Inoi T, Tozawa H, Yamamoto N, Hinuma Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I (HTLV-I) International Journal of Cancer. 1985;36:549–555. doi: 10.1002/ijc.2910360506. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey WR, Fagnoni FF, Harara MA, Buck D, Engleman EG. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–762. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baum PR, Gayle RB, 3rd, Ramsdell F, Srinivasan S, Sorensen RA, Watson ML, Seldin MF, Baker E, Sutherland GR, Clifford KN, et al. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. Embo J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Compaan DM, Hymowitz SG. The crystal structure of the costimulatory OX40-OX40L complex. Structure. 2006;14:1321–1330. doi: 10.1016/j.str.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 23.Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol. 185:4856–4862. doi: 10.4049/jimmunol.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann R, Yousefi S, Simon D, Russmann S, Mueller C, Simon HU. Functional expression of CD134 by neutrophils. Eur J Immunol. 2004;34:2268–2275. doi: 10.1002/eji.200424863. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–1826. [PubMed] [Google Scholar]
- 26.Nohara C, Akiba H, Nakajima A, Inoue A, Koh CS, Ohshima H, Yagita H, Mizuno Y, Okumura K. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol. 2001;166:2108–2115. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- 27.Chen AI, McAdam AJ, Buhlmann JE, Scott S, Lupher ML, Jr, Greenfield EA, Baum PR, Fanslow WC, Calderhead DM, Freeman GJ, Sharpe AH. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 28.Stuber E, Neurath M, Calderhead D, Fell HP, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 29.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- 30.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka T, Nakajima A, Akiba H, Ishiwata T, Asano G, Yoshino S, Yagita H, Okumura K. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–2823. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Murata K, Nose M, Ndhlovu LC, Sato T, Sugamura K, Ishii N. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169:4628–4636. doi: 10.4049/jimmunol.169.8.4628. [DOI] [PubMed] [Google Scholar]
- 34.Blazar BR, Sharpe AH, Chen AI, Panoskaltsis-Mortari A, Lees C, Akiba H, Yagita H, Killeen N, Taylor PA. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood. 2003;101:3741–3748. doi: 10.1182/blood-2002-10-3048. [DOI] [PubMed] [Google Scholar]
- 35.Malmstrom V, Shipton D, Singh B, Al-Shamkhani A, Puklavec MJ, Barclay AN, Powrie F. Cd134l expression on dendritic cells in the mesenteric lymph nodes drives colitis in t cell-restored scid mice. J Immunol. 2001;166:6972–6981. doi: 10.4049/jimmunol.166.11.6972. [DOI] [PubMed] [Google Scholar]
- 36.Redmond WL, Weinberg AD. Targeting OX40 and OX40L for the treatment of autoimmunity and cancer. Crit Rev Immunol. 2007;27:415–436. doi: 10.1615/critrevimmunol.v27.i5.20. [DOI] [PubMed] [Google Scholar]
- 37.Stuber E, Von Freier A, Marinescu D, Folsch UR. Involvement of OX40-OX40L interactions in the intestinal manifestations of the murine acute graft-versus-host disease. Gastroenterology. 1998;115:1205–1215. doi: 10.1016/s0016-5085(98)70092-7. [DOI] [PubMed] [Google Scholar]
- 38.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW, Ware CF, Croft M. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T Cells: A CD4-dependent mechanism. J Immunol. 2007;179:2195–2202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, Urba WJ, Alvord G, Bunce C, Shields J. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg AD, English M, Swain SL. Distinct regulation of lymphokine production is found in fresh versus in vitro primed murine helper T cells. J Immunol. 1990;144:1800–1807. [PubMed] [Google Scholar]
- 41.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 42.Swain SL, Weinberg AD, English M. CD4+ T cell subsets. Lymphokine secretion of memory cells and of effector cells that develop from precursors in vitro. J Immunol. 1990;144:1788–1799. [PubMed] [Google Scholar]
- 43.Swain SL, Huston G, Tonkonogy S, Weinberg A. Transforming growth factor-beta and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol. 1991;147:2991–3000. [PubMed] [Google Scholar]
- 44.Olsson T. Cytokines in neuroinflammatory disease: role of myelin autoreactive T cell production of interferon-gamma. J Neuroimmunol. 1992;40:211–218. doi: 10.1016/0165-5728(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 45.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 46.Wekerle H, Sun D, Oropeza-Wekerle RL, Meyermann R. Immune reactivity in the nervous system: modulation of T-lymphocyte activation by glial cells. J Exp Biol. 1987;132:43–57. doi: 10.1242/jeb.132.1.43. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg AD, Whitham R, Swain SL, Morrison WJ, Wyrick G, Hoy C, Vandenbark AA, Offner H. Transforming growth factor-beta enhances the in vivo effector function and memory phenotype of antigen-specific T helper cells in experimental autoimmune encephalomyelitis. J Immunol. 1992;148:2109–2117. [PubMed] [Google Scholar]
- 48.Weinberg AD, Wyrick G, Celnik B, Vainiene M, Bakke A, Offner H, Vandenbark AA. Lymphokine mRNA expression in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis is associated with a host recruited CD45R hi/CD4+ population during recovery. J Neuroimmunol. 1993;48:105–117. doi: 10.1016/0165-5728(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg AD, Wallin JJ, Jones RE, Sullivan TJ, Bourdette DN, Vandenbark AA, Offner H. Target organ-specific up-regulation of the MRC OX-40 marker and selective production of Th1 lymphokine mRNA by encephalitogenic T helper cells isolated from the spinal cord of rats with experimental autoimmune enciphalomyelitis. Journal of Immunology. 1994;152:4712–4721. [PubMed] [Google Scholar]
- 50.Offner H, Buenafe AC, Vainiene M, Celnik B, Weinberg AD, Gold DP, Hashim G, Vandenbark AA. Where, when, and how to detect biased expression of disease-relevant V beta genes in rats with experimental autoimmune encephalomyelitis. J Immunol. 1993;151:506–517. [PubMed] [Google Scholar]
- 51.Gold DP, Offner H, Sun D, Wiley S, Vandenbark AA, Wilson DB. Analysis of T cell receptor beta chains in Lewis rats with experimental allergic encephalomyelitis: conserved complementarity determining region 3. J Exp Med. 1991;174:1467–1476. doi: 10.1084/jem.174.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buenafe AC, Weinberg AD, Culbertson NE, Vandenbark AA, Offner H. V beta CDR3 motifs associated with BP recognition are enriched in OX-40+ spinal cord T cells of Lewis rats with EAE. J Neurosci Res. 1996;44:562–567. doi: 10.1002/(SICI)1097-4547(19960615)44:6<562::AID-JNR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 53.Weinberg AD, Bourdette DN, Sullivan TJ, Lemon M, Wallin JJ, Maziarz R, Davey M, Palida F, Godfrey W, Engleman E, Fulton RJ, Offner H, Vandenbark AA. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2:183–189. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- 54.Dijkstra CD, De Groot CJ, Huitinga I. The role of macrophages in demyelination. J Neuroimmunol. 1992;40:183–188. doi: 10.1016/0165-5728(92)90132-5. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg AD. OX40: targeted immunotherapy--implications for tempering autoimmunity and enhancing vaccines. Trends Immunol. 2002;23:102–109. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 56.Vetto JT, Lum S, Morris A, Sicotte M, Davis J, Lemon M, Weinberg A. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am J Surg. 1997;174:258–265. doi: 10.1016/s0002-9610(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 57.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 58.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 59.Allison JP, Hurwitz AA, Leach DR. Manipulation of costimulatory signals to enhance antitumor T-cell responses. Curr Opin Immunol. 1995;7:682–686. doi: 10.1016/0952-7915(95)80077-8. [DOI] [PubMed] [Google Scholar]
- 60.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 61.Weinberg AD, Vella AT, Croft M. OX-40: life beyond the effector T cell stage. Semin Immunol. 1998;10:471–480. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- 62.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–112. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 63.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 64.Prell RA, Evans DE, Thalhofer C, Shi T, Funatake C, Weinberg AD. OX40-mediated memory T cell generation is TNF receptor-associated factor 2 dependent. J Immunol. 2003;171:5997–6005. doi: 10.4049/jimmunol.171.11.5997. [DOI] [PubMed] [Google Scholar]
- 65.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8(+) memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37:157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 66.Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181:7969–7976. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bandyopadhyay S, Long M, Qui HZ, Hagymasi AT, Slaiby AM, Mihalyo MA, Aguila HL, Mittler RS, Vella AT, Adler AJ. Self-antigen prevents CD8 T cell effector differentiation by CD134 and CD137 dual costimulation. J Immunol. 2008;181:7728–7737. doi: 10.4049/jimmunol.181.11.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrancois L, Borst J, Sugamura K, Ishii N. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salek-Ardakani S, Moutaftsi M, Sette A, Croft M. Targeting OX40 Promotes Lung-Resident Memory CD8 T Cell Populations That Protect against Respiratory Poxvirus Infection. J Virol. 85:9051–9059. doi: 10.1128/JVI.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salek-Ardakani S, Flynn R, Arens R, Yagita H, Smith GL, Borst J, Schoenberger SP, Croft M. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J Clin Invest. 121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Redmond WL, Gough MJ, Weinberg AD. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur J Immunol. 2009;39:2184–2194. doi: 10.1002/eji.200939348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bekiaris V, Gaspal F, Kim MY, Withers DR, Sweet C, Anderson G, Lane PJ. Synergistic OX40 and CD30 signals sustain CD8+ T cells during antigenic challenge. Eur J Immunol. 2009;39:2120–2125. doi: 10.1002/eji.200939424. [DOI] [PubMed] [Google Scholar]
- 73.Ueki T, Murata S, Kitamura N, Mekata E, Tani T. Pre-treatment with cyclophosphamide or OX40 (CD134) costimulation targeting regulatory T cell function enhances the anti-tumor immune effect of adoptively transferred CD8+ T cells from wild-type mice. Mol Med Report. 2009;2:615–620. doi: 10.3892/mmr_00000146. [DOI] [PubMed] [Google Scholar]
- 74.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 75.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the Acquisition of CD8 T Cell Effector Function after Priming with Tumor or Soluble Antigen Can Be Overcome by the Addition of an OX40 Agonist. J Immunol. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 76.Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J Immunol. 2001;167:6669–6677. doi: 10.4049/jimmunol.167.11.6669. [DOI] [PubMed] [Google Scholar]
- 77.Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, Wong S, Axthelm MK, Picker LJ, Urba WJ. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–585. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 78.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 79.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, Sievers EL, Hughes SD, DeVries TA, Hausman DF. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 80.Nada A, Somberg J. First-in-Man (FIM) clinical trials post-TeGenero: a review of the impact of the TeGenero trial on the design, conduct, and ethics of FIM trials. Am J Ther. 2007;14:594–604. doi: 10.1097/MJT.0b013e31813737dd. [DOI] [PubMed] [Google Scholar]
- 81.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr, Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Jr, Lifson JD, Maino VC, Axthelm MK, Villinger F. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 86.Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT, Hu HM, Redmond WL, Holland J, Weinberg AD. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother. 33:798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 33:369–383. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 88.Morris NP, Peters C, Montler R, Hu HM, Curti BD, Urba WJ, Weinberg AD. Development and characterization of recombinant human Fc:OX40L fusion protein linked via a coiled-coil trimerization domain. Mol Immunol. 2007;44:3112–3121. doi: 10.1016/j.molimm.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 90.Ruby CE, Yates MA, Hirschhorn-Cymerman D, Chlebeck P, Wolchok JD, Houghton AN, Offner H, Weinberg AD. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 92.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vezys V, Penaloza-Macmaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R. 4-1BB Signaling Synergizes with Programmed Death Ligand 1 Blockade To Augment CD8 T Cell Responses during Chronic Viral Infection. J Immunol. 187:1634–1642. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, Mittler RS, Vella AT. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 95.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Totterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 33:225–235. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 96.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 97.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 37:430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]