Abstract
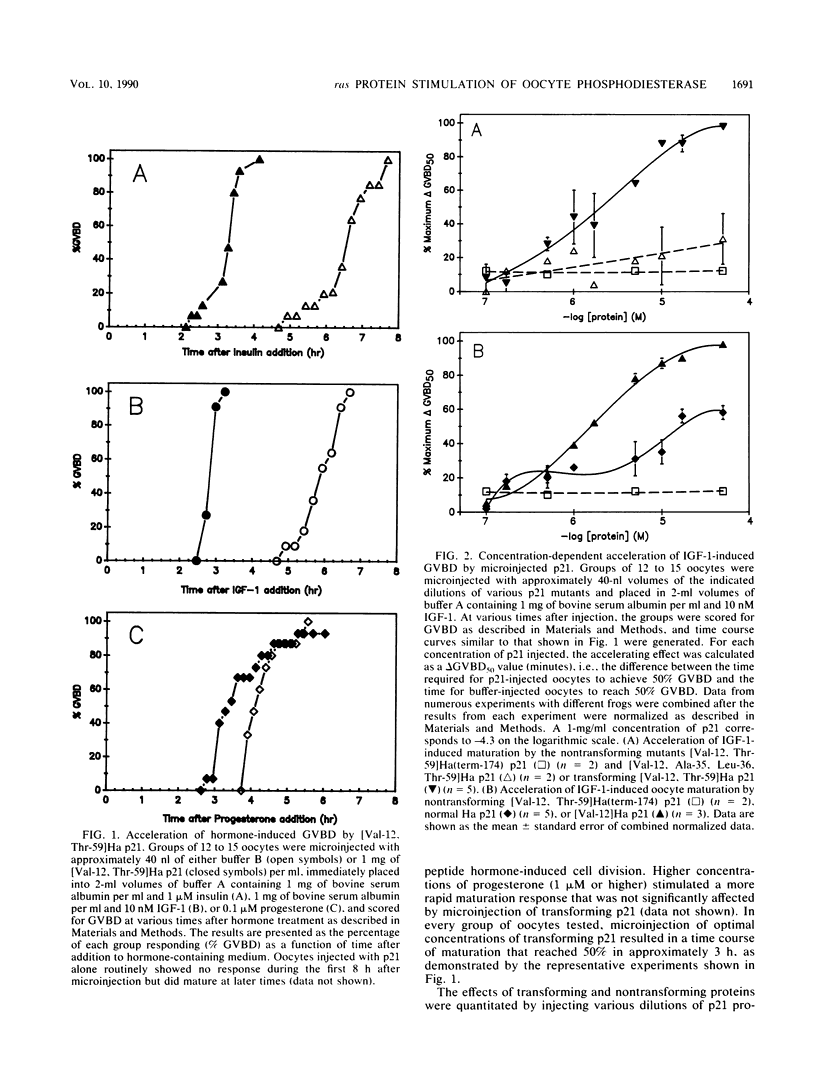
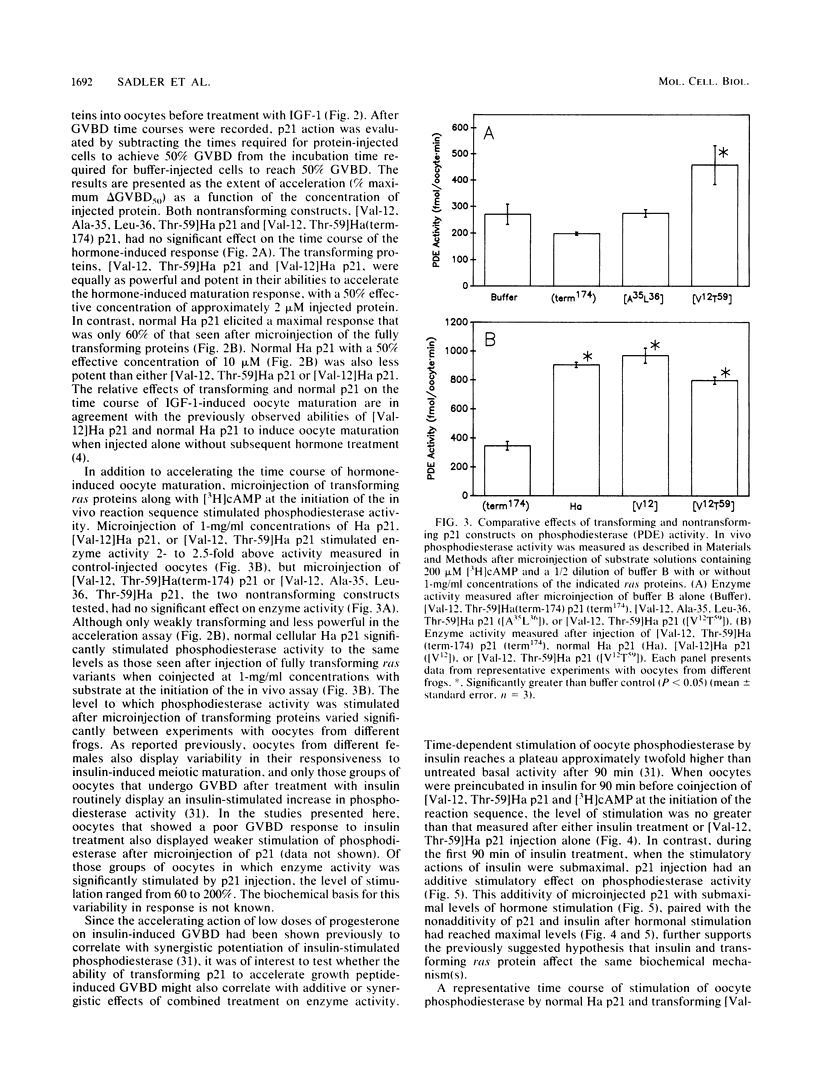
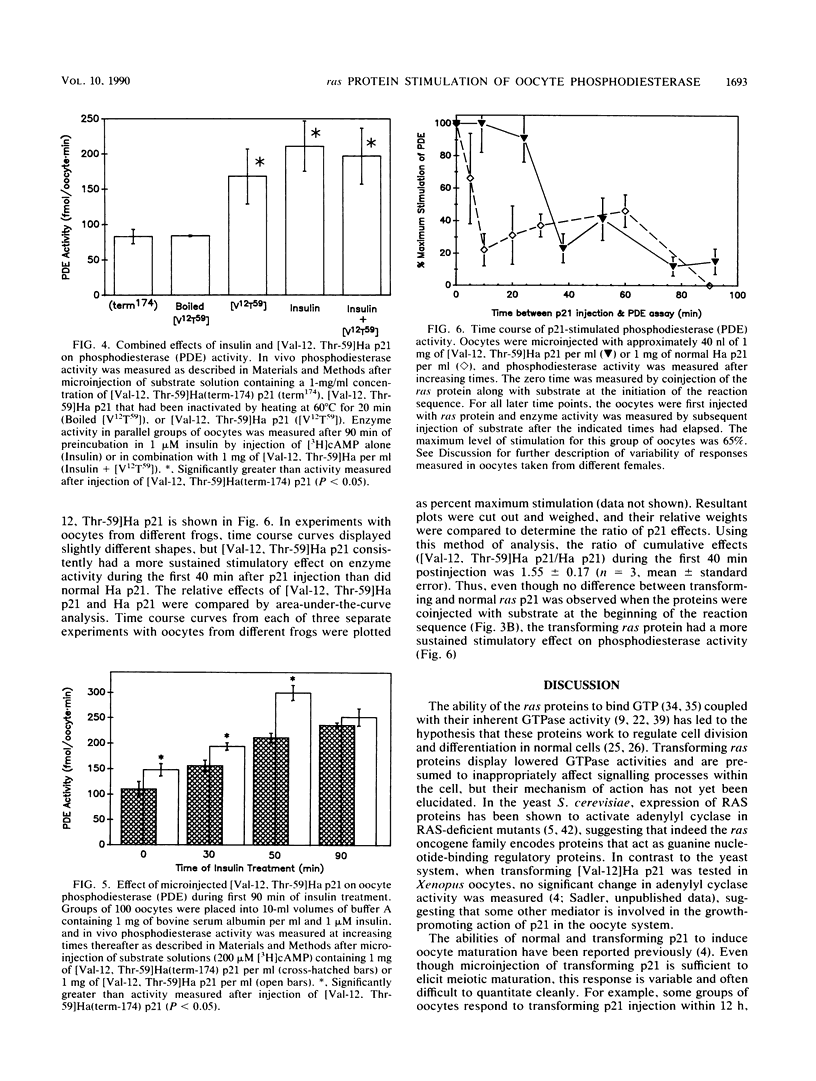
Transforming Harvey (Ha) ras oncogene products accelerated the time course of Xenopus oocyte maturation induced by insulin, insulinlike growth factor 1, or progesterone. The transforming constructs, [Val-12]Ha p21 and [Val-12, Thr-59]Ha p21, displayed equal potency and efficacy in their abilities to accelerate the growth peptide-induced response. Normal Ha p21 was only 60% as powerful and one-fifth as potent as the mutants containing valine in the 12 position. In contrast, two nontransforming constructs, [Val-12, Ala-35, Leu-36, Thr-59]Ha p21 and [Val-12, Thr-59]Ha(term-174) p21, had no effect on the time course of hormone-induced maturation. Effects of the transforming ras proteins on hormone-induced maturation correlated with their abilities to stimulate in vivo phosphodiesterase activity measured after microinjection of 200 microM cyclic [3H] AMP. When p21 injection followed 90 min of insulin treatment, there was no increase in phosphodiesterase activity over that measured after hormone treatment or p21 injection alone, but additive effects of p21 and insulin on enzyme activity were observed during the first 90 min of insulin treatment. Even though normal Ha p21 and transforming [Val-12, Thr-59]Ha p21 stimulated oocyte phosphodiesterase to equal levels when coinjected with substrate at the initiation of the in vivo assay, the transforming protein elicited a more sustained stimulation of enzyme activity. These results suggest that stimulation of a cyclic AMP phosphodiesterase activity associated with insulin-induced maturation is involved in the growth-promoting actions of ras oncogene products in Xenopus oocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adari H., Lowy D. R., Willumsen B. M., Der C. J., McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988 Apr 22;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Allende C. C., Bravo R., Allende J. E. Comparison of in vivo and in vitro properties of cyclic adenosine 3':5'-monophosphate phosphodiesterase of amphibian oocytes. J Biol Chem. 1977 Jul 10;252(13):4662–4666. [PubMed] [Google Scholar]
- Baulieu E. E., Schorderet-Slatkine S. Steroidal and peptidic control mechanisms in membrane of Xenopus laevis oocytes resuming meiotic division. J Steroid Biochem. 1983 Jul;19(1A):139–145. [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Calés C., Hancock J. F., Marshall C. J., Hall A. The cytoplasmic protein GAP is implicated as the target for regulation by the ras gene product. Nature. 1988 Apr 7;332(6164):548–551. doi: 10.1038/332548a0. [DOI] [PubMed] [Google Scholar]
- Deshpande A. K., Kung H. F. Insulin induction of Xenopus laevis oocyte maturation is inhibited by monoclonal antibody against p21 ras proteins. Mol Cell Biol. 1987 Mar;7(3):1285–1288. doi: 10.1128/mcb.7.3.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finidori-Lepicard J., Schorderet-Slatkine S., Hanoune J., Baulieu E. E. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981 Jul 16;292(5820):255–257. doi: 10.1038/292255a0. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Goodhardt M., Ferry N., Buscaglia M., Baulieu E. E., Hanoune J. Does the guanine nucleotide regulatory protein Ni mediate progesterone inhibition of Xenopus oocyte adenylate cyclase? EMBO J. 1984 Nov;3(11):2653–2657. doi: 10.1002/j.1460-2075.1984.tb02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S., Le Goascogne C., Baulieu E. E. Induction of germinal vesicle breakdown in Xenopus laevis oocytes: response of denuded oocytes to progesterone and insulin. Dev Biol. 1983 Nov;100(1):214–221. doi: 10.1016/0012-1606(83)90213-0. [DOI] [PubMed] [Google Scholar]
- Hughes S. M. Are guanine nucleotide binding proteins a distinct class of regulatory proteins? FEBS Lett. 1983 Nov 28;164(1):1–8. doi: 10.1016/0014-5793(83)80006-4. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Siebel C. W., McCormick F., Roth R. A. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987 May 15;236(4803):840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., de la Peña P., Moscat J., Garcia-Barreno P., Anderson P. S., Aaronson S. A. Rapid stimulation of diacylglycerol production in Xenopus oocytes by microinjection of H-ras p21. Science. 1987 Oct 23;238(4826):533–536. doi: 10.1126/science.2821623. [DOI] [PubMed] [Google Scholar]
- Le Goascogne C., Hirai S., Baulieu E. E. Induction of germinal vesicle breakdown in Xenopus laevis oocytes: synergistic action of progesterone and insulin. J Endocrinol. 1984 Apr;101(1):7–12. doi: 10.1677/joe.0.1010007. [DOI] [PubMed] [Google Scholar]
- Le Goascogne C., Sananès N., Gouézou M., Baulieu E. E. Testosterone-induced meiotic maturation of Xenopus laevis oocytes: evidence for an early effect in the synergistic action of insulin. Dev Biol. 1985 May;109(1):9–14. doi: 10.1016/0012-1606(85)90340-9. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Butcher F. R., Krebs E. G. Early effect of progesterone on levels of cyclic adenosine 3':5'-monophosphate in Xenopus oocytes. J Biol Chem. 1979 Feb 10;254(3):579–582. [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Smith L. D. Inhibition of oocyte maturation by theophylline: possible mechanism of action. Dev Biol. 1976 Sep;52(2):318–322. doi: 10.1016/0012-1606(76)90249-9. [DOI] [PubMed] [Google Scholar]
- Olate J., Allende C. C., Allende J. E., Sekura R. D., Birnbaumer L. Oocyte adenylyl cyclase contains Ni, yet the guanine nucleotide-dependent inhibition by progesterone is not sensitive to pertussis toxin. FEBS Lett. 1984 Sep 17;175(1):25–30. doi: 10.1016/0014-5793(84)80562-1. [DOI] [PubMed] [Google Scholar]
- Papageorge A. G., Willumsen B. M., Johnsen M., Kung H. F., Stacey D. W., Vass W. C., Lowy D. R. A transforming ras gene can provide an essential function ordinarily supplied by an endogenous ras gene. Mol Cell Biol. 1986 May;6(5):1843–1846. doi: 10.1128/mcb.6.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Weinberg R. A. Presence of a Kirsten murine sarcoma virus ras oncogene in cells transformed by 3-methylcholanthrene. Mol Cell Biol. 1983 Dec;3(12):2298–2301. doi: 10.1128/mcb.3.12.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. A similar pool of cyclic AMP phosphodiesterase in Xenopus oocytes is stimulated by insulin, insulin-like growth factor 1, and [Val12,Thr59]Ha-ras protein. J Biol Chem. 1989 Jan 15;264(2):856–861. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L., Cooper D. M. Progesterone inhibition of Xenopus oocyte adenylate cyclase is not mediated via the Bordetella pertussis toxin substrate. Mol Pharmacol. 1984 Nov;26(3):526–531. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. J Biol Chem. 1982 Jan 10;257(1):355–361. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. In vivo regulation of cyclic AMP phosphodiesterase in Xenopus oocytes. Stimulation by insulin and insulin-like growth factor 1. J Biol Chem. 1987 Aug 5;262(22):10644–10650. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Inhibition of Xenopus oocyte adenylate cyclase by progesterone and 2',5'-dideoxyadenosine is associated with slowing of guanine nucleotide exchange. J Biol Chem. 1983 Jul 10;258(13):7935–7941. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Inhibition of Xenopus oocyte adenylate cyclase by progesterone: a novel mechanism of action. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:179–194. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem. 1981 Jun 25;256(12):6368–6373. [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Stokes P. E., Smythers G. W., Dhar R., Oroszlan S. Characterization of the phosphorylation sites and the surrounding amino acid sequences of the p21 transforming proteins coded for by the Harvey and Kirsten strains of murine sarcoma viruses. J Biol Chem. 1982 Oct 10;257(19):11767–11773. [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Temeles G. L., Wolanski B. S., Socher S. H., Scolnick E. M. Mutant ras-encoded proteins with altered nucleotide binding exert dominant biological effects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):952–956. doi: 10.1073/pnas.83.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speaker M. G., Butcher F. R. Cyclic nucleotide fluctuations during steroid induced meiotic maturation of frog oocytes. Nature. 1977 Jun 30;267(5614):848–850. doi: 10.1038/267848a0. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Temeles G. L., Gibbs J. B., D'Alonzo J. S., Sigal I. S., Scolnick E. M. Yeast and mammalian ras proteins have conserved biochemical properties. Nature. 1985 Feb 21;313(6004):700–703. doi: 10.1038/313700a0. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Papageorge A. G., Hubbert N., Bekesi E., Kung H. F., Lowy D. R. Transforming p21 ras protein: flexibility in the major variable region linking the catalytic and membrane-anchoring domains. EMBO J. 1985 Nov;4(11):2893–2896. doi: 10.1002/j.1460-2075.1985.tb04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


