Abstract
Human neuroimaging is expanding our understanding of the biological processes that are essential for healthy brain function. Methods such as diffusion weighted imaging provide insights into white matter fascicles, growth and pruning of dendritic arbors and axons, and properties of glia. This review focuses on what we have learned from diffusion imaging about these processes and the development of reading circuitry in the human brain. Understanding reading circuitry development may suggest ways to improve how we teach children to read.
Introduction
A generation of cognitive neuroscientists has pursued the idea that the neurobiological principles of perception, learning and memory can be understood by analyzing synaptic properties in simple model organisms, or by measuring action potentials in small collections of neurons [1]. The emphasis on synapse and spiking is reflected in computational theories, which give a central role to synaptic efficacy [2,3].
Human neuroimaging methods inform us about brain processes beyond synapses and spikes. Functional MRI (fMRI) measures integrative metabolic signals [4]; EEG/MEG methods measure extrasynaptic mean field potentials [5,6]. Diffusion weighted imaging and tractography measure the long-range axonal projections that carry signals between distant cortical regions [7]; quantitative MRI methods [8] can assess tissue properties of neurons and also the nearby glia, whose function are significant throughout the lifespan [9]. For example, glia have an essential role in cortical circuit formation [10–12], glial properties are shaped by experience during critical periods [13], and glia influence axonal transmission [14,15]. Just like the synapse, the properties of tracts and tissue influence cognition and behavior [16–20].
Given the expanded opportunity to measure such processes, what might be learned from these measurements? Some behaviors, such as psychological tests of performance during brief trials, may be best understood by measuring synaptic activity or spikes. But other important behaviors, such as learning to read, acquiring a second language, or learning to regulate emotions, take place over longer time periods and may depend on biological processes such as cell development, growth and pruning of dendritic arbors, the proliferation and activity of glia, axon myelination and pruning, and vascular development. Ultimately, neuroscientists and clinicians will need to account for the entire range of processes to understand circuit function in health and disease.
This review focuses on how one of the new neuroimaging modalities, diffusion weighted magnetic resonance imaging, informs us about reading circuitry in the human brain. The neurobiology of reading has been an active research area [21–26] because many scientists would like to understand how the integration of signals across visual, auditory and language circuits implements this uniquely human cognitive process. Furthermore, there is a hope that understanding the reading circuits will lead us to develop ways to improve how we teach children to read. Here we provide a brief and opinionated discussion of recent findings centered on the information provided by diffusion-weighted imaging (DWI) about reading circuitry. We conclude by discussing how these findings might matter for society.
Background
Instrumentation and algorithms to measure white matter connections in the living human brain advanced dramatically in the 1990s; diffusion-weighted MRI coupled with tractography algorithms provided spatially resolved measurements of specific white matter pathways in the living human brain [27–30]. Perhaps Klingberg and colleagues [31], were the first to take advantage of the opportunity to relate white matter properties to cognition. The idea of the measurement still seems remarkable: Place a subject’s head in a scanner and measure how water diffuses through the white matter fascicles. Then, bring the subject to a behavioral testing room to measure reading skills. Klingberg et al. found that diffusion measurements from good and poor readers differ, and the size of the difference is largest within a region within the left hemisphere temporo-parietal white matter. Few researchers could have predicted that variation in a cognitive skill such as reading could be associated with how efficiently water diffuses in the white matter. Now there is no doubt, and this finding has been replicated several times [22,32–34].
The initial report gave rise to many questions: Can these diffusion differences be localized to specific fascicles in the reading circuitry? Are these differences a product of a lifetime of poor reading experience, or are they present at an early age, constraining each child’s ability to learn to read? Can additional measurements clarify whether differences arise because of myelin, or glia, specific macromolecules, or axonal membrane properties? Can the diffusion differences be incorporated into a quantitative cognitive model that includes functional and behavioral measures of reading? We discuss these topics in the following sections.
Reading circuitry
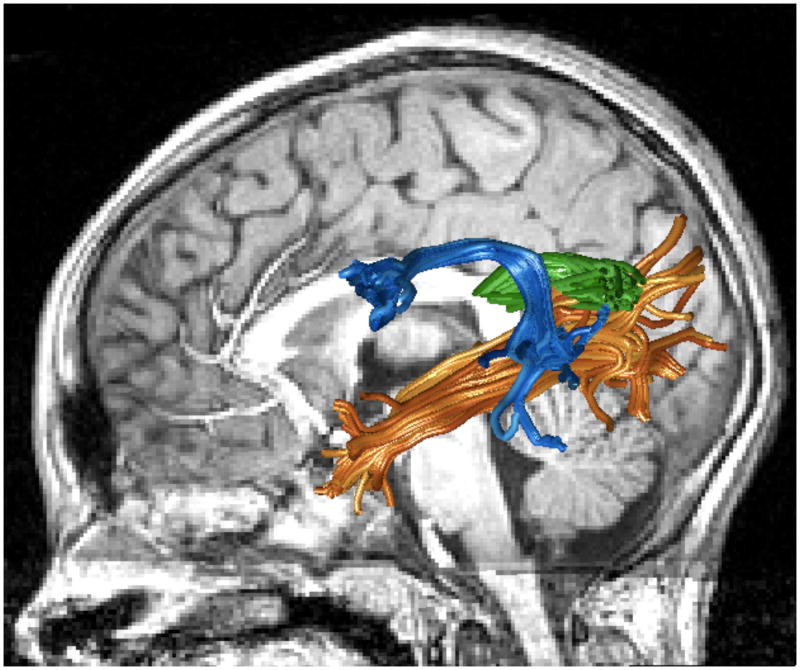
Diffusion weighted imaging measures suggest that several large fascicles beyond the early visual pathways are part of the reading circuitry. Specifically, diffusion measures consistently reveal associations between diffusion and reading skills in three major tracts: the posterior corpus callosum, the arcuate fasciculus and the inferior longitudinal fasciculus (Figure 1). These are large fascicles that contain axons between many different cortical regions, and they certainly carry information beyond that required for reading.
Figure 1. Reading circuitry.
Arcuate fasciculus (blue), inferior longitudinal fasciculus (orange) and temporal callosal projections for a representative subject. See https://github.com/jyeatman/reading_circuits for the software and data used to create this image.
Corpus callosum
In his seminal work on acquired alexia, Jules Dejerine observed lesions in the posterior corpus callosum [35,36]. He hypothesized that the damaged axons carry signals between the left and right hemisphere angular gyrus, a region he believed was essential for interpreting word forms. The significance of posterior callosal lesions in alexia was explored by Damasio and Damasio [37] and Binder and Mohr [38] in more recent analyses and these investigations confirmed Dejerine’s original observation.
Dougherty et al. [39] used diffusion imaging and tractography to investigate covariation between callosal tissue diffusivity and reading skills in typically developing children. They found a negative correlation between reading skills and fractional anisotropy in the posterior callosum, within a region that contains axons that connect with the temporal lobe. This finding has been replicated at least twice. Frye et al. [40] found that dyslexic adults have higher fractional anisotropy (FA) in the posterior portion of the corpus callosum than do typical readers; Odegard et al. [41] found a negative correlation between posterior callosal FA and reading measures. Unpublished observations in our lab suggest that these fibers are destined for the angular gyrus, as Derjerine would have predicted.
Arcuate fasciculus
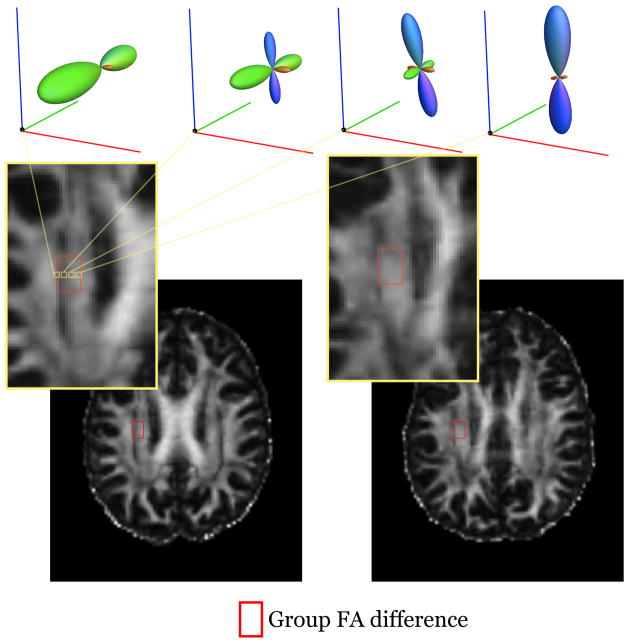
Klingberg et al. [31] used whole brain, voxel-based analyses to compare diffusion in good and poor readers. They found that the voxels differentiating good and poor readers were primarily oriented in the anterior-posterior direction and reasoned that these voxels might contain the arcuate fasciculus. Other groups suggested that the differences were not within the arcuate fasciculus, but rather in a nearby tract, the corona radiata [22,32,42]. It is impossible to confidently assign a voxel from a template to a specific tract in individual subjects; the observed voxel-based differences consistently fall on the border between the corona radiata and the arcuate fasciculus/superior longitudinal fasciculus [34]. Because fractional anisotropy decreases substantially in regions containing fibers tracts with different orientations (Figure 2), the difference between good and poor readers may be due to between-group difference in the relative position or size of these fascicles rather than tissue properties of the axons within a tract [22].
Figure 2. Group differences in FA are located at the juncture of two tracts.
The peak group FA differences (from a meta-analysis by Vandermoosten et al.[34]) are outlined by a red box; each subject’s image is aligned to the MNI template. For the subject in the left panel the region includes voxels with very low FA because the box includes crossing fibers where the corona radiata and superior longitudinal fasciculus intersect. Fiber orientation distribution functions are shown for 4 voxels within the ROI. For the subject in the right panel the corresponding region is centered near the core of the corona radiata, and the FA values are all relatively high. The differences in these voxels for subject 1 compare to subject 2 reflect the relative size and position of these two tracts.
An alternative to voxel-based group analysis is to measure specific tracts in individual subjects. Yeatman et al. [19] identified the arcuate fasciculus in individual children and measured diffusion within a segment of the tract that was clear of major crossing fibers [19]. They found a modest correlation (r ~0.35) between diffusivity in the left arcuate fasciculus and phonological awareness, a key measure of reading readiness. No significant correlation was found with diffusion in the adjacent superior longitudinal fasciculus or the right arcuate or the corona radiata. This correlation replicated in an independent sample of children [43].
Two additional studies support the hypothesis that the arcuate fasciculus tissue properties influence phonological processing. Rolheiser et al. [44] compared lesion locations in the white matter of stroke patients. They found that lesions in the vicinity of the arcuate fasciculus produced deficits in phonological skills. Vandermosten et al. [45] identified the arcuate fasciculus and found reductions in FA in dyslexic adults compared to typical readers.
Occipital lobe pathways: Inferior longitudinal fasciculus and the vertical occipital fasciculus
Three recent pieces of evidence suggest that the ILF, too, is part of the reading circuitry. First, the ILF contains axons that project to a region of ventral occipital temporal (VOT) cortex – the visual word form area – that is critically involved in skilled reading [46]. It appears that the ILF carries signals between the occipital lobe and the temporal pole, medial temporal lobe and regions on the ventral surface of the temporal lobe.
The ILF is important for a number of visual functions [47,48], and it is also specifically implicated in reading. Epelbaum et al. [49] showed that a lesion to a specific portion of the ILF immediately adjacent to the VWFA produces reading impairment. They localized the VWFA in a patient with epilepsy and observed that resection of adjacent white matter containing ILF axons produced alexia and eliminated the responsivity of the previously identified VWFA.
Two studies reported correlations between FA in the ILF and measures of reading skills. A voxel based analysis by Steinbrink et al. [50] reported a positive FA-reading correlation in temporal lobe white matter voxels in the vicinity of the ILF. Yeatman et al. [51] tracked the ILF in a sample of children ages 7–12 years. Following these children longitudinally for 3 years with yearly reading and diffusion measurements, they found correlations between diffusion changes in the ILF and reading skills.
The portion of the ILF near the VWFA is relatively small, and the fraction of axons within the ILF that are part of the reading circuitry may be correspondingly small. The vertical occipital fasciculus (VOF), a tract adjacent to the ILF, projects more extensively into the VWFA [46]. This tract was analyzed by Greenblatt, who observed that a lesion to this tract can produce alexia [52,53]. Yeatman et al. [46] describe how to identify the VOF using diffusion imaging and tractography. The VOF contains fibers that connect to the angular gyrus as well al lateral and dorsal occipital regions.
Circuit and tissue development
At what age do the neurobiological differences between good and poor readers arise? The Klingberg et al. findings in adult were replicated in two independent samples of children [32,33]. Hence, these differences are present at an early age and do not change substantially over the course of reading instruction. Earlier in this article we suggested that the diffusivity differences reported by Klingberg et al. might be explained by the relative size and position of the corona radiata, arcuate fasciculus and superior longitudinal fasciculus. The stability of the difference through development is consistent with the stability of major anatomical characteristics of the white matter, which develop early and are fixed by specific molecular mechanisms [54]. It is possible that the size and position of these fascicles predict the ability to extract information about the sounds of speech or to learn to associate sounds with visual forms, or that differences in tract size and position are the product of other developmental factors that predispose children to poor reading development.
In contrast to the stability of tract position, tissue properties of the reading-related tracts change during development. Yeatman et al. [51] followed subjects in a longitudinal study (children aged 7–15). They found that FA development follows different trajectories between individuals: The FA values increase with development in good readers and decrease in poor readers. This difference explains why the FA correlation with reading reverses with age and emphasizes the importance of following individual children longitudinally. While the size and position of the major fascicles are established early in development, biological properties at a finer scale, such as myelination and pruning, remain dynamic during childhood and adolescence. The biological mechanisms that drive myelination and pruning are plastic and can be modified by experience and the rates of these processes vary between children.
What biological factors account for the developmental FA differences?
Beginning in the late prenatal period and continuing through infancy and childhood, oligodendrocytes form the myelin sheaths that surround axons. This process depends both on intrinsic genetic codes and extrinsic environmental factors [55]. The myelination process is plastic; the level of electrical activity of an axon in part drives myelination [56,57]. Myelination speeds conduction between distant cortical regions, however the axons occupy more space as each wrap of myelin increases the overall diameter of the axon. Myelination and related processes may increase FA.
During development, some axons grow and stabilize while other axons are eliminated; a process called pruning. La Mantia and Rakic [58,59] demonstrated that at birth a rhesus monkey’s corpus callosum contains 3.5 times more axons than in adulthood. The pruning process is also in part driven by experience [60]. Underused axons are pruned away during childhood and the remaining axons are increasingly myelinated. Pruning and related processes may decrease FA.
Dual-process account of the joint development of white matter and skilled reading
Diffusion MRI cannot yet establish the specific biological changes, but it is possible to describe the variations in FA development using a phenomenological model. Some biological processes tend to increase FA (such as myelination) and others tend to decrease FA (such as pruning). Yeatman et al. [51] captured this opponency in a dual-process model of white matter development. According to this model, the development of FA is described by the same equations that balance opposing processes and govern critical damping in homeostatic systems. In this model, the net development of FA depends on the parameters of these two opposing processes.
The two curves in Figure 3A show that the observed variation in FA developmental trajectories can be explained by adjusting the relative rate and timing of the two opposing processes. One curve illustrates the case when the two processes are synchronous throughout development: the FA value monotonically increases and approaches its mature level. This pattern of FA development is consistent with the data from good readers. In the second case, the two processes are asynchronous. Early development is dominated by increasing FA. However the growth process asymptotes prior to the pruning process and later development is dominated by decreasing FA. This pattern of FA development is consistent with the data from relatively poor readers.
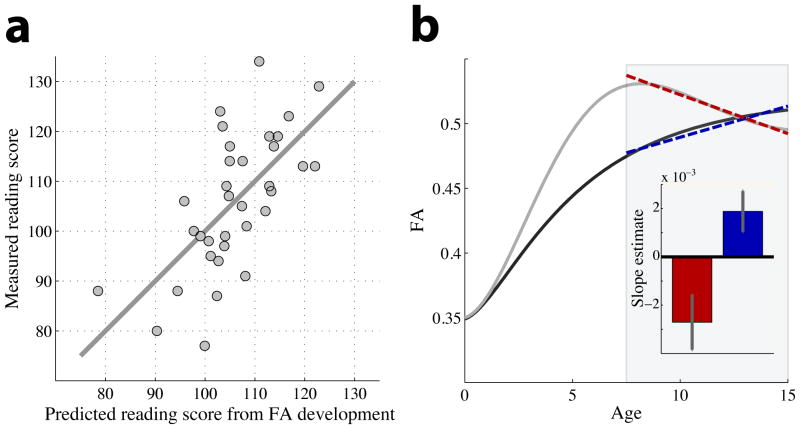
Figure 3. A dual process model of white matter development.
(a) FA-development is illustrated for two cases. Throughout white matter and in all children FA is low at birth and generally increases with age. Tract development combines processes that increase and decrease FA, and the balance between these opposing processes differs between children. The balance can be modeled by classic homeostatic equations that govern development over time; the parameters of the developmental processes for the two curves were chosen to model the characteristics of the above-average readers (black curve) and below-average readers (gray curve). The model for the above-average group has synchronous development of the processes, while the below-average group is modeled with slightly asynchronous development. For both groups the FA-developmental rate is approximately linear during the age range of 7–15 years (shaded region) but the specific rate of development differs (dotted lines). (b) During development (7–15 years) FA increases in above-average readers and declines FA in below-average readers for both the left arcuate and inferior longitudinal fasciculus. Combining FA development rates from the arcuate and ILF into an additive model predicts reading proficiency.
One consequence of the different developmental patterns is that FA is negatively correlated with reading performance at early ages and positively correlated with reading performance at older ages. However, the key prediction of the model is that measuring development rates is more informative of the underlying mechanisms than measuring an instantaneous value.
There is another important difference between these two cases. In synchronous development, both processes are active in early development and thus both processes are guided by common experiential factors. In the asynchronous development, the process that increases FA is influenced by experience at early ages and the process that decreases FA is influenced by experience at later ages. Hence, the two processes are guided by different experiential factors.
The data we describe are simply an association between a biological measurement and behavior; we have no data to determine the causal factors that influence the onset of the processes. These causal factors may be intrinsic to the biology, such as genetics, or they may be environmental, such as the quality of early-life language input, reading experience, or the timing of reading instruction. The causal mechanisms and interaction with education are important to address in future research.
Prediction and prevention of poor reading
There has been good progress developing simple behavioral measures to predict which children are at risk for a reading disability. Children in kindergarten and 1st grade who have difficulty naming letters and little ability to distinguish or blend individual sounds are likely to be poor readers in the 4th grade [61]. We are less skilled at selecting interventions to help children who are poor readers at the end of first grade become average readers by the end of elementary school [62,63]. How might neurobiological measures provide specific diagnoses and recommendations for the best intervention for a poor reader?
First, neurobiological measures should be applicable to individuals rather than group comparisons. A child may have difficulty learning to read for many reasons; helpful neurobiological measures require characterizing each child, not a representative group. Second, neurobiological measures should distinguish among the different reasons for poor reading and use these differences to select a targeted intervention. An important goal is to understand the reading pathways broadly so that we can measure the individual’s circuitry with respect to the typical reading population.
Hoeft et al. [64] used fMRI measures to guide the choice of targeted interventions. Children with certain responses to reading stimuli are more likely to benefit from an intervention, and equally important children without these responses are not good candidates for the intervention. Yeatman et al. [51] showed that the diffusivity development rates in the arcuate fasciculus and ILF can be combined to predict the current and future levels of reading (Figure 3B). It remains an important open question as to whether we can use diffusion measures to predict the success of specific interventions.
The idea of using diffusion-weighted MR for diagnosis in Education may seem unlikely. But, note that an hour’s worth of MR scanning in our facility is about $400; a measurement takes about 30 minutes. The software for analyzing these data and comparing an at-risk individual to norms established in a control group is freely distributed and the data processing pipeline has been completely automated [43]. We might suppose that the entire cost of the analysis is under $2000. The cost of a special education intervention for a child is more than ten times this price, and the cost to the family in self-esteem and stress is also very high. Developing a diagnostic seems worth investigating, and a useful complement to our motivation, which is to achieve a clear scientific understanding.
Conclusions
Brain computations operate over a range of temporal and spatial scales. Understanding action potentials and synaptic efficacy is essential for understanding certain aspects of performance. Other forms of behavior, perhaps learning that takes place over the time scale of cognitive development, may depend on multiple biological processes including those that operate over time scales from hours to days to years. Cognitive neuroscientists can now measure and consider the influence of a wide range of factors - cell development, growth and pruning of dendritic arbors and axons, the status of the glia, and the status of the vasculature – as well as spikes and synaptic efficacy.
The relationship between cognition and white matter tissue properties is one example of how these factors may set important limits on both cognition and affect. The neuroimaging tools for exploring these processes in the living human brain continue to improve over time; understanding how synaptic and extrasynaptic factors combine to determine human cognition, affect, and performance promises to help us understand how the whole brain functions.
Highlights.
Cognitive skills, such as reading, depend on long-term development of axons and glia
Diffusion weighted MRI can assess tissue properties of specific reading circuits
Biological models of the reading circuitry can predict children’s reading skills.
Acknowledgments
We thank Franco Pestilli and Jon Winawer for comments. Supported by NIH R01-EY15000 (BAW) and NSF Graduate Research Fellowship (JDY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kandel ER. Principles of neural science. 5. New York: McGraw-Hill; 2012. [Google Scholar]
- 2.Rumelhart DE, McClelland JL University of California San Diego. PDP Research Group. Parallel distributed processing: explorations in the microstructure of cognition. Cambridge, Mass: MIT Press; 1986. [Google Scholar]
- 3.McClelland JL, Rumelhart DE. Explorations in parallel distributed processing: a handbook of models, programs, and exercises. Cambridge, Mass: MIT Press; 1989. [Google Scholar]
- 4.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra S, Cross RW, van der Meer MA. Theta phase precession beyond the hippocampus. Reviews in the neurosciences. 2012;23:39–65. doi: 10.1515/revneuro-2011-0064. [DOI] [PubMed] [Google Scholar]
- 6.Ritaccio A, Boatman-Reich D, Brunner P, Cervenka MC, Cole AJ, Crone N, Duckrow R, Korzeniewska A, Litt B, Miller KJ, et al. Proceedings of the Second International Workshop on Advances in Electrocorticography. Epilepsy & Behavior. 2011;22:641–650. doi: 10.1016/j.yebeh.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tofts P. Quantitative MRI of the brain: measuring changes caused by disease. Chichester, UK: John Wiley & Sons Ltd; 2003. [Google Scholar]
- 9**.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. This important review paper describes the role of glia in synapse formation, development, and cortical signaling. [DOI] [PubMed] [Google Scholar]
- 10.Antic SD, Zhou WL, Moore AR, Short SM, Ikonomu KD. The decade of the dendritic NMDA spike. Journal of neuroscience research. 2010;88:2991–3001. doi: 10.1002/jnr.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields RD. White matter matters. Sci Am. 2008;298:42–49. [PubMed] [Google Scholar]
- 15.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- 17.Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 18.Tsang JM, Dougherty RF, Deutsch GK, Wandell BA, Ben-Shachar M. Frontoparietal white matter diffusion properties predict mental arithmetic skills in children. Proc Natl Acad Sci U S A. 2009;106:22546–22551. doi: 10.1073/pnas.0906094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, Ben-Shachar M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cogn Neurosci. 2011;23:3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. Mean diffusivity was measured before and after spatial learning in a sample of humans and a sample of rats. Learning changed mean diffusivity in the hippocampus in both species. In humans the amount of change correlated with the amount of learning. Histological analysis of rat hippocampus identified structural changes in astrocytes as a leading candidate to explain the change. [DOI] [PubMed] [Google Scholar]
- 21.Shaywitz SE, Morris R, Shaywitz BA. The education of dyslexic children from childhood to young adulthood. Annu Rev Psychol. 2008;59:451–475. doi: 10.1146/annurev.psych.59.103006.093633. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr Opin Neurobiol. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Gabrieli JD. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 2009;325:280–283. doi: 10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- 24.Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- 25.Wandell BA, Rauschecker AM, Yeatman JD. Learning to see words. Annu Rev Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- 27.Basser P. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomedicine. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 28.Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori S. Introduction to diffusion tensor imaging. Amsterdam; Boston, MA: Elsevier; 2006. [Google Scholar]
- 30.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- 32.Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 33.Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- 34.Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Dejerine J. Sur un cas de cecite verbale avec agraphie, suivi d’autopsie. Mémoires de la Société Biologique. 1891;3:197–201. [Google Scholar]
- 36.Dejerine J. Contribution a l’étude anatomoclinique et clinique des differentes varietes de cecite verbal. Compte Rendu Hebdomadaire des Séances et Mémoires de la Société de Biologie. 1892;4:61–90. [Google Scholar]
- 37.Damasio AR, Damasio H. The anatomic basis of pure alexia. Neurology. 1983;33:1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- 38.Binder JR, Mohr JP. The topography of callosal reading pathways. A case-control analysis. Brain. 1992;115 (Pt 6):1807–1826. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- 39.Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci U S A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frye RE, Hasan K, Xue L, Strickland D, Malmberg B, Lieberman J, Papanicolaou A. Splenium microstructure is related to two dimensions fo reading skill. Neuroreport. 2008;19:1627–1631. doi: 10.1097/WNR.0b013e328314b8ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47:1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006 doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber-tract quantification. PloS one. 2012;7:e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J Neurosci. 2011;31:16949–16957. doi: 10.1523/JNEUROSCI.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquiere P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135:935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- 46.Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area. Adjacent cortical circuits and long-range white matter connections. Brain Lang. 2012 doi: 10.1016/j.bandl.2012.04.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 48.Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 49**.Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44:962–974. doi: 10.1016/j.cortex.2008.05.003. Analysis of white matter pre- and post-surgery show that disruption of the ILF can cause of pure alexia. [DOI] [PubMed] [Google Scholar]
- 50.Steinbrink C, Vogt K, Kastrupo A, Müller HP, Juengling FD, Kassubek J, Riecker A. The contribution of white and gray matter differences to development dyslexia: Insights from DTI and VBM at 3. 0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 51**.Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3045–3053. doi: 10.1073/pnas.1206792109. The first longitudinal study to measure joint development of white matter and reading skills in a cohort of elementary school children. These data reveal wide variability in individual children’s growth curves and suggest that two opposing biological processes contribute to change in tissue properties within the reading circuitry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Greenblatt SH. Alexia without agraphia or hemianopsia. Anatomical analysis of an autopsied case. Brain. 1973;96:307–316. doi: 10.1093/brain/96.2.307. An important neurological paper on lesions giving rise to alexia. The paper identifies the vertical occipital fasciculus, a pathway diagrammed by Dejerine that connects occipitotemporal sulcus with dorsal occipital-parietal cortex (posterior and adjacent to the angular gyrus), as essential for reading. We find that this pathway terminates in the VWFA. [DOI] [PubMed] [Google Scholar]
- 53.Greenblatt SH. Subangular alexia without agraphia or hemianopsia. Brain Lang. 1976;3:229–245. doi: 10.1016/0093-934x(76)90019-5. [DOI] [PubMed] [Google Scholar]
- 54.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 55.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 56.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 57.Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58**.LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. Histological analysis of the corpus callosum in developing rhesus monkeys elucidates two main developmental processes in the white matter; axonal myelination which increases the overall volume of each axon and axonal pruning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaMantia AS, Rakic P. Axon overproduction and elimination in the anterior commissure of the developing rhesus monkey. J Comp Neurol. 1994;340:328–336. doi: 10.1002/cne.903400304. [DOI] [PubMed] [Google Scholar]
- 60.Yamahachi H, Marik SA, McManus JN, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–729. doi: 10.1016/j.neuron.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61**.Torgesen JK. Preventing Early Reading Failure. American Educator. 2004;28 Children who get off to a poor start in reading rarely catch up, highlighting the importance of early identification and remediation. [Google Scholar]
- 62.Juel C, Leavell JA. Retention and nonretention of at-risk readers in first grade and their subsequent reading achievement. J Learn Disabil. 1988;21:571–580. doi: 10.1177/002221948802100910. [DOI] [PubMed] [Google Scholar]
- 63.Shaywitz SE, Fletcher JM, Holahan JM, Shneider AE, Marchione KE, Stuebing KK, Francis DJ, Pugh KR, Shaywitz BA. Persistence of dyslexia: the Connecticut Longitudinal Study at adolescence. Pediatrics. 1999;104:1351–1359. doi: 10.1542/peds.104.6.1351. [DOI] [PubMed] [Google Scholar]
- 64.Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Lyytinen H, Whitfield-Gabrieli S, Glover GH, Reiss AL, et al. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci U S A. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]