Abstract
The germline and embryo of the nematode Caenorhabditis elegans have emerged as powerful model systems to study membrane dynamics in an intact, developing animal. In large part, this is due to the architecture of the reproductive system, which necessitates de novo membrane and organelle biogenesis within the stem cell niche to drive compartmentalization throughout the gonad syncytium. Additionally, membrane reorganization events during oocyte maturation and fertilization have been demonstrated to be highly stereotypic, facilitating the development of quantitative assays to measure the impact of perturbations on protein transport. This review will focus on regulatory mechanisms that govern protein trafficking, which have been elucidated using a combination of C. elegans genetics, biochemistry, and high-resolution microscopy. Collectively, studies using the simple worm highlight an important niche that the organism holds to define new pathways that regulate vesicle transport, many of which appear to be absent in unicellular systems but remain highly conserved in mammals.
Keywords: autophagy, embryo, endocytosis, germline, oocyte, secretion
INTRODUCTION
While the core components of most trafficking pathways have been well defined, regulatory factors that augment or attenuate transport networks remain poorly characterized. This deficiency is most obvious in the context of metazoan development, which cannot be addressed through the use of mammalian cells grown in culture. Instead, studies must be conducted in animal models, including mice, zebrafish, Drosophila, and C. elegans. Although each system possesses specific benefits and drawbacks, the genetic and biochemical tractability of the simple worm have helped to cement its position at the forefront of the membrane biology field (1–3). Several features of the C. elegans reproductive system in particular are highly amenable to the study of membrane trafficking and enable quantitative analysis of cargo transport and organelle remodeling during development. First, the organization of the syncytial gonad (Figure 1) permits the use of RNA interference (RNAi) to generate a tissue that is reproducibly depleted of a specific target protein, independently of its intrinsic turnover rate (4). Protein present following dsRNA delivery is systematically removed by continual packaging into oocytes, which are fertilized every 22–24 minutes and exit the reproductive system (5). Within 48 hours after dsRNA administration, newly formed oocytes typically contain <5% of a targeted protein, allowing for analysis of cargo sorting in its absence. Shorter timecourses enable partial depletion of target proteins, which are critical for germline formation, enabling an analysis of embryonic development under partial loss-of-function conditions. Second, hundreds of C. elegans mutant strains that exhibit defects in transport through the endomembrane system are currently available for examination (6). Importantly, this collection contains animals that harbor deletion alleles, which are predicted to completely eliminate protein function. In contrast to siRNA- or morpholino-based studies, the use of null alleles in C. elegans eliminates the possibility of inefficient knock down confounding functional analysis of targets. Third, membrane reorganization events during germline development, oocyte maturation and fertilization are highly stereotypic, which has facilitated the establishment of quantitative assays to measure the impact of perturbations on protein transport (7,8). Fourth, C. elegans is highly amenable to genetic manipulation and can be engineered to stably express fluorescently tagged proteins, including secretory and endocytic cargoes that can be monitored by live cell microscopy (9,10). Fifth, large quantities of intact animals or embryos can be generated in liquid culture, enabling the biochemical purification of trafficking complexes during different stages of development (11). Finally, sequence analysis of C. elegans trafficking components demonstrates that they are closely related to those present in mammals, strongly suggesting that findings in worms will be directly applicable to human biology (12; Tables S1 and S2). Collectively, these attributes have been exploited to define new regulatory components that function in the secretory, endocytic, and autophagic pathways. Although this review will focus mostly on studies conducted in the C. elegans germline and early embryo, work performed in other tissues will also be highlighted to further emphasize the importance of the worm to the field of membrane trafficking.
Figure 1.
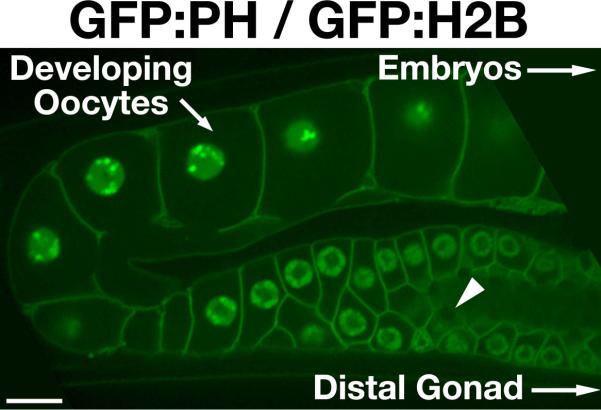
Organization of the C. elegans syncytial gonad. Swept field confocal optics were used to image a transgenic animal co-expressing GFP fusions to histone H2B (HIS-58), to mark the DNA, and the PH domain of rat PLC1δ, which localizes to the plasma membrane. The gonad is a syncytial tube that contains multiple nuclei at various cell cycle stages. In the distal region (not shown), mitotic nuclei continually proliferate, feeding additional germ cells into the system. As the nuclei progress and mature, they enter different stages of meiotic prophase and produce mRNA. Translated messages generate protein that is loaded into developing oocytes through structures resembling ring canals (example highlighted by an arrowhead). Only the final 4–5 oocytes are diffusionally restricted from the syncytium. As oocytes pass through the spermatheca, they are fertilized and resume cell cycle progression. Ovulation is highly stereotypic, occurring every 22–24 minutes in fertile animals. Introduction of dsRNA triggers degradation of target mRNA in the gonad. Remaining protein present at the time of dsRNA delivery is depleted by continual packaging of gonad cytoplasm into developing oocytes. Within 36–48 hours following dsRNA treatment, the majority of target protein is depleted (typically >95% following injection of dsRNA), and the first embryonic cell division can be analyzed. Scale bar, 10 μm.
The Secretory Pathway
Biosynthetic cargoes leave the endoplasmic reticulum (ER) in COPII coated vesicles, which are generated at specialized lipid subdomains marked by the peripheral membrane protein Sec16 (13,14). Based on its purification from embryos, C. elegans SEC-16 forms a complex with several COPII subunits (SEC-23, SEC-24, NPP-20/SEC-13, and SEC-31) and promotes their recruitment onto ER membranes (15). Depletion studies indicate that loss of SEC-16 or any individual COPII subunit results in early embryonic lethality or sterility of the treated animal (15–18). Embryos that are produced under partial loss-of-function conditions are strongly osmotically sensitive, likely resulting from a defect in eggshell formation, a phenotypic hallmark of disrupting secretory transport. The C. elegans eggshell is a trilaminar structure comprised of an outer vitelline layer, a medial layer enriched in chitin, and an inner layer that contains chondroitin proteoglycans (19,20). An additional permeability barrier also forms between the embryo plasma membrane and the eggshell, which restricts the flow of small molecules (21). Live-cell imaging experiments revealed that the inner chondroitin layer of the eggshell forms during anaphase of the first meiotic cell cycle, which is triggered by oocyte fertilization, and is coincident with the exocytosis of cortical granules that contain caveolin-1 (21–23). Consistent with these findings, two chondroitin proteoglycans (CPG-1 and CPG-2) appear to be cargoes of these granules (21). Although the precise requirements for cortical granule formation remain unclear, analysis of caveolin-1 trafficking suggests that the early secretory pathway plays a significant role (22). Thus, disruption of ER/Golgi transport inhibits normal eggshell formation, resulting in embryo osmotic sensitivity.
Since eggshell formation is severely compromised in the absence of ER export, analysis of COPII function during later stages of embryogenesis has been challenging. However, in a screen for mutants defective for extracellular matrix formation, an allele of sec-23 (ij13) was identified, implicating COPII-mediated vesicle transport in cuticular collagen secretion (17). The ij13 mutation introduces a premature stop codon into the coding sequence, resulting in a non-functional, truncated form of SEC-23 lacking its carboxyl-terminal gelsolin domain, which is critical for association with the COPII components SAR-1 and SEC-31 (24–28). Homozygous mutant embryos generated by heterozygous hermaphrodites contain sufficient maternally deposited SEC-23 for early stages of embryogenesis. However, during embryo elongation, the lack of functional SEC-23 causes rupturing from the hypodermal surface, due to a disruption in collagen secretion. Additionally, formation of the pharynx fails, followed rapidly by a general loss of embryo structural integrity, suggestive of a defect in cell adhesion (17). Consistent with these effects, loss of function mutations in Drosopholia COPII components also disrupt cuticle formation and organogenesis (29,30). Together, these data indicate that COPII function remains essential throughout embryogenesis by regulating key morphogenesis steps during development.
Germline maintenance also requires ongoing COPII-mediated vesicle trafficking. In particular, organization within the distal portion of the germline requires constitutive membrane biogenesis to partition nuclei formed within the mitotic stem cell niche (31). In the absence of normal COPII assembly, compartmentalization fails, resulting in multinucleation, another hallmark of secretory pathway dysfunction (Figure 2). Moreover, this defect is propagated throughout the reproductive system, visualized by the apparent premature expansion of individual compartments at the turn region of the gonad arm. Based on a high throughput RNAi-based screen, this phenotype is not only shared among many components of the COPII machinery (SAR-1, SEC-12, and SEC-24.1), but also several additional factors that function in the early secretory pathway including Arf and Rab-type GTPases (RAB-1, ARF-1 and ARF-3), subunits of the COPI coat, which mediate cargo recycling to the ER, components of the TRAPP (Transport Protein Particle) tethering complexes, factors required for SNARE-mediated membrane fusion (NSF-1, SNAP-1, SNAP-29, and SYX-18), lipid-modifying enzymes required for the acylation of numerous trafficking components (NMT-1 and GGTB-1), and the SEC-61 complex that mediates the translocation of secretory pathway cargoes into the ER (31). Interestingly, depletion of SEC-24.2, a second isoform of the COPII cargo selection factor, results in a distinct phenotype, in which oocytes fail to bud away from the syncytium efficiently, but still causes sterility in adult animals (31). These data strongly suggest that each SEC-24 isoform regulates a unique set of cargoes required for different stages of germline development. Importantly, the findings also highlight C. elegans as an attractive, tractable setting to define mechanisms that underlie the ability of SEC-24 isoforms to distinguish cargo molecules, which may be conserved in other systems (from yeast to man) that all express multiple Sec24 homologs.
Figure 2.
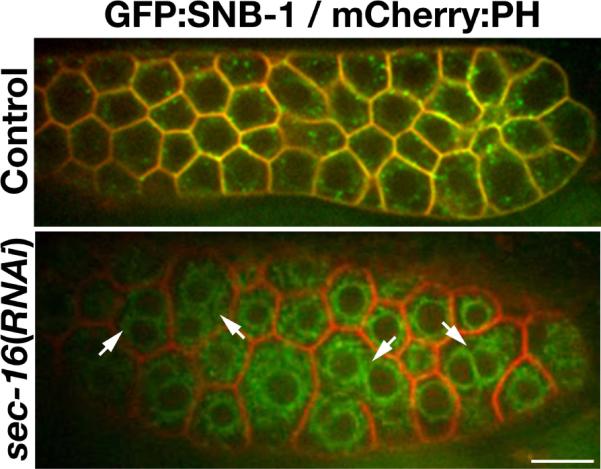
A defect in secretory pathway function results in multinucleation within the distal region of the germline. Swept field confocal optics were used to image a transgenic animal co-expressing a GFP fusion to the v-SNARE SNB-1, a cargo of the secretory pathway, and a mCherry fusion to the PH domain of rat PLC1δ, which localizes to the plasma membrane. In control animals (top), the majority of the v-SNARE localizes to the cell surface, indicating normal function of the secretory system. However, in animals depleted of SEC-16, a critical component of the early secretory pathway, the v-SNARE becomes trapped throughout the ER and fails to co-localize with the PH domain of PLC1δ at the plasma membrane. In many cases, multiple nuclei are observed within an individual membrane compartment (highlighted by arrows), likely resulting from a failure to secrete sufficient membrane to generate partitions following germline mitosis. Scale bar, 10 μm.
In addition to the well-characterized factors described above, several additional proteins were implicated in secretory pathway function as a result of their phenotypes following depletion. In particular, animals lacking TFG-1 exhibit premature compartment expansion within the germline and embryos produced are osmotically sensitive (31). Further analysis indicated that TFG-1 binds directly to SEC-16 and assembles into a matrix that extends away from sites of COPII vesicle biogenesis toward the ER-Golgi intermediate compartment (ERGIC; 15). Biochemical and live-cell imaging studies further demonstrated that depletion of TFG-1 disrupts COPII complex formation, thereby inhibiting protein secretion (15). Notably, TFG-1 function is conserved in human cells, but is apparently absent in unicellular organisms including yeast. Therefore, these studies underscore the importance of C. elegans in identifying new, conserved components of the early secretory pathway. In a similar manner, the conserved ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs)-like metalloprotease GON-1 has also been implicated in secretory pathway function. Although its direct contribution to protein trafficking remains unclear, depletion of GON-1 dramatically alters ER structure, disrupts germline architecture, and induces an ER stress response, similar to the impact of inhibiting COPII vesicle secretion (32). Notably, similar effects on ER organization and protein secretion were observed in human cells following depletion of the GON-1 homolog ADAMTS9 (32). These findings further highlight the importance of C. elegans in the identification of conserved trafficking genes that are important for development in metazoans.
While powerful, it is important to point out that high throughput, reverse genetics-based screens possess limitations, as some targets are refractory to RNAi. For example, while depletion of PIGA-1, the catalytic subunit of the phosphatidylinositol N-acetylglucosaminyltransferase complex that catalyzes the first step of GPI-anchor synthesis, fails to affect viability, isolation of a deletion mutation within piga-1 demonstrated that GPI biogenesis is essential for germline maintenance and eggshell formation (33). In the future, the use piga-1 mutant animals should enable the identification of all proteins in C. elegans, which are normally subject to GPI-linkage. The effects of individually depleting the modified proteins will then permit determination of key GPI-anchored factors necessary for germline morphogenesis and embryogenesis. Importantly, with several new technologies becoming available to generate targeted deletions in C. elegans (6,34), it should be feasible to confirm and extend many findings made previously using RNAi-based studies.
Although C. elegans ER morphology bears a strong resemblance to that observed in human cells (35), the organization and distribution of the Golgi in worms is distinct. As opposed to a juxtanuclear ribbon-like architecture, individual Golgi ministacks assemble throughout the C. elegans cytoplasm, invariably found adjacent to sites of COPII vesicle biogenesis, forming an integrated secretory unit (15). Although few studies have directly examined requirements for Golgi structure during early development in C. elegans, its role in cargo modification and sorting is clear. In particular, analysis of animals lacking the normal function of the conserved oligomeric Golgi (COG) complex revealed a key role for this tethering factor in the formation of fucose-rich N-glycans and fucosylation of terminal residues on N-glycan branches (36,37). Furthermore, inhibition of the COG complex causes a defect in the migration of distal tip cells (DTCs) within the germline, which leads to aberrant gonad morphogenesis (38,39). In part, this phenotype is due to a defect in the glycosylation of another ADAMTS-like protease (MIG-17). More importantly however, these studies helped to define the importance of the COG complex in protein glycosylation during organ development, which is likely conserved in mammals as a mutation in one of its subunits was previously implicated in a human congenital disorder of glycosylation (CDG; 40). Patients with a defect in COG function exhibit multisystem developmental abnormalities, often leading to death due to multiple organ failure. These findings suggest that C. elegans may serve as an ideal model system to dissect the precise molecular function of the COG complex in human disease (41).
Similar to other systems, post-Golgi secretion in C. elegans depends on a set of Rab-type GTPases, SNAREs, lipids, tethering factors and coat proteins. RAB-8 and RAB-10 appear to function redundantly in germline secretion, together with their effector EHBP-1, a calponin homology (CH) domain containing protein (42,43). Interestingly, unlike most other Rab effectors, EHBP-1 also functions to recruit its Rab partner onto membranes, a relationship that is shared by one of its mammalian counterparts, MICAL-L1, and may represent a new theme in Rab-effector associations that govern membrane transport steps (44). In addition to the constitutive secretory pathway, both Rab6 and Rab11 isoforms have been implicated in regulated cortical granule exocytosis. C. elegans RAB-11.1 contributes to the targeting of cortical granules to the cell surface, potentially in coordination with the SNAREs, SYN-4 and SNB-1 (23). In contrast, RAB-6.1 and RAB-6.2 function independently of RAB-11.1 to target the C. elegans isoform of separase (SEP-1) to cortical granules, where it appears to play a role in membrane fusion (45,46). Although a mechanistic understanding of SEP-1 function in membrane trafficking remains elusive, studies in other organisms have confirmed a role for separase in protein secretion (47,48). Defining the substrates of separase on membranes will be critical for understanding its function in this pathway.
The endocytic pathway and autophagy
The uptake of nutrients and other macromolecules into cells typically requires the active process of endocytosis, which is initiated at the plasma membrane, often in a receptor-dependent manner. In oocytes, the internalization of yolk lipoprotein particles and cholesterol has been studied extensively and is dependent upon RME-2, a member of the LDL receptor superfamily, which is named for its role in receptor mediated endocytosis (49). Mutations in RME-2 reduce oocyte viability and cause a defect in ovulation, suggesting multiple requirements for lipid uptake during early development (16,49). Using transgenic animals expressing a GFP-tagged yolk protein, genetic screens have identified numerous regulators of endocytic transport (49,50). Based on these and other studies, clathrin-mediated endocytosis has proven to be the predominant route by which extracellular materials are internalized in the germline and embryos. In contrast, simultaneous loss-of-function mutations in both C. elegans caveolin isoforms (CAV-1 and CAV-2) fail to significantly impact viability or embryonic development, although the rate of egg laying is slightly reduced in these animals (23).
Experiments to inhibit clathrin heavy chain function, either through the use of RNAi or a temperature sensitive mutant, indicate a key role for endocytic transport in the proximal germline and also during embryogenesis (16,31,51). By contrast, the distal region of the gonad is not significantly disrupted in the absence of clathrin function, and even oocytes continue to form. However, the permeability barrier surrounding embryos fails to assemble normally under these conditions, potentially due to the lack of internalized lipid species that are critical for this process, resulting in osmotic sensitivity (21). Surprisingly, animals harboring a null mutation in the only clathrin light chain isoform identified in C. elegans do not exhibit a strong phenotype, suggesting that the heavy chain is capable of generating coated pits and endocytic vesicles without the regulatory function of a light chain. In a similar vein, individual deletion mutations in several clathrin adaptor proteins, which link clathrin to membranes and cargoes, are also well tolerated, including components of the AP-2 complex (52), Eps15 (EHS-1; 53,54), intersectin (ITSN-1; 53,54), stonin (UNC-41; 55), AP180 (UNC-11; 56), Disabled (DAB-1; 57), and NUMB (NUM-1; 58). By contrast, a deletion mutation in the gene encoding epsin (epn-1) is lethal. However, RNAi experiments indicate that depletion of epsin fails to phenocopy the effect of clathrin heavy chain inhibition during germline formation and embryonic growth (16). Collectively, these data argue against models in which a single adaptor protein or adaptor complex is essential for all forms of clathrin-mediated endocytosis, and instead indicates a high level of redundancy among these factors, especially during embryogenesis. The further analysis of animals lacking the function of specific adaptors will be useful in delineating their unique roles throughout development.
Based on effects in the germline and early embryo, clathrin heavy chain depletion most strongly resembles inhibition of the Rab-type GTPase RAB-5, suggesting that they function in a common pathway (16,42,51). Consistent with this finding, the RAB-5 exchange factor RME-6 is recruited to sites of clathrin-mediated endocytosis, via an interaction with the AP-2 complex, and activates RAB-5 at nascent pits or vesicles prior to uncoating (59). As suggested by its name, RME-6 was discovered in a genetic screen for C. elegans mutants that exhibit a defect in receptor-mediated endocytosis (49). Subsequent studies in human cells confirmed these findings and further demonstrated that hRME-6 and Rab5 promote AP-2 disassembly (60). In the same genetic screen, a DENN domain protein, which was named RME-4, was also identified and shown to interact with AP-2. Moreover, RME-4 is responsible for recruiting another Rab-type GTPase (RAB-35) to clathrin-coated pits and/or vesicles to act in subsequent cargo recycling steps (61). The RME screen further isolated mutations in RME-1, an EH-domain protein, and RME-8, a DnaJ protein, which both function in a conserved fashion to regulate endosomal trafficking (62–65). Notably, the majority of RME proteins discovered using C. elegans lack orthologs in lower eukayotes, again highlighting the important role played by worms in deciphering mechanisms of membrane transport in multicellular organisms.
Studies in the C. elegans germline, embryo, and other tissues have also influenced the current view of cargo movement through the endo-lysosomal system. Work in human cells initially illustrated a key role for Rab5 to Rab7 conversion in redefining endosome identity (66). However, regulatory mechanisms underlying this process remained poorly defined. Analysis of a mutation in C. elegans SAND-1 shed new light on the conversion process. Originally identified as a regulator of RAB-7 in oocytes and coelomocytes (67), SAND-1 was subsequently shown to interact with the RAB-5 exchange factor RABX-5 to promote its dissociation from endosomal membranes, thereby driving endosome maturation. In the absence of SAND-1 function, early endosomes swell due to constitutive Rab5-mediated homotypic fusion events. Additionally, SAND-1 interacts with components of the HOPS (homotypic fusion and vacuole protein sorting) complex, which further facilitates Rab conversion (68). Notably, the role for SAND-1 in endosome maturation is also conserved in human cells (68). In parallel with these studies, animals harboring a mutation in the RAB-5 GAP TBC-2 were shown to similarly accumulate enlarged endosomes within intestinal cells (69). However, in this case, the endosomes were RAB-7 positive, suggesting a role for TBC-2 in terminating RAB-5 function following Rab conversion. Consistent with this idea, TBC-2 co-localizes with RAB-7 on late endosomes (69).
In addition to studies focused on Rab conversion, the regulation of early endosome homotypic fusion has also been investigated using C. elegans. Similar to work conducted in mammalian cells, overexpression of a GTP-locked isoform of RAB-5 (Q78L) causes a dramatic increase in endosome size in oocytes, embryos, and other tissues (42,70). In contrast, inhibition of Rab5-mediated fusion, through inactivation of the RAB-5 effector RABS-5 (Rabenosyn-5), leads to the formation of smaller endosomes in coelomocytes (70). Analysis of an animal carrying a deletion mutation in the SM protein VPS-45 revealed phenotypes very similar to that exhibited by worms lacking RABS-5 expression. Moreover, loss of VPS-45 suppressed the effect of overexpressing GTP-locked RAB-5, consistent with a role for the SM protein in homotypic early endosome fusion (70). Together, these studies highlight the multiple routes by which Rab5 and Rab7 activities are regulated, which are likely conserved in mammals.
Rab conversion is accompanied by the formation of intralumenal vesicles within endosomes, generating a specialized compartment known as the multivesicular endosome (Figure 3). The formation of these organelles is necessary for the turnover of integral membrane proteins within lysosomes and requires a set of 5 protein complexes collectively known as the ESCRT machinery (71,72). Early acting ESCRT components (ESCRT-0, ESCRT-I, and ESCRT-II) participate in ubiquitin-dependent cargo recruitment and potentially the initiation of inward membrane bending. The downstream factors (ESCRT-III and the Vps4 complex) drive membrane scission to release vesicles into the endosomal lumen (73,74). Consistent with findings in other systems, inhibition of individual ESCRT subunits in C. elegans results in a defect in endosomal trafficking to lysosomes and perturbations to endosome morphology during embryogenesis. In particular, depletion of the ESCRT-III subunit VPS-32 arrests embryonic development during body elongation, suggesting a defect in epidermal morphogenesis (75). Electron microscopy-based studies revealed that the lumen of the pharynx and the intestine fail for form without ESCRT function, likely resulting from a failure in epidermal cell alignment during organogenesis (75). In vitro, VPS-32 assembles into filaments nucleated by a complex of ESCRT-II and the ESCRT-III subunit VPS-20. Interestingly, analysis of reconstituted filaments using atomic force microscopy showed that they exhibit potent sensitivity to membrane curvature (76). These findings using recombinant C. elegans proteins demonstrated that the ESCRT machinery is capable of recognizing highly curved membranes, similar to those found at nascent vesicle bud necks, and likely directs ESCRT scission activity specifically to sites of vesicle formation on endosomes.
Figure 3.
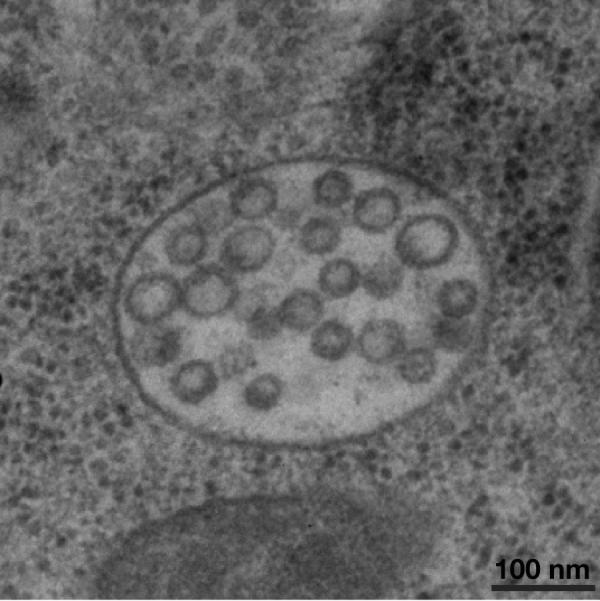
Morphology of multivesicular endosomes that form within the C. elegans one-cell stage embryo. Intact animals were subject to high pressure freezing, followed by freeze substitution and embedding in plastic. Thin sections (60 nm) were cut using an ultramicrotome, and processed for imaging using a Philips CM120 electron microscope. Embryos at the one cell stage contain numerous multivesicular endosomes that range in size from 400–500 nm in diameter. The majority of intralumenal vesicles observed were 47–50 nm in diameter. The stereotypic ovulation of oocytes in C. elegans, which delivers newly fertilized embryos into the uterus directly adjacent to the spermatheca every 22–24 minutes provides the spatial cues necessary to study multivesicular endosome dynamics following high pressure freezing and EM analysis of intact animals. Artificial stimulation of receptor downregulation, a commonly used technique in other metazoan systems to analyze requirements for protein transport to the lysosome, is unnecessary in C. elegans. Oocyte maturation and fertilization reproducibly trigger the internalization and degradation of multiple transmembrane cargoes in one-cell stage embryos. Scale bar, 100 nm.
In addition to its function on endosomes, analysis of a C. elegans mutant defective in lipid distribution revealed that the ESCRT machinery can also function at the cell surface in embryos. Specifically, loss of the P-type ATPase TAT-5 perturbs the asymmetric accumulation of phospholipids in the plasma membrane and promotes ESCRT recruitment (77). At this site, the ESCRT machinery can promote membrane bending away from the cytoplasm and thereby drives the budding of vesicles into the extracellular space. These studies suggest that lipid asymmetry plays an important role during ESCRT-mediated vesicle budding, a concept that may have a significant impact on the future study of multivesicular endosome formation in all organisms.
While the ESCRT machinery plays an essential role to turnover ubiquitin-modified integral membrane proteins, an alternative pathway known as autophagy is utilized for the degradation of other materials, such as aging organelles and intracellular pathogens. In C. elegans, P granule components also undergo autophagy specifically in somatic cells during embryogenesis, to restrict their localization exclusively to germline precursor cells (78). Taking advantage of this phenomenon, a genetic screen uncovered four metazoan-specific autophagy genes (EPG-2, EPG-3, EPG-4, and EPG-5). Of these, three possess homologs in humans, which were also found to function at various steps of autophagosome formation (79). Subsequent studies have identified additional EPG proteins, which similarly bear homology to conserved components of the autophagy system in mammalian cells (80–82). Thus, these studies provide yet another example in which C. elegans has played an important role in understanding the molecular basis of an essential trafficking pathway, and will likely be useful in the future to provide mechanistic insights into this process during development. Finally, recent work using C. elegans as a model also established a key role for autophagy in selectively degrading paternal mitochondria following fertilization (83). These studies provide a potential mechanism by which maternal inheritance of mitochondrial DNA is ensured during reproduction.
Concluding Remarks
Over the past several decades, tremendous advances have been made in our understanding of membrane trafficking. Genetic screens conducted in yeast, combined with biochemical assays to explore pathways in cultured mammalian cells, have generated a map of the core machinery necessary to transport cargoes throughout cells. However, regulatory systems that govern membrane trafficking during development have been more difficult to dissect. To address this issue, several multicellular organisms became popular for the study of membrane dynamics. In particular, the C. elegans germline and embryo offered convenient, genetically tractable settings for discovery. Based on recent findings, it has become clear that the metazoan trafficking network has evolved beyond that found in lower eukaryotes, a necessity to accommodate changes in cellular function that occur subsequent to developmental cues, and C. elegans provides an excellent model for further exploration. While the secretory pathway plays a prominent role to establish germline tissue, endocytosis becomes more crucial later in development to foster oocyte viability. The oocyte-to-embryo transition is accompanied by dramatic changes in organelle architecture and function, which enables the shift from a relatively quiescent maturation stage to a highly active state of embryonic division and organogenesis. These aspects of C. elegans development have been exploited using genetics, biochemistry, and live animal imaging to define new, conserved regulators of secretion, endocytosis and autophagy. Future work that takes advantage of these characteristics will undoubtedly yield additional new information regarding the plasticity of membrane transport, which is necessary during growth and differentiation.
Supplementary Material
ACKNOWLEDGEMENTS
Work performed in the laboratory of A. Audhya is supported in part by the National Institutes of Health (grant 1R01GM088151). Additional support comes from the American Heart Association (grant SDG3720032), the American Cancer Society (grant 123268-RSG-12-139-01-CSM), and the Greater Milwaukee Foundation (MSN133969).
REFERENCES
- 1.Wang Z, Sherwood DR. Dissection of genetic pathways in C. elegans. Methods Cell Biol. 2011;106:113–157. doi: 10.1016/B978-0-12-544172-8.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moresco JJ, Carvalho PC, Yates JR. Identifying components of protein complexes in C. elegans using co-immunoprecipitation and mass spectrometry. J Proteomics. 2010;73:2193–2204. doi: 10.1016/j.jprot.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BD, Sato M. In: Intracellular trafficking (January 21, 2006) Wormbook, editor. WormBook; The C. elegans Research Community: doi/10.1895/wormbook.1.77.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oegema K, Hyman AA. In: Cell division (January 19, 2006) WormBook, editor. WormBook; The C. elegans Research Community: doi/10.1895/wormbook.1.72.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenstein D. In: Control of oocyte meiotic maturation and fertilization (December 28, 2005) WormBook, editor. WormBook; The C. elegans Research Community: doi/10.1895/wormbook.1.53.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moerman DG, Barstead RJ. Towards a mutation in every gene in Caenorhabditis elegans. Brief Funct Genomic Proteomic. 2008;7:195–204. doi: 10.1093/bfgp/eln016. [DOI] [PubMed] [Google Scholar]
- 7.Audhya A, McLeod IX, Yates JR, Oegema K. MVB-12, a fourth subunit of metazoan ESCRT-I, functions in receptor downregulation. PLoS ONE. 2007;2:e956. doi: 10.1371/journal.pone.0000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcello MR, Singaravelu G, Singson A. Fertilization. Adv Exp Med Biol. 2013;757:321–350. doi: 10.1007/978-1-4614-4015-4_11. [DOI] [PubMed] [Google Scholar]
- 9.Frokjaer-Jensen C, Davis MW, Ailion M, Jorgensen EM. Improved Mos1-mediated transgenesis in C. elegans. Nat Methods. 2012;9:117–118. doi: 10.1038/nmeth.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Praitis V, Maduro MF. Transgenesis in C. elegans. Methods Cell Biol. 2011;106:161–185. doi: 10.1016/B978-0-12-544172-8.00006-2. [DOI] [PubMed] [Google Scholar]
- 11.Audhya A, Desai A. Proteomics in Caenorhabditis elegans. Brief Funct Genomic Proteomic. 2008;7:205–210. doi: 10.1093/bfgp/eln014. [DOI] [PubMed] [Google Scholar]
- 12.Shaye DD, Greenwald I. OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One. 2011;6:e20085. doi: 10.1371/journal.pone.0020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budnik A, Stephens DJ. ER exit sites-localization and control of COPII vesicle formation. FEBS Lett. 2009;583:3796–3803. doi: 10.1016/j.febslet.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2011;13:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 15.Witte K, Schuh AL, Hegermann J, Sarkeshik A, Mayers JR, Schwarze K, Yates JR, Eimer S, Audhya A. TFG-1 function in protein secretion and oncogenesis. Nat Cell Biol. 2011;13:550–558. doi: 10.1038/ncb2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sönnichsen B, Koski L, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume A, Artelt J, Bettencourt P, Cassin E, Hewitson M, Holz C, Khan M, Lazik S, Martin C, Nitzsche B, Ruer M, Stamford J, Winzi M, Heinkel R, Roder M, Finell J, Hantsch H, Jones S, Jones M, Piano F, Gunsalus K, Oegema K, Gönczy P, Coulson A, Hyman AA, Echeverri CJ. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- 17.Roberts B, Clucas C, Johnstone IL. Less of SEC-23 in Caenorhabditis elegans causes defects in oogenesis, morphogenesis and extracellular matrix secretion. Mol Biol Cell. 2003;14:4414–4426. doi: 10.1091/mbc.E03-03-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galy V, Mattaj IW, Askjaer P. Caenorhabditis elegans nucleoporins Nup95 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol Biol Cell. 2003;14:5104–5115. doi: 10.1091/mbc.E03-04-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wharton D. Nematode egg-shells. Parasitology. 1980;81:447–463. doi: 10.1017/s003118200005616x. [DOI] [PubMed] [Google Scholar]
- 20.Johnston WL, Dennis JW. The eggshell in the C. elegans oocyte-to-embryo transition. Genesis. 2012;50:333–349. doi: 10.1002/dvg.20823. [DOI] [PubMed] [Google Scholar]
- 21.Olson SK, Greenan G, Desai A. Muller-Reichert T, Oegema K. Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J Cell Biol. 2012;198:731–748. doi: 10.1083/jcb.201206008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato K, Sato M, Audhya A, Oegema K, Schweinsberg P, Grant BD. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol Biol Cell. 2006;17:3085–3094. doi: 10.1091/mbc.E06-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Grant BD, Harada A, Sato K. Rab11 is required for synchronous secretion of chondroitin proteoglycans after fertilization in Caenorhabditis elegans. J Cell Sci. 2008;121:3177–3186. doi: 10.1242/jcs.034678. [DOI] [PubMed] [Google Scholar]
- 24.Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23-Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635–645. doi: 10.1016/j.devcel.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 26.Stagg SM, LaPointe P, Razvi A, Gurkan C, Potter CS, Carragher B, Balch WE. Structural basis for cargo regulation of COPII coat assembly. Cell. 2008;134:474–484. doi: 10.1016/j.cell.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell C, Stagg SM. New insights into the structural mechanisms of the COPII coat. Traffic. 2010;11:303–310. doi: 10.1111/j.1600-0854.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee MC, Miller EA. Molecular mechanisms of COPII vesicle formation. Sem Cell Dev Biol. 2007;18:424–434. doi: 10.1016/j.semcdb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Norum M, Tang E, Chavoshi T, Schwarz H, Linke D, Uv A, Moussian B. Trafficking through COPII stabilizes cell polarity and drives secretion during Drosophila epidermal differentiation. PLoS One. 2010;5:e10802. doi: 10.1371/journal.pone.0010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forster D, Armbruster K, Luschnig S. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr Biol. 2010;20:62–68. doi: 10.1016/j.cub.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 31.Green RA, Kao HL, Audhya A, Arur S, Mayers JR, Fridolfsson HN, Schulman M, Schloissnig S, Niessen S, Laband K, Wang S, Starr DA, Hyman AA, Schedl T, Desai A, Piano F, Gunsalus KC, Oegema K. A high-resolution C. elegans essential gene network based on phenotypic profiling of a complex tissue. Cell. 2011;145:470–482. doi: 10.1016/j.cell.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshina S, Sakaki K, Yonezumi-Hayashi A, Gengyo-Ando K, Inoue H, Iino Y, Mitani S. Identification of a novel ADAMTS9/GON-1 function for protein transport from the ER to the Golgi. Mol Biol Cell. 2012;23:1728–1741. doi: 10.1091/mbc.E11-10-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murara D, Nomura KH, Dejima K, Mizuguchi S, Kawasaki N, Matsuishi-Nakajima Y, Ito S, Gengyo-Ando K, Kage-Nakadai E, Mitani S, Nomura K. GPI-anchor synthesis is indispensable for the germline development of the nematode Caenorhabditis elegans. Mol Biol Cell. 2012;23:982–995. doi: 10.1091/mbc.E10-10-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frojaer-Jensen C, Davis MW, Hollopeter G, Taylor J, Harris TW, Nix P, Lofgren R, Prestgard-Duke M, Bastiani M, Moerman DG, Jorgensen EM. Targeted gene deletions in C. elegans using transposon excision. Nat Methods. 2010;7:451–453. doi: 10.1038/nmeth.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poteryaev D, Squirrell JM, Campbell JM, White JG, Spang A. Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans. Mol Biol Cell. 2005;16:2139–2153. doi: 10.1091/mbc.E04-08-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubota Y, Sano M, Goda S, Suzuki N, Nishiwaki K. The conserved oligomeric Golgi complex acts in organ morphogenesis via glycosylation of an ADAM protease in C. elegans. Development. 2006;133:263–273. doi: 10.1242/dev.02195. 2006. [DOI] [PubMed] [Google Scholar]
- 37.Struwe WB, Reinhold VN. The conserved oligomeric Golgi complex is required for fucosylation of N-glycans in Caenorhabditis elegans. Glycobiology. 2012;22:863–875. doi: 10.1093/glycob/cws053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishiwaki K. Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics. 1999;152:985–997. doi: 10.1093/genetics/152.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blelloch R, Kimble J. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 1999;399:586–590. doi: 10.1038/21196. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Steet RA, Bohorov O, Bakker J, Newell J, Krieger M, Spaapen L, Kornfeld S, Freeze HH. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med. 2004;10:518–523. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- 41.Kubota Y, Nishiwaki K. C. elegans as a model system to study the function of the COG complex in animal development. Biol Chem. 2006;387:1031–1035. doi: 10.1515/BC.2006.127. [DOI] [PubMed] [Google Scholar]
- 42.Audhya A, Desai A, Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. J Cell Biol. 2007;178:43–56. doi: 10.1083/jcb.200701139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi A, Chen CC, Banerjee R, Glodowski, Audhya A, Rongo C, Grant BD. EHBP-1 functions with RAB-10 during endocytic recycling in Ceanorhabditis elegans. Mol Biol Cell. 2010;21:293–2943. doi: 10.1091/mbc.E10-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma M, Giridharan SS, Rahajeng J, Naslavsky N, Caplan S. MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol Biol Cell. 2009;20:5181–5194. doi: 10.1091/mbc.E09-06-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura K, Kimura A. Rab6 required for the exocytosis of cortical granules and the recruitment of separase to the granules during the oocyte-to-embryo transition in Caenorhabditis elegans. J Cell Sci. 2012 doi: 10.1242/jcs.116400. in press. [DOI] [PubMed] [Google Scholar]
- 46.Bembenek JN, White JG, Zheng Y. A role for separase in the regulation of RAB-11-positive vesicles at the cleavage furrow and midbody. Curr Biol. 2010;20:259–264. doi: 10.1016/j.cub.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bacac M, Fusco C, Planche A, Santodomingo J, Demaurex N, Leemann-Zakaryan R, Provero P, Stamenkovic I. Securin and separase modulate membrane traffic by affecting endosomal acidification. Traffic. 2011;12:615–626. doi: 10.1111/j.1600-0854.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- 48.Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, Perrimon N, Malhotra V. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439:604–607. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- 49.Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 51.Sato K, Ernstrom GG, Watanabe S, Weimer RM, Chen CH, Sato M, Siddiqui A, Jorgensen EM, Grant BD. Differential requirements for clathrin in receptor-mediated endocytosis and maintenance of synaptic vesicle pools. Proc Natl Acad Sci USA. 2009;106:1139–1144. doi: 10.1073/pnas.0809541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu M, Schuske K, Watanabe S, Liu Q, Baum P, Garriga G, Jorgensen EM. Mu2 adaptin facilitates but is not essential for synaptic vesicle recycling in Caenorhabditis elegans. J Cell Biol. 2008;183:881–892. doi: 10.1083/jcb.200806088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salcini AE, Hilliard MA, Croce A, Arbucci S, Luzzi P, Tacchetti C, Daniell L, De Camilli P, Pelicci PG, Di Fiore PP, Bazzicalupo P. The Eps15 C. elegans homogue EHS-1 is implicated in synaptic vesicle recycling. Nat Cell Biol. 2001;3:755–760. doi: 10.1038/35087075. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Bouhours M, Gracheva EO, Liao EH, Xu K, Sengar AS, Xin X, Roder J, Boone C, Richmond JE, Zhen M, Egan SE. ITSN-1 controls vesicle recycling at the neuromuscular junction and functions in parallel with DAB-1. Traffic. 2008;9:742–754. doi: 10.1111/j.1600-0854.2008.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mullen GP, Grundahl KM, Gu M, Wantanabe S, Hobson RJ, Crowell JA, McManus JR, Mathews EA, Jorgensen EM, Rand JB. UNC-41/stonin functions with AP2 to recycle synaptic vesicles in Caenorhabditis elegans. PLoS One. 2012;7:e40095. doi: 10.1371/journal.pone.0040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, Wei L, Hartwieg E, Jorgensen EM, Alfonso A. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol Biol Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holmes A, Flett A, Coudreuse D, Korswagen HC, Pettitt J. C. elegans Disabled is required for cell-type specific endocytosis and is essential in animals lacking the AP-3 adaptor complex. J Cell Sci. 2007;120:2741–2751. doi: 10.1242/jcs.03474. [DOI] [PubMed] [Google Scholar]
- 58.Nilsson L, Conradt B, Ruaud AF, Chen CC, Hatzold J, Bessereau JL, Grant BD, Tuck S. Caenorhabditis elegans num-1 negatively regulates endocytic recycling. Genetics. 2008;179:375–387. doi: 10.1534/genetics.108.087247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato M, Sato K, Fonarev P, Huang CJ, Liou W, Grant BD. Caenorhabditis elegans RME-6 is a novel regulator of RAB-5 at the clathrin-coated pit. Nat Cell Biol. 2005;7:559–569. doi: 10.1038/ncb1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semerdjieva S, Shortt B, Maxwell E, Singh S, Fonarev P, Hansen J, Schiavo G, Grant BD, Smythe E. Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J Cell Biol. 2008;183:499–511. doi: 10.1083/jcb.200806016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato M, Sato K, Liou W, Pant S, Harada A, Grant BD. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. EMBO J. 2008;27:1183–1196. doi: 10.1038/emboj.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grant B, Zhang Y, Paupard MC, Lin SX, Hall DH, Hirsh D. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol. 2001;3:573–579. doi: 10.1038/35078549. [DOI] [PubMed] [Google Scholar]
- 63.Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Grant B, Hirsh D. RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol Biol Cell. 2001;12:2011–2021. doi: 10.1091/mbc.12.7.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girard M, Poupon V, Blondeau F, McPherson PS. The DnaJ-domain protein RME-8 functions in endosomal trafficking. J Biol Chem. 2005;280:40135–40143. doi: 10.1074/jbc.M505036200. [DOI] [PubMed] [Google Scholar]
- 66.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 67.Poteryaev D, Fares H, Bowerman B, Spang A. Caenorhabditis elegans SAND-1 is essential for RAB-7 function in endosomal traffic. EMBO J. 2007;26:301–312. doi: 10.1038/sj.emboj.7601498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Chotard L, Mishra AK, Sylvain MA, Tuck S, Lambright DG, Rocheleua CE. TBC-2 regulates RAB-5/RAB-7-mediated endosomal trafficking in Caenorhabditis elegans. Mol Biol Cell. 2010;21:2285–2296. doi: 10.1091/mbc.E09-11-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gengyo-Ando K, Kuroyanagi H, Kobayashi T, Murate M, Fujimoto K, Okabe S, Mitani S. The SM protein VPS-45 is required for RAB-5 dependent endocytic transport in Caenorhabditis elegans. EMBO Rep. 2007;8:152–157. doi: 10.1038/sj.embor.7400882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;19:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 72.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 73.Mayers JR, Audhya A. Vesicle formation within endosomes: An ESCRT marks the spot. Commun Integr Biol. 2012;5:50–56. doi: 10.4161/cib.18208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hurley JH. The ESCRT complexes. Crit Rev Biochem Mol Biol. 2010;45:463–487. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michelet X, Alberti A, Benkemoun L, Roudier N, Lefebvre C, Legouis R. The ESCRT-III protein CeVPS-32 is enriched in domains distinct from CeVPS-27 and CeVPS-23 at the endosomal membrane of epithelial cells. Biol Cell. 2009;101:599–615. doi: 10.1042/BC20090025. [DOI] [PubMed] [Google Scholar]
- 76.Fyfe I, Schuh AL, Edwardson JM, Audhya A. Association of the endosomal sorting complex ESCRT-II with the Vps20 subunit of ESCRT-III generates a curvature-sensitive complex capable of nucleating ESCRT-III filaments. J Biol Chem. 2011;286:34262–34270. doi: 10.1074/jbc.M111.266411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wehman AM, Poggioli C, Schweinsberg P, Grant BD, Nance J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol. 2011;21:1951–1959. doi: 10.1016/j.cub.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Yan L, Zhou Z, Yang P, Tian E, Zhang K, Zhao Y, Li Z, Song B, Han J, Miao L, Zhang H. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell. 2009;136:308–321. doi: 10.1016/j.cell.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 79.Tian Y, Li Z, Hu W, Ren H, Tian E, Zhao Y, Lu Q, Huang X, Yang P, Li X, Wang X, Kovacs AL, Yu L, Zhang H. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell. 2010;141:1042–1055. doi: 10.1016/j.cell.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 80.Yang P, Zhang H. The coiled-coil protein EPG-8 plays an essential role in the autophagy pathway in C. elegans. Autophagy. 2011;7:159–165. doi: 10.4161/auto.7.2.14223. [DOI] [PubMed] [Google Scholar]
- 81.Lu Q, Yang P, Huang X, Hu W, Guo B, Wu F, Lin L, Kovacs AL, Yu L, Zhang H. The WD40 repeat PtdIns(3)P-binding protein EPG-6 regulates progression of omegasomes to autophagosomes. Dev Cell. 2011;21:343–357. doi: 10.1016/j.devcel.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 82.Liang Q, Yang P, Tian E, Han J, Zhang H. The C. elegans ATG101 homolog EPG-9 directly interacts with EPG-1/Atg13 and is essential for autophagy. Autophagy. 2012;8:1426–1433. doi: 10.4161/auto.21163. [DOI] [PubMed] [Google Scholar]
- 83.Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334:1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


