Abstract
The incidence of chronic kidney disease (CKD) in liver transplant recipients has been estimated to be from 18 to 28% at 10 years after transplantation. As outcomes from liver transplantation continue to improve, long-term native kidney function in these recipients becomes more critical to patient survival.
Methods
We analyzed 1151 adult, deceased-donor, single-organ primary liver transplantations performed at our center between 7/17/84 and 12/31/07. Analysis of renal function was performed on 972 patients with liver allograft survival >1 year.
Results
Kaplan-Meier analysis revealed that 3%, 7%, and 18% of liver transplant recipients with allograft survival > 1 year developed ESRD at 5, 10, and 20 years, respectively. Significant independent risk factors for ESRD included dialysis during the transplant hospitalization, the stage of CKD at one year, hypercholesterolemia, non-Caucasian race, and hepatitis C as the primary indication for liver transplantation. The initial immunosuppression of essentially all recipients was a calcineurin-inhibitor based regimen.
Conclusion
Close, long-term follow-up of liver transplant recipients permits optimal management of liver allograft and native renal function, and can lead to excellent long-term outcomes despite a calcineurin inhibitor-based immunosuppressive regimen.
Keywords: End-stage renal disease, liver transplantation
INTRODUCTION
The incidence of chronic kidney disease (CKD) in liver transplant recipients has been estimated to be up to 28% at 10 years after transplantation.[1] A number of single-center studies[2–4] have reported dissimilar outcomes, but comparison between studies is made difficult by differential definitions of chronic kidney disease, as well as variable patient exclusion criteria. Reporting of the development of end-stage renal disease (ESRD), defined as dialysis-dependence or renal transplantation, has been more consistent. Renal dysfunction is a widely accepted complication of liver transplantation, and has been hypothesized to be secondary to calcineurin inhibitor use. [3, 5–10] For this reason, many centers have altered their traditional maintenance immunosuppression in an effort to reduce the incidence of CKD and ESRD.[11–18] It is clear that renal dysfunction following liver transplantation is multifactorial, and known risk factors for ESRD in the general public such as hypertension, hyperlipidemia, and diabetes mellitus[19–20] remain critical in transplant recipients. Furthermore, the liver transplant recipient has additional risk factors, including a higher prevalence of hepatitis C positivity, the operative procedure itself, and a high incidence of perioperative acute renal failure.[21–25]
As improved surgical techniques, better intensive care capabilities, and novel immunosuppressants continue to improve outcomes from liver transplantation, long-term native kidney function in these recipients assumes a more critical role in patient survival. Since the inception of our liver transplant program in 1984, we have relied on a calcineurin inhibitor-based maintenance immunosuppressive regimen. We have the unique opportunity of providing the primary follow-up care for the vast majority of our liver transplant recipients. The long-term post-operative management of our patients is routine in our center. Here we report the incidence of ESRD, in all adult, single-organ liver transplant recipients from our center since the beginning of our program. In addition, we have identified significant risk factors for the development of ESRD in these patients.
PATIENTS AND METHODS
After Institutional Review Board approval was obtained, we retrospectively reviewed all adult (age ≥ 18 years), primary, deceased-donor, single-organ liver transplants (n=1151) performed between July 17, 1984 and December 31, 2007. The analysis was conducted utilizing the University of Wisconsin prospectively-collected transplant database. Limited data from transplants performed before 1994 were obtained with retrospective chart review. Analysis of renal function was performed on 972 patients with liver allograft survival >1 year. Patients who returned to their country of origin, and lacked 1-year follow-up despite a functioning liver allograft (n=34) were excluded. The primary endpoint in this study was the onset of ESRD. Transplant year was divided into 5-year eras (1984–1989, 1990–1994, 1995–1999, 2000–2004, and 2005–2007).
Due to a significant expansion in the variables captured by our database, we were able to analyze a number of additional risk factors for ESRD from 1994 onward, and a subset analysis of these patients (n=725) was conducted to identify other potential risk factors for ESRD. Pre-operative and intraoperative risk factors, as well as factors occurring during the first year after transplant, were assessed. As this study spanned greater than 20 years, a number of medical advances have resulted in new diagnoses such as hepatorenal syndrome (HRS) and novel techniques such as international normalized ratio (INR). Thus, in analysis of these variables, transplants occurring prior to the origination of these variables were excluded from analysis. Physiologic Model for End-stage Liver Disease (MELD) scores were calculated for patients in the pre-MELD era from 1997 onward, when our institution began recording INR. GFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation.[26] Patients were assigned to a stage of CKD based on the National Kidney Foundation Kidney Disease Outcomes Quality Initiative as follows: Stage 1 GFR ≥ 90 ml/min/1.73m2, Stage 2 GFR 60–89 ml/min/1.73m2, Stage 3 GFR 30–59 ml/min/1.73m2, Stage 4 GFR 15–29 ml/min/1.73m2, Stage 5 GFR < 15 ml/min/1.73m2 or dialysis-dependence. ESRD was defined as dialysis-dependence or undergoing renal transplantation.
Immunosuppression regimens evolved over the 20-year study period as novel agents were introduced. All patients received dexamethasone or methylprednisolone at the time of implantation, and steroids were typically transitioned during the transplant hospitalization to prednisone (20 mg/day). This dose was tapered further over the first post-operative months to a baseline of 5–10 mg/day. A subset of patients was weaned completely off steroids at the discretion of their hepatologist by the first year (36%). Induction therapy was used at the discretion of the surgeon. A variety of induction therapies were used throughout the study period, including Minnesota anti-lymphocyte globulin (University of Minnesota, n=142), basiliximab (Simulect, Novartis, n=124), muromonab (OKT3, Ortho Biotech Products, n=109), alemtuzumab (Campath-1H, ILEX, n=19), anti-thymocyte globulin (Thymoglobulin, Genzyme, n=7), and daclizumab (Zenapax, Roche, n=4). Five hundred forty-five patients were maintained on an antimetabolite, either azathioprine (Imuran, GlaxoSmithKline, n=226) in the earlier years of the study period, or a mycophenolate derivative (CellCept, Roche or Myfortic, Novartis) in the later period of the study (n=319). Nine hundred thirty-one patients (99%) were maintained on a calcineurin inhibitor. During the study period, our group transitioned from a cyclosporine-based maintenance regimen (Neoral, Novartis, n=322) to a tacrolimus-based regimen (Prograf, Fujisawa, n=609). Reported drug levels are 12-hour trough levels. Presently, patients receive basiliximab induction if they have pre-operative renal dysfunction, and are maintained on mycophenolic acid, tacrolimus, and low-dose prednisone post-operatively.
Cytomegalovirus (CMV) prophylaxis evolved over time as well. Initially, ganciclovir was utilized for CMV-negative recipients of CMV-positive donor organs. Since 2004, valganciclovir has been used on these high-risk recipients. Acyclovir was used for all other donor-recipient combinations for three months. In recent years, trimethoprim/sulfamethoxazole (160 mg/800 mg daily) was used for pneumocystis jiroveci prophylaxis for one year and oral nystatin or clotrimazole tablets were used for mucosal candidiasis prophylaxis for three months.
Unexplained elevations in liver function test values were initially evaluated with duplex ultrasonography of the liver allograft to assess vascular patency. If hepatic vascular flow was normal, percutaneous liver biopsy was performed and evaluated using hematoxylin and eosin staining.
Mean follow-up was 7.6±4.6 years. Forty-eight patients (5%) were lost to follow-up during the study period. In the remaining group, direct patient contact occurred in 95% of surviving patients in the 16 months preceding data collection.
Statistics
Rates of rejection and patient and graft survival were estimated by Kaplan-Meier analysis. Time-dependent variables were analyzed over the course of the first year post-transplant. Group comparisons were performed by a log-rank test. Cox proportional hazards analyses were performed to identify potential risk factors leading to ESRD. Factors found to be significant in univariate analysis were used to construct a multivariate model. Those variables that were non-significant on univariate analysis were excluded. Factors analyzed on multivariate analysis included: acute dialysis, race, CKD stage, hepatitis C, hepatorenal syndrome, hypercholesterolemia, post-transplant diabetes, pre-transplant diabetes, and pre-transplant hypertension. Continuous data are presented as mean±standard deviation. Statistical analysis was performed with SAS software. P-values<0.05 were considered significant.
RESULTS
Demographics
The mean recipient age at the time of transplant was 51.5±10.5 years. Recipients were more commonly male (n=573, 61%). Caucasian race was most common (n=876, 93%), with Asian (n=21) and African-American (n=20) contributing smaller numbers. Mean recipient body-mass index (BMI) was 27.7±6.4 kg/m2. Diabetes (22%) and hypertension (30%) were frequently noted in recipients prior to transplantation (Table 1).
Table 1.
Demographics
| Age (years) | 51.5±10.5 |
|
| |
| Male gender (n, %) | 573 (61%) |
|
| |
| Race (n, %) | |
| African-American | 20 (2%) |
| Asian | 21 (2%) |
| Caucasian | 876 (93%) |
| Other | 21 (2%) |
|
| |
| BMI (kg/m2) | 27.7±6.4 |
|
| |
| Physiologic MELD | 22.2±6.5 |
|
| |
| Donor (n, %) | |
| DBD | 893 (95%) |
| DCD | 44 (5%) |
|
| |
| Cold ischemic time (hours) | 9.0±3.2 |
|
| |
| Pretransplant diabetes (%) | 22 |
|
| |
| Pretransplant HTN (%) | 30 |
|
| |
| Indication for Transplant (%) | |
| Alcoholic liver disease | 29 |
| Hepatitis C | 18 |
| Alcoholic liver disease and Hepatitis C | 5 |
| Primary sclerosing cholangitis | 11 |
| Non-alcoholic steatohepatitis | 10 |
| Primary biliary sclerosis | 8 |
| Autoimmune hepatitis | 4 |
| Inherited genetic disorders | 4 |
| Hepatitis B | 2 |
| Other | 9 |
Abbreviations: BMI: body mass index, MELD: Model for End-stage Liver Disease, DBD: donation after brain death, DCD: donation after cardiac death, HTN: hypertension.
The vast majority of donor livers were from donation after brain death (DBD) donors (n=893, 95%) compared to donation after cardiac death (DCD) donors (n=44, 5%). Mean donor age was 35.3±16.0 years. Donors were more often male (n=599, 64%) and Caucasian (n=894, 96%). Donor BMI was 26.2±6.0 kg/m2. Mean cold ischemic time was 9.0±3.2 hours.
Indication for transplantation
The indications for liver transplantation are listed in Table 1. The most common primary indications were alcoholic liver disease (n=319), hepatitis C (n=170), primary sclerosing cholangitis (n=105), primary biliary cirrhosis (n=74), and cryptogenic cirrhosis (n=52). Less common indications included steatohepatitis (n=40), inherited metabolic disorders (n=39), autoimmune hepatitis (n=31), other viral hepatitis (n=25), hepatitis B (n=21), neoplasm (n=16), fulminant failure (n=11), and other causes (n=34). Although not always considered the primary indication for transplantation in all cases, 225 patients (24%) were hepatitis C positive.
Development of ESRD
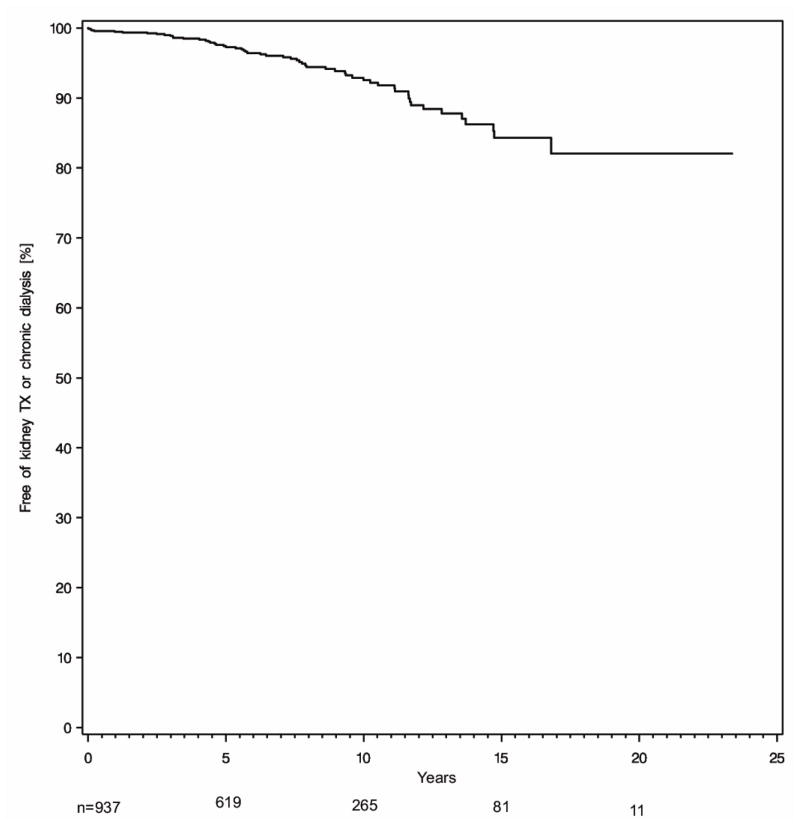
Fifty-six patients developed ESRD, and, of these, 17 patients underwent renal transplantation. Five patients were transplanted preemptively. Kaplan-Meier estimated rates of ESRD were 0.5%, 2.6%, 7.5%, and 18.0% at 1, 5, 10, and 20 years following transplantation (Figure 1).
Figure 1.
Development of ESRD. ESRD, defined as either renal transplantation or dialysis-dependence, is not uncommon following liver transplantation. The incidence of ESRD was 3%, 7%, and 16% at 5, 10, and 15 years post-transplant, respectively. The number of patients at risk is depicted beneath the time points.
Maintenance of GFR
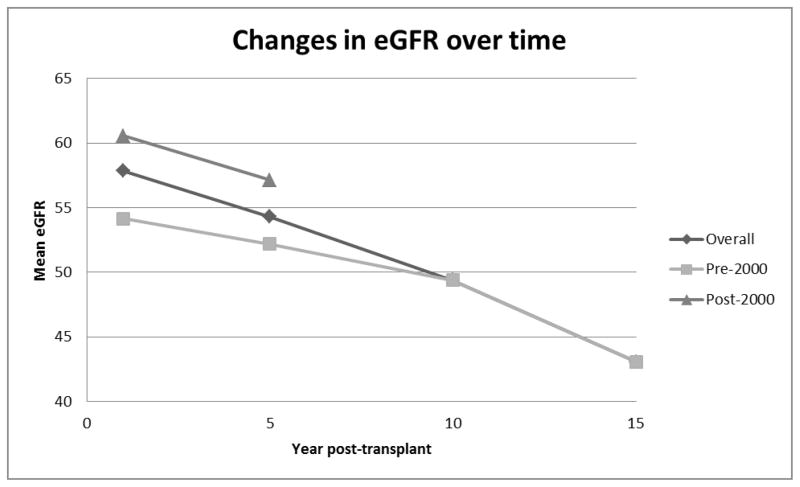
The mean estimated GFR decreased over time. Figure 2 shows that the mean estimated GFR for the entire cohort is 57 ml/min/1.73 m2 at one year. This decreases to 54 ml/min/1.73 m2, 49 ml/min/1.73 m2, and 43 ml/min/1.73 m2 at 5, 10, and 15 years (p = 0.0003). The estimated GFR is better preserved in patients undergoing liver transplantation in the latter decade of the study (post-2000) as compared to patients undergoing transplant prior to the year 2000. In the post-2000 group, estimated GFR was 60 ml/min/1.73 m2 and 57 ml/min/1.73 m2 at one and 5 years, as compared to 54 ml/min/1.73 m2 and 52 ml/min/1.73 m2 in the earlier group (p = 0.001).
Figure 2.
The estimated GFR decreases over time. The GFR of the overall cohort was 57 ml/min/1.73 m2, 54 ml/min/1.73 m2, 49 ml/min/1.73 m2, and 43 ml/min/1.73 m2 at 1, 5, 10, and 15 years, respectively. The effect is more pronounced in patients undergoing liver transplantation in the earlier period of the study (prior to the year 2000).
Preoperative risk factors for ESRD
A subset univariate analysis of risk factors for the development of ESRD following liver transplantation was performed in 725 patients undergoing transplantation after 1994 due to an expansion in the variables captured by our database. (Table 2)
Table 2.
Univariate analysis of risk factors for ESRD. Acute hemodialysis, cessation of calcineurin inhibitors, non-Caucasian race, CKD stage at 1 year, hepatitis C, hepatorenal syndrome, hypercholesterolemia, PRBC transfusion, pre- and overall post-transplant DM, and pre-transplant HTN were significant risk factors for subsequent ESRD on univariate analysis
| Risk Factor | Hazard ratio | Confidence Interval | p-value |
|---|---|---|---|
| Acute hemodialysis | 5.33 | 2.40–11.82 | <0.0001 |
| Age at transplant | 1.00 | 0.98–1.03 | 0.68 |
| Alcoholic liver disease | 1.65 | 0.98–2.80 | 0.06 |
| BMI | 1.01 | 0.97–1.05 | 0.73 |
| Calcineurin stopped within 1 year | 6.75 | 1.56–29.15 | 0.01 |
| Caucasian race | 0.38 | 0.16–0.90 | 0.03 |
| CKD stage at 1 year | 3.38 | 2.47–5.95 | <0.0001 |
| Cold ischemia time | 1.06 | 0.99–1.14 | 0.10 |
| Cyclosporine in first year | 0.64 | 0.19–2.11 | 0.46 |
| Donor age | 1.00 | 0.98–1.02 | 0.90 |
| Donor BMI | 1.03 | 0.96–1.10 | 0.49 |
| Donor gender | 0.93 | 0.54–1.61 | 0.81 |
| Donor race | 1.19 | 0.29–4.87 | 0.81 |
| Gender | 0.92 | 0.54–1.56 | 0.75 |
| Hemoglobin A1c > 6.0 | 1.73 | 0.82–3.65 | 0.15 |
| Hemoglobin A1c at 1 year | 1.21 | 0.89–1.65 | 0.22 |
| Hepatitis B | 0.98 | 0.14–7.12 | 0.99 |
| Hepatitis C | 1.82 | 1.01–3.26 | 0.05 |
| Hepatorenal syndrome | 2.35 | 1.25–4.41 | 0.008 |
| Hypercholesterolemia | 3.04 | 1.27–7.25 | 0.01 |
| MELD at transplant | 1.08 | 0.99–1.18 | 0.09 |
| MELD era | 1.54 | 0.68–3.50 | 0.30 |
| Physiologic MELD | 1.05 | 1.00–1.11 | 0.07 |
| Physiologic MELD >25 | 2.52 | 0.98–6.50 | 0.06 |
| Post-transplant DM (New-onset) | 1.27 | 0.49–3.32 | 0.62 |
| Post-transplant DM (Overall) | 2.22 | 1.10–4.47 | 0.03 |
| Post-transplant HTN | 1.41 | 0.64–3.14 | 0.40 |
| PRBC transfusions | 1.02 | 1.01–1.03 | 0.001 |
| Pre-transplant DM | 2.20 | 1.06–4.53 | 0.03 |
| Pre-transplant HTN | 2.56 | 1.27–5.14 | 0.008 |
| Rejection within 1 year | 0.89 | 0.52–1.51 | 0.66 |
| Reoperation within 90 days | 1.12 | 0.55–2.29 | 0.76 |
| Steroid withdrawal in 1st year | 1.23 | 0.53–2.82 | 0.63 |
| Tacrolimus in first year | 1.10 | 0.51–2.29 | 0.82 |
Abbreviations: BMI: body mass index, CKD: chronic kidney disease, DM: diabetes mellitus, HTN: hypertension, MELD: Model for End-stage Liver Disease, PRBC: packed red blood cells.
Hepatorenal syndrome and acute dialysis during hospitalization
Since 2001, the clinical diagnosis of HRS was added to our database. Our analysis revealed that 130 patients carried this diagnosis prior to liver transplantation. A preoperative diagnosis of HRS significantly impacted the likelihood of subsequent ESRD (HR 2.35, CI 1.25–4.41, p=0.008). Acute dialysis during the liver transplant hospitalization, captured in the database since 1994, (n=33) also increased the risk of subsequent ESRD (HR 5.33, CI 2.40–11.8, p<0.0001) and 8 of these patients ultimately developed ESRD.
Risk factors are similar to the general population
Two hundred twenty-five patients (24%) were hepatitis C positive and this was a significant risk factor for ESRD (HR 1.82, CI 1.01–3.26, p=0.05). The presence of pretransplant diabetes (HR 2.20, CI 1.06–4.53, p=0.03) and hypertension (HR 2.56, CI 1.27–5.14, p=0.008) were also significant risk factors for the development of ESRD. Both diabetes (22%) and hypertension (30%) were common in the recipient population pretransplant. Caucasian recipient race was a protective factor for the development of ESRD (HR 0.38, CI 0.16–0.90, p=0.03).
There were no significant differences in the rate of ESRD in patients when analyzed by recipient age, recipient gender, recipient BMI, transplant era, donor age, donor gender, donor race, donor BMI, type of donor liver (DBD versus DCD), or cold ischemic time. Indications for transplantation other than hepatitis C did not predict subsequent ESRD.
MELD
Since the introduction of the MELD system in 2002, 345 transplants were performed at our center. In that group, the mean UNOS-listed MELD at transplant was 22.2±6.5. The mean physiologic MELD of patients transplanted after 1997 was 18.7±7.8. As indicated in Table 2, neither the UNOS-listed MELD at transplant nor transplantation during the MELD era was predictive of subsequent ESRD. Both the physiologic MELD score at transplant and a MELD score greater than 25 approached, but did not reach, statistical significance.
Operative risk factors
The mean number of packed red blood cells (PRBC) transfused in the operating room and within the first 48 hours post-operatively was 15.1±16.2 units. The total number of PRBC transfused was predictive of ESRD (HR 1.02, CI 1.01–1.03, p=0.001). One hundred sixty-eight patients (18%) required a return to the operating room in the first 90 days following transplant, but this did not predict subsequent ESRD.
Univariate risk factors at one year for ESRD
Stage of CKD
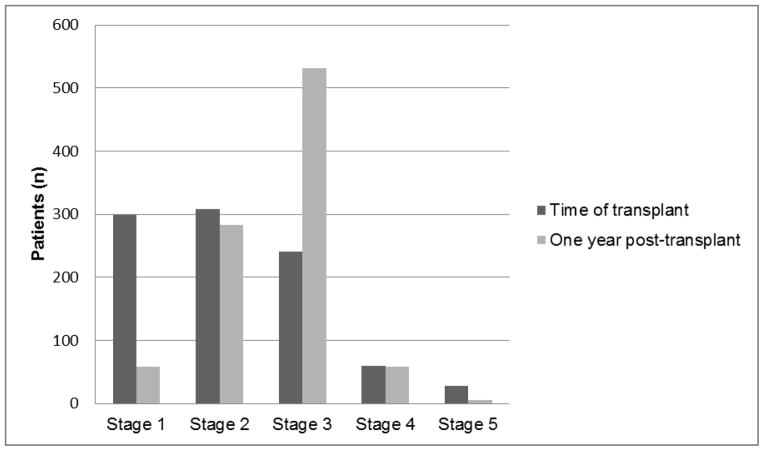
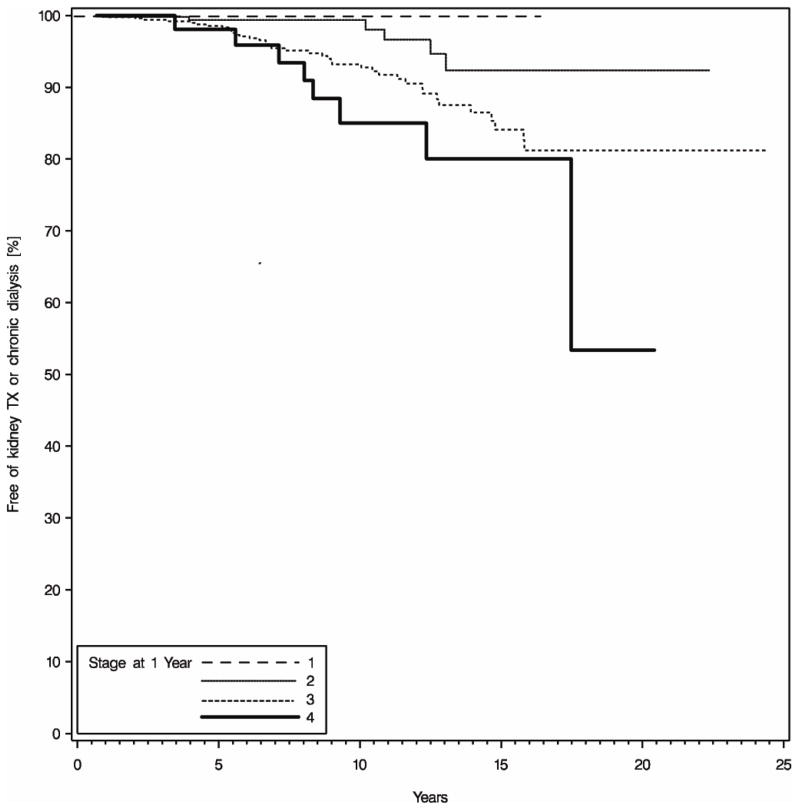
The stage of CKD at one year was predictive of subsequent ESRD, and the risk of ESRD increased with each stage of CKD (HR 3.38, CI 2.47–5.95, P<0.0001). The majority of patients had developed Stage 2 (n=283) or Stage 3 (n=532) CKD at one year following liver transplantation (Figure 3). Fewer patients were in Stage 1 (n=58) or Stage 4 (n=58). Six patients had developed Stage 5 CKD within the first year of liver transplant. No patients that were CKD stage 1 developed ESRD. Although only 5 patients (1.8%) in CKD stage 2 developed ESRD, 38 patients (7.1%) in Stage 3, and 8 patients (13.8%) in Stage 4 progressed to ESRD. Five of 6 patients (83.3%) classified as Stage 5 at one year developed ESRD. Kaplan-Meier estimates of the rates of development of ESRD by the stage of CKD at one year are shown in Figure 4.
Figure 3.
Progression of CKD stage between time of transplant and one year. Stage progression of CKD occurs in the majority of patients during the first year following liver transplantation. At the time of transplant, the majority of patients are evenly distributed between CKD Stage 1, 2, and 3. However, by one year, there is a preponderance of patients with Stage 2 and 3 CKD.
Figure 4.
Stage of CKD at one year, and the risk of the subsequent development of ESRD. The stage of CKD at one year was a significant risk factor for ESRD. Although no patients with Stage 1 CKD developed ESRD, Kaplan-Meier estimates indicate that nearly 20 percent of patients with Stage 4 CKD will develop ESRD by fifteen years post-transplant.
Calcineurin inhibitor drug levels
Tacrolimus (n=609) was utilized more frequently than cyclosporine (n=322) for maintenance immunosuppression. Cyclosporine levels in the first year were typically maintained between 100–200 ng/ml. Tacrolimus levels were typically maintained between 5–10 ng/ml.
Cessation of calcineurin inhibitor therapy was associated with a significantly increased risk of ESRD (HR 6.75, CI 1.56–29.15, p=0.01). The choice of calcineurin inhibitor was not associated with an increased risk of ESRD (p=0.38). Weaning patients off steroids (n=251) within the first year was not associated with ESRD.
Effects of induction therapy
Induction agents were utilized in 378 patients. The most common agents included Minnesota antilymphocyte globulin (n=120), muromonab (n=105), and basiliximab (n=124). Although the subsequent development of ESRD was associated with the use of induction therapy (HR 2.24, CI 1.23–3.96, p=0.005), there was no statistical difference detected between induction agents (p=0.15).
Univariate medical risk factors for ESRD occurring during the first year after transplant
Diabetes Mellitus
One hundred twenty-three (17%) patients developed post-transplant diabetes, defined as new-onset permanent (greater than 30 days) insulin dependence. Twenty-two percent of patients had pre-transplant diabetes. Two hundred forty-three (34%) patients had diabetes at one year following transplant. A small portion of patients that had insulin dependence pre-transplant did not require insulin therapy at one year. At one year, twenty-two percent of patients had a hemoglobin A1c (HgA1c) >6%. Although univariate analysis revealed that pre-transplant diabetes (HR 2.22. CI 1.10–4.47, p=0.03) and diabetes at one year (HR 1.27, CI 1.10–4.47, p=0.03) was associated with ESRD, neither HgA1c>6% at one year, nor new-onset post-transplant diabetes within the first post-operative year was a significant risk factor for ESRD. HgA1c was also not a significant factor when treated as a continuous variable.
Hypertension
Hypertension, defined as a blood pressure requiring permanent (greater than 30 days) outpatient treatment with one or more anti-hypertensives, was present in 215 patients (30%) pretransplant, and another 314 (41%) at one year. At one year post-transplant 529 patients (73%) required at least one anti-hypertensive medication. Although pretransplant hypertension was associated with ESRD, new-onset post-operative hypertension within the first year was not associated with ESRD.
Hypercholesterolemia
Hypercholesterolemia, defined as treatment with statin therapy, during the first year (n=101, 14%) proved to be a risk factor for ESRD on univariate analysis (HR 3.04, CI 1.27–7.25, p=0.01).
Rejection
Biopsy-proven rejection occurred in 510 patients (54%) in the first year following transplant. There was no significant difference in the rate of ESRD in patients when analyzed by episodes of rejection.
Multivariate analysis
In multivariate analysis, recipient race, hepatitis C positivity, acute dialysis during the transplant hospitalization, hypercholesterolemia, and the stage of CKD at the end of the first year remained significant independent risk factors for ESRD following liver transplantation. (Table 3).
Table 3.
Multivariate analysis of risk factors for ESRD. Acute hemodialysis, non-Caucasian race, CKD stage at 1 year, hepatitis C, and hypercholesterolemia were significant risk factors for subsequent ESRD on multivariate analysis
| Risk Factor | Hazard Ratio | Confidence Interval | p-value |
|---|---|---|---|
| Acute dialysis | 3.99 | 1.35–11.8 | 0.012 |
| Caucasian race | 0.20 | 0.07–0.59 | 0.003 |
| CKD stage at 1 year | 8.69 | 4.73–16.0 | <0.0001 |
| Hepatitis C | 2.92 | 1.41–6.08 | 0.004 |
| Hepatorenal syndrome | 1.00 | 0.37–2.72 | 0.99 |
| Hypercholesterolemia | 3.83 | 1.41–10.42 | 0.009 |
| Post-transplant diabetes (Overall) | 1.56 | 0.61–3.98 | 0.35 |
| Pre-transplant diabetes | 1.03 | 0.37–2.91 | 0.95 |
| Pre-transplant hypertension | 1.59 | 0.70–3.64 | 0.27 |
Patient and liver allograft survival
In the cohort of patients surviving at least one year, Kaplan-Meier estimates of patient survival were 87%, 69%, 55%, and 35% at 5, 10, 15, and 20 years, respectively. Estimates of liver allograft survival were 86%, 65%, 51%, and 30% at the same time points.
Cause of death
At the time of analysis, 673 patients (72%) were alive. Common causes of death included malignancy (n=69, 26%), infection (n=44, 17%), cardiac (n=26, 10%), and cerebrovascular accident (n=13, 5%). Sudden death with unknown cause occurred in 47 patients (18%) and liver failure developed in 15 patients (6%). A variety of other causes led to the remaining 50 deaths (19%).
Post-transplant long-term management of known modifiable risk factors for ESRD
Using our cohort of patients undergoing liver transplantation from 1994 onwards, management patterns in regards to known risk factors for ESRD were assessed.
Cholesterol, HDL, and triglyceride levels
Using our cohort of patients undergoing liver transplantation from 1994 onwards, the mean adjusted total cholesterol level at 1, 5, and 10 years was 183.6, 177.8, and 172.0 respectively (p = 0.03). The mean adjusted triglyceride level was 192.7, 179.2, and 165.8 at the same time points (p = 0.07). The mean adjusted HDL levels were 46.9 and 48.3 at one and five years (p = 0.16).
Hypertension
Seventy-three percent of patients were maintained on anti-hypertensive drug therapy at one year post-transplant (n=529), and 94% of patients were on anti-hypertensive agents by 5 years (n=384). This rate increased over time, and 96% of patients were being treated for hypertension by 10 years (n=152).
BMI
The mean adjusted BMI of liver transplant recipients did not increase over time. The mean BMI was 28.8 at one year, 29.3 at 5 years, and 29.4 at ten years (p=0.09).
HgA1c
HgA1c levels were monitored in diabetic patients, and increased over time. The mean adjusted HgA1c level at 1, 5, and 10 years were 5.8%, 6.6%, and 6.8% (p < 0.001).
DISCUSSION
As many of the technical and perioperative challenges in liver transplantation have been overcome in the past twenty years, attention is being directed to long-term patient and allograft survival. Increasingly, physicians are faced with liver transplant recipients who develop CKD, and a subset of these patients progress to ESRD. Although earlier studies have estimated that the rate of ESRD in liver transplant recipients at 10 years ranges from 4.2% to 7.9%, [2–3] and increases to nearly 10% at 13 years, [2] there have been no reports with 20 years of follow-up from a center utilizing calcineurin inhibitors in essentially all recipients. Here, we describe acceptable long-term native kidney function up to 20 years after liver transplantation. While these outcomes are better than those seen in large database studies, the overall rate of ESRD might, in fact, be lower than that published in this report because we included only patients who survived one year post-transplant. Native kidney function was maintained in the vast majority of appropriately managed patients despite calcineurin inhibitor use. Levels of calcineurin inhibitors were maintained within the standard therapeutic range in the majority of patients. Calcineurin inhibitors were generally weaned as patients approached CKD stages 4 and 5. As patients in higher stages of CKD were more likely to develop ESRD, and because calcineurin inhibitors were stopped in these recipients, cessation of calcineurin inhibitors was associated with ESRD. This was most certainly reflective of the tendency to eliminate calcineurin inhibitor therapy in patients with decreasing renal function. The initial choice of calcineurin inhibitor (cyclosporine versus tacrolimus) did not affect the rate of subsequent ESRD. As induction therapy tended to be used in patients with decreased renal function at the time of liver transplantation, an association between the use of induction therapy and subsequent ESRD was detected. We feel that that this is a reflection of our clinical tendency to utilize induction therapy in patients with higher CKD stage (decreased renal function) or acute renal failure. As acute renal failure at the time of liver transplant was among the strongest predictors of subsequent ESRD, it was not surprising that induction therapy, which is used most prevalently in these patients, was associated with ESRD.
As expected, risk factors for the development of ESRD in liver transplant recipients were similar to those seen in the general public. Patients with pretransplant diabetes and pretransplant hypertension were more likely to develop ESRD following liver transplantation. Surprisingly, the development of these conditions post-operatively did not predict ESRD. It is possible that this is because the native kidneys of patients with these conditions had already suffered a significant insult by the time of liver transplantation. It is feasible that appropriate recognition and management of these conditions, particularly new-onset diabetes, in the post-transplant period could stave off the development of ESRD. Furthermore, the particularly high prevalence of hypertension may make it difficult to detect an effect of hypertension on the development of ESRD: nearly 96% of all recipients ultimately required anti-hypertensive medication. Certainly, the post-operative long-term management of these patients plays a critical role in the long-term freedom from ESRD.
Patients who had demonstrated renal dysfunction at the time of liver transplant, requiring hemodialysis for acute renal failure, were more likely to develop ESRD. Furthermore, the stage of CKD at one-year post-transplant predicted subsequent ESRD, and patients with progressive stages of CKD were more likely to ultimately develop ESRD.
The stage of CKD at one year post-transplant was the most significant risk factor for the subsequent development of ESRD following liver transplant on multivariate analysis. Patients with Stage 1 or 2 CKD were unlikely to develop ESRD, while nearly 5% of patients with Stage 3, 10% of patients with Stage 4 CKD, and five of six patients with Stage V CKD developed ESRD during the study period. While this is intuitive, it does suggest that the use of CNI-based immunosuppression in closely-monitored patients with Stage 1 and 2 CKD is safe. We have not attempted to wean or minimize calcineurin inhibitor therapy in this group. Furthermore, it demonstrates that providers must be particularly judicious and attentive to the renal function of patients who have the latter stages of CKD at one year post-transplant. In this patient group, minimizing and monitoring the other risk factors for ESRD might prove particularly critical.
This study represents the entirety of our experience in adult single-organ liver transplantation at the University of Wisconsin, and as such, is highly reflective of our patient demographic. This population is predominantly Caucasian, and the rate of hepatitis C is 24%, which is lower than that reported nationally. This rate may be underestimated by the lack of serological testing for hepatitis C in the early years of the study. Furthermore, the prevalence of hepatitis C in the early years of liver transplantation was lower than it is presently. Both recipient race and the low prevalence of hepatitis C in our patient population would be expected to favorably influence native kidney outcomes. On the other hand, this favorable effect may be counterbalanced by the rates of pretransplant hypertension and pretransplant diabetes which were much higher than that reported in other studies.[1] Both of these factors influenced the development of CKD more strongly than the rate of hepatitis C in another large study.[1] A further limitation of the retrospective nature of the study, particularly given the duration of the study period, is the limited available dataset which may be analyzed. In particular, although native renal biopsy data would have proven enlightening, biopsies were performed in less than fifteen percent of patients who developed ESRD, which makes it difficult to draw any meaningful conclusion from the results.
Comparison of our findings to other reports of CKD in liver transplant recipients is made complicated by differing definitions of CKD and variable endpoints. The majority of studies employed CKD as the primary end-point, and lacked a similar cohort of patients or a comparable duration of follow-up. The Baylor group reported 834 patients who underwent liver transplantation between 1985 and 1994, and who survived at least 6 months. In their experience, the incidence of ESRD was 1.6%, 3.0%, and 9.5% at 1, 5, and 13 years, respectively.[2] This rate may be underreported, as the length of follow-up was variable. Post-operative creatinine at 1 and 3 months, and at 1 year, correlated with subsequent CKD. This is similar to our finding that CKD stage at 1 year is a risk factor for subsequent ESRD.
Although Ojo et al. reported a 4.8% incidence of ESRD in recipients of non-renal transplants with 10 years of follow-up, the rate of ESRD specific to liver transplant recipients was not reported.[1] As the rate of CKD in liver transplant recipients (18% at 5 years) was among the highest reported, the rate of ESRD in this population would be expected to be higher than the overall rate in non-renal transplant recipients.
Although another study has shown an association between the MELD at transplant and an increased risk of CKD, [27] we did not find this in our analysis. There are a number of potential explanations for this finding. It may be simply because 63% of the liver transplants in this study were performed in the pre-MELD era, and given the low overall incidence of ESRD, there were not a sufficient number of events to detect an effect. Additionally, the average MELD in our region is lower than that noted in other regions, and patients may be transplanted more rapidly here, prior to the development of HRS or acute renal failure. Furthermore, less time has elapsed since transplantation in patients undergoing transplantation in the MELD era. This lack of a perceived effect on ESRD may be a time bias.
The patient and liver allograft survival was calculated for the patients undergoing analysis, which includes only patients with liver allograft survival for greater than one year. Patients who either expired or lost their graft in the first year were excluded from this analysis.
In this study we have demonstrated that excellent long-term liver allograft and patient survival can be obtained in recipients maintained on a calcineurin inhibitor-based regimen. The use of this regimen need not be associated with poor native kidney function or an unacceptably high rate of ESRD. It is critical to follow these patients closely and maintain meticulous attention to the management of known risk factors for the development of ESRD. This practice can lead to the long-term preservation of renal function in liver transplant recipients.
Acknowledgments
Funding sources: The project described was supported by the Clinical and Translational Science Award (CTSA) program (D.P.F. and J.D.M.), through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors wish to thank Barbara Voss and Glen Leverson for assistance in the analysis of data and the preparation of this manuscript.
Footnotes
Conflicts of interest: no conflict of interest
AUTHOR CONTRIBUTIONS
Designed study: JCL, AD, JDP, DPF
Performed study: JCL, AD, JDP, DPF
Collected and analyzed data: JCL, DPF
Composed manuscript: JCL, DPF
Reviewed and edited manuscript: JCL, JDM, LAF, AMD, AD, AIM, JDP, DPF
References
- 1.Ojo AO, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 2.Gonwa TA, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72(12):1934–9. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 3.Fisher NC, et al. Chronic renal failure following liver transplantation: a retrospective analysis. Transplantation. 1998;66(1):59–66. doi: 10.1097/00007890-199807150-00010. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz V, et al. Chronic renal dysfunction following liver transplantation. Clin Transplant. 2008;22(3):333–40. doi: 10.1111/j.1399-0012.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett WM. Insights into chronic cyclosporine nephrotoxicity. Int J Clin Pharmacol Ther. 1996;34(11):515–9. [PubMed] [Google Scholar]
- 6.Bennett WM, et al. Chronic cyclosporine nephropathy: the Achilles’ heel of immunosuppressive therapy. Kidney Int. 1996;50(4):1089–100. doi: 10.1038/ki.1996.415. [DOI] [PubMed] [Google Scholar]
- 7.A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331(17):1110–5. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 8.Iwatsuki S, et al. Nephrotoxicity of cyclosporine in liver transplantation. Transplant Proc. 1985;17(4 Suppl 1):191–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Platz KP, et al. Nephrotoxicity following orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation. 1994;58(2):170–8. [PubMed] [Google Scholar]
- 10.de Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am J Kidney Dis. 2000;35(2):333–46. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 11.Creput C, et al. Long-term effects of calcineurin inhibitor conversion to mycophenolate mofetil on renal function after liver transplantation. Liver Transpl. 2007;13(7):1004–10. doi: 10.1002/lt.21170. [DOI] [PubMed] [Google Scholar]
- 12.Herrero JI, et al. Conversion of liver transplant recipients on cyclosporine with renal impairment to mycophenolate mofetil. Liver Transpl Surg. 1999;5(5):414–20. doi: 10.1002/lt.500050513. [DOI] [PubMed] [Google Scholar]
- 13.Schlitt HJ, et al. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet. 2001;357(9256):587–91. doi: 10.1016/s0140-6736(00)04055-1. [DOI] [PubMed] [Google Scholar]
- 14.Reich DJ, Clavien PA, Hodge EE. Mycophenolate mofetil for renal dysfunction in liver transplant recipients on cyclosporine or tacrolimus: randomized, prospective, multicenter pilot study results. Transplantation. 2005;80(1):18–25. doi: 10.1097/01.tp.0000165118.00988.d7. [DOI] [PubMed] [Google Scholar]
- 15.Koch RO, et al. Long-term efficacy and safety of mycophenolate mofetil in liver transplant recipients with calcineurin inhibitor-induced renal dysfunction. Transpl Int. 2004;17(9):518–24. doi: 10.1007/s00147-004-0749-9. [DOI] [PubMed] [Google Scholar]
- 16.Jain A, et al. Long-term outcome of adding mycophenolate mofetil to tacrolimus for nephrotoxicity following liver transplantation. Transplantation. 2005;80(6):859–64. doi: 10.1097/01.tp.0000173994.63299.63. [DOI] [PubMed] [Google Scholar]
- 17.Raimondo ML, et al. Long-term mycophenolate mofetil monotherapy in combination with calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Transplantation. 2003;75(2):186–90. doi: 10.1097/01.TP.0000041702.31262.CD. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida EM, et al. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low-dose tacrolimus regimen vs. a standard-dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial. Liver Transpl. 2005;11(9):1064–72. doi: 10.1002/lt.20490. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal AR, et al. Barriers to adequate delivery of hemodialysis. Am J Kidney Dis. 1998;31(4):593–601. doi: 10.1053/ajkd.1998.v31.pm9531174. [DOI] [PubMed] [Google Scholar]
- 20.Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S142–56. doi: 10.1053/ajkd.1998.v32.pm9820472. [DOI] [PubMed] [Google Scholar]
- 21.Velidedeoglu E, et al. Early kidney dysfunction post liver transplantation predicts late chronic kidney disease. Transplantation. 2004;77(4):553–6. doi: 10.1097/01.tp.0000114609.99558.41. [DOI] [PubMed] [Google Scholar]
- 22.Newell GC. Cirrhotic glomerulonephritis: incidence, morphology, clinical features, and pathogenesis. Am J Kidney Dis. 1987;9(3):183–90. doi: 10.1016/s0272-6386(87)80053-7. [DOI] [PubMed] [Google Scholar]
- 23.Amore A, et al. Experimental IgA nephropathy secondary to hepatocellular injury induced by dietary deficiencies and heavy alcohol intake. Lab Invest. 1994;70(1):68–77. [PubMed] [Google Scholar]
- 24.Sabry AA, et al. A comprehensive study of the association between hepatitis C virus and glomerulopathy. Nephrol Dial Transplant. 2002;17(2):239–45. doi: 10.1093/ndt/17.2.239. [DOI] [PubMed] [Google Scholar]
- 25.Weir MR, Fink JC. Risk for posttransplant Diabetes mellitus with current immunosuppressive medications. Am J Kidney Dis. 1999;34(1):1–13. doi: 10.1016/s0272-6386(99)70101-0. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, et al. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009;15(9):1142–8. doi: 10.1002/lt.21821. [DOI] [PubMed] [Google Scholar]