Abstract
Background
Our previous work showed a beneficial therapeutic effect on blepharospasm using slow repetitive transcranial magnetic stimulation which produces a long-term depression-like effect. High-frequency supraorbital electrical stimulation, asynchronous with the R2 component of the blink reflex, can also induce long-term depression-like effects on the blink reflex circuit in healthy subjects. Patients with blepharospasm have reduced inhibition of their blink recovery curves; therefore, a long-term depression-like intervention might normalize the blink reflex recovery and have a favorable therapeutic effect.
Methods
This is a randomized, sham-controlled, observer-blinded prospective study. In 14 blepharospasm patients, we evaluated the effects of high-frequency supraorbital stimulation on three separate treatment days. We applied 28 trains of 9 stimuli, 400 Hertz, either BEFORE or AFTER the R2 or used SHAM stimulation. The primary outcome was the blink rate, number of spasms rated by a blinded physician and patient rating before, immediately after and 1 hour after stimulation while resting, reading, and talking; secondary outcome was the blink reflex recovery.
Results
Stimulation-BEFORE and stimulation-AFTER the R2 both showed a similar improvement as SHAM-stimulation in physician rating, but patients felt significantly better with the BEFORE condition. Improvement in recovery of the blink reflex was seen only in the BEFORE condition. Clinical symptoms differed in the three baseline conditions (resting, reading, talking).
Conclusions
Stimulation BEFORE R2 increased inhibition in trigeminal blink reflex circuits in blepharospasm toward normal values and produced subjective but not objective improvement. Inhibition of the blink reflex pathway by itself appeared to be insufficient for a useful therapeutic effect.
Keywords: blepharospasm, dystonia, plasticity, clinical trials randomized controlled, blink reflex
In patients with benign essential blepharospasm, a common form of focal dystonia characterized by excessive involuntary closure of the eyelids, changes in excitability of cortical regions, brainstem, cerebellum and basal ganglia have been found.1 Non-invasive low-frequency magnetic stimulation to cortical motor regions can induce an increase in inhibition.2 However, clinical improvements in blepharospasm depend on the appropriate stimulation technique to the proper brain area.3 Two controlled studies investigated long-term depression-like (LTD) transcranial magnetic stimulation in focal dystonia.4,5 In both studies, clinical improvements lasting an hour or longer were seen in both writer's cramp and blepharospasm. In the study investigating blepharospasm, electrophysiological changes in the blink reflex recovery curve were also demonstrated. The blink reflex recovery curve indicates the state of excitability of facial motor neurons and bulbar interneurons.6 Patients with blepharospasm, cervical dystonia and also generalized dystonia exhibit diminished habituation (enhanced recovery) of the R2 component of the blink reflex as a result of decreased inhibition.7,8
In earlier studies, stimulus paradigms were described that produced long-term potentiation (LTP) or long-term depression (LTD) of the trigeminal blink reflex. Low-intensity, electrical high-frequency stimulation to the supraorbital branch of the trigeminal nerve modified subsequent reflex blinks in healthy volunteers if electrical high-frequency stimulation was appropriately paired with movement feedback from the eyelid.9 When electrical stimulation occurred concurrently with R2 (DURING condition), the procedure potentiated subsequent blinks (LTP), whereas when stimulation preceded the blink (BEFORE condition), this treatment suppressed subsequent blinks (LTD) for 30 min. Electrical stimulation immediately after the blink did not alter subsequent blinks (AFTER condition).9 In a later study, electrical stimulation in the DURING condition in blepharospasm patients showed enhanced R2 responses compared to healthy controls.10 We hypothesized that electrical high-frequency stimulation in the BEFORE condition may result in LTD-like plasticity of trigeminal blink reflex circuits leading to improved clinical symptoms in blepharospasm patients.
PATIENTS AND METHODS
Primary research question
In this study, we investigated whether repeated trains of electrical high-frequency stimulation in the BEFORE condition improves clinical symptoms of blepharospasm in a controlled design and whether this procedure can normalize the blink reflex recovery curve.
Patients
This is a prospective, randomized, sham-controlled, observer-blinded study. According to our sample size calculation, we included 14 right-handed patients with blepharospasm. Five males and 9 females, age 63.2±8.6 years, duration of disease 8.7±3 years, Blepharospasm Disability Scale 11.9±3.9, Blepharospasm Movement Scale 5.9±2.4, Severity Rating Scale 2.1±0.7)11 were recruited from our Movement Disorders Clinic. They all met the following inclusion criteria: clinical diagnosis of blepharospasm or cranial dystonia with eyelid involvement (Meige syndrome); age between 18 and 80 years; normal physical and neurological examinations (except for blepharospasm); no prior use of neuroleptics; no treatment with antidepressants, anti-epileptic medication, anticholinergic drugs, benzodiazepines and muscle relaxants within the prior 4 weeks. All patients except patient #5 had a history of botulinum toxin injections with good (n=8), moderate (n=4) or modest (n=1) response; however, the last injection was at least 3 months (5.8±2.6 months) prior to participation. Patients received general information about stimulation techniques and stimulation patterns but no specific information before each particular experiment. Before inclusion in the study, written informed consent was obtained from all patients. A signed Patient Consent-to-Disclose Form was obtained for videos of any recognizable patient. This study was approved by the NIH Institutional Review Board.
Methods
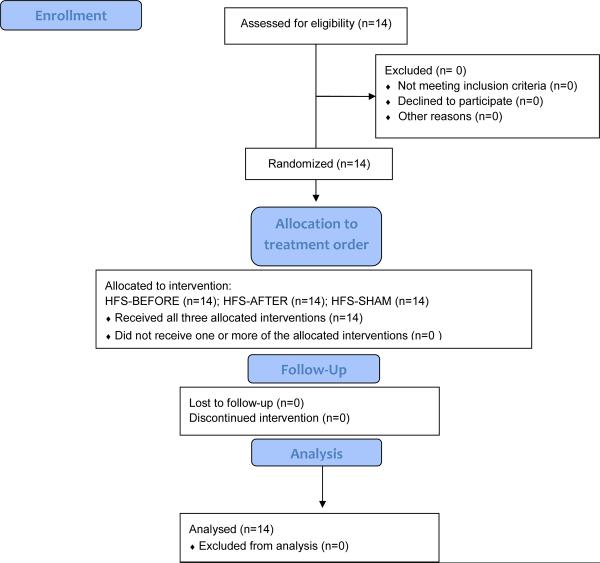
Every patient came for three visits, each separated by at least 2 days. In random order, we performed electrical high-frequency stimulation over the supraorbital nerve with three different stimulation paradigms (Table 1, Consort-Diagram). Patients were assigned by the primary investigator to one of 6 possible treatment orders by having the patient draw a slip from a hat.
Table 1.
CONSORT 2010 Flow Diagram
|
For electrical stimulation of the supraorbital nerve, we used silver chloride disc surface electrodes (square wave pulse with a pulse width of 200 μs). The cathode was placed over the right supraorbital foramen and the anode 2 cm above. Stimulus intensity was set at three times the threshold to evoke a consistent R2 response. The R2 threshold was determined as the minimum intensity required to evoke a reliable R2 response with amplitude of ≥50μV. We applied four treatment blocks with a 5-min interblock-interval. Each block lasted for 1 min and consisted of 7 electrical high-frequency stimulation trains (9 stimuli with 400 Hertz) repeated every 10 sec, manually triggered. Before applying each train, a single conditioning stimulus to the supraorbital nerve was given to evoke a blink. In the BEFORE treatment condition, electrical high-frequency stimulation was terminated before the onset of R2-EMG activity (stimulation started 5 ms after the conditioning stimulus). In the AFTER treatment condition, electrical high-frequency stimulation was given after the R2-EMG activity (stimulation started 60 ms after the conditioning stimulus). In the SHAM condition, the subjects did not receive any electrical high-frequency stimulation after the conditioning stimulus.
Outcome measures
Clinical benefits on blepharospasm symptoms were the primary outcome measure. Five-minute videos of eye blinks each during resting, reading and talking (15-min recording) before and after stimulation were assessed by a blinded rater (P.L.). Physician rating was expressed in percent (%) change before and after stimulation, considering the eye blink rate and the number of sustained spasms equally. An eye blink was defined as any visible, bilateral, and synchronous contraction of the orbicularis oculi (OO) muscle, causing eyelid drop. The eye blink rate was expressed as blinks per minute. Sustained spasms of the OO muscle were not considered blinks and were counted separately.
Subjective rating by the patients was the secondary outcome. Patients were instructed to rate their symptoms before and after stimulation on a 7-point ordinal scale: 1) excellent, 2) very good, 3) good, 4) average, 5) slightly worse than usual, 6) bad, and 7) very bad.
The primary and secondary outcome measures tested clinical changes of the blepharospasm. The blink reflex recovery curve was used as a third measure to evaluate neurophysiological correlates to the clinical endpoint measures (paired stimuli, intensity three times the sensory threshold, interstimulus interval 0.2 sec; we computed the average of 6 trials; the rest period was 25–35 sec between trials). All responses were stored digitally. In an offline analysis procedure performed fully automatically (NI LabView software), reflex responses were digitally band pass-filtered. Then the responses were full-wave rectified, and we computed the average of six trials. Peak amplitude of R2 was calculated within a window from 30 to 60 ms to avoid stimulation artifacts. We obtained R2 recovery values by dividing the size of R2test [R2t] by the size of conditioning response [R2c].
Evaluation was performed before (T1), immediately after (T2), and 1 hr after stimulation (T3). Outcome measures were evaluated in all patients in the same order: blink reflex recovery, video rating, patient rating. The same three outcome measures were used in a recent study where they are described in more detail.5
Statistics
Since the physician rating was measured in percentages, an arc sine transformation was used prior to further statistical testing. We conducted two-way repeated-measures ANOVAs using time (T1, T2, T3) and stimulation condition (BEFORE, AFTER, SHAM) as repeated factors adjusted for baseline values, and subjects as the random factor. According to Akaike's information criterion (indicates model fit by also penalizing for the number of model parameters)12, repeated measurements were modeled using an unstructured covariance for the outcome measure patient rating and blink reflex recovery curve, and a compound symmetry covariance structure for physician rating. Interaction effects between stimulation technique and time were dropped in case of non-significance. For significant main effects, post hoc pairwise comparisons were corrected using Fishers' least significant difference procedure in accordance with the “closed test principle”; i.e., post hoc comparisons were declared non-significant if the global p-value of the main effect (testing equality of all three simulation conditions simultaneously) was non-significant, but carried out without further correction in case of a significant global main effect. The level of significance was set to p ≤ 0.05. SPSS version 15.0 for Windows was used for statistical computations.
RESULTS
Baseline values for the three outcome parameters did not differ between the three stimulation days (all p>0.1). However, there was a difference at the baseline physician rating for the three clinical evaluation conditions `resting', `reading' and `talking' (F [2,13] = 9.76; p = 0.003) with the strongest symptoms of blepharospasm during `resting' and the fewest symptoms during `reading' (Figure 1). The stimulation intensity for electrical high-frequency stimulation did not differ between the three stimulation paradigms and was 10.5 ± 5.1 mA in the BEFORE condition, 10.8 ± 3.3 mA in the AFTER condition and 10.9 ± 3.9 mA in the SHAM condition.
FIG. 1. Baseline physician rating.
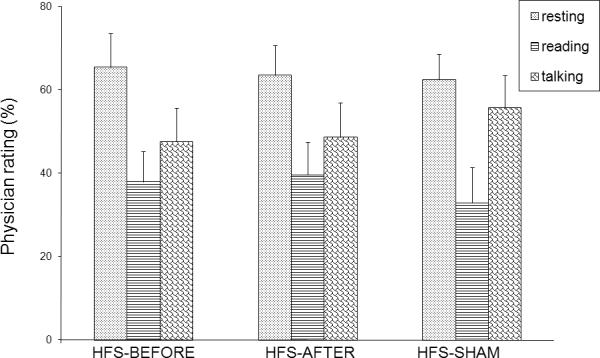
Physician rating prior to electrical high-frequency stimulation, (HFS-BEFORE, HFS-AFTER, HFS-SHAM) is on the X-axis. The Y-axis represents physician rating (expressed in percent (%) change between different tasks). The dotted bars represent patients at rest; striped bars patients reading; scaled bars patients talking.
Clinical improvements did not differ between the three stimulation conditions. For physician rating and patient rating, ANOVA showed a main effect of time (physician rating: F [2,103] = 7.48; p = 0.001; patient rating: F [2,13] = 32.30; p< 0.001) but no main effect of stimulation condition and no interaction between stimulation condition and time. The absence of interaction indicates a global stimulation-related effect over time that did not depend on the specific stimulation condition. In patient rating, however, an exploratory post-hoc analysis of the three time points, separated for each stimulation condition, revealed that only the BEFORE condition led to a significant improvement over time (Figure 2).
FIG. 2. Patient rating.
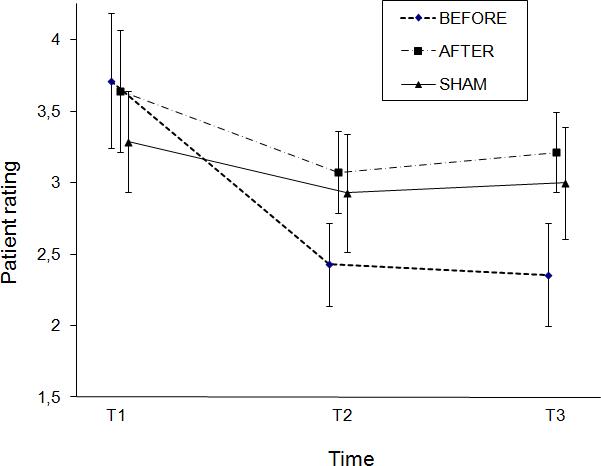
X-axis: Time: T1 = baseline, evaluation before stimulation; T2 = evaluation immediately after stimulation; T3 = evaluation 1 hr after stimulation. Y-axis: Patient rating = PatR (ordinal scale). Error bars represent standard errors for the estimated least-squares means. .
In the blink reflex recovery curve, we found a main effect of time (F [2,13] = 8.58; p = 0.004), no main effect of intervention (F [2,13] = 1.66; p > 0.1) over all time points, but an interaction between intervention and time F[4,13] = 11.69; p < 0.001). A post-hoc separate analysis for each stimulation condition revealed only an effect of time in the BEFORE condition (F [2,13] = 27.37; p < 0.001), indicating a decrease of blink reflex recovery between T1 and T2 (p < 0.001) and trend- wise between T1 and T3 (p = 0.076) (Figure 3).
FIG. 3. Blink reflex recovery (BRR).
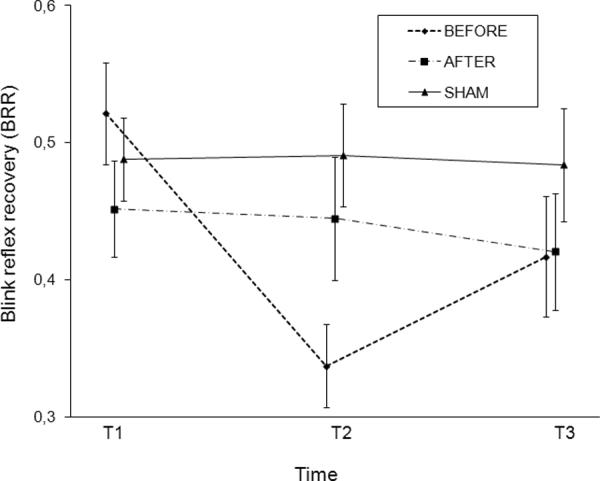
We obtained BRR by dividing the size of R2test [R2t] by the size of conditioning response [R2c]. X-axis: Time: T1 = baseline, evaluation before stimulation; T2 = evaluation immediately after stimulation; T3 = evaluation 1 hr after stimulation. Y-axis: Blink reflex recovery = BRR. Error bars represent standard errors for the estimated least-squares means.
DISCUSSION
This study was performed to test the clinical and electrophysiological effects of electrical high-frequency stimulation in patients with blepharospasm. To this end, we appropriately paired electrical high-frequency stimulation with movement feedback from the eyelid and applied stimulation before and after the R2 of the blink reflex. Earlier studies showed that, in healthy subjects, stimulation BEFORE R2 induced LTD like plasticity of the blink reflex, stimulation DURING R2 induced LTP, and stimulation AFTER R2 did not alter subsequent blinks9. There is strong evidence that blepharospasm is associated with abnormal plasticity of the neuronal circuits mediating reflex blinks, and it was demonstrated that electrical high-frequency stimulation DURING R2 induced LTP in blepharospasm with greater facilitation of the R2 response in patients relative to controls.10 In this study, we applied electrical high-frequency stimulation BEFORE R2, and tested whether this stimulation could induce LTD as a basic principle in blepharospasm patients, and beyond that, if stimulation leads to improved clinical symptoms. For the controls, we used SHAM stimulation as well as electrical high-frequency stimulation AFTER R2.
We found that only the BEFORE condition induced LTD of the blink reflex recovery immediately after stimulation. However, this did not result in a clear clinical improvement of blepharospasm symptoms. Whereas patients subjectively felt that only the stimulation BEFORE R2 improved their clinical symptoms, this could not be demonstrated in the objective blinded physician rating in any of the testing conditions, i.e., resting, reading, or talking. This may be because the clinical impact of stimulation BEFORE R2 was too small (relative to the marked placebo response) and only just perceivable to the patients, who felt less affected by their symptoms after stimulation. This is important because patients had no way of knowing which stimulation paradigm they received. Electrical high-frequency stimulation in any stimulation paradigm cannot be distinguished by sensation from one another or from placebo stimulation and the stimulation procedure was performed identically for all stimulation conditions. The clinical differences at baseline in our study, with the strongest symptoms during rest and the mildest during reading, confirm the results of an earlier study where similar blinking patterns were found in a large patient sample.13 This indicates that talking or reading may reduce the excitability of eyelid closure. The lack of clinical changes after stimulation in any stimulation paradigm was similar in the three clinical conditions.
A recent study failed to demonstrate LTP or LTD in blepharospasm after electrical high-frequency stimulation.14 We used the basic protocol that was also originally used in healthy subjects9; however, we stimulated with higher intensities than in that recent study (3 times the threshold to evoke reliable R2 responses compared to 2 times) and used a higher number of stimulation trains (7×4 stimulation trains instead of 4×3 trains). We assumed that in blepharospasm, compared to healthy subjects, it might be more difficult to increase the amount of blink reflex inhibition and therefore, we increased both the stimulation intensity and time. Moreover, we only measured paired-pulse inhibition of the R2 response (instead of adding single pulses) and minimized the number of R2 measurements (6 instead of 15) following stimulation which might have reduced habituation effects. Currently, it cannot be ascertained which of those differences was the determining factor. A further difference, the frequency of R2 measurements before and after electrical high-frequency stimulation might be relevant, because short intervals (measurements every 10 sec; we measured every 30±5 sec) may reduce the susceptibility to LTD-stimulation, as the authors discussed in their recent paper.14
Our data suggest that abnormal plasticity of the neuronal blink-circuit might not be the main pathology for clinical symptoms of blepharospasm. In fact, current concepts of dystonia assume the involvement of a wide range of cerebral structures with deficient inhibition at various levels of the nervous system.15 These include, among others, impaired cerebello-thalamo-cortical loops and a loss of reciprocal inhibition at the spinal and brainstem level. Basal ganglia dysfunction with increased striato-pallidal inhibition results in disinhibited thalamo-frontal projections with increased cortical excitability. Hyperexcitability of the primary and secondary cortical areas is a characteristic finding in dystonia. Whereas most of these electrophysiological findings are well documented, it is currently unclear which of them are primary and which are secondary. Changing pathological activity in defined brain regions toward normal levels and restoring normal plasticity seem to be a logical therapeutic principle derived from pathophysiology. It is, however, unclear what target regions should be primarily addressed to improve clinical symptoms. Within the basal ganglia, deep brain stimulation, e.g., of the globus pallidus internus, can improve dystonia presumably by disconnecting disorganized pallido-thalamo-cortical loops.16 Another current more experimental approach aims to directly modulate cortical activity. Earlier studies showed that reducing cortical excitability improved blepharospasm symptoms and also normalized R2 recovery of the blink reflex.3,5 On the other hand, directly inhibiting R2 recovery in this study did not result in distinct clinical improvements. Although R2 recovery values that patients reached in our study after the BEFORE stimulation condition were within the range of published values of healthy subjects, we do not know for certain whether we completely normalized R2 recovery, since we did not test healthy subjects. An incomplete inhibition of R2 recovery could potentially explain the lack of distinct clinical improvement after the BEFORE stimulation condition. Diminished habituation of the R2 component of the blink reflex is a characteristic finding in blepharospasm. Recently, it was proposed as a distinguishing factor to differentiate between organic and psychogenic forms of blepharospasm.17 However, improvement in brainstem inhibition may be a necessary but insufficient condition for a significant clinical improvement and improvement at different levels of the nervous system might be needed.
Table 2.
Blink reflex recovery (BRR)
| Pat. Nr. | Tl | T2 | T3 |
|---|---|---|---|
| 1 | 0,67 | 0,43 | 0,41 |
| 2 | 0,60 | 0,43 | 0,44 |
| 3 | 0,65 | 0,35 | 0,32 |
| 4 | 0,42 | 0,35 | 0,45 |
| 5 | 0,40 | 0,09 | 0,87 |
| 6 | 0,31 | 0,18 | 0,24 |
| 7 | 0,51 | 0,44 | 0,50 |
| 8 | 0,33 | 0,29 | 0,26 |
| 9 | 0,61 | 0,43 | 0,42 |
| 10 | 0,69 | 0,50 | 0,60 |
| 11 | 0,49 | 0,30 | 0,31 |
| 12 | 0,70 | 0,38 | 0,40 |
| 13 | 0,35 | 0,23 | 0,35 |
| 14 | 0,57 | 0,31 | 0,26 |
|
| |||
| Mean | 0,52 | 0,34 | 0,42 |
| SEM | 0,037 | 0,030 | 0,044 |
BRR = [R2t] / [R2c] for each patient (Pat.Nr.) at the three different time points (T1, T2, T3) for the HFS-BEFORE condition (Mean = mean BRR for each time point, SEM = standard error for the estimated least-squares means for each time point)
Acknowledgments
G. Kranz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Devera Schoenberg, M.Sc. (National Institutes of Health) for help in editing the manuscript.
Disclosure Study Funding: Supported by the NIH/NINDS Human Motor Control Section and the Max Kade Foundation, New York (G.K.). Dr. Kranz received funding for travel from the NIH/NINDS and Ipsen; received speaker honoraria from Allergan, Inc., Ipsen, and Merz Pharmaceuticals, LLC; and received research support from the Max Kade Foundation and the NIH/NINDS (Intramural Grant). Dr. Shamim received research support from the NIH/NINDS (Intramural Grant); and holds stock in Amgen, Pfizer Inc, and Medtronic, Inc; received support from the Kinetic Foundation; participated in Allergan-sponsored trials and was one of the site PIs for CD probe and COMPEL studies. Dr. Lin has received research support from the NIH/NINDS (Intramural Grant). Mr. Kranz reports no disclosures. Dr. Hallett serves as Chair of the Medical Advisory Board and receives funding for travel from the Neurotoxin Institute; serves as Chair of the Medical Advisory Board of the Benign Essential Blepharospasm Foundation; may accrue revenue on patents re: Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders and H-Coil for Magnetic Stimulation and methods for using the same; receives royalties from publishing from Blackwell Publisher, Cambridge University Press, Springer Verlag, Taylor & Francis Group, Oxford University Press, John Wiley & Sons, and Elsevier; receives research support from Ariston Pharmaceuticals for studies of essential tremor, NIH/NINDS (Intramural Program); has received license fee payments from the NIH (from Brainsway) for licensing the patent for the H-coil.
Footnotes
Author contributions: Study concept and design (Drs. Hallett, G. Kranz, and Shamim);acquisition of data (Drs. G. Kranz, Shamim, and Lin); analysis and interpretation of data (Drs. G. Kranz, Hallett, Shamim, and G.S. Kranz); drafting of the manuscript (Drs. G. Kranz, Hallett, Shamim, and G.S. Kranz); critical revision of the manuscript for important intellectual content (Drs. Hallett, Shamim, Lin, G.S. Kranz); statistical expertise (G.S. Kranz); administrative, technical, or material support (Drs. Shamim, Lin, and Hallett); study supervision (Dr. Hallett).
REFERENCES
- 1.Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: Report from the BEBRF International Workshop. Neurology. 2008;71:1275–1282. doi: 10.1212/01.wnl.0000327601.46315.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 3.Kranz G, Shamim EA, Lin P, et al. Blepharospasm and the modulation of cortical excitability in primary and secondary motor areas. Neurology. 2009;73:2031–2036. doi: 10.1212/WNL.0b013e3181c5b42d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murase N, Rothwell JC, Kaji R, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain. 2005;128:104–115. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- 5.Kranz G, Shamim EA, Lin PT, et al. Transcranial magnetic brain stimulation modulates blepharospasm: a randomized controlled study. Neurology. 2010;75:1465–1471. doi: 10.1212/WNL.0b013e3181f8814d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauletti G, Berardelli A, Cruccu G, et al. Blink reflex and masseter inhibitiory reflex in patients with dystonia. Mov Disord. 1993;8:495–500. doi: 10.1002/mds.870080414. [DOI] [PubMed] [Google Scholar]
- 7.Aramideh M, Eekhof LA, Bour LJ, et al. Electromyography and blink reflex recovery in involuntary eyelid closure: A comparative study. J Neurol Neurosurg Psychiatry. 1995;58:692–698. doi: 10.1136/jnnp.58.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eekhof JLA, Aramideh M, Bour JL, et al. Blink reflex recovery curves in blepharospasm, torticollis spasmodica and hemifacial spasm. Muscle Nerve. 1996;19:10–15. doi: 10.1002/(SICI)1097-4598(199601)19:1<10::AID-MUS2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 9.Mao JB, Evinger C. Long-term potentiation of the human blink reflex. J Neurosci. 2001;21:RC151. doi: 10.1523/JNEUROSCI.21-12-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quartarone A, Sant'Angelo A, Battaglia F, et al. Enhanced long-term potentiation-like plasticity of the trigeminal blink reflex circuit in blepharospasm. J Neurosci. 2006;26:716–721. doi: 10.1523/JNEUROSCI.3948-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindeboom R, De Haan R, Aramideh M, Speelman JD. The blepharospasm disability scale: an instrument for the assessment of functional health in blepharospasm. Mov Disord. 1995;10:444–449. doi: 10.1002/mds.870100407. [DOI] [PubMed] [Google Scholar]
- 12.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 13.Bentivoglio AR, Daniele A, Albanese A, et al. Analysis of blink rate in patients with blepharospasm. Mov Disord. 2006;8:1225–1229. doi: 10.1002/mds.20889. [DOI] [PubMed] [Google Scholar]
- 14.Zeuner KE, Knutzen A, Al-Ali A, et al. Associative stimulation of the supraorbital nerve fails to induce timing-specific plasticity in the human blink reflex. PLoS One. 2010;5:e13602. doi: 10.1371/journal.pone.0013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallett M, Rothwell J. Milestones in clinical neurophysiology. Mov Disord. 2011;26:958–967. doi: 10.1002/mds.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008;5:320–330. doi: 10.1016/j.nurt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwingenschuh P, Katschnig P, Edwards MJ, et al. The blink reflex recovery cycle differs between essential and presumed psychogenic blepharospasm. Neurology. 2011;76:610–614. doi: 10.1212/WNL.0b013e31820c3074. [DOI] [PMC free article] [PubMed] [Google Scholar]


