Abstract
The hippocampal CA1 region is most susceptible to cerebral ischemia in both rodents and humans, whereas CA3 is remarkably resistant. Here, we investigated the possible role of membrane lipids in differential susceptibility in these regions. Transient ischemia was induced in rats via bilateral occlusion of common carotid arteries and membrane lipids were analyzed by mass spectrometry. While lipid profile differences between the intact CA1 and CA3 were rather minor, ischemia caused significant pyramidal cell death with concomittant reduction of phosphatidylserine, phosphatidylinositol, phosphatidylethanolamine, plasmalogen and sphingomyelin only in CA1. The phospholipid loss was evenly distributed in most molecular species. Ischemia also significantly increased cell death mediator ceramides only in CA1. Our data suggests that differential susceptibility to ischemia between CA1 and CA3 is not linked to their unique phospholipid profile. Also, selective activation of phospholipase A2, which primarily releases polyunsaturated fatty acids, might not be characteristic to cell death in CA1.
Supplementary key words: polyunsaturated fatty acids, ischemia, hippocampus, phospholipids
Introduction
One of the most susceptible brain regions to ischemia in rodent models [1, 2] and humans [3] is the hippocampus, particularly the CA1 region, while CA3 region remain remarkably resistant to ischemia. These two regions have long been the targets of interest because investigating the neurodegenerative and/or protective mechanism of these two areas might provide us preventive and/or therapeutic intervention for brain ischemia.
CA1 and CA3 were known to react similarly to ischemia; for example, Mitani et al [4] reported that CA1 and CA3 did not show any differences in the time-course of ischemia-induced glutamate release and the levels of glutamate. Excitatory postsynaptic currents during energy deprivation were also similar in pyramidal cells in both areas [5]. During cerebral ischemia, neuronal transporters release glutamate, instead of removing extracellular glutamate to protect neurons in CA1 [6] and CA3 [5]. Anatomically, CA3 pyramidal cells are bigger than those of CA1, and input and output pathways of signals are different [7]. Ouyang et al [8] found that CA1 astrocytes were more sensitive to ischemia than those of the dentate gyrus. It has been also suggested that tyrosine kinase and phosphatase, located down-stream of N-Methyl-D-aspartate (NMDA) activation might be different between CA1 and CA3 [9].
The involvement of phospholipids (PL) in susceptibility of CA1 to ischemia has also been suggested. Kubota M, et al [10] reported that the concentration of plasmalogen (PE-pl) in CA1 was higher than CA3. Nevertheless, the reason for the differential susceptibility has not been clearly understood. It is well accepted that ischemia increases free fatty acid concentrations, particularly polyunsaturated fatty acids released from membrane PL [11, 12] through activation of phospholipase A2 (PLA2) [13]. There are also many reports on degradation of various PL molecular species during ischemia [14–23] and reperfusion [15, 19–22, 24, 25] in the brain regions including hippocampus. However, no report has demonstrated concurrent changes in PL molecular species induced by ischemia in both CA1 and CA3 areas. To gain insight on the role of membrane lipids in differential susceptibility of these two areas, we investigated the PL profile and molecular species in CA1 and CA3 in relation to ischemic damage.
Materials and methods
Animals
Sprague-Dawley rats (269–343g, male) were purchased from Charles River Laboratories (Wilmington, MA, USA). They were maintained in our animal facility under conventional conditions with controlled temperature (23 ± 1°C) and illumination (12 hour; 6:00–18:00); water was provided ad libitum. The rats were fed with an NIH chow diet, which contains approximately 2% each of docosahexaenoic and eicosapentaenoic acids as well as 0.2% of arachidonic acid. All experimental procedures were approved by the Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, National Institute of Health (LMS-HK02).
Surgery
Rats were deprived of food for approximately 18 h overnight before bilateral occlusion of the common carotid arteries (2VO) or sham surgery under halothane anesthesia. The tail artery was cannulated for blood pressure monitoring. During surgery, rats were placed in a supine position on a heated pad together with an overhead heating lamp. The body temperature was maintained at 37.0°C using a rectal thermometer. The neck was incised in the ventral midline to expose the common carotid arteries. Both common carotid arteries were occluded simultaneously for 20 min with nontraumatic micro-arterial clips, and blood pressure was reduced to and maintained between 50 and 55 mmHg during the surgery by means of fine adjustment of halothane dosage [26]. Four days after surgery, when neuronal death was supposedly completed in the hippocampal CA1 region [27], rats were euthanized to obtain brains for histology (right hemisphere) and lipid analysis (left hemisphere).
Histology
The extent of ischemic injury was assessed with coronal hippocampal sections stained with toluidine blue. Surviving pyramidal cells per millimeter in the dorsal hippocampal CA1 region were counted in four 10 μm sections 120 μm apart, from and caudal to −4.2 mm relative to bregma. The four values were summed for each rat.
Tissue preparation and lipid extraction
The hippocampal tissue was cut in 300 μm at −4.2 mm relative to bregma with a cryostat. CA1 and CA3 were located according to “The Rat Brain in Stereotaxic Coordinates” (5th edition) [28]. The frozen sections of these regions were scraped off by a small metallic spatula from the slides on dry ice under microscope and homogenized in ice-cold HEPES buffer (pH 7.4). Aliquots were subjected to protein assay and lipid analysis. Total lipids were extracted according to the method of Bligh and Dyer [29].
Phospholipid analysis
The PL molecular species were separated and analyzed using reversed-phase high performance liquid chromatography-electrospray ionization-mass spectrometry (LC/MS) with a C18 column (Prodigy, 150 × 2.0 mm, 5 μm; Phenomenex, Torrance, CA, USA) as described previously [30]. The separation was accomplished using a linear solvent gradient (water:0.5% ammonium hydroxide in methanol:hexane), changing from 12:88:0 to 0:88:12 in 17 min after holding the initial solvent composition for 3 min at a flow rate of 0.4 mL/min [31]. An Agilent 1100 LC/MSD instrument (Palo Alto, CA, USA) was used to detect the separated PL molecular species. For electrospray ionization, the drying gas temperature was 350°C; the drying gas flow rate and nebulizing gas pressure were 11 L/min and 45 p.s.i., respectively. The capillary and fragmentor voltages were set at 4500 and 300 V, respectively. Identification of individual PL molecular species was based on the monoglyceride, diglyceride and protonated molecular ion peaks [30]. As internal standards representing each PL class, we used 1-d35-stearoyl-2-docosapentaenoyl-glycerophosphoserine (d3518:0,22:5-PS), 1-d35-stearoyl-2-arachidonoyl-glycerophosphoethanolamine (d3518:0,20:4-PE) and 1-d35-stearoyl-2-linoleoyl-glycerophosphocholine (d3518:0,18:2-PC). Quantitation of PL species was based on the area ratio calculated against the deuterium-labeled internal standards using diglyceride ions for PS and PE, and protonated molecular ions for PC. For quantitation of phosphatidylinositol (PI), plasmalogen (PE-pl) and ceramides (CM), d3518:0,20:4-PE was used as an internal standard and d3518:0,18:2-PC was used for sphingomyelin (SM).
Statistical analysis
Data are expressed as means ± SD. Statistical difference between experimental groups was assessed using Student’s t-test. p< 0.05 was considered significant.
Results
Comparison of PLs between CA1 and CA3
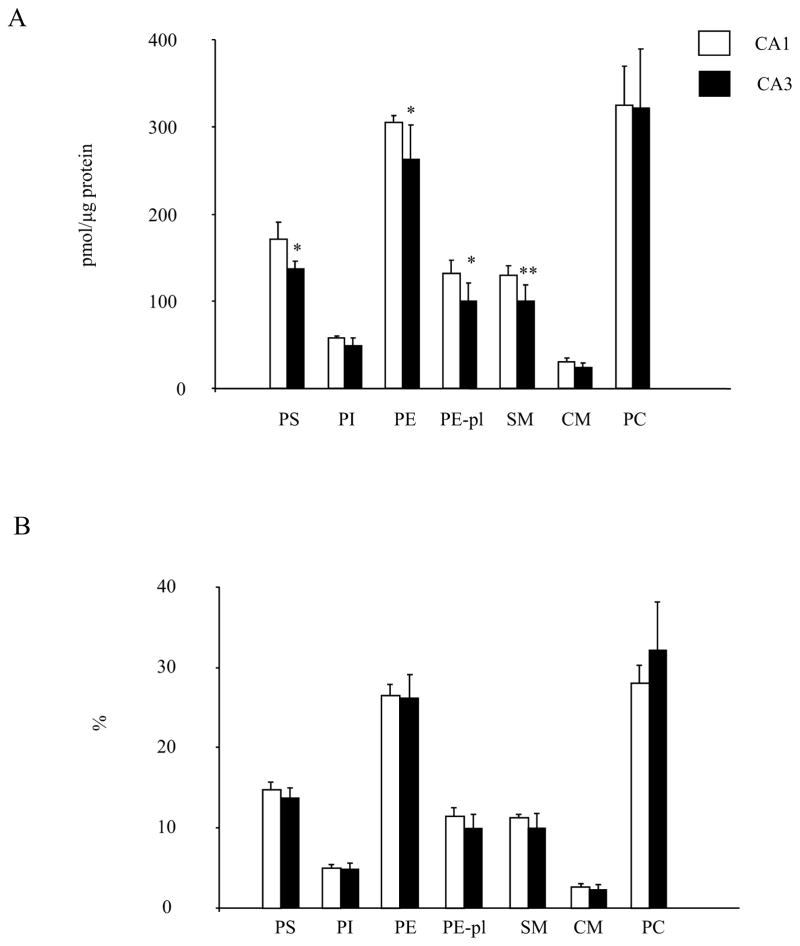
The PL profile in the CA1 and CA3 areas obtained from sham-operated control animals assessed by LC/MS are shown in Fig 1. PC was the most abundant PL species followed by PE and PS, which was consistent with a previous report using rat hippocampus [32]. The absolute levels of PL normalized to protein amount appeared to be slightly but significantly higher in CA1 than those in CA3. The PS, PE, PE-pl and SM contents in CA1 were higher by 20% (p=0.02), 14% (p=0.02), 24% (p=0.02), 23% (p=0.01) and 14% (p=0.02), respectively (Fig. 1A). Nevertheless, this difference between CA1 and CA3 became statistically insignificant when individual PL classes were expressed with % distribution, indicating that the PL profile in these regions is similar (Fig. 1B).
Fig 1.
Comparison of the absolute level (A) and proportion (B) of phospholipid classes in the CA1 and CA3 region. PLs were measured by LC/MS (n=6). Data are mean±SD. *p < 0.05, **p < 0.01versus CA1.
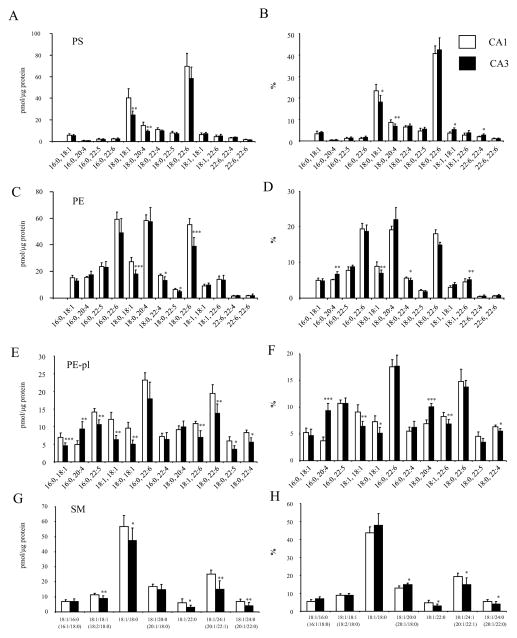
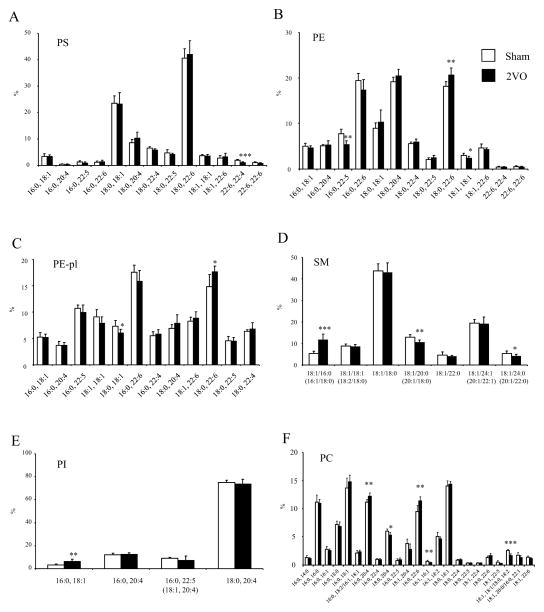
The subtle differences in the phospholipoid classes between CA1 and CA3 regions were further analyzed at the molecular species level (Fig. 2). The molecular species profiles of PS, PE and PC in these regions were similar to the report from the entire hippocampus [32]. The 18:0,18:1- and 18:0,22:6-PS were the two most abundant species in PS while the 16:0,22:6, 18:0,20:4 and 18:0,22:6 species were most abundant in PE. Similar to PE, 16:0,22:6 and 18:0,22:6 were the two prominent species in PE-pl while 18:1, 18:0 species was most abundant in SM.
Fig 2.
Comparison of individual phospholipid molecular species between CA1 and CA3 regions (n=6). Data are mean±SD of contents for (A) PS, (C) PE, (E) PE-pl and (G) SM, and proportions for (B) PS, (D) PE, (F) PE-pl, and (H) SM. *p < 0.05, **p < 0.01, ***p < 0.001 versus CA1.
The differences observed for PS were mainly due to lower levels of 18:0,18;1-PS (40.3±8.7 vs. 24.6±3.7 pmol/μg protein, p<0.01) and 18:0,20:4-PS (14.8±3.0 vs. 9.3±1.2 pmol/μg protein, p< 0.01) in CA3 compared to CA1 (Fig. 2A). For PE, higher 18:0,22:6-PE (55.3±4.6 vs. 38.9±6.6 pmol/μg protein, p<0.001) and 18:0,18:1-PE (27.3±3.5 vs. 18.2±2.8 pmol/μg protein, p<0.001) in CA1 contributed to the differences between two regions (Fig. 2C). Interestingly, almost all the PE-pl molecular species were significantly higher in CA1 except for the species containing arachidonic acid (20:4n-6) (Fig. 2E). The 16:0,20:4 plasmalogen species was actually higher in CA3 (4.9±1.1 vs. 9.3±2.1 pmol/μg protein, p<0.001). The differences in SM were derived mainly from higher levels of 18:1,22:0-SM (6.1±2.5 vs. 3.1±1.4 pmol/μg protein, p<0.05), 18:1,24:1-SM (25.1±2.7 vs. 15.0±5.7 pmol/μg protein, p<0.01) and 18:1,24:0-SM (6.9±1.5 vs. 4.1±2.1 pmol/μg protein, p<0.01) in CA1 in comparison to CA3 (Fig. 2G). The significance of the differences for these molecular species was maintained when the data were expressed as % distribution (Fig. 2B, D, F, H). Nevertheless, the relative differences in the phospholipid profile in these two regions were rather minor (within 20% difference) with the exception of 16:0,20:4-PE-pl which showed a greater proportion in CA3 compared to CA1 by more than two fold (Fig. 2F).
Effect of ischemic injury on hippocampal cell death
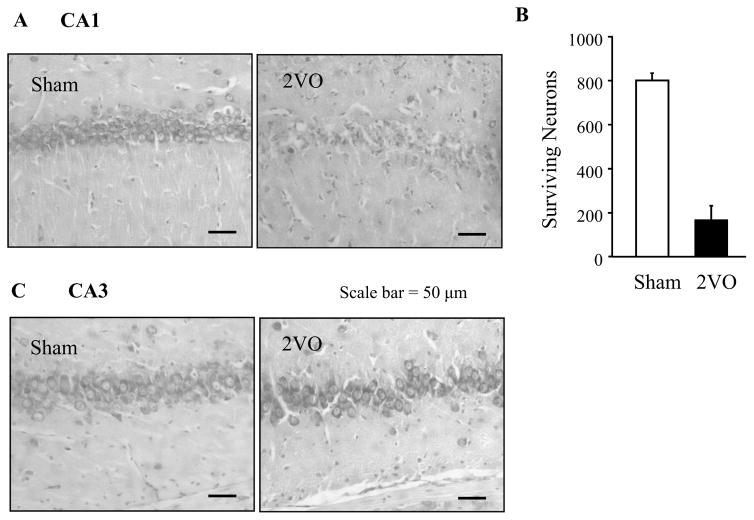
To verify the effects of transient ischemia by 2VO on pyramidal cell death, histologic analysis of hippocampal cells was performed for the coronal section of CA1 (Fig. 3A) and CA3 regions (Fig. 3C) after toluidine blue staining. Surviving pyramidal cells were counted in the CA1 region after sham operation or ischemia. Mean total pyramidal cell numbers per millimeter of dorsal hippocampal CA1 after sham operation and ischemia were 799±35 and 165±68, respectively (Fig. 3B). After ischemia only 21% of pyramidal cells survived in the CA1 region in comparison to the sham operated group. In contrast, essentially no changes were observed in the numbers of surviving pyramidal cells in the CA3 and granule cells in the dentate gyrus region (greater than 90% survial) after 2VO (Fig. 3C).
Fig 3.
Surviving CA1 hippocampal cells 4 days after the bilateral occlusion of common carotid arteries (2VO). (A) Photomicrograph showing a representative CA1 region from a sham and 2VO-treated rat. (B) Surviving Pyramidal cells per millimeter in the dorsal hippocampal CA1 region were counted in four 10 μm sections 120 μm apart, from and caudal to −4.2 mm relative to the bregma. The four values were summed for each rat. Data for each group of rats were expressed as the mean±SD (n=6). (C) A representative CA3 region from a sham and 2VO-treated rat.
Alteration of PL molecular species in CA1 and CA3 after ischemic injury
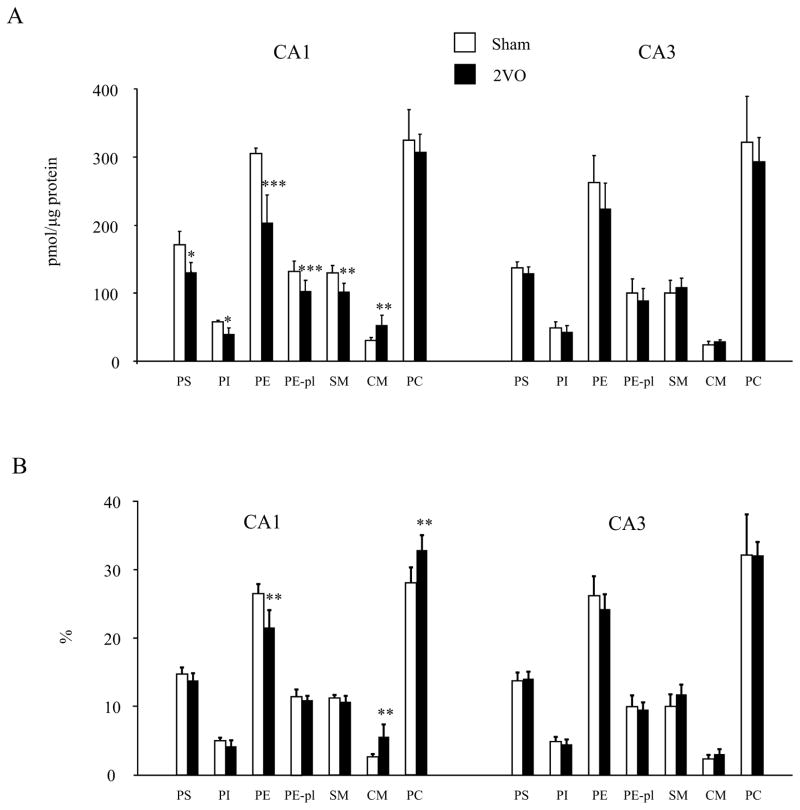
Ischemia-reperfusion injury induced by 2VO resulted in significant decreases in PS, PI, PE, PE-pl and SM contents in CA1 by 24% (p=0.002), 31% (p=0.002), 33% (p=0.0001), 22% (p=0.002) and 22% (p=0.002), respectively, compared to the sham operated group (Fig. 4A). When the PL data was expressed as the proportion (% distribution), only the reduction of PE remained statistically significant (Fig. 4B), indicating that the extent of decrease in PE was greater than other PL classes. In addition, PC was the only class of PL that was not reduced by the injury in CA1. Accordingly, the proportion of PC increased significantly when normalized to the total phospholipid content. Notably, the ceramide content increased in CA1 by 71% (p=0.01) after injury. In the CA3 region, where no cell death was detected, no significant alteration of PL level or proportion was noted.
Fig 4.
Effect of transient ischemia on individual phospholipid classes in CA1 and CA3. PLs were measured by LC/MS (n=6). Data are mean±SD of (A) contents and (B) proportion. **p < 0.01, ***p < 0.001 versus the sham operated group.
Alteration of PL molecular species after ischemic injury
The absolute amount of phospholipids decreased by the ischemic injury was evenly distributed across most molecular species regardless of the unsaturation status, and the molecular species distribution within individual PL classes remained almost identical (Fig. 5). A few exceptions include the proportion of 18:0, 22:6-PE, 18:0, 22:6-PE-pl, 16:0, 22:6-PC and 16:0,20:4-PC which were significantly increased after injury (Fig 5). In SM, the proportion of 18:1/16:0 species was also increased at the expense of 18:1/20:0 species.
Fig 5.
Effect of transient ischemia on the composition of individual PL molecular species in CA1. PLs were measured by LC/MS (n=6). Data are mean±SD of proportion for (A) PS (B) PE, (C) PE-pl, (D) SM, (E) PI and (F) PC. *p < 0.05, **p < 0.01, ***p < 0.001 versus the sham operated group.
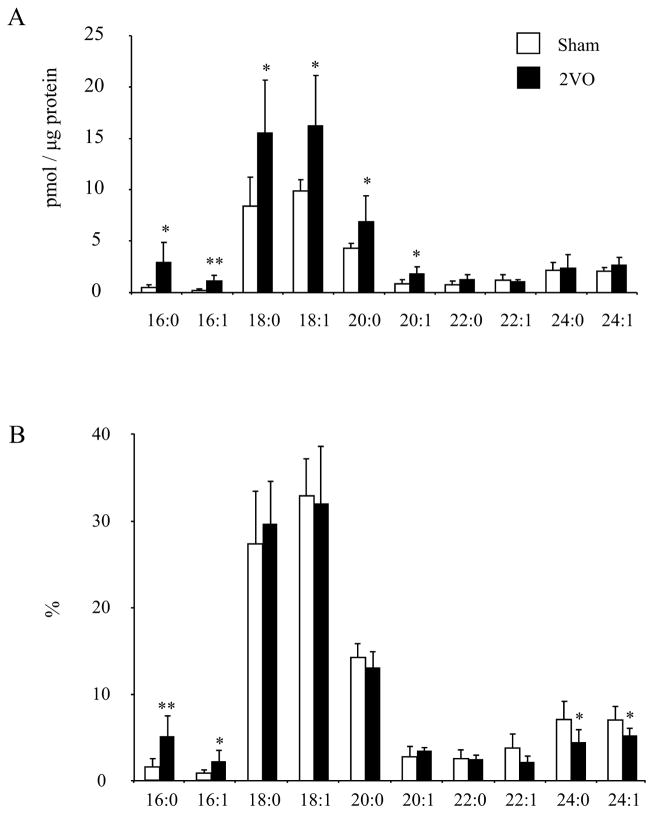
Ceramide molecular species increased after ischemic injury
The most abundant ceramide molecular species in CA1 are 18:0, 18:1 and 20:0 fatty acid containing species. After cerebral ischemia the CM level was significantly increased (Fig. 4), particularly the molecular species containing shorter carbon chain fatty acid (C16 through C20) including the most abundant species (Fig. 6). The longer carbon chain species (longer than C20) did not show significant changes, indicating some molecular specificity in ischemia-induced production of ceramides in CA1. When ceramide molecular species were expressed as % distribution, only 16:0 and 16:1 species showed significant increases while the reduced proportion of 24:0 and 24:1 was apparent.
Fig 6.
Effect of transient ischemia on the contents (A) and proportions (B) of individual ceramide molecular species in CA1 measured by LC/MS (n=6). Data are meanrSD. *p < 0.05, **p < 0.01 versus the sham operated group.
Discussion
The present study tested the possible role of membrane lipids in CA1 and CA3 in the differential susceptibility of pyramidal cells to ischemia in these regions. The phospholipid molecular species in CA1 and CA3 regions were compared for the sham-operated control or ischemic injury-inflicted animals. We found that the PL profile in these regions from sham-operated control animals is similar although CA1 has a bit higher PL to protein ratio than CA3. After transient ischemia, the CA3 region was preserved without cell death or PL changes. In contrast, ischemia induced significant cell death and lowered the level of all lipid classes in CA1 with the exception of PC. The loss of PL was evenly distributed in most molecular species (Fig 4), suggesting that cell death in CA1 may not be specifically associated with selective activation of PLA2, which leads to the preferred release of polyunsaturated fatty acids from PL. Ischemic injury significantly induced the cell death mediator ceramides in CA1.
Docosahexaenoic acid (DHA) is known to attenuate cerebral ischemic injury by downregulating apoptosis and, in turn, promoting cell survival [33]. Recently, it has been also reported to provide protection against postischemic inflammation/innate immune responses [34]. A recent meta-analysis shows an inverse association between fish consumption and the risk of stroke in prospective studies [35], although no beneficial effects of n-3 fatty acids was apparent in another study for a secondary prevention of cardiovascular disease (including transient ischemic attack and stroke) [36]. Interestingly, in the current study, DHA containing PE was significantly higher in CA1 than in CA3. Despite the susceptibility of DHA to oxidation, DHA containing species were not reduced disproportionately, but sustained in PS, and even significantly increased in PE, PE-pl and PC (Fig 5A–C, F).
Goto et al [14] reported that during 60 min of ischemia, polyunsaturated molecular species in PC were more vulnerable than saturated and monounsaturated species; polyunsaturated molecular species became 58.1% of the value before ischemia, and saturated plus monounsaturated became 68.3%. The similar decrease was seen in PE (34.1% versus 45.5%, respectively). This can be explained by the fact that PLA2 was activated after ischemia. However, the present study revealed that the PL reduction in CA1 was evenly distributed across most molecular species of PS, PE and PE-pl (Fig. 5). Moreover, when PL molecular species were evaluated as the polyunsaturated and the saturated plus monounsaturated, no specific reduction in polyunsaturated species were observed (76.7% vs.74.2% in PS; 66.5% vs. 67.4% in PE; 83.2% vs. 79.4% in PC for polyunsaturated vs. saturated plus monounsaturated species, respectively). These findings suggested that selective activation of PLA2 might not be directly involved in CA1 susceptibility to ischemia-reperfusion injury. The discrepancy between Goto’s study [14] and ours maybe explained by the extent of the damage in the blood-brain barrier which depends on the duration of ischemia and the severity of injury [37]. When the blood–brain barrier is disrupted by ischemia, albumin, a carrier of free fatty acids, can be increased in the brain [38]. It is possible that the cell membranes were replenished with DHA [39] and AA [40], which appeared to be preferentially taken up by brain cells, during four days of reperfusion.
Kubota et al [10] showed that the concentration of PE-pl in the CA1 region was 2.6- and 2.7- fold higher than that in the CA3 region and cerebral cortex, respectively. Up to date, this is the only report that shows the difference of PL profiles between the CA1 and CA3 regions. The reconstituted Na+–Ca2+ exchanger (NCX) with PE-pl-containing proteoliposomes was reported to have higher activity than the control [41]. NCX is a plasma membrane anti-porter which exchanges three Na+ for one Ca2+ and can either accumulate Ca2+ (reverse mode) or extrude it (forward mode) depending on the concentration of each ion on both sides of the membranes and membrane potential [42]. NCX is also known to be regulated by ATP. When ATP levels are sufficient, the forward mode is activated; however, when ATP is exhausted under ischemic conditions, the reverse mode is activated, which results in intracellular Ca2+ overload. Kubota et al [10] hypothesized that NCX played an important role in the vulnerability of CA1 to ischemia due to its higher level of PE-pl. Our study also found significantly higher levels of PE-pl in CA1 (24%) which might have contributed to NCX activity.
PC is the major membrane PL in the brain, and its loss alone induces cell death [43]. Previous reports showed that PC levels decreased in the brain during ischemia [14, 21] and reperfusion [20, 21, 25, 44]. On the other hand, some studies showed no changes in PC levels during ischemia [15, 17], and even an increase during reperfusion [15]. The marked difference between the studies reporting decreases and those with no changes plus an increase was partly due to the difference in experimental models as well as the severity of the ischemic insult. Lukácová et al [20] also investigated the change of PC in the hippocampus, and they found that there was a significant decrease 15 min after reperfusion and this decrease was abolished after 30 min. Our data did not show any significant change in PC (Fig. 4); however, PC might have been decreased in an early time point of reperfusion and returned to a normalized level through PC synthesis afterward.
CM, a hydrolyzed product of SM catalyzed by sphingomyelinase, is known to play an important role in apoptosis [45, 46]. Before the onset of cell death CM level has been shown to be increased [47]. Sphingomyelinase was reported to affect the size of cerebral infarction in mice [48] and newborn rats [49]. It has been shown that ischemia decreases SM levels and increases CM levels in the gerbil hippocampus [24] and rat cortex [16]. In our study, we also observed significant decrease in SM after ischemia in CA1 but not in CA3, suggesting that sphingomyelinase was activated selectively in CA1 in response to ischemic injury. Curiously, CM containing only shorter carbon chain (C16 through C20) increased significantly after injury in CA1 (Fig. 6) without specific decrease in those molecular species in SM (Fig. 5). To clarify the underlying mechanism, further investigation is needed.
We have previously reported that DHA promoted PS biosynthesis and accumulation [50], and inhibited apoptosis of neuronal cells in a PS-dependent manner [50–53]. DHA depletion by an n-3 fatty acid-deficient diet decreased the total PS levels in neuronal tissues [54, 55], and inhibited cell survival by impeding Akt signaling [52, 53]. The evidence suggested that membrane PL components, especially DHA and PS, might contribute to differential neuronal survival in the hippocampus. Nevertheless, a higher PS content was observed in CA1 (Fig. 1) which is more susceptible to ischemic injury than CA3 (Fig. 3), indicating that CA1 vulnerability to ischemia-induced cell death is not due to the low level of PS. It is possible that a higher concentration of PS in the hippocampal CA1 may serve to protect neurons against vulnerability caused by other factors yet to be defined. In other words, it can be hypothesized that decreased PS in the CA1 may exacerbate the ischemia-induced injury in CA1. This area of research is still at an early stage and further investigation is needed.
In conclusion, we document the changes of PL molecular species after cerebral ischemia in CA1 and CA3 regions concurrently for the first time. We demonstrated that PS, PE, PE-pl and SM were higher in CA1 in comparison to CA3, and all these phospholipids were decreased after injury specifically in CA1. No disproportionate reduction of AA or DHA species was observed. Instead, the proportion of DHA-containing PE or PE-pls species became greater after injury, suggesting that selective loss of polyunsaturated fatty acids may not be associated with ischemia-reperfusion injury.
Acknowledgments
The authors thank Dr. Sarah Spencer (University of Calgary, Department of Physiology and Biophysics), Dr. Sandor Batkai (NIAAA/Laboratory of Physiological Studies), Dr. Lee Chedester (NIAAA/Animal care unit) for technical advice of surgeries and Dr. Norio Horiguchi (NIAAA/Laboratory of Physiological Studies) and Dr. Douglas Osei-hyiaman (NIAAA/Laboratory of Physiological Studies) for technical advice of histology. This research was supported by the intramural program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Abbreviations
- AA
arachidonic acid
- CM
ceramide
- DHA
docosahexaenoic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PE-pl
plasmalogen
- PI
phosphatidylinositol
- PLA2
phospholipase A2
- PS
phosphatidylserine
- SM
sphingomyelin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 2.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 3.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitani A, Andou Y, Kataoka K. Selective vulnerability of hippocampal CA1 neurons cannot be explained in terms of an increase in glutamate concentration during ischemia in the gerbil: brain microdialysis study. Neuroscience. 1992;48:307–313. doi: 10.1016/0306-4522(92)90492-k. [DOI] [PubMed] [Google Scholar]
- 5.Jabaudon D, Scanziani M, Gahwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc Natl Acad Sci U S A. 2000;97:5610–5615. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 7.Johnston D, Amaral DG. The synaptic organization of the brain. 5. Oxford University Press; Oxford; New York: 2004. p. 455.p. 498. [Google Scholar]
- 8.Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gee CE, Benquet P, Raineteau O, Rietschin L, Kirbach SW, Gerber U. NMDA receptors and the differential ischemic vulnerability of hippocampal neurons. Eur J Neurosci. 2006;23:2595–2603. doi: 10.1111/j.1460-9568.2006.04786.x. [DOI] [PubMed] [Google Scholar]
- 10.Kubota M, Nakane M, Nakagomi T, et al. Regional distribution of ethanolamine plasmalogen in the hippocampal CA1 and CA3 regions and cerebral cortex of the gerbil. Neurosci Lett. 2001;301:175–178. doi: 10.1016/s0304-3940(01)01631-7. [DOI] [PubMed] [Google Scholar]
- 11.Bazan NG., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970;218:1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem Res. 1997;22:759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- 13.Edgar AD, Strosznajder J, Horrocks LA. Activation of ethanolamine phospholipase A2 in Brain during ischemia. J Neurochem. 1982;39:1111–1116. doi: 10.1111/j.1471-4159.1982.tb11503.x. [DOI] [PubMed] [Google Scholar]
- 14.Goto Y, Okamoto S, Yonekawa Y, et al. Degradation of phospholipid molecular species during experimental cerebral ischemia in rats. Stroke. 1988;19:728–735. doi: 10.1161/01.str.19.6.728. [DOI] [PubMed] [Google Scholar]
- 15.Enseleit WH, Domer FR, Jarrott DM, Baricos WH. Cerebral phospholipid content and Na+, K+-ATPase activity during ischemia and postischemic reperfusion in the mongolian gerbil. J Neurochem. 1984;43:320–327. doi: 10.1111/j.1471-4159.1984.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 16.Kubota M, Narita K, Nakagomi T, et al. Sphingomyelin changes in rat cerebral cortex during focal ischemia. Neurol Res. 1996;18:337–341. doi: 10.1080/01616412.1996.11740432. [DOI] [PubMed] [Google Scholar]
- 17.Abe K, Kogure K, Yamamoto H, Imazawa M, Miyamoto K. Mechanism of arachidonic acid liberation during ischemia in gerbil cerebral cortex. J Neurochem. 1987;48:503–509. doi: 10.1111/j.1471-4159.1987.tb04121.x. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda M, Yoshida S, Busto R, Santiso M, Ginsberg MD. Polyphosphoinositides as a probable source of brain free fatty acids accumulated at the onset of ischemia. J Neurochem. 1986;47:123–132. doi: 10.1111/j.1471-4159.1986.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishida A, Shimazaki K, Kawai N. Ischemia-induced changes in PIP2 levels of gerbil hippocampus. Neurosci Res. 1992;15:305–309. doi: 10.1016/0168-0102(92)90053-f. [DOI] [PubMed] [Google Scholar]
- 20.Lukacova N, Gottlieb M, Marsala J. Lipid peroxidation and phospholipid composition in rat brain regions after ischemia and in early perfusion periods. Arch Ital Biol. 1998;136:167–180. [PubMed] [Google Scholar]
- 21.Drgova A, Likavcanova K, Dobrota D. Changes of phospholipid composition and superoxide dismutase activity during global brain ischemia and reperfusion in rats. Gen Physiol Biophys. 2004;23:337–346. [PubMed] [Google Scholar]
- 22.Rehncrona S, Westerberg E, Akesson B, Siesjo BK. Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem. 1982;38:84–93. doi: 10.1111/j.1471-4159.1982.tb10857.x. [DOI] [PubMed] [Google Scholar]
- 23.Bhakoo KK, Crockard HA, Lascelles PT. Regional studies of changes in brain fatty acids following experimental ischaemia and reperfusion in the gerbil. J Neurochem. 1984;43:1025–1031. doi: 10.1111/j.1471-4159.1984.tb12839.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakane M, Kubota M, Nakagomi T, et al. Lethal forebrain ischemia stimulates sphingomyelin hydrolysis and ceramide generation in the gerbil hippocampus. Neurosci Lett. 2000;296:89–92. doi: 10.1016/s0304-3940(00)01655-4. [DOI] [PubMed] [Google Scholar]
- 25.Adibhatla RM, Hatcher JF. Secretory phospholipase A2 IIA is up-regulated by TNF-alpha and IL-1alpha/beta after transient focal cerebral ischemia in rat. Brain Res. 2007;1134:199–205. doi: 10.1016/j.brainres.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Spencer SJ, Auer RN, Pittman QJ. Rat neonatal immune challenge alters adult responses to cerebral ischaemia. J Cereb Blood Flow Metab. 2006;26:456–467. doi: 10.1038/sj.jcbfm.9600206. [DOI] [PubMed] [Google Scholar]
- 27.Abe K, Aoki M, Kawagoe J, et al. Ischemic delayed neuronal death. A mitochondrial hypothesis Stroke. 1995;26:1478–1489. doi: 10.1161/01.str.26.8.1478. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Amsterdam; London: Elsevier Academic; 2004. [Google Scholar]
- 29.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 30.Kim HY, Wang TC, Ma YC. Liquid chromatography/mass spectrometry of phospholipids using electrospray ionization. Anal Chem. 1994;66:3977–3982. doi: 10.1021/ac00094a020. [DOI] [PubMed] [Google Scholar]
- 31.Ma YC, Kim HY. Development of the on-line high-performance liquid chromatography/thermospray mass spectrometry method for the analysis of phospholipid molecular species in rat brain. Anal Biochem. 1995;226:293–301. doi: 10.1006/abio.1995.1228. [DOI] [PubMed] [Google Scholar]
- 32.Wen Z, Kim HY. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J Neurochem. 2004;89:1368–1377. doi: 10.1111/j.1471-4159.2004.02433.x. [DOI] [PubMed] [Google Scholar]
- 33.Bazan NG, Eady TN, Khoutorova L, et al. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp Neurol. 2012;236:122–130. doi: 10.1016/j.expneurol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalancette-Hebert M, Julien C, Cordeau P, et al. Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke. 2011;42:2903–2909. doi: 10.1161/STROKEAHA.111.620856. [DOI] [PubMed] [Google Scholar]
- 35.Larsson SC, Orsini N. Fish consumption and the risk of stroke: a dose-response meta-analysis. Stroke. 2011;42:3621–3623. doi: 10.1161/STROKEAHA.111.630319. [DOI] [PubMed] [Google Scholar]
- 36.Kwak SM, Myung SK, Lee YJ, Seo HG. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012;172:686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 37.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–1664. doi: 10.1161/01.str.25.8.1658. discussion 1664–1655. [DOI] [PubMed] [Google Scholar]
- 38.Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 39.DeGeorge JJ, Nariai T, Yamazaki S, Williams WM, Rapoport SI. Arecoline-stimulated brain incorporation of intravenously administered fatty acids in unanesthetized rats. J Neurochem. 1991;56:352–355. doi: 10.1111/j.1471-4159.1991.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 40.Rabin O, Chang MC, Grange E, et al. Selective acceleration of arachidonic acid reincorporation into brain membrane phospholipid following transient ischemia in awake gerbil. J Neurochem. 1998;70:325–334. doi: 10.1046/j.1471-4159.1998.70010325.x. [DOI] [PubMed] [Google Scholar]
- 41.Ford DA, Hale CC. Plasmalogen and anionic phospholipid dependence of the cardiac sarcolemmal sodium-calcium exchanger. FEBS Lett. 1996;394:99–102. doi: 10.1016/0014-5793(96)00930-1. [DOI] [PubMed] [Google Scholar]
- 42.Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- 43.Cui Z, Houweling M. Phosphatidylcholine and cell death. Biochim Biophys Acta. 2002;1585:87–96. doi: 10.1016/s1388-1981(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 44.Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao FH. CDP-choline significantly restores phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP: phosphocholine cytidylyltransferase after stroke. J Biol Chem. 2006;281:6718–6725. doi: 10.1074/jbc.M512112200. [DOI] [PubMed] [Google Scholar]
- 45.Brugg B, Michel PP, Agid Y, Ruberg M. Ceramide induces apoptosis in cultured mesencephalic neurons. J Neurochem. 1996;66:733–739. doi: 10.1046/j.1471-4159.1996.66020733.x. [DOI] [PubMed] [Google Scholar]
- 46.Casaccia-Bonnefil P, Aibel L, Chao MV. Central glial and neuronal populations display differential sensitivity to ceramide-dependent cell death. J Neurosci Res. 1996;43:382–389. doi: 10.1002/(SICI)1097-4547(19960201)43:3<382::AID-JNR13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 47.Yen CL, Mar MH, Zeisel SH. Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. FASEB J. 1999;13:135–142. [PubMed] [Google Scholar]
- 48.Soeda S, Tsuji Y, Ochiai T, et al. Inhibition of sphingomyelinase activity helps to prevent neuron death caused by ischemic stress. Neurochem Int. 2004;45:619–626. doi: 10.1016/j.neuint.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Feng Y, LeBlanc MH. Treatment of hypoxic-ischemic brain injury in newborn rats with TPCK 3 h after hypoxia decreases caspase-9 activation and improves neuropathologic outcome. Dev Neurosci. 2003;25:34–40. doi: 10.1159/000071466. [DOI] [PubMed] [Google Scholar]
- 50.Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–35223. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- 51.Akbar M, Kim HY. Protective effects of docosahexaenoic acid in staurosporine-induced apoptosis: involvement of phosphatidylinositol-3 kinase pathway. J Neurochem. 2002;82:655–665. doi: 10.1046/j.1471-4159.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 52.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang BX, Akbar M, Kevala K, Kim HY. Phosphatidylserine is a critical modulator for Akt activation. J Cell Biol. 2011;192:979–992. doi: 10.1083/jcb.201005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamilton L, Greiner R, Salem N, Jr, Kim HY. n-3 fatty acid deficiency decreases phosphatidylserine accumulation selectively in neuronal tissues. Lipids. 2000;35:863–869. doi: 10.1007/s11745-000-0595-x. [DOI] [PubMed] [Google Scholar]
- 55.Murthy M, Hamilton J, Greiner RS, Moriguchi T, Salem N, Jr, Kim HY. Differential effects of n-3 fatty acid deficiency on phospholipid molecular species composition in the rat hippocampus. J Lipid Res. 2002;43:611–617. [PubMed] [Google Scholar]