Abstract
The realization that pregnant and infant monkeys were challenged by high nutritional needs for iron led vendors to markedly increase iron concentrations in commercial diets. Yet, no systematic research was conducted to investigate the consequences of this important dietary change. Hematology and iron panels were determined for 142 infant rhesus monkeys gestated and reared on 3 different diets varying in iron concentration (180, 225 or 380 mg Fe/kg). Anemia was significantly more prevalent in offspring from females fed the 180 and 225 mg Fe/kg diets (32–41% versus 0 for the 380 mg Fe/kg diet, P<0.001). Higher hepcidin levels were protective against iron overload in infants from the 380 mg Fe/kg condition. These findings indicate a highly fortified diet during pregnancy continues to have postnatal benefits for the growing infant. However, for those interested in iron deficiency, lower iron diets provide a reliable way to generate anemic infant monkeys for research.
Keywords: iron deficiency, anemia, pregnancy, infancy, primate, monkey, hepcidin
1. Introduction
Pregnant females and infants of many animal species are challenged by the dietary need to obtain high levels of iron and thus are prone to developing an iron deficiency anemia (IDA), with the risk comparable to that seen in women and young children (Allen, 2005; Bothwell, 2000, Looker et al., 1997). The likelihood of IDA in nonhuman primates, as well as in litter-bearing rodents and some farm animals, led to increased fortification of commercial diets. Since 1972 the recommended Minimal Daily Requirements have been updated repeatedly by advisory panels in the U.S. (National Research Council, 2003). Iron concentrations in most primate diets today are over twice the level from two decades ago, and almost fourfold higher than the amount considered adequate for a non-pregnant adult monkey (i.e., 100 mg Fe/kg). Given the diverse effects of iron, including on oxidative metabolism and the synthesis of neurotransmitters and myelin, these changes in nutriture over time are likely to have impacted the health of monkeys in zoos and laboratories and the findings of many studies (Rao et al., 2002; Ortiz et al., 2004). Highlighting this type of concern, it was found previously that vitamin A levels had actually been excessively high in primate diets, with toxic hepatic effects evident upon histological exam and from elevated liver enzyme values (Penniston and Tanumihardjo, 2006).
Two hematological surveys documented that the occurrence of anemia in infant monkeys used to be very common in research facilities (Bicknese et al., 1993; Kriete et al., 1995). The 30–40% occurrence of anemia was comparable to the prevalence in American children prior to the fortification of infant formula and cereals, and concurs with the widespread IDA still evident in non-industrialized countries (Zetterstrom, 2004). While lactoferrin, the primary source of iron in breast milk, is readily absorbed, it is also critical that substantial amounts of maternal iron be acquired transplacentally before birth in order to meet the infant's growth needs for iron (Davidson et al., 1990; Golub et al., 2006). Monkeys born with low storage iron, as indexed by serum ferritin, are at increased risk to become anemic by the end of the nursing period at 4–6 months of age. In keeping with the importance of this prenatal iron acquisition, adult female monkeys fed a low iron diet during pregnancy are predisposed to birth infants that will progress to a clinical anemia (Lubach and Coe, 2006). Moreover, primiparous monkeys are less likely to provide sufficient iron than are multiparous dams, a maternal/fetal conflict also seen in adolescent human pregnancies (Iannotti et al., 2005; Meier et al., 2003).
Although the infant's hematology gradually improves with the consumption of solid food, some lingering effects of the iron deficiency may remain evident, including on brain dopamine and norepinephrine activity and myelination (Coe et al., 2009; Lozoff et al., 2006; Patton et al., 2012). Iron transport proteins, such as transferrin and divalent metal transporter, also stay up-regulated in cerebrospinal fluid, suggesting that the acquisition of brain iron takes longer to be completely normalized (Geguchadze et al., 2008). Thus, it is important to better understand the maternal factors that influence the occurrence of infant anemia and to evaluate when the protracted effects on hematology and iron biology resolve. A second aim of our study was to assess if there is evidence of iron overload in monkeys consuming a very fortified diet (Tanno and Miller, 2010). Iron absorption should be tightly regulated, particularly by the peptide hepcidin, and from heme feedback signals including transferrin saturation (TSAT) (Ganz, 2011; Kemna et al., 2008). Prior research in rodents had also suggested that low hepcidin in an iron-deficient infant could result in a rebound overshoot of iron absorption during rapid repletion (Hegde et al., 2011).
To address these issues, hematology and iron measures were evaluated in infant monkeys born to females fed 3 different diets: 1) the current diet used by most facilities today, 2) a diet formulation similar to the standard one used prior to 1995, and 3) a customized diet with intermediate iron levels. Serum hepcidin levels were determined because of its known role in the regulation of iron absorption and tissue storage. A final aim was to assess if parity influenced the effects of maternal diet, which was investigated by screening infants of primiparous and multiparous monkeys in each diet condition.
2. Methods
2.1. Subjects
This research determined the hematology and iron status of 142 young rhesus monkeys (Macaca mulatta) reared and housed under standardized indoor conditions at the Harlow Primate Laboratory and adjacent Wisconsin National Primate Research Center. Light/dark schedules were regulated and constant across the year; room temperature was controlled at 21 °C; and the monkeys lived in stainless steel caging, which was cleaned daily and completely sanitized every 2 weeks. Similar veterinary care and clinical treatments (if needed) were provided to all animals, and all monkeys received annual physical exams to monitor their general health status. The mothers were fed one of 3 diets during pregnancy and nursing (180 mg/kg [N=73], 225 mg/kg, [N=29], 380 mg/kg [N=40]). All infants were healthy, from full term, singleton births and delivered naturally, with primiparous and multiparous dams represented in each diet condition (N=51, and 91, respectively). It was also possible to consider the possible influence of infant gender (78 males, 64 females).
Blood samples were collected twice from infants in the two lower iron diet conditions, at 6–7 months and 12 months of age, because some were iron-deficient and it was important to determine if their anemia resolved. For the 40 infants from the high iron diet condition, half were assessed at each age point, with parity and gender completely balanced. Samples were collected from infants of 10 primiparous and 10 multiparous dams at each time point, with each parity condition comprised of 5 males and 5 females. All procedures were reviewed and approved by the Animal Care and Use Committee at the University of Wisconsin.
2,2. Diet
Three different diets varying in iron concentration were assessed (Table 1). The low iron diet (180 mg Fe/kg) was comparable to the standard diet used in the U.S. prior to 1995 (Purina 5L1Q, PMI Nutrition International, St. Louis, MO). The high iron biscuit (380 mg Fe/kg) is the diet commonly used by research facilities and primate centers today (Harlan Teklad 2050, Madison, WI). In addition, with the assistance of the vendor, we specifically created an intermediate level of iron fortification (225 mg Fe/kg), while maintaining other constituents comparable to the low iron diet (Purina 5LFD). Ferrous sulfate was used during manufacture to supplement the natural ingredients up to the appropriate iron level. Each day the monkeys were given a specified number of biscuits. A small amount of fruit was provided in the afternoon as part of an environmental enrichment program required by federal regulations.
TABLE 1.
Mineral and vitamin concentrations, as well as ingredient composition, for the 3 diets fed to the rhesus monkeys in this study.1
| Fe concentration | 180 | 225 | 380 |
|---|---|---|---|
| Ingredients | |||
| Protein, % | 15.6 | 15.7 | 20.0 |
| Carbohydrate, % | 68.9 | 68.7 | 40.1 |
| Fat, % | 6.0 | 6.0 | 5.4 |
| Ash, % | 5.2 | 5.3 | 6.1 |
| Fiber (crude), % | 4.6 | 4.5 | 8.1 |
| Minerals | |||
| Iron2, mg/kg | 180 | 225 | 380 |
| Zinc, mg/kg | 114 | 110 | 72 |
| Copper, mg/kg | 20 | 21 | 14 |
| Vitamins | |||
| A, IU/g | 20.0 | 20.0 | 19.5 |
| B12, mg/kg | .022 | .073 | .040 |
| C, mg/kg | 500 | 500 | 910 |
The vender sources were Purina (5L1Q and 5LFD for the 180 and 225 mg Fe/kg biscuits, respectively) and Harlan Teklad (2050) for the 380 mg Fe/kg diet.
Based on typical daily consumption (220g), each day the adult female monkeys were provided approximately 39.6, 49.5, or 83.6 mg of Fe, respectively, from the 3 diets.
2.3. Hematology and iron Panel
Blood samples (< 4 mL) were obtained via femoral or saphenous venipuncture in order to generate the panel of hematological and iron measures. Results are presented for two red blood cell (RBC) parameters with known clinical cutoffs for IDA in monkeys, mean corpuscular volume (MCV, for IDA <60 fL) and hemoglobin (Hb, for IDA <110 g/L). Three measures from the iron panel are also summarized: 1) serum ferritin, 2) iron level in circulation and 3) TSAT (serum iron/total iron binding capacity × 100). In addition, serum hepcidin levels were analyzed at each age point from a representative subset of 21 infants, including 6 from the high iron diet as well as for 15 iron-sufficient (IS) and IDA monkeys from the 225 mg Fe/kg diet condition (N = 8 and 7, respectively).
2.4 Hepcidin assay
Hepcidin-25 (Hep-25) levels were analyzed with an established competitive radioimmunoassay, which has been described in detail previously (Busbridge et al., 2009). Rabbit anti-Hep-25 polyclonal antibody was generated using synthetic hepcidin (Bachem Ltd, UK), and the displacement of Hep-25 radiolabeled with iodine (125I) used to quantify serum titers. Prior comparisons had verified that Hep-25 values determined by this RIA were highly correlated with established SELDI-TOF-Mass Spectrometry methods (r = 0.96) (Ashby et al., 2010). The lower limit of detection was 0.22 nmol/L; intra-assay precision averages 7.2%, and the inter-assay coefficient of variation averages 7.6%. The sequence homology of Hep-25 is conserved across species, with the Hep-25 of rhesus monkeys differing by only one amino acid, a threonine for an alanine. From these structural data, it was reasonable to assume that the antibody against human Hep-25 crossreacts with monkey Hep-25.
2.5. Statistical analysis
The effect of diet on the infants' iron status and hematology was examined with analyses of variance (ANOVA). Infant gender was included initially as a nested factor in 3-factor models, but proved not to have a major influence on outcomes, other than for body weight. Maternal parity (primparous vs. multiparous) was also included in the initial models, but the effects of dietary iron were similar, and thus the presented results do not focus in detail on parity. The primary ANOVAs were two-factor tests comparing the 3 diets and two age points, and/or differences between IS and anemic infants. Post hoc comparisons were conducted with Fisher's Protected LSD test with alpha set at <0.05. Significant differences in the prevalence of anemia were verified by Chi square analysis. The potential influence of growth rate on iron utilization was considered by examining body weights at 6–7 months and one year of age, as well as the weight increment from birth to 6–7 months, which is the age by which female monkeys wean the infant.
3. Results
3.1. Hematology
The hematology panels of the infants at 6–7 months of age revealed a clear effect of the diet fed to the adult females during pregnancy and lactation (Figure 1). At this point, which corresponds to the end of the nursing period, the mean MCV values for infants from both the 180 and 225 mg Fe/kg diet conditions were significantly lower than for monkeys from the 380 mg Fe/kg diet (P<0.005). Similar differences were evident for Hb, with infants from both low iron diets having mean Hb values below those from the 380 mg Fe/kg diet (P<0.028). By one year of age, the hematological differences had lessened, because of post-weaning improvements as all monkeys consumed solid food independently. At this point, MCV indices were similar across the 3 diet conditions. The lower mean Hb values for infants consuming the 180 and 225 mg Fe/kg diets were reduced to a statistical trend below the 380 mg Fe/kg diet (P<0.06), an effect driven primarily by those infants that had been anemic at 6–7 months of age.
Figure 1.
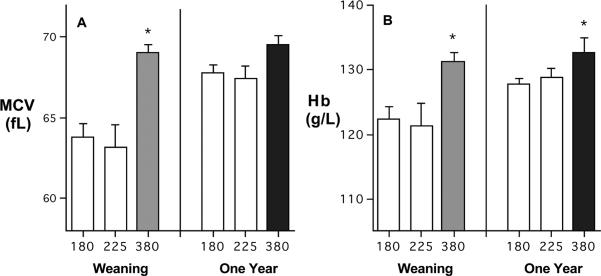
Mean (SEM) hematology values for offspring from female monkeys fed 3 different diets varying in iron concentration (180, 225, and 380 mg Fe/kg). At 6–7 months of age, infants from the high iron diet had significantly higher MCVs (Panel A). Hb values were still significantly higher at one year of age (Panel B). For the 180 mg/kg diet, 23 of 73 met hematological criteria for anemia; for the 225 mg/kg diet, 12 of 29 were anemic at 6–7 months, which is the point when monkey mothers wean the infant.
3.2. Prevalence of Anemia
Differences between the 3 dietary conditions were strongly influenced by a large number of overtly anemic infants from dams fed the two low iron diets. Based on standard diagnostic criteria for IDA in monkeys, the prevalence of anemia at the end of the nursing period was significantly greater in these diet conditions: 32% and 41% for the 180 and 225 mg Fe/kg condition, respectively (X2=19.3, P<0.001). None of the 40 infants from dams fed the high iron diet were anemic. The other iron indices confirmed this categorization: both serum iron (P<0.001) and TSAT values (P<0.001) were significantly lower in infants designated as anemic (Table 2). However, by one year of age, the iron status of all infants had improved, even for the previously anemic ones. It was noteworthy that the former IDA infants now consuming the 225 mg Fe/kg diet, and becoming replenished, evinced high serum iron and uniquely elevated TSAT values, significantly above the other yearling monkeys (P<0.03 and P<0.02, respectively) (see Table 2).
TABLE 2.
Mean (SEM) hematology and iron values at weaning and one year of age for the 3 diet conditions. Between 32–41% of infants born to dams consuming the two lower iron diets were anemic at weaning, but their values normalized with the consumption of solid foods.
| Dietary Fe (mg/kg) | 180 | 225 | 380 | |||
|---|---|---|---|---|---|---|
| Age1 | Weaning | Yearling | Weaning | Yearling | Weaning | Yearling |
| Total N | 73 | 73 | 29 | 29 | 20 | 20 |
| Anemic (n) | 23 | 0 | 12 | 0 | 0 | 0 |
| MCV2, fL | ||||||
| IS | 67.9 (.5) | 68.1 (.5) | 67.6 (1.3) | 63.8 (.8) | 69.0 (.5) | 69.5 (.6) |
| IDA/former IDA | 54.7 (.8)* | 67.1 (.8) | 53.7 (2.3)* | 66.9 (1.3) | - | - |
| Hb3, g/L | ||||||
| IS | 130.8 (1.6) | 129.1 (1.0) | 134.5 (3.5) | 128.7 (1.1) | 131.1 (1.5) | 132.7 (2.2) |
| IDA/former IDA | 103.8 (2.7)* | 125.0 (1.7) | 97.2 (7.0)* | 129.2 (2.4) | - | - |
| Iron4, μM/L | ||||||
| IS | 20.7 (.9) | 23.2 (.9) | 25.3 (1.6) | 21.1 (.9) | 20.3 (1.4) | 21.0 (1.5) |
| IDA/former IDA | 14.8 (1.7)* | 18.8 (1.1) | 15.4 (2.4)* | 23.1 (2.4) | - | - |
| TSAT5, % | ||||||
| IS | 31.3 (1.7) | 32.5 (1.0) | 34.3 (2.6) | 32.4 (2.5) | 31.4 (1.9) | 29.7 (2.5) |
| IDA/former IDA | 18.8 (2.1)* | 27.3 (1.6) | 16.4 (2.1)* | 37.9 (5.1) | - | - |
| Weight6, g | ||||||
| IS | 1395 (20) | 2236 (36) | 1341 (42) | 2150 (62) | 1347 (29) | 2470 (63) |
| IDA/former IDA | 1392 (28) | 2152 (51) | 1279 (60) | 2119 (60) | - | |
Infants were assessed at 6–7 months of age and also at one year of age after consuming solid foods.
MCVs for the 380 mg Fe/kg diet were significantly higher at weaning, due to anemic infants in the 2 other diet conditions.
Hb was significantly higher for 380 mg Fe/kg, driven by significant differences in anemic monkeys at both ages, although the post hoc testing of Hb differences in just yearlings reached only P<0.06.
Serum iron was lower in anemic infants at weaning, but improved markedly by 12 months of age.
TSAT was low in IDA infants at weaning, but rose dramatically after consumption of solid food.
The growth of IS and IDA infants was similar. By one year, males consuming the 380 mg Fe/kg diet tended to be heavier than males in the two other diet conditions. Males were larger than females on all diets.
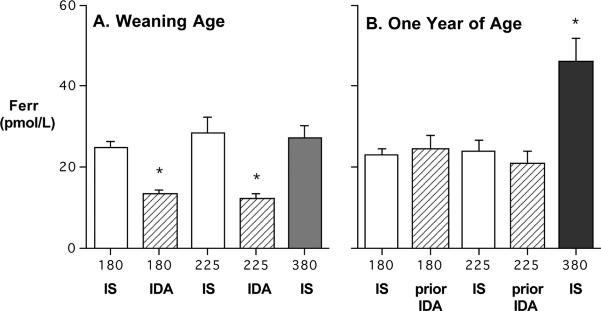
3.3. Serum Ferritin
The ferritin results paralleled these other indices. Serum ferritin levels were significantly lower at 6–7 months of age than in those categorized as anemic, both for the 180 mg Fe/kg and 225 mg Fe/kg diets (P< 0.001) (Figure 2). After consuming solid food, ferritin increased in most yearlings, even in those that had been anemic. The largest increase was evident in those monkeys fed the 380 mg Fe/kg diet. At one year of age, their ferritin levels were significantly above the other two dietary conditions, due to almost a doubling of their ferritin values (P<0.001).
Figure 2.
Mean (SEM) ferritin levels at 6–7 months and one year of age in monkeys from 3 diet conditions (Panel A and B, respectively). The IDA infants had significantly lower ferritin at 6–7 months (23 of 73 for the 180 mg/kg diet, 12 of 29 for the 225 mg/kg diet), which improved by one year after eating solid food. Yearlings from the 380 mg Fe/kg diet had the highest ferritin levels.
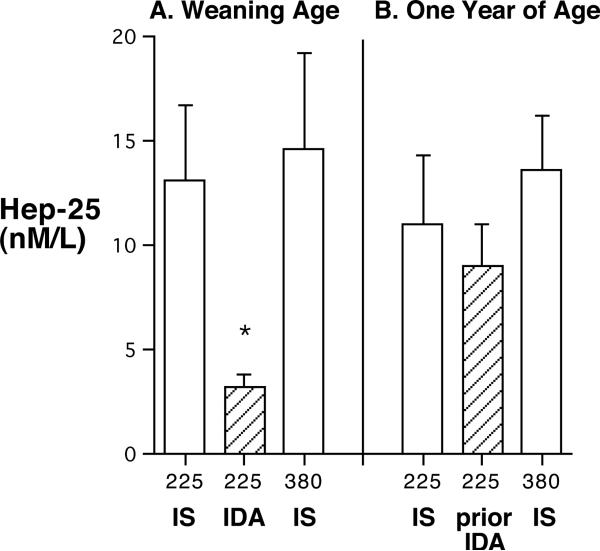
3.4. Hepcidin
At 6–7 months of age, the Hep-25 levels in anemic infants were significantly below the values for IS infants, both from the intermediate and high iron diets (P<0.05) (Figure 3). By one year of age, Hep-25 levels were more similar across conditions. However, the increment in Hep-25 for the previously anemic ones still had not reached a statistically significant improvement above the previously low values evident at 6–7 months of age (P>0.065).
Figure 3.
Mean (SEM) serum Hep-25 levels in IS and IDA infants at 6–7 months of age, and subsequently at one year of age when both IS and former IDA monkeys had normal hematological values. Hep-25 in anemic infants at 6–7 months (n=7) was significantly below IS infants (n=14). No monkeys in the 380 mg Fe/kg diet condition became iron deficient.
3.5. Growth
The strong influence of maternal diet on the infants' iron status was not attributable to a differential effect on growth. Body weights were similar at 6–7 months of age across the 3 diet conditions (Table 2). The birth weights and rate of growth from birth-to-weaning were also not different in anemic infants when compared to the IS infants from dams fed the 180 and 225 mg Fe/kg diet. However, this similar stature of anemic infants may indicate that equivalently high growth-related demands for iron had contributed to the depletion of their ferritin reserves and thus their poorer hematological profile. The large additional weight increases through one year of age indicate that infant monkeys continue to sustain this rapid growth after being weaned from the mother and, in our rearing conditions, following the re-housing with peers (Table 2). A modest but statistically significant effect of consuming the 380 mg Fe/kg diet was evident in the yearlings (larger by a mean 0.27 kg, P<0.001, equivalent to a 2% difference in body weight). In addition, across the 3 diets and at both age points, male infants tended to be heavier than females (by 0.8 kg P<0.06), and the weight gain in males consuming the 380 mg Fe/kg was greater than for the other males (P<0.05). On average, infants from primiparous females were born slightly smaller and weighed less at 6–7 months of age than those raised by multiparous dams (by 0.9 kg at 6–7 months, P<0.02).
4. Discussion
These results demonstrate that the higher iron content of today's commercial primate diets ensures against the occurrence of anemia in infant monkeys. In contrast, if adult female rhesus monkeys are fed a less fortified diet throughout pregnancy and the nursing period (i.e., typical of the nutrition provided in the past), a significant percent of their infants became anemic by 6–7 months of age, which is when monkeys typically decrease nursing and infants shift to solid foods. The prevalence of anemia in our study is similar to reports from two decades ago when this type of diet was widely used at primate facilities (Bicknese et al., 1993; Kriete et al., 1995). In fact, our laboratory has experimentally utilized the 180 mg Fe/kg diets to reliably generate IDA infant monkeys for research projects (Coe et al., 2009; Patton et al., 2012). In the current analysis, the 225 mg Fe/kg also did not appear to be an iron-adequate diet during pregnancy, although it remains to be determined if it is somewhat more effective at protecting against the postnatal iron depletion seen in a fast-growing infant. Although it would have been of value to verify that the iron differences were already evident at birth, we know from previous research that low ferritin in newborn infants and declining ferritin levels during growth are the best determinants of which monkey will become anemic (Lubach and Coe, 2006). In monkeys approximately 50% of the iron needed for postnatal growth must be acquired prenatally via transplacental transfer of maternal iron (Golub et al., 2006).
Further analyses are still needed to determine the optimal amount of iron required for the gravid monkey, including by employing more sophisticated methods to directly quantify iron absorption and transmission to the fetus, as has been done in pregnant women and human neonates (Hallberg and Hulthen, 2000; Milman et al., 2005). The absolute concentration of iron in the biscuits will be just one factor to consider, because it is known that other nutrients and substances can interfere with iron bioavailability. Iron absorption can be affected by several micronutrients, such as zinc, as well as by the phytates in grains and cereals used in commercial biscuits, and the tannins common in natural food items eaten by monkeys (Lonnerdal et al., 1990; Lonnerdal et al., 1997). It is possible that the current formulation of the 380 mg Fe/kg diet, which includes more Vitamin C and less zinc (a promoter and inhibitor of iron absorption, respectively) may contribute to its effectiveness in preventing iron deficiency.
The course of hematological restoration and normalizing of iron status when the weaned monkeys began to eat solid food exclusively is also of interest. Even infants from dams fed the most iron had relatively low ferritin by 6–7 months of age, both compared to the high ferritin values in neonates at birth, and also with respect to the rebound in serum ferritin by one year of age. The upward ferritin trend in yearlings was not as robust for the two lower iron diets, suggesting that relatively more iron was being allocated to erythropoiesis and growth than to storage. A distinctive sequence of repletion was also evident in the formerly anemic animals consuming the 225 mg Fe/kg diet. They evinced high circulating levels of iron and an elevated TSAT, which may reflect enhanced absorption, facilitated by their lower hepcidin. Our previous research on anemic monkeys provided several other examples of how iron can be differentially partitioned across tissues, with heme measures recovering more quickly than the central nervous system, where neurochemical and protein indictors of low iron linger for months (Geguchadze et al., 2008; Patton et al., 2012).
The evidence for a tissue prioritization during repletion, as well as some protracted effects, concurs with the conclusions from rodent models of anemia (Lozoff and Georgieff, 2006). The slower recovery of brain iron is also of potential clinical concern for anemic children given reports of persistent cognitive, motor, sensory and emotional deficits, with some effects still evident at school age and even into adolescence (Chang et al., 2011). Sustained anemia during early rearing may thus have serious developmental and behavioral ramifications in both animals and humans (Golub et al., 2007). Given that it can be readily remedied by consuming iron-rich foods or iron supplementation, there continues to be a need for closer surveillance of both animal and human infants likely to become IDA (Baker et al., 2010). Among the prominent predictors of risk are low ferritin stores at delivery, which is caused by low iron in the diet, premature birth or the occurrence of maternal anemia, diabetes or high parasite load during pregnancy. It should be emphasized that infant monkeys destined to become anemic are not necessarily small at birth or stunted in their growth, but rather are often the larger animals. We found previously that the co-occurrence of low ferritin at birth combined with a rapid postnatal growth rate is the best predictor of which infant monkey will become anemic (Coe et al., 2007). Rapid growth in human infants has also been reported to compound the consequences of being born with low ferritin (Georgieff et al., 2002). Conversely, in the current study, the slower growth of infant monkeys born to primiparous mothers may actually have been somewhat protective, and could account for why they were not more affected by low iron diets than the infants of multiparous females.
To our knowledge, this is the first research to assess the 25-amino acid peptide hepcidin in young monkeys. The range of monkey Hep-25 values generated by this assay was comparable to human levels (Kroot et al., 2009). In keeping with expectations, hepcidin was significantly reduced in anemic infants, which would augment iron absorption via the intestine, reduce macrophage retention of iron, and facilitate hemoglobin-iron recycling within the blood compartment (Theurl et al., 2009). Low Hep-25 in the IDA monkey also appeared to create a permissive state for iron absorption when fed an iron-rich diet after they were re-housed with peers. In a complementary manner, the higher Hep-25 in the IS infants appeared to protect against excessive iron uptake. There wasn't any evidence that the most fortified diet resulted in iron overload or abnormal erythocytosis. As many others have concluded, iron absorption and erythopoiesis are tightly regulated and responsive to body iron stores and red blood cell needs.
In conclusion, the highly fortified diet used at most facilities today meets the needs of pregnant female monkeys and serves as a vital nutrient source for the rapidly growing infant. On the other hand, the traditional monkey diet with lower iron levels offers an invaluable tool for researchers interested in generating animal models of gestational and pediatric deficiency. Both diet regimens still provide more iron than recommended for non-gravid adult monkeys, and these iron concentrations are all much higher than found in the typical diets of most other species. Thus, when selecting a diet for a nonpregnant monkey, additional husbandry and health factors should also be considered. Some enteric pathogens are prone to thrive in an iron-rich gastrointestinal environment (Zimmerman et al., 2010), and diarrheal symptoms are often more common in primate colonies fed the most fortified diets. In addition, we reported recently that signs of excessive iron uptake and deposition can be visualized with MRI scans in the brains of aged monkeys (Kastman et al., 2010), similar to the abnormal iron deposition in the striatum of individuals with dementia and Alzheimer's Disease (Zecca et al., 2004). Thus, the optimal diet formulation will depend upon the age and reproductive characteristics of the population at the primate facility and the goals of the program.
Acknowledgments
Our analyses benefited from suggestions given by several investigators involved in a NICHD-sponsored Program Project Grant (P01 HD39386, P.I.: B. Lozoff). However, the listed authors are solely responsible for this paper's content; the conclusions do not reflect the official view of the NIH. The research was also enabled by support from R01 HD057064 to CLC and the primate resources of the WNPRC (P51 RR000167).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supported by grant awards from NICHD (R01 HD057064; P01 HD39386), with additional resources from P51 RR000167 to the WNRPC. The authors have no financial or proprietary conflicts of interest to report.
Literature Cited
- Allen LH. Multiple micronutrients in pregnancy and lactation: an overview. American Journal of Clinical Nutrition. 2005;81:1206S–1212S. doi: 10.1093/ajcn/81.5.1206. [DOI] [PubMed] [Google Scholar]
- Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, Taube DH, Bloom SR, Tam FWK, Chapman R, Maxwell PH, Choi P. Erythopoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica. 2010;95:505–508. doi: 10.3324/haematol.2009.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RD, Greer FR. Committee on Nutrition American Academy of Pediatrics: Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics. 2010;126:1040–1050. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- Bicknese EJ, George JW, Hird DW, Paul-Murphy J, Anderson JA, Roberts JR. Prevalence and risk factors for iron deficiency anemia in weanling rhesus macaques. Laboratory Animal Science. 1993;43:434–438. [PubMed] [Google Scholar]
- Bothwell T,H. Iron requirements in pregnancy and strategies to meet them. American Journal of Clinical Nutrition. 2000;72:257S–264S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- Busbridge M, Griffiths C, Ashby D, Gale D, Jayantha A, Sanwaiya A, Chapman RS. Development of a novel immunoassay for the iron regulatory peptide hepcidin. British Journal of Biomedical Science. 2009;66:150–157. doi: 10.1080/09674845.2009.11730263. [DOI] [PubMed] [Google Scholar]
- Chang S, Wang L, Wang Y, Brouwer ID, Kok FJ, Lozoff B, Chen C. Iron-deficiency anemia in infancy and social emotional development in preschool-aged Chinese children. Pediatrics. 2011;127:e927–933. doi: 10.1542/peds.2010-1659. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Bianco L, Beard JL. A history of iron deficiency anemia during infancy alters brain monoamine activity later in juvenile monkeys. Developmental Psychobiology. 2009;5:301–309. doi: 10.1002/dev.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatric Research. 2007;61(5):520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Litov RE, Lönnerdal B. Iron retention from lactoferrin-supplemented formulas in infant rhesus monkeys. Pediatric Research. 1990;27:176–180. doi: 10.1203/00006450-199002000-00018. [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;119(17):4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geguchadze RN, Coe CL, Lubach GR, Clardy TW, Beard JW, Connor RJ. CSF proteomic analysis reveals persistent iron deficiency-induced alterations in nonhuman primate infants. Journal of Neurochemistry. 2008;105:127–136. doi: 10.1111/j.1471-4159.2007.05113.x. [DOI] [PubMed] [Google Scholar]
- Georgieff MK, Wewerka SW, Nelson CA, deRegnier R-A. Iron status at 9 months of infants with low iron stores. Journal of Pediatrics. 2002;141:405–409. doi: 10.1067/mpd.2002.127090. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann S. Iron deprivation during fetal development changes the behavior of juvenile monkeys. Journal of Nutrition. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE,, Tarantal AF, Germann SL, Beard JL, Georgieff MK, Calatroni A, Lozoff B. Diet induced iron deficiency anemia and pregnancy outcomes in rhesus monkeys. American Journal of Clinical Nutrition. 2006;83(3):647–656. doi: 10.1093/ajcn.83.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg L, Hulthén L. Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. American Journal of Clinical Nutrition. 2000;71:1147–1160. doi: 10.1093/ajcn/71.5.1147. [DOI] [PubMed] [Google Scholar]
- Hegde NV, Jensen GL, Unger EL. A postweaning iron-adequate diet following neonatal iron deficiency affects iron homeostasis and growth in young rats. Journal of Nutrition. 2011;141:386–390. doi: 10.3945/jn.110.133363. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, O'Brien KO, Chang S-C, Mancini J, Schulman-Nathanson M, Liu S, Harris Z,L, Witter FR. Iron deficiency anemia and depleted body iron reserves are prevalent among pregnant African-American adolescents. Journal of Nutrition. 2005;135:2572–2577. doi: 10.1093/jn/135.11.2572. [DOI] [PubMed] [Google Scholar]
- Kastman EK, Willette AA, Coe CL, Bendlin BB, Kosmatka KJ, McLaren DG, Guofan X, Canu E, Field AS, Alexander AL, Voytko ML, Beasle TM, Colman RJ, Weindruch RH, Johnson SC. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. Journal of Neuroscience. 2010;20:7940–7947. doi: 10.1523/JNEUROSCI.0835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemna EH, Tjalsma T, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haemotologica. 2008;93:90–97. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- Kreite MF, Champoux M, Suomi S. Development of iron deficiency anemia in infant rhesus monkeys. Laboratory Animal Science. 1995;45:15–21. [PubMed] [Google Scholar]
- Kroot JJ, Kemma EH, Bansal SS, Busbridge M, Campostrini N, Girelli D, Hider RC, Koliaraki V, Mamalaki A, Olbina G, Tomosugi N, Tselepis C, Ward DG, Ganz T, Hendriks JC, Swinkels DW. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94:1748–1752. doi: 10.3324/haematol.2009.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnerdal B, Jayawickrama L, Lien EL. Effect of reducing the phytate content and of partially hydrolyzing the protein in soy formula on zinc and copper absorption and status in infant rhesus monkeys and rat pups. American Journal of Clinical Nutrition. 1999;69:490–496. doi: 10.1093/ajcn/69.3.490. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B, Keen CL, Hendrickx AG, Golub MS, Gershwin ME. Influence of dietary zinc and iron in pregnant rhesus monkeys and their infants. Obstetrics and Gynecology. 1990;75:369–374. [PubMed] [Google Scholar]
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. Journal of the American Medical Association. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schaller T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutrition Reviews. 2006;64:S34–43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK. Iron deficiency and brain development. Seminars in Pediatric Neurology. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lubach GR, Coe CL. Preconception maternal iron status is a risk factor for iron deficiency in infant rhesus monkeys (Macaca mulatta) Journal of Nutrition. 2006;136:2345–2349. doi: 10.1093/jn/136.9.2345. [DOI] [PubMed] [Google Scholar]
- Meier PR, Nicerkson J, Olson MS, Berg MS, Meyer JA. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clinical Medical Research. 2003;1:29–36. doi: 10.3121/cmr.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman N, Bergholt T, Eriksen L, Bey KE, Pedersen P, Hertz J. Iron prophylaxis during pregnancy. How much is needed: A randomized dose-response study of 20–80 mg ferrous iron daily in pregnant women. Acta Obstetrica Gynecologica Scandinavica. 2005;84:238–247. doi: 10.1111/j.0001-6349.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient requirements of nonhuman primates. 2nd Revised Edition The National Academies Press; Washington DC: 2003. p. 286. [Google Scholar]
- Ortiz E, Pasquini JM, Thompson K, Felt BT, Butkus G, Beard JL, Connor JR. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. Journal of Neuroscience Research. 2004;77:681–689. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- Patton SM, Coe CL, Lubach GR, Connor JR. Quantitative proteomic analyses of cerebrospinal fluid using iTRAQ in a primate model of iron deficiency anemia. Developmental Neuroscience. 2012 doi: 10.1159/000341919. Published online September, 26, 2012, DOI:1159/000341919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston KL, Tanumihardjo SA. Vitamin A intake of captive rhesus monkeys exceeds National Research Council recommendations. American Journal of Primatology. 2006;68:1114–1149. doi: 10.1002/ajp.20311. [DOI] [PubMed] [Google Scholar]
- Rao R, Georgieff MK. Perinatal aspects of iron metabolism. Acta Paediatrica Supplement. 2002;91:124–129. doi: 10.1111/j.1651-2227.2002.tb02917.x. [DOI] [PubMed] [Google Scholar]
- Sandstrom B. Micronutrient interactions: effects on absorption and bioavailability. British Journal of Nutrition. 2001;85(Suppl 2):S181–185. [PubMed] [Google Scholar]
- Tanno T, Miller JL. Iron loading and overloading due to ineffective erythropoiesis. Advances in Hematology. 2010 doi: 10.1155/2010/358283. 2010, 358383 Epub Article ID 404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Witche DR, Murphy AT, Wroblewski VJ, Wurz E, Datz C, Weiss G. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- Young MF, Griffin I, Pressman E, McIntyre A, Cooper E, McNanley T, Harris L, Westerman M, O'Brien KO. Utilization of iron from an animal-based iron source is greater than that of ferrous sulfate in pregnant and non-pregnant women. Journal of Nutrition. 2010;140:2162–2166. doi: 10.3945/jn.110.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Youdim MBH, Connor JR, Crichton RR. Iron brain ageing and neurodegenerative disorders. Nature Reviews Neuroscience. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zetterstrom R. Iron deficiency and iron deficiency anaemia during infancy and childhood. Acta Paediatrica. 2004;93:436–439. doi: 10.1080/08035250410027535. [DOI] [PubMed] [Google Scholar]
- Zimmerman MB, Chassard C, Rohner F, N'goran EK, Nindjin C, Dostal A, Utzinger A, Ghattas H, Lacrox C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. American Journal of Clinical Nutrition. 2010;92:1406–1415. doi: 10.3945/ajcn.110.004564. [DOI] [PubMed] [Google Scholar]