Abstract
Voltage gated calcium channels (VGCCs) play a major role during the development of the central nervous system (CNS). Ca2+ influx via VGCCs regulates axonal growth and neuronal migration as well as synaptic plasticity. Specifically, L-type VGCCs have been well characterized to be involved in the formation and refinement of the connections within the CA3 region of the hippocampus. The majority of the growth, formation, and refinement in the CNS occurs during the human third trimester. An equivalent developmental time period in rodents occurs during the first two weeks of post-natal life, and the expression pattern of L-type VGCCs during this time period has not been well characterized. In this study, we show that Cav1.2 channels are more highly expressed during this developmental period compared to adolescence (post-natal day 30) and that L-type VGCCs significantly contribute to the overall Ca2+ currents. These findings suggest that L-type VGCCs are functionally expressed during the crucial developmental period.
Keywords: Hippocampus, Calcium, Calcium Channels, Postnatal, Development
1. INTRODUCTION
In the central nervous system (CNS), Ca2+ influx through voltage-gated Ca2+ channels (VGCCs) is essential for a number of processes, including vesicular release of neurotransmitters, intracellular signaling pathways, gene expression and synaptic plasticity (Turner et al., 2011). Furthermore, VGCCs are tightly coupled to and modulate other ion channels as well as G-protein coupled receptors (Turner et al., 2011). VGCCs are comprised of a pore-forming α subunit and auxiliary β, γ, and δ subunits (Catterall, 2011). VGCCs are subdivided into five different types: L, N, P/Q, R, and T. In this study, we focused on L-type VGCCs, which can include one of four α-subunit variants: Cav1.1, Cav1.2, Cav1.3 or Cav1.4 (Catterall, 2011). Neurons within the CNS predominantly express Cav1.2 and Cav1.3 in an overlapping pattern that includes both somatic and dendritic compartments (Zuccotti et al., 2011). VGCCs containing Cav1.2 display the typical characteristics of L-type channels, including high-voltage activation, slow activation kinetics, and high sensitivity to dihydropyridines (Lipscombe et al., 2004). In contrast, recombinant studies show that Cav1.3 channels have a lower voltage activation threshold, activate with faster kinetics and are less sensitive to dihydropyridines (Lipscombe et al., 2004).
Alterations in L-type VGCCs containing Cav 1.2 or Cav1.3 have been linked to neurodevelopmental disorders. A missense gain-of-function mutation in Cav1.2 (G406R) increases membrane expression and substantially decreases voltage dependent inactivation, leading to a multisystem disorder known as Timothy syndrome (Splawski et al., 2004). The neuropsychological alterations linked to this syndrome involve language deficits and impairments in social skills that have led to the diagnosis of autism-spectrum disorder in many of these patients (Liao and Soong, 2010). Mice deficient in Cav1.3 exhibit congenital deafness and neuronal circuit abnormalities that are particularly pronounced in brainstem auditory centers (Hirtz et al., 2011; Platzer et al., 2000). Cav1.3 knockout mice also display deficits in the consolidation of contextual fear conditioning suggesting functional alterations in the neurons of the hippocampus and basolateral amygdala (Gamelli et al., 2011; McKinney and Murphy, 2006; McKinney et al., 2009). In addition, levels of serotonin, glutamate, γ-aminobutyric acid (GABA), and taurine were shown to be elevated in the striatum of Cav1.3 knockout mice (Sagala et al., 2012). Although these studies clearly indicate that L-type VGCCs containing Cav1.2 and Cav1.3 subunits play an important role in CNS development, the precise mechanisms responsible for the alterations in neuronal circuit development produced by L-type VGCC dysfunction are not fully understood.
The 3rd trimester of human development is a period of significant brain development, gyrification (cortical folding), and synaptogenesis (formation of synapses) (Lodygensky et al., 2010). The equivalent neuronal developmental period in rodents is during the first two weeks of post-natal life (Cudd, 2005; Lodygensky et al., 2010). L-type VGCCs have been shown to be involved in several processes required for the formation and refinement of neuronal circuits. Prior to synapse formation, Ca2+ influx via L-type VGCCs regulates axonal growth and neuronal migration (Hutchins and Kalil, 2008; Takahashi and Magee, 2009; Tang et al., 2003). Subsequently, L-type VGCCs participate in the formation and refinement of synaptic connections, which has been particularly well-characterized in the CA3 hippocampal region. This hippocampal region integrates inputs from the dentate gyrus, entorhinal cortex, and neighboring CA3 pyramidal neurons via mossy fibers, perforant path, and recurrent collateral pathways, respectively. CA3 pyramidal neurons also receive inhibitory connections from local interneurons that play a role of feed-forward inhibition (Lawrence and McBain, 2003). During the 3rd trimester equivalent, L-type VGCC-dependent retrograde release of brain-derived neurotrophic factor (BDNF) is thought to contribute to the stabilization of interneuron-CA3 pyramidal neuron synapses via a long-term potentiation-like mechanism (Gubellini et al., 2005; Kuczewski, Langlois, et al., 2008; Kuczewski, Porcher, et al., 2008). L-type VGCC/BDNF-dependent plasticity has been shown to play a similar role in mossy fiber-CA3 pyramidal neuron synapse maturation (Kasyanov et al., 2004; Sivakumaran et al., 2009; Spitzer et al., 2004). L-type VGCC/BDNF-dependent plasticity of GABAergic transmission at CA3 pyramidal neurons has been shown to be inhibited by alcohol exposure during the 3rd trimester equivalent, a deficit that may contribute to the hippocampal dysfunction that characterizes fetal alcohol spectrum disorders (Zucca and Valenzuela, 2010). Despite the importance of L-type VGCCs in the formation of CA3 hippocampal neuronal circuits, the developmental expression patterns and function of these channels have not been systematically characterized in this region.
Here, we investigated the developmental expression and distribution of Cav1.2 and Cav1.3 subunits in the CA3 hippocampal region using western immunoblotting and immunohistochemistry. Using whole-cell voltage-clamp slice electrophysiology and Ca2+ imaging, we assessed the function of L-type VGCCs in developing CA3 pyramidal neurons. We show that Cav1.2 expression transiently peaks during the 3rd trimester equivalent compared to adolescence (post-natal day 30), whereas, Cav 1.3 is expressed at lower levels, but relatively constant, throughout development and in adolescence. Functionally, 30-40% of total VGCC currents in CA3 pyramidal neurons were mediated by L-type channels between P4 and P15. L-type VGCC-mediated Ca2+ transients could be detected in both dendrites and soma of developing CA3 pyramidal neurons.
2. EXPERIMENTAL PROCEDURES
2.1. Animals and Slice preparation
The University of New Mexico Health Sciences Center Institutional Care and Use Committee approved all animal procedures. Pregnant Sprague Dawley dams between gestational day 12 and 17 were received from Harlan Laboratories (Indianapolis, IN). Both male and female pups were used for all experiments except for the post-natal day 30 time points when only male offspring were used. Animals were heavily anesthetized with ketamine followed by decapitation. Brain tissue was removed and incubated for 2-4 minutes in oxygenated ice cold cutting solution (in mM): sucrose, 220; KCl, 2; NaH2PO4, 1.3; NaHCO3, 26; MgSO4, 12; CaCl2, 0.2; glucose, 10; ketamine, 1 mg/ml. Coronal slices were generated using a vibrating slicer (1000 Plus Vibratome, Leica, Bannockburn, Illinois) at a thickness of 300 μm. Slices were incubated in oxygenated artificial cerebral spinal fluid (ACSF) (in mM): NaCl, 125; KCl, 2; NaH2PO4, 1.3; NaCO3, 26; glucose, 10, CaCl2, 2; MgSO4, 10 at 35°C for 40 minutes and allowed to recover at room temperature (21-22°C) for at least 30 minutes prior to recording or dissection. All chemicals were purchased from Sigma-Aldrich (St Louis, MO) unless specified.
2.2. Electrophysiology Recordings
Slices were maintained in ACSF during recording at approximately 32°C. CA3 pyramidal neurons were identified by morphology under video monitoring of infra-red Differential Interference Contrast Microscopy using an BX51WI upright microscope (Olympus, Center Valley, PA) and a LUMPlan Fl/IR 40× water immersion lens 0.8 N.A. Recording electrodes where pulled using a DMZ-Universal Puller (Zeitz Instruments, Martinsreid, Germany) resulting in resistances between 2 and 5 MΩ. Electrodes were filled with internal solution containing the following (in mM): CsCl, 135; MgCl, 4; HEPES, 10; EGTA, 10; MgATP, 4; NaGTP, 0.3. Internal solution pH was adjusted to 7.2 with CsOH. Recordings were obtained with a Multiclamp 700B amplifier connected to a Digidata 1440A and data were acquired with pClamp 10 software (Molecular Devices Sunnyvale, CA). CA3 pyramidal neurons were filled using regular recording electrodes containing in mM: K-gluconate, 135; NaCl, 8; MgCl2, 1; HEPES, 10 (pH 7.2 with KOH); MgATP, 2; EGTA, 0.05, and Alexa-488 hydrazide, 1 (Invitrogen, Grand Island, NY). In the whole-cell configuration, we compensated for membrane capacitance and 70% of series resistance. VGCC currents were isolated with 1μM TTX (Tocris, Bristol, United Kingdom), 10μ M GABazine (Tocris), 50μM DL-APV (Tocris), 10mM TEA (Sigma), and 1mM kynurenic acid (Sigma).
2.3. Calcium Imaging
In the whole-cell patch-clamp configuration, CA3 pyramidal neurons were filled with the CsCl internal solution (described above) supplemented with Bis-Fura-2 hexapotassium salt (150 μM) (Invitrogen). Neurons were allowed to dialyze for 20 minutes prior to Ca2+ imaging. Ca2+ transients were acquired using a Polychrom V imaging system from TILL photonics (Grafelfig, Germany) equipped with a monochromator and an optical fiber to deliver light to the microscope. Within the imaging path a 500 nm dichroic mirror and emission filter 525/50 nm were used to image the fluorescence of Fura-2 at both 350 nm and 380 nm. Data acquisition and analysis were performed with TILLvisION imaging software version 4.5.41 (TILL Photonics). Images were acquired at 350 nm and 380 nm at approximately 4 Hz. Ca2+ transients were measured at the soma and dendrites (approximately 30-60 μm from the soma). Three Ca2+ transients were induced and averaged together with each transient normalized to the pre-depolarization baseline fluorescence. Fluorescence intensities at 350 nm were divided by the fluorescence at 380 nm with background subtracted. Ratiometric images were used to measure the Ca2+ transients in both the soma and dendritic compartments. Ca2+ transients were normalized to pre-depolarization fluorescence baseline.
2.4. Western Blot
Hippocampal slices were prepared as described above and the CA3 region was micro-dissected from those slices in ice cold Hank’s Buffered Saline Solution (Invitrogen). Tissue was immediately placed in homogenization buffer containing the following (in mM): HEPES, 25; NaCl, 500; EDTA, 2; DTT, 1; PMSF, 1; NaF, 20; cyclosporin, 0.005; 10% Tween-20 (v/v); 1× Phosphatase inhibitor cocktail 3 (Sigma); 1× SigmaFAST protease inhibitor tablet (Sigma). Tissue was probe sonicated, aliquoted and stored at −80°C. The heart from a P15 rat was homogenized under the same conditions and used as a positive control to aid in the identification of the L-type VGCC subunits. Protein concentration was assessed using the Quick Start Bradford assay kit (Bio-Rad Hercules, CA). Proteins samples were diluted with 5× loading buffer: 250 mM Tris-HCl, 10% sodium dodecyl sulfate, 30% glycerol, 5% β-mercaptoethanol, 0.02% bromophenol blue, and boiled for 5 min. Samples were loaded (10 μg/well) and separated using 4-15% Tris-glycine TGX precast gels (Bio-Rad Hercules, CA) at 110 V for 60 – 70 minutes. Proteins were transferred onto Immobilon-FL polyvinylidene difluoride membrane at 100 V for 70 minutes. Non-specific binding was blocked with Odyssey Blocking Buffer (Li-Cor, Lincoln, NE) for 2 hours at room temperature. Membranes were incubated in primary antibody; either rabbit polyclonal anti- Cav1.2 (1:500) (Millipore, Billerica, MA #ab5156) or rabbit polyclonal anti- Cav1.3 (1:500) (Millipore #ab5158) with mouse anti-β actin (1:15,000) (Sigma # A1978) overnight at 4°C. Membranes were incubated with goat anti-rabbit IR-Dye 800CW (Li-Cor # 926-32211) and goat anti-mouse IR-Dye 680 (Li-Cor # 926-32220) secondary antibodies for 2 hours at room temperature, and imaged on an ODYSSEY fluorescent imager (Li-Cor).
2.5. Immunohistochemistry
Brains were removed and immediately fixed with 4% paraformaldehyde for 24-48 hours at 4°C. After fixation tissue was incubated in 30% sucrose in PBS until the tissue sank. Tissue was submerged in Optimal Cutting Temperature (OCT) compound (Sakura Finetek USA, Torrance, CA) compound and flash frozen in 2-methyl butane. Tissue was stored at −80°C and 16 μm thick coronal sections were cut using a HM505E cryostat (Microm International, Germany). Sections were stained with the above-described anti-Cav1.2 (1:500) or anti-Cav1.3 (1:500) antibodies. Non-specific binding was assessed by incubating sections with primary antibody and equal amounts of the immunogen peptides (Millipore). Sections were incubated in goat ant-rabbit IgG conjugated to Alexa-555 (1:1000) (Invitrogen, # A21434) and 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen # D1306) prior to mounting with Vectashield (Vector Laboratories, Burlingame, CA). Sections were imaged using a Zeiss 510 meta inverted microscope (Carl Zeiss, Germany) with a 543 nm HeNe and 405 nm lasers and a Plan-Neofluor 20× 0.5 N.A. or a Plan-Apochromat 63× 1.4 N.A. oil immersion DIC objective. Blue and red fluorescence were detected through 420-480 nm and 561-657 nm band pass filters, respectively. NIH-ImageJ was used to quantify fluorescence.
2.6. Statistical Analysis
All graphs and statistical analyses were performed with Graph Pad Prism 5.0 (San Diego, CA). The level of significance was p<0.05. A statistical unit (n) was considered an animal; results obtained with multiple cells or tissue sections from a single animal were averaged to yield a unit of determination.
3. RESULTS
3.1. Developmental expression of Cav1.2 and 1.3 in the hippocampal CA3 region
In rodents the majority of synaptogenesis and synaptic refinement occurs during the first 2 weeks of post-natal life. Using western immunoblotting techniques, we investigated the expression of Cav1.2 and Cav1.3 subunits during this period (Fig 1A and B). For comparison, we also assessed expression of these subunits in adolescent rats (P30). Within the CA3, we detected two Cav1.2 isoforms ~240 and ~170 kDa. We found that the 240 kDa isoform is transiently expressed during this developmental time period with peak levels observed at P8 and the lowest levels detected at P30 (F(3,3) = 9.3, p = 0.0115). Conversely, the 170 kDa isoform expression remained relatively stable (F(3,3) = 2.1, p = 0.6489). For Cav1.3 we detected only one isoform at ~270 kDa that was expressed at levels lower relative to Cav1.2, but remained constant from P4-30.
Figure 1. Western immunoblot analysis of L-type VGCC α subunit expression during post-natal development in CA3 hippocampal region homogenates.
(A) Top panel, example of western blots obtained with brain samples from P4-30 rats. A sample from the heart of a P15 rat was also assayed as a reference. Developmental expression of Cav1.2 isoforms 240 kDa and 170 kDa at the indicated postnatal days (P) are shown. The lower band corresponds to β-actin, which was used to normalize for differences in lodading across lanes. (B) Top and Bottom Panels, same as above but for Cav1.3. Integrated intensities were measured and Cav1.2 and 1.3 expression were normalized to that of β-actin. Possibly due to minor defects in the pre-cast polyacrylamide gels that we used, we experienced some distortions in our immunoblots but this did not limit our ability to identify the proteins of interest or quantify their expression levels. Homogenates from P15 heart tissue was used for a positive control for Cav1.2 and 1.3. Asterisks indicate a p value less than 0.05 (n = 4 in all cases; unit of determination = average of results obtained with a single animal).
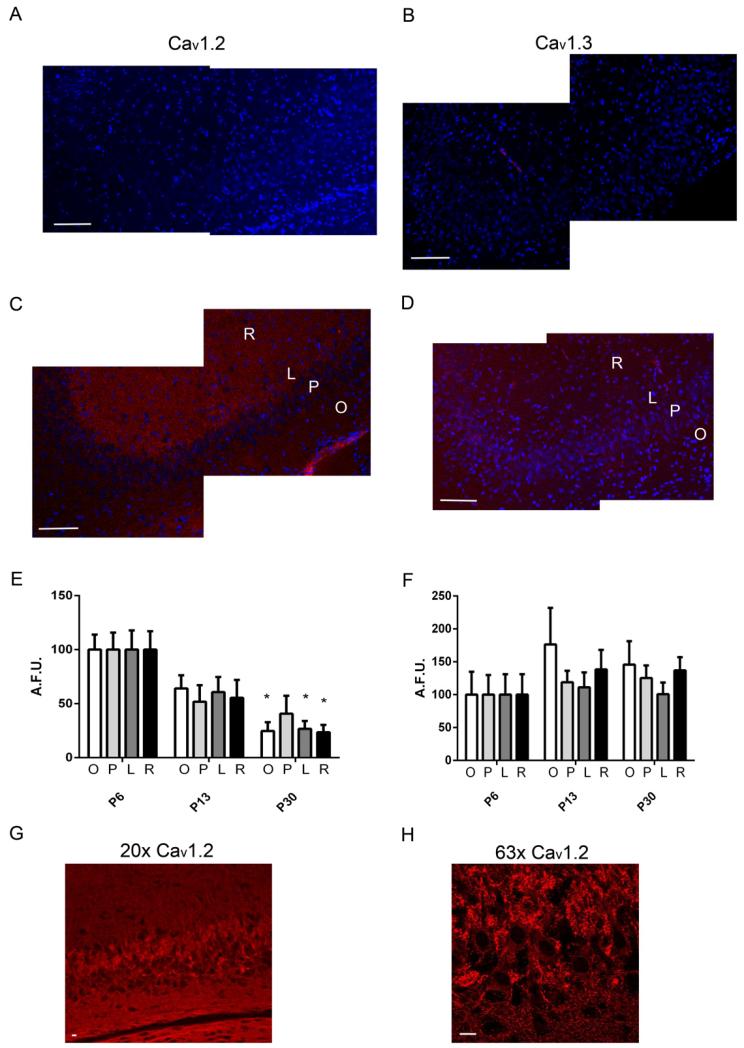
To complement these western immunoblotting studies, we used immunohistochemistry to assess the distribution of both Cav1.2 and 1.3 in the developing CA3 region. Cryostat sectioned hippocampal slices were used to stain for Cav1.2 and 1.3. For each protein, the confocal settings were determined from images obtained from slides incubated with the immunogen peptide (Fig 2A and B) and maintained for all developmental time points. Representative confocal images at P6 are shown for both Cav1.2 and 1.3 in Figure 2C and D, respectively. Expression levels for both Cav1.2 and 1.3 were quantified by averaging the pixel intensities throughout each given region, and average intensities from each hemisphere were averaged together for each animal. The regions were compared individually across ages using a one-way ANOVA with post-hoc analysis. In agreement with the western immunoblotting results, the expression of Cav1.2 was higher at P6 compared to P30, within the Striatum Oriens (F(2,4) = 13.83, p = 0.011), lucidum (F(2,4) = 8.37, p = 0.033), and Radiatum (F(2,4) = 7.68, p = 0.037) (Fig 2E). Some sections exhibited high levels of Cav1.2 expression within the lucidum compared to other regions within the CA3 (Fig 2G). Cav1.3 expression levels across development were not significantly different among the P6, P13 and P30 developmental time points, similar to our immunoblotting results (Fig 2F). However, Cav1.3 levels were much lower than those of Cav1.2 and had to be imaged at a higher detector gain. The distribution of Cav1.3 was relatively uniform throughout the hippocampal layers. Higher magnification images of Cav1.2 revealed a clustering distribution around the stratum lucidum and pyramidal (Fig 2G and H).
Figure 2. Immunohistochemical analysis of L-type VGCC α subunit expression during post-natal development in the CA3 hippocampal region.
Representative confocal images of tissue sections from a P6 rat that were incubated with anti-Cav1.2 or anti-Cav1.3 antibodies in the presence (A and B) or absence (C and D) of the immunogen peptide. Cav1.2 and 1.3 are represented by the red fluorescence and the blue fluorescence represents the nuclear DAPI stain. The location of the Stratum Oriens (O), Stratum Pyramidale (P), Stratum Lucidum (L), and Stratum Radiatum (R) are indicated. Scale Bars = 100 μm. Summary graphs of the average pixel intensities from each region within the CA3 for Cav1.2 (E) and Cav1.3 (F) (n = 5). Unit of determination = average of results obtained with tissue sections from a single animal. Asterisks indicate a significant (p < 0.05) difference between P30 and P6 for each region determined for each group by a one-way ANOVA. Higher magnification images at 20× (G) and 63× (H) of Cav1.2 at P6 show clustering of Cav1.2 within the soma and proximal dendrites. Scale bars = 10 μm.
3.2. CA3 pyramidal neuron morphology and electrophysiological properties significantly change during the third trimester-equivalent
Influx of Ca2+ via L-type VGCCs is a key regulator in dendritic branching (Urbanska et al., 2008). Therefore, we tested if the expression of Cav1.2 coincided with morphological changes in CA3 pyramidal neurons. To assess CA3 pyramidal neuron morphology, we used the whole cell configuration with an internal solution containing Alexa-488 hydrazide. Neurons were allowed to fill for approximately 20 minutes, then the slices were fixed and confocal images were collected. Montage images were generated using individual z-projection images at P4 and P15 (Fig 3A). Dendritic branching was assessed by counting the number of dendrites that intersected with concentric circles centered on the cell body with each circle having a radius 40 μm larger than the previous (Sholl analysis). Although, the total dendritic length did not significantly change between P4-6 and P13-15 (Fig 3B) our sholl analysis showed differences in dendritic morphology. At P4-6, we observed that P4-6 there were multiple apical dendritic branches that extended past 280 μm from the cell body, whereas at ages P13-15 there was only one apical dendrite that extended past 240 μm (Fig 3C, left panel). Conversely, we found more basal dendrites extending farther at P13-15 compared to P4-6 (Fig 3C, right panel).
Figure 3. Morphological Characteristics of CA3 pyramidal neurons.
(A) Representative images of CA3 pyramidal neurons filled with Alexa-488 hydrazide in the whole-cell patch-clamp configuration at P4 and P15. Scale bars = 10 μm. Multiple Z-axis confocal images were projected as a single image and combined to form montage images. (B) Summary graph of the total CA3 dendritic length for the apical and basal dendrites (n=5). (C) Sholl analysis of apical and basal dendrites in CA3 pyramidal neurons from P4 (n=5) and P15 (n=5; unit of determination = average of results obtained with slices from an individual animal).
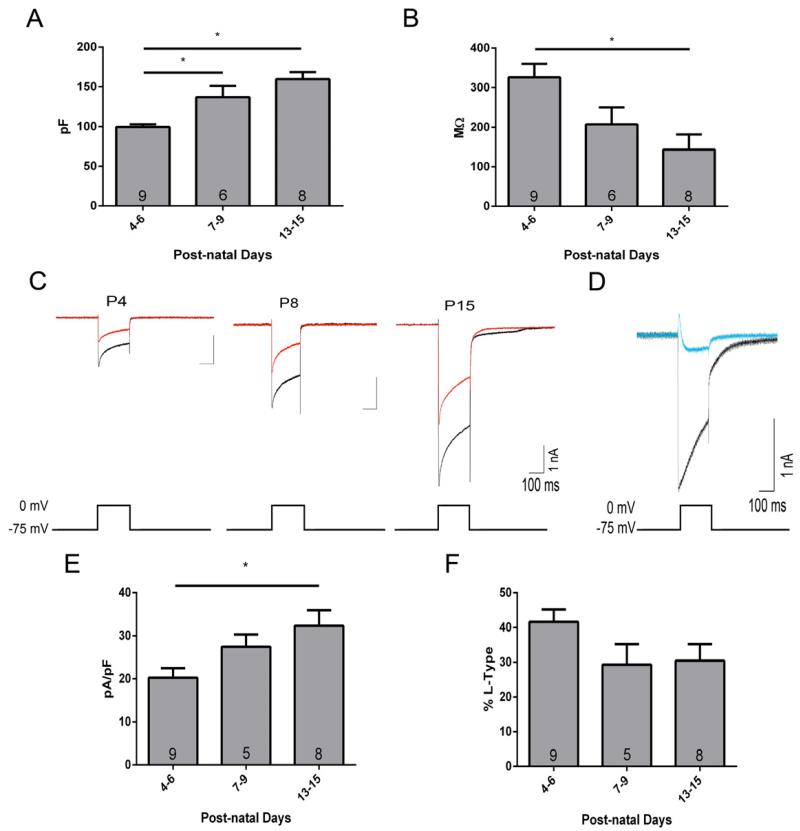
For our electrophysiological studies, we focused on the 3rd trimester-equivalent. Using whole-cell voltage-clamp electrophysiology, we measured the membrane capacitance (Cm) and membrane resistance (Rm). We found that the Cm nearly doubled from P4-6 to P7-15 (F(2,20) = 14.20, p = 0.001) (Fig 4A), consistent with an increase in neuronal size and dendritic complexity. This increase in Cm was mirrored by a ~60% reduction in Rm rom P4-6 to P13-15 (F(2,20) = 6.545, p = 0.0065) (Fig 4B), suggesting an increase in total open channel density within the plasma membrane at the latter developmental time points. Whole-cell voltage-clamp electrophysiology was used to record VGCC currents (pharmacologically isolated with 1 μM TTX, 10 μM gabazine, 50 μM DL-APV, 10 mM TEA, and 1 mM kynurenic acid). The membrane potential was held at −75 mV then depolarized to 0 mV for 200 ms. Representative Ca2+ currents from P4, P8, and P15 are shown in Figure 4C. The depolarization-induced currents were significantly blocked with 50 μM CdCl2 (Fig 4D). Total VGCC currents increased from P4-6 to P7-9 and P13-15 (F(2,20) = 11.39, p = 0.0005). VGCC currents were expressed as current density (peak amplitudes divided by the cell capacitance) to correct for developmental increases in cell size. We found that VGCC current densities significantly increased by ~60% between P4-6 and P13-15 (F(2.20) = 4.692, p = 0.022) (Fig 4E). Next, we assessed the contribution of L-type VGCCs to the total VGCC currents using the L-type VGCC blocker verapamil (100 μM). This agent significantly reduced the VGCC currents by ~30 - 40% at P4-6 (t(8) = 8.14, p < 0.0001), P7-9 (t(4) = 7.348, p = 0.0018) and P13-15 (t(7) = 8.38, p < 0.0001) (Fig 4F). The raw L-type current peak amplitude did not significantly change as a function of development (P4-6, 851 ± 102 pA; P7-9, 901 ±169 pA; P13-15, 742 ± 214 pA) consistent with our immunohistological studies. These results demonstrate the presence of Ca2+ currents in CA3 pyramidal neurons during this developmental time period and the contribution of L-type VGCCs.
Figure 4. Functional analysis of the contribution of L-type VGCCs to total VGCC currents as a function of development.
Summary graphs of cell capacitance (A) and membrane resistance (B) for CA3 pyramidal neurons during development measured in the whole-cell voltage-clamp configuration. (C) Representative VGCC currents evoked by a 200 ms voltage-step from −75 mV to 0 mV are shown in in the absence (black) and presence (red) of the L-type VGCC antagonist, verapamil (100 μM). (D) VGCC currents recorded in the absence (black) and presence (blue) of 50 μM CdCl2. Summary graph of the VGCC current densities (E) and the percent of L-type contribution (F) during development. Asterisks indicate a p value less than 0.05. Numbers in bar graphs represent the number of determinations (i.e., average of results obtained with slices from an individual animal).
3.3. L-type VGCCs significantly contribute to somatic and dendritic Ca2+ transients
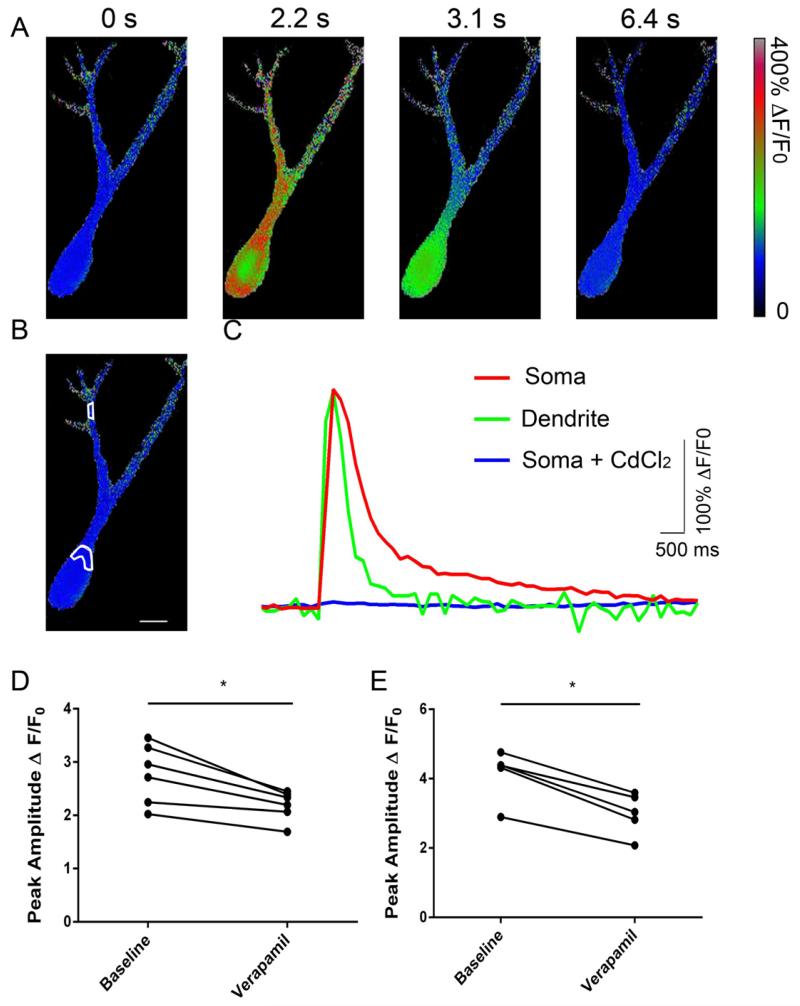
CA3 pyramidal neurons from P7-9 rats were filled with cell impermeant Fura-2 via the patch pipette and voltage clamped at −75 mV. Neurons were stepped from −75 mV to 0 mV and VGCC responses were pharmacologically isolated as mentioned above for the electrophysiological VGCC current recordings. The depolarizing potentials on average resulted in a ~3-fold increase in fluorescence in the soma and a ~4-fold increase in the proximal dendrites. Representative ratiometric images are shown in Figure 5A during a single depolarization. The ratiometric fluorescence was measured in the regions indicated (Fig 5B). Figure 5C shows the time-course of dendritic and somatic Ca2+ transients, illustrating that the transients decayed faster in the dendritic compartment. In the presence of 50 μM CdCl2 there were no significant Ca2+ transients upon depolarization (Fig 5C). Verapamil (100 μM) significantly reduced the peak amplitude of the Ca2+ transients in both the soma (t(5) = 4.491, p = 0.0065) (Fig 5D) and dendrites (t(5) = 10.13, p = 0.0002) (Fig 5E). The percent inhibition of the Ca2+ transients was similar in soma (21.07 ± 3.77%) and dendrites (27.87 ± 2.408%). These data indicate that L-type VGCCs contribute to Ca2+ influx both in the soma and dendrites of developing CA3 hippocampal pyramidal neurons.
Figure 5. L-type VGCCs contribute to both somatic and dendritic Ca2+ transients.
(A) Representative ratiometric images of an individual CA3 pyramidal neuron from a P9 rat filled with fura-2 in the whole-cell patch-clamp configuration. Ca2+ transients were evoked by a 200 ms voltage step from −75 mV to 0 mV. Images were collected at ~4 Hz and representative images are shown at the times indicated above each panel. (B) Representative image indicating the regions of interest that were selected for analysis in the soma and dendrites. (C) Ca2+ transients recorded from representative images in A within the somatic and dendritic regions indicated in B. The fluorescence Ca2+ transients were blocked in the presence of 50 μM CdCl2 (C). Summary graph illustrating the peak amplitude of the Ca2+ transients recorded in the soma (D) and dendrites (E) in the absence and presence of 100 μM verapamil. Asterisks indicate a p value less than 0.05. Each symbol represents a unit of determination (average of results obtained with slices from an individual animal).
4. DISCUSSION
In this study, we show that both Cav1.2 and Cav1.3 are expressed in the CA3 hippocampal region of developing rats, albeit with different patterns of expression. Cav1.2 expression levels peak toward the end of the first week of life, a period that is considered equivalent to the 3rd trimester of fetal human development. The Cav1.3 subunit is expressed at lower levels than Cav1.2 and its expression levels remain constant during this developmental period. Expression of both Cav1.2 and Cav1.3 was detected in the strata oriens, pyramidale, lucidum and radiatum. Functional studies revealed a developmental increase in current densities for VGCCs that coincided with a significant increase in Cm and dendritic complexity, and a decrease in Rm. Importantly, the contribution of L-type VGCCs to total VGCC currents remained relatively constant throughout this developmental time period. Collectively, these findings are consistent with published reports, showing L-type VGCCs play a central role in the development of synaptic connections at CA3 pyramidal neurons (Cherubini et al., 2011; Kuczewski et al., 2010).
4.1. Different patterns of expression of Cav1.2 and 1.3 in the developing CA3 region
In the CA3 hippocampal region of adult rats, expression of both Cav1.2 and 1.3 was previously demonstrated using immunohistochemical techniques (Hell et al., 1993). More recently, Veng and Browning, (2002) used immunoblotting techniques to demonstrate that Cav1.2 is expressed at similar levels in the CA3 hippocampal region of adult (4 month-old) vs. aged (24 month-old) rats; in contrast, Cav1.3 was expressed at lower levels in the CA3 region of aged rats. Here, we report that protein expression of Cav1.2 (240 kDa isoform) in the CA3 region is dynamically regulated during rat postnatal development, demonstrating that Cav1.2 levels peak during the 3rd trimester equivalent period. In contrast, Cav1.3 levels remain relatively constant during P4-30. Our findings are similar to those of previous studies showing that Cav1.2 protein levels, albeit different isoforms, reach a maximum between P4-8 in the CA1 region of the rat hippocampus (Kramer et al., 2012; Nuñez and McCarthy, 2007). However, Kramer et al. found that different Cav1.3 isoform levels progressively increase between P1 and P20 in the CA1 region, suggesting that the expression of specific isoforms is differentially regulated between the CA1 and CA3 region during postnatal development. Developmental regulation of Cav1.2 levels was also demonstrated in cortical membrane extracts from rat brains, where levels increased from P1 to P8 followed by a decline at P21 and adulthood (Gomez-Ospina et al., 2006). Collectively, these results suggest that L-type Ca2+ channels are expressed during a critical period of brain development, with channels containing the Cav1.2 subunit being particularly abundant during the middle of the 3rd trimester-equivalent developmental period.
Our immunohistochemical studies revealed expression of both Cav1.2 and 1.3 across somatic and dendritic fields of the CA3 region at all developmental stages. Similar to the results obtained by Hell et al., (1993), we found that Cav1.2 was distributed in clusters throughout the strata lucidum and pyramidale in the CA3 hippocampal region of P6 rats, as previously described (Zhou et al., 2004). Veng and Browning, (2002) observed patterns of expression for Cav1.2 and 1.3 that are comparable to those reported here and also demonstrated that Cav1.3 levels decrease in the stratum radiatum of the CA3 region of aged rats. Functional and electron microscopy studies suggest that L-type VGCCs are not only expressed postsynaptically in the CA3 region, but also presynaptically, particularly in large mossy fiber buttons that connect with pyramidal neuron dendrites (Tokunaga et al., 2004, Tippens et al., 2008). Therefore, it will be interesting to determine whether the subcellular localization of Cav1.2 and 1.3 changes as a function of development in this region.
In the CA3 region, it has been shown that the excitatory actions of GABAA receptors contribute to the generation of a network-driven pattern of oscillatory neuronal activity known as giant depolarizing potentials (GDPs) (Ben-Ari, 2002; Ben-Ari et al., 2012; Bregestovski and Bernard, 2012). GDPs are associated with Ca2+ transients mediated in part by L-type VGCCs and these are thought to drive neuronal growth and synapse formation (Cherubini et al., 2011). Our data show that Cav1.2 expression peaks during the first post-natal week, which coincides with the presence of GDPs, suggesting that L-type VGCCs containing this subunit may contribute to the dendritic Ca2+ influx that is essential for synaptogenesis. The expression of Cav1.2 in the strata lucidum and radiatum further supports the involvement of Cav1.2 in synapse remodeling. Importantly, expression of Cav1.2 peaks during a period of enhanced synaptic plasticity in CA3 pyramidal neurons. During P4-8, L-type VGCC-driven retrograde release of BDNF from CA3 pyramidal neurons induces long-term potentiation of GABAA receptor-mediated transmission at both interneuron-pyramidal neuron and mossy fiber-pyramidal neuron synapses (Gubellini et al., 2005; Sivakumaran et al., 2009). Interestingly, the ability of CA3 pyramidal neurons to undergo this form of synaptic plasticity decreases after P8 and future studies should examine whether this is, at least in part, a consequence of decreased in Cav1.2 expression.
4.2. L-type VGCC expression peaks during a period of intense dendritic remodeling
L-type VGCCs play a major role in dendritic growth and branching via the down stream signaling of calcium/calmodulin-dependent prtein kinase (CaCMK) I and II (Urbanska et al., 2008). Blockade of L-type VGCCs decrease dendritic arborization in primary cortical and hippocampal cultured neurons (Ramakers et al., 2001; Shitaka et al., 1996). We show that the peak of Cav1.2 expression coincides with an increase in both apical and basal dendritic branching. Interestingly, Shitaka and colleagues noted that L-type VGCCs were localized to branch points in cultured hippocampal neurons (Shitaka et al., 1996). Furthermore, the current peak amplitude of L-type channels slightly increased from P4-P9 that coincided with the increase in cell capacitance. Furthermore, dendritic length and complexity decrease our ability to record VGCC currents from those regions that most likely contain Cav1.2 channels. Nonetheless, future experiments are needed to directly link Cav1.2 to dendritic branching.
In this study, we show that CA3 pyramidal neurons from rats undergo morphological changes between P4 and P15. Pillai and colleagues showed that CA3 dendritic length and branching in 7-week-old mice is similar to the dendritic characteristics of CA3 pyramidal neurons from P15 rats, suggesting that the dendritic growth and branching stabilizes at the end of the 3rd trimester-equivalent (Pillai et al., 2012). This would suggest that the majority of the dendritic arborization occurs during the developmental time period when the expression of Cav1.2 is higher relative to adolescence (P30). Our electrophysiological recordings showed a developmental increase in membrane capacitance coinciding with the increase in dendritic complexity and this was mirrored by a reduction in membrane resistance. Tyzio and colleagues showed a very similar inverse relationship between membrane capacitance and membrane resistance that also coincided with a decrease in the resting membrane potential at P15 compared to P0 (Tyzio et al., 2003). In pyramidal neurons of the CA3, the less negative membrane potentials in the earlier portion of the 3rd trimester-equivalent (~P0-8) are thought to facilitate the activation of VGCCs and NMDA receptors (Leinekugel, 2003). In sum, high expression of L-type VGCCs-containing the Cav1.2 subunit coincides with a period of significant dendritic growth in CA3 pyramidal neurons, suggesting that these channels may play a role in the maturation of the dendritic tree of these neurons.
4.3. L-type channels contribute to VGCC currents in developing CA3 pyramidal neurons
Our electrophysiology recordings corroborated our previous findings that the expression of L-type channels is relatively stable during this developmental period. We found that L-type VGCC currents contributed ~30 - 40% to total VGCC currents in CA3 pyramidal neurons between P4 and P15. The contribution of L-type to the total VGCC currents remained relatively stable during P4-15 in agreement with our western blotting and immunohistochemical data. Unfortunately, we were unable to reliably record VGCC currents in neurons from P30 rats due to inadequate space-clamp. These findings are in close agreement with those of Avery and Johnston, (1996) who reported that L-type channels contribute to ~30% of the total VGCC currents in acutely dissociated CA3 hippocampal neurons from P7-14 rats. Results are also consistent with a previous study from our laboratory where nifedipine reduced total VGCC currents by 45% in CA3 pyramidal neurons from P4-6 pups and the L-type contribution decreased to ~20% at P20-25 (Zucca and Valenzuela, 2010). We previously showed that ~50% of the nifedipine-resistant VGCC currents in CA3 pyramidal neurons are mediated by N-type VGCC channels with little contribution from P/Q type channels (Zucca and Valenzuela, 2010). The remaining VGCC current is most likely mediated by T-type (Avery and Johnston, 1996) and perhaps R-type VGCCs. In vitro studies with cultured hippocampal neurons found a gradual increase in N-type channel expression in the somatodendritic region that coincides with synaptic maturation (Pravettoni et al., 2000). Therefore, it is possible that the increase in VGCC current density is due to an increase in N-type channels.
The functional properties of Cav1.2 subunit-containing L-type VGCCs include high potential of activation, slow inactivation, and large single channel conductance (Catterall et al., 2005). In contrast, Cav1.3 subunit-containing channels are activated at lower membrane potentials and inactivate slightly faster than Cav1.2 (Helton et al., 2005). Therefore, Cav1.2 subunit-containing channels are activated by strong membrane potential depolarization, for example induced by a back propagating action potential or by CA3 pyramidal neuron firing that coincides with a depolarizing postsynaptic potential, making this class of L-type VGCCs ideal mediators of release of retrograde messengers (e.g., BDNF) in response to robust neuronal activity (Kuczewski, Porcher, et al., 2008; Sivakumaran et al., 2009). Moreover, L-type VGCCs are activated by GDPs and Ca2+-dependent K+ channels play a role in the termination of these events (Ben-Ari, 2002; Leinekugel et al., 1997; Sipilä et al., 2006). Pairing of GDPs with mossy fiber stimulation was shown to induce long-term potentiation at mossy fiber-to-CA3 pyramidal neuron synapses via a mechanism involving L-type VGCCs that likely contain Cav1.2 subunits (Kasyanov et al., 2004). The slower inactivation of Cav1.2 allows a relatively large Ca2+influx that is large enough to activate protein kinases and transcription factors. Consequently, the relatively higher abundance of Cav1.2-containing L-type VGCCs at P8 would be advantageous to developing CA3 pyramidal neurons because these channels play a central role in coupling neuronal firing with activation of synaptic plasticity mechanisms and downstream signaling cascades required for development. Conversely, Cav1.3 subunit-containing channels are activated by stimuli that induce subthreshold depolarization of the membrane potential (Lipscombe et al., 2004). Indeed, studies have clearly demonstrated that Cav1.3 subunit-containing L-type VGCCs are important mediators of substhreshold Ca2+ signaling that controls oscillations in substantia nigra dopaminergic neurons (Chan et al., 2007). Interestingly, repeated Ca2+ influx via Cav1.3 subunit-containing L-type VGCCs during pacemaker firing of these neurons was shown to cause oxidative stress, which may be involved in the pathophysiology of Parkinson’s disease (Chan et al., 2007). Thus, we speculate that the relatively low expression levels of Cav1.3 in developing CA3 pyramidal neurons may be a protective factor against oxidative stress and related insults.
4.4. L-type VGCCs generate Ca2+ transients in both dendrites and soma of developing CA3 pyramidal neurons
Our Fura-2 imaging experiments demonstrated that somatic stimulation elicited VGCC-mediated Ca2+ transients in both the dendritic and somatic compartments of CA3 pyramidal neurons from P7-9 rats. Verapamil reduced these transients by 20-30%, in agreement with the results of our electrophysiological experiments. Our experimental conditions limited how far into the distal dendritic compartments we could image Ca2+ transients; however, dendritic Ca2+ responses were reproducibly inhibited by verapamil, suggesting L-type channel involvement. VGCC-mediated Ca2+ transients in CA3 pyramidal neurons were characterized in a previous study with organotypic hippocampal slice cultures prepared from 4 day-old rats and maintained 10-18 days in vitro (Elliott et al., 1995). This study reported that L-type VGCCs predominantly mediate somatic Ca2+ transients evoked by strong stimulation trains delivered either to the soma or the distal apical dendrite, and suggested that the role of L-type VGCCs in these transients decreases with age. Similarly, our findings indicate that L-type VGCC significantly contribute to both somatic and dendritic Ca2+ transients in P7-9 rats. An uncertainty that must be kept in mind is that verapamil may have reduced dendritic Ca2+ transients indirectly via blockade of somatic L-type VGCCs leading to a reduction in the propagation of the depolarization to the dendrites. Future experiments must confirm that L-type VGCCs indeed mediate dendritic transients in CA3 pyramidal neurons, possibly by, testing the effect of focal dendritic stimulation. However, L-type VGCCs in dendritic Ca2+ transients have been documented in other studies; Christie and colleagues (Christie et al., 1995) showed in the CA1 region that nimodipine blocked approximately 25-30 % of back-propagating Ca2+ currents at 25 μm away from the soma. The results of our immunohistochemistry experiments indicating that Cav1.2 and 1.3 are expressed in the soma and dendrites of CA3 pyramidal neurons further support that L-type VGCCs indeed mediate Ca2+ influx into the dendritic compartment of these cells.
4.5. Conclusion
Our studies provide evidence suggesting that L-type VGCCs, particularly those containing the Cav1.2 subunit, are abundantly expressed in the CA3 hippocampal pyramidal neurons during the 3rd trimester-equivalent period of development in rats. These findings are consistent with the results of studies showing that L-type VGCCs play an essential role in the mechanism of synaptic plasticity involved in the maturation and refinement of CA3 hippocampal neuronal circuits. Studies from our laboratory indicate that developmental ethanol exposure impairs L-type VGCC-dependent plasticity in this hippocampal region, a mechanism that could play a role in the pathophysiology of fetal alcohol spectrum disorders (Zucca and Valenzuela, 2010). Future studies should investigate the potential role of alterations in the expression and/or function of L-type VGCC in the pathophysiology of other neurodevelopmental disorders.
Highlights.
L-type calcium channel expression peaks during the first rat post-natal week.
L-type channels are expressed throughout all stratum layers of the CA3.
In neonates, L-type VGCCs significantly contribute to total Ca2+ currents.
In the CA3, L-type channels contribute to ~25% of the dendritic Ca2+ transients.
Acknowledgements
This work was supported by NIGMS K12-GM088021 (RAM), the UNM-School of Medicine-Undergraduate Pipleline Network (MSN) and R37-AA15614 (CFV). Present address for MSN: Department of Chemistry University of Wyoming, Laramie, WY, 82071. Present address for CCV: Cellular, Molecular and Biophysical Studies-Graduate School of Arts and Sciences, Columbia University, New York, NY 10032.
ABBREVIATIONS
- VGCC
voltage gated calcium channel
- CNS
central nervous system
- BDNF
brain-derived neurotrophic factor
- GABA
γ-aminobutyric acid
- P
postnatal day
- DAPI
4′,6-diamidino-2-phenylindole
- GDP
giant depolarizing potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:5567–82. doi: 10.1523/JNEUROSCI.16-18-05567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature reviews. Neuroscience. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Woodin M. a, Sernagor E, Cancedda L, Vinay L, Rivera C, Legendre P, Luhmann HJ, Bordey A, Wenner P, Fukuda A, Van den Pol AN, Gaiarsa J-L, Cherubini E. Refuting the challenges of the developmental shift of polarity of GABA actions: GABA more exciting than ever! Frontiers in cellular neuroscience. 2012;6:35. doi: 10.3389/fncel.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P, Bernard C. Excitatory GABA: How a Correct Observation May Turn Out to be an Experimental Artifact. Frontiers in pharmacology. 2012;3:65. doi: 10.3389/fphar.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. a. Voltage-gated calcium channels. Cold Spring Harbor perspectives in biology. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and Structure-Function Relationships of Voltage-Gated Calcium Channels. Pharmacological Reviews. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. “Rejuvenation” protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–6. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Griguoli M, Safiulina V, Lagostena L. The depolarizing action of GABA controls early network activity in the developing hippocampus. Molecular neurobiology. 2011;43:97–106. doi: 10.1007/s12035-010-8147-z. [DOI] [PubMed] [Google Scholar]
- Christie BR, Eliot LS, Ito K, Miyakawa H, Johnston D. Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. Journal of neurophysiology. 1995;73:2553–7. doi: 10.1152/jn.1995.73.6.2553. [DOI] [PubMed] [Google Scholar]
- Cudd T. a. Animal model systems for the study of alcohol teratology. Experimental biology and medicine (Maywood, N.J.) 2005;230:389–93. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Elliott EM, Malouf T, Catterall A. Role of Calcium Channel Subtypes Hippocampal CA3 Neurons in Calcium Transients in 15. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamelli AE, McKinney BC, White J. a, Murphy GG. Deletion of the L-type calcium channel Ca(V) 1.3 but not Ca(V) 1.2 results in a diminished sAHP in mouse CA1 pyramidal neurons. Hippocampus. 2011;21:133–41. doi: 10.1002/hipo.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Ben-Ari Y, Gaïarsa J-L. Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5796–802. doi: 10.1523/JNEUROSCI.0824-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall W. a. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. The Journal of cell biology. 1993;123:949–62. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton TD, Xu W, Lipscombe D. Neuronal L-type calcium channels open quickly and are inhibited slowly. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10247–51. doi: 10.1523/JNEUROSCI.1089-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz JJ, Boesen M, Braun N, Deitmer JW, Kramer F, Lohr C, Müller B, Nothwang HG, Striessnig J, Löhrke S, Friauf E. Cav1.3 calcium channels are required for normal development of the auditory brainstem. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8280–94. doi: 10.1523/JNEUROSCI.5098-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins BI, Kalil K. Differential outgrowth of axons and their branches is regulated by localized calcium transients. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:143–53. doi: 10.1523/JNEUROSCI.4548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3967–72. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A. a., Ingraham NE, Sharpe EJ, Mynlieff M. Levels of CaV1.2 L-Type Ca2+ Channels Peak in the First Two Weeks in Rat Hippocampus Whereas CaV1.3 Channels Steadily Increase through Development. Journal of Signal Transduction. 2012;2012:1–11. doi: 10.1155/2012/597214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczewski N, Langlois A, Fiorentino H, Bonnet S, Marissal T, Diabira D, Ferrand N, Porcher C, Gaiarsa J-L. Spontaneous glutamatergic activity induces a BDNF-dependent potentiation of GABAergic synapses in the newborn rat hippocampus. The Journal of physiology. 2008;586:5119–28. doi: 10.1113/jphysiol.2008.158550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Ferrand N, Fiorentino H, Pellegrino C, Kolarow R, Lessmann V, Medina I, Gaiarsa J-L. Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7013–23. doi: 10.1523/JNEUROSCI.1673-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Gaiarsa J-L. Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity. The European journal of neuroscience. 2010;32:1239–44. doi: 10.1111/j.1460-9568.2010.07378.x. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends in neurosciences. 2003;26:631–40. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Leinekugel X. Developmental patterns and plasticities: the hippocampal model. Journal of physiology, Paris. 2003;97:27–37. doi: 10.1016/j.jphysparis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–55. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Liao P, Soong TW. CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflügers Archiv : European journal of physiology. 2010;460:353–9. doi: 10.1007/s00424-009-0753-0. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. Journal of neurophysiology. 2004;92:2633–41. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Lodygensky G. a, Vasung L, Sizonenko SV, Hüppi PS. Neuroimaging of cortical development and brain connectivity in human newborns and animal models. Journal of anatomy. 2010;217:418–28. doi: 10.1111/j.1469-7580.2010.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learning & memory (Cold Spring Harbor, N.Y.) 2006;13:584–9. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Sze W, Lee B, Murphy GG. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiology of learning and memory. 2009;92:519–28. doi: 10.1016/j.nlm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez J, McCarthy M. Evidence for an extended duration of GABA†mediated excitation in the developing male versus female hippocampus. Developmental neurobiology. 2007:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AG, De Jong D, Kanatsou S, Krugers H, Knapman A, Heinzmann J-M, Holsboer F, Landgraf R, Joëls M, Touma C. Dendritic morphology of hippocampal and amygdalar neurons in adolescent mice is resilient to genetic differences in stress reactivity. PloS one. 2012;7:e38971. doi: 10.1371/journal.pone.0038971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer a, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Pravettoni E, Bacci a, Coco S, Forbicini P, Matteoli M, Verderio C. Different localizations and functions of L-type and N-type calcium channels during development of hippocampal neurons. Developmental biology. 2000;227:581–94. doi: 10.1006/dbio.2000.9872. [DOI] [PubMed] [Google Scholar]
- Ramakers GJ, Avci B, Van Hulten P, Van Ooyen a, Van Pelt J, Pool CW, Lequin MB. The role of calcium signaling in early axonal and dendritic morphogenesis of rat cerebral cortex neurons under non-stimulated growth conditions. Brain research. Developmental brain research. 2001;126:163–72. doi: 10.1016/s0165-3806(00)00148-6. [DOI] [PubMed] [Google Scholar]
- Sagala FSP, Harnack D, Bobrov E, Sohr R, Gertler C, James Surmeier D, Kupsch A. Neurochemical characterization of the striatum and the nucleus accumbens in L-type Ca(v)1.3 channels knockout mice. Neurochemistry international. 2012;60:229–32. doi: 10.1016/j.neuint.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Shitaka Y, Matsuki N, Saito H, Katsuki H. Basic fibroblast growth factor increases functional L-type Ca2+ channels in fetal rat hippocampal neurons: implications for neurite morphogenesis in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:6476–89. doi: 10.1523/JNEUROSCI.16-20-06476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipilä ST, Huttu K, Voipio J, Kaila K. Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+ -activated K+ current. The European journal of neuroscience. 2006;23:2330–8. doi: 10.1111/j.1460-9568.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- Sivakumaran S, Mohajerani MH, Cherubini E. At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2637–47. doi: 10.1523/JNEUROSCI.5019-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends in neurosciences. 2004;27:415–21. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Magee JC. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron. 2009;62:102–11. doi: 10.1016/j.neuron.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Tang F, Dent EW, Kalil K. Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:927–36. doi: 10.1523/JNEUROSCI.23-03-00927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippens AL, Pare JF, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A. Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. Journal of Comparative Neurology. 2008;4:596–583. doi: 10.1002/cne.21567. [DOI] [PubMed] [Google Scholar]
- Tokunaga T, Miyazaki K, Koseki M, Mobarakeh JI, Ishizuka T, Yawo H. Pharmacological dissection of calcium channel subtype-related components of strontium inflow in large mossy fiber boutons of mouse hippocampus. Hippocampus. 2004;14:570–85. doi: 10.1002/hipo.10202. [DOI] [PubMed] [Google Scholar]
- Turner RW, Anderson D, Zamponi GW. Signaling complexes of voltage-gated calcium channels. Channels (Austin, Tex.) 2011;5:440–8. doi: 10.4161/chan.5.5.16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben-Ari Y, Khazipov R. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. Journal of neurophysiology. 2003;90:2964–72. doi: 10.1152/jn.00172.2003. [DOI] [PubMed] [Google Scholar]
- Urbanska M, Blazejczyk M, Jaworski J. Molecular basis of dendritic arborization. Acta neurobiologiae experimentalis. 2008;68:264–88. doi: 10.55782/ane-2008-1695. [DOI] [PubMed] [Google Scholar]
- Veng LM, Browning MD. Regionally selective alterations in expression of the alpha(1D) subunit (Ca(v)1.3) of L-type calcium channels in the hippocampus of aged rats. Brain research. Molecular brain research. 2002;107:120–7. doi: 10.1016/s0169-328x(02)00453-9. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kim S-A, Kirk E. a, Tippens AL, Sun H, Haeseleer F, Lee A. Ca2+-binding protein-1 facilitates and forms a postsynaptic complex with Cav1.2 (L-type) Ca2+ channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4698–708. doi: 10.1523/JNEUROSCI.5523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca S, Valenzuela CF. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6776–81. doi: 10.1523/JNEUROSCI.5405-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotti A, Clementi S, Reinbothe T, Torrente A, Vandael DH, Pirone A. Structural and functional differences between L-type calcium channels: crucial issues for future selective targeting. Trends in pharmacological sciences. 2011;32:366–75. doi: 10.1016/j.tips.2011.02.012. [DOI] [PubMed] [Google Scholar]