Abstract
Objective
To examine whether preterm very low birth weight (VLBW) infants have an increased risk of late-onset sepsis (LOS) following early-onset sepsis (EOS).
Study design
Retrospective analysis of VLBW infants (401-1500 g) born September 1998 through December 2009 who survived >72 hours and were cared for within the NICHD Neonatal Research Network. Sepsis was defined by growth of bacteria or fungi in a blood culture obtained ≤72 hr of birth (EOS) or >72 hr (LOS) and antimicrobial therapy for ≥5 days or death <5 d while receiving therapy. Regression models were used to assess risk of death or LOS by 120d and LOS by 120d among survivors to discharge or 120d, adjusting for gestational age and other covariates.
Results
Of 34,396 infants studied 504 (1.5%) had EOS. After adjustment, risk of death or LOS by 120d did not differ overall for infants with EOS compared with those without EOS [RR:0.99 (0.89-1.09)] but was reduced in infants born at <25wk gestation [RR:0.87 (0.76-0.99), p=0.048]. Among survivors, no difference in LOS risk was found overall for infants with versus without EOS [RR:0.88 (0.75-1.02)], but LOS risk was shorter in infants with BW 401-750 g who had EOS [RR:0.80 (0.64-0.99), p=0.047].
Conclusions
Risk of LOS after EOS was not increased in VLBW infants. Surprisingly, risk of LOS following EOS appeared to be reduced in the smallest, most premature infants, underscoring the need for age-specific analyses of immune function.
Keywords: Very low birth weight, early-onset sepsis, late-onset sepsis
Sepsis is a common, costly, and deadly condition that affects all age groups of intensive care patients. Preterm neonates are highly vulnerable to sepsis due to physiologic immaturity at birth, invasive interventions, and prolonged hospitalization. The fetal immune system often is unchallenged in the protected uterine environment, and even the immune system of the term neonate does not or cannot respond to sepsis ex utero in the same manner as older more mature children(1).
An increased risk of subsequent infection follows sepsis in adults and older children and is associated with high mortality(2, 3). In adults, multiple epidemiologic and functional immunology studies performed over the last 35 years show consistent post-sepsis alterations in host immune response. These alterations, termed immunoparalysis, are associated with an increased risk of subsequent infection(4, 5). Post-sepsis epidemiologic and immune function studies are rare in preterm neonates. The high rate of infection in the most preterm infants (up to 60%) coupled with their distinct immune function which is similar at birth to post-sepsis suppressed immunologic function seen in adults, suggests that early infection in preterm infants will increase risk of subsequent infections(6-11). If true, this might indicate development of post-sepsis immune system alterations that could have a long-lasting impact by affecting not only response to subsequent infectious challenges, but also response to immunizations.
To quantify the risk of subsequent or late-onset sepsis (LOS) after early-onset sepsis (EOS) in premature neonates, we examined data collected from very low birth weight (VLBW) infants hospitalized at centers in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) during an 11-year period.
Methods
All very low birth weight infants (VLBW, birth weight 401-1500g) studied were born between September 1, 1998 and December 31, 2009, admitted <14 days of age to a neonatal intensive care unit affiliated with the NRN, and included in a registry of VLBW infants maintained by the NRN(6). Beginning in 2008, eligibility for the NRN registry was limited to inborn infants with birth weight (BW) 401-1000 grams (g) or gestational age (GA) 22-28 weeks (wk) or infants enrolled in an NRN study in order to focus on the most vulnerable infants. Data collected included maternal and delivery information and infant data collected prospectively until death, hospital discharge, transfer, or 120 days (d). Limited data were collected for infants still in the hospital at 120 days including final status up to one year of age. The registry was approved by the Institutional Review Boards at each participating center.
Analysis was limited to infants who survived >3d in order to include those at risk for LOS. Sepsis was defined by growth of a bacterial or fungal organism on a blood culture obtained within 72 hours of birth (EOS) or after 72 hours (LOS) and antibiotic therapy for ≥5d or death <5d while receiving therapy. Propionibacterium spp, Corynebacterium spp, Micrococcus spp, Penicillium spp, and Alcaligenes spp were predefined as probable contaminants and single organism cultures positive for these pathogens were treated as negative and not indicative of sepsis (n=89). Blood cultures that grew three pathogens were included if the infant died within three days of the culture date (n=7), but all others were considered contaminated and were excluded, regardless of organisms isolated (n=84). Cultures that grew two pathogens were excluded if at least one organism was a predefined contaminant (n=17). Single cultures positive for coagulase-negative staphylococcus (CoNS) if blood was taken within four days after one or more samples positive for a non-CoNS organism were presumed to be contaminants(n=170) and the infection was considered caused by the non-CoNS organism only. The total number of excluded cultures represented approximately 5% of the positive blood cultures reported over the entire study period. After exclusions, positive cultures taken 0-4 days apart were considered part of the same episode.
Statistical analyses
Statistical significance for unadjusted comparisons between infants with and without EOS was determined by Fisher exact or chi-square tests for categorical variables and the Wilcoxon test for continuous variables. Because infants who die cannot develop LOS, the primary outcome was the composite of death or LOS by hospital discharge/ transfer or 120d. Other outcomes examined were death by 120d and LOS by discharge/transfer or 120d among survivors. Three similar secondary outcomes based on death or LOS by 28 days were defined in order to examine outcomes after a common exposure period as the majority of infants were still in the hospital at 28 days. Survivors included infants discharged to home (either from the birth or transfer hospital) and infants still in the hospital at the specified time. Infants with unknown final status after transfer, or for whom the date of discharge to home after transfer was unknown, were excluded from analysis of survivors.
Outcomes were first defined based on LOS due to any pathogen and again based on LOS due to non-CoNS pathogens only. Poisson regression models with robust variance estimators(12) were used to examine the adjusted risk of death or LOS for infants with EOS compared with infants without EOS, and the risk of LOS among survivors. All models included an EOS indicator and effects for study center, GA (<25, 25-28, 29+wk), BW (401-750, 751-1000, 1001-1250, 1251-1500g), male sex, and race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other). Separate models that included interaction terms between GA category and the EOS indicator or between BW category and the EOS indicator in addition to the other covariates were used to estimate adjusted relative risks for infants in each GA and BW category. EOS pathogens were classified as CoNS or other gram-positive, gram-negative, or fungal pathogens. Separate models included an EOS pathogen group indicator (CoNS, other gram positive, gram negative, fungal pathogens, or no EOS) instead of the binary EOS indicator to allow for pairwise comparisons between infants with each type of EOS and infants without EOS. Adjusted relative risks, 95% confidence intervals, and Score or Wald chi-square tests are reported from each model. Analyses were considered hypothesis-generating and p-values were not adjusted for multiple comparisons.
Results
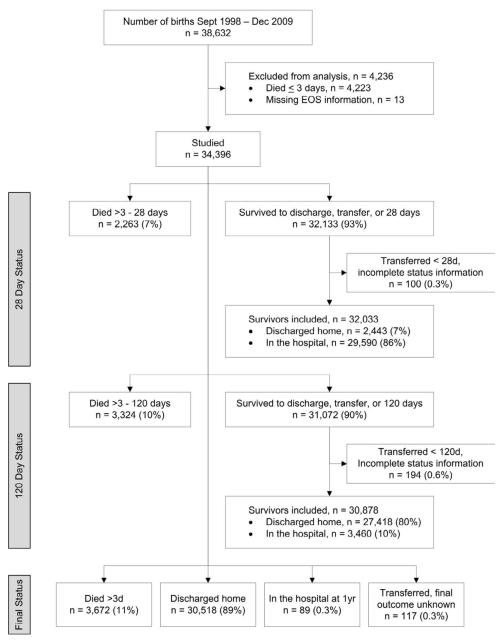
Among 38,632 infants born September 1, 1998 through December 31, 2009 and enrolled in the NRN registry at one of twenty-three study centers, 34,396 who survived >3 days were studied (Figure). Of these, 504 (1.5%) had EOS. The proportion with EOS ranged from 0.81% to 2.57% across study centers (p<0.001). Nearly all infants were preterm (34,331, 99.8%; GA 20-36 weeks); however, fifty-eight were term infants with low BW (GA 37-42 weeks).
Figure.
Neonatal Research Network VLBW cohort studied. Infants were followed to death, discharge/transfer, or 120 days. Limited information was collected for infants still in the hospital at 120 days, including final status up to one year of age. Final status after transfer (discharged to home, death) was recorded for infants transferred to another hospital if known. Transfers with incomplete status information included infants with unknown final status after transfer and infants known to have been discharged to home but with missing discharge date. The latter are shown with final status discharged home.
Infants who had EOS generally were more premature at birth than infants who did not have EOS, with earlier GA and lower BW (Table I). A larger proportion of infants with EOS than without had mothers who were given antibiotics during the delivery admission (74% vs. 63%, p<0.001).
Table 1. Characteristics of VLBW infants in the NRN born Sept. 1, 1998 – Dec.31, 2009 who survived > 3 days—overall and with and without early onset sepsis (EOS).
| Characteristic, N (%) or Median (25th-75th percentile) 1/ |
Overall N=34396 |
Infants with EOS N=504 |
Infants without EOS N=33892 |
p-value 2/ |
|---|---|---|---|---|
| Mother’s age (years) | ||||
| Median (25th-75th p) | 27 (22-32) | 29 (23-34) | 27 (22-32) | <0.001 |
| Antenatal antibiotics | 21674 ( 64) | 370 ( 74) | 21304 ( 63) | <0.001 |
| Antenatal steroids | 27124 ( 79) | 386 ( 78) | 26738 ( 79) | 0.4 |
| Cesarean delivery | 22031 ( 64) | 294 ( 58) | 21737 ( 64) | 0.008 |
| Multiple birth | 8792 ( 26) | 84 ( 17) | 8708 ( 26) | <0.001 |
| Histologic chorioamnionitis 3/ | 2908/7514 (39) | 92/118 (78) | 2816/7396 (38) | <0.001 |
| Birth weight (grams) | ||||
| Median (25th-75th p) | 1039 (796-1280) | 909 (702-1170) | 1040 (800-1280) | <0.001 |
| l401-750 | 7123 ( 21) | 167 ( 33) | 6956 ( 21) | |
| l751-1000 | 8885 ( 26) | 134 ( 27) | 8751 ( 26) | |
| l1001-1250 | 8858 ( 26) | 116 ( 23) | 8742 ( 26) | |
| l1251-1500 | 9530 ( 28) | 87 ( 17) | 9443 ( 28) | |
| Gestational age (weeks) | ||||
| Median (25th-75th p) | 28 (26-30) | 27 (25-28) | 28 (26-30) | <0.001 |
| l<25 | 3965 ( 12) | 122 ( 24) | 3843 ( 11) | |
| l25-28 | 16157 ( 47) | 273 ( 54) | 15884 ( 47) | |
| l29+ | 14267 ( 41) | 109 ( 22) | 14158 ( 42) | |
| Small for gestational age4/ | 7210 ( 21) | 42 ( 8) | 7168 ( 21) | <0.001 |
| Male | 17231 ( 50) | 273 ( 54) | 16958 ( 50) | 0.07 |
| Race/ethnicity | ||||
| lNon-Hispanic black | 13183 ( 38) | 182 ( 36) | 13001 ( 38) | 0.27 |
| lNon-Hispanic white | 13781 ( 40) | 195 ( 39) | 13586 ( 40) | |
| lHispanic | 5794 ( 17) | 99 ( 20) | 5695 ( 17) | |
| lOther | 1574 ( 5) | 27 ( 5) | 1547 ( 5) | |
Information was missing for mother’s age: 11 infants, antenatal antibiotics during the delivery admission: 275, antenatal steroids: 160, cesarean delivery: 20, gestational age: 7, small for gestational age: 10, male sex: 3, race/ethnicity: 64.
P-value for a difference on the distribution of each characteristic in infants with EOS versus no EOS by the Wilcoxon test (median mother’s age, birth weight, gestational age), Fisher exact test or the general association chi-square test.
Beginning in 2006, information was collected about whether placental pathology was performed and chorioamnionitis documented. Numbers shown are among those with placental pathology performed (7576 of 9694 infants with information, 78%; 22% of the total cohort) for whom results were known [7514 infants; results were unavailable for 62 infants with placental pathology performed].
Growth charts developed by Alexander (Alexander, 1996) were used to classify infants as small for GA at birth (SGA), defined by a BW <10th percentile for sex and GA. Because eligibility for the NRN registry was based on low BW alone for most years studied, the proportion of infants SGA increased with increasing GA in this cohort. Thus, the percent SGA was lower among those with EOS than without.
At least one episode of LOS was diagnosed by the time of death, discharge/transfer, or 120d in 8232 (24%) infants; LOS due to a non-CoNS pathogen was diagnosed in 4779 (14%). Median time from birth to first LOS episode was 17d (25th-75th percentiles: 11-28 days) and did not differ for those with and without EOS (median 18d and 17d respectively, p=0.72). The proportion of infants with LOS decreased with increasing GA (<25 wk: 52%; 25-28 wk: 30%; 29+ wk: 10%).
Risk of death or LOS by 120 days of life
Of the 34,396 infants studied, 80% had been discharged home, 10% were still hospitalized, and 10% had died by 120d (Figure). For those who had been discharged home, median hospital stay was longer for infants who had EOS compared with those who did not (72 vs. 59 d, p<0.001).
By 120d after birth, 18% of those with EOS and 10% of those without EOS had died [adjusted RR: 1.25 (1.04-1.50), p=0.03, Table II]. Median day of death for those with EOS was 15 [interquartile range (IQR): 7-28] and 18 [IQR: 10-35] for those without EOS. The percent of infants with and without EOS who died within 120d was similar for those born at <25 wk GA (33% of both groups) but higher for those with EOS among infants born at older GA (25-28 wk GA: 15% vs. 10%; 29+ wk GA: 8% vs. 3%).
Table 2. Mortality and late onset sepsis (LOS) in the first 120 days of life in VLBW infants with early onset sepsis (EOS) compared with other VLBW infants in the NRN born Sept. 1, 1998-Dec. 31, 2009 who survived > 3 days.
| Outcome, n (%) | Infants with EOS |
Infants without EOS |
Adjusted RR (95% CI) for outcome, infants with vs. without EOS 1/ |
Adjusted p-value 1/ |
|---|---|---|---|---|
| Infants who survived > 3 days | N=504 | N=33892 | ||
|
| ||||
| Death or LOS by 120 days (all pathogens) Overall |
196/504 (39) | 9906/33892 (29) | 0.99 (0.89-1.09) | 0.79 |
| By gestational age (weeks) 2/ | ||||
| <25 | 76/122 (62) | 2651/3843 (69) | 0.87 (0.76-0.99) | 0.048 |
| 25-28 | 105/273 (38) | 5608/15884 (35) | 1.07 (0.93-1.24) | 0.37 |
| 29+ | 15/109 (14) | 1645/14158 (12) | 1.14 (0.71-1.83) | 0.58 |
|
| ||||
| Death or LOS by 120 days (non-CoNS pathogens) Overall |
148/504 (29) | 6896/33892 (20) | 1.04 (0.91-1.18) | 0.58 |
| By gestational age (weeks) 2/ | ||||
| <25 | 64/122 (52) | 2118/3843 (55) | 0.92 (0.78-1.09) | 0.34 |
| 25-28 | 72/273 (26) | 3743/15884 (24) | 1.10 (0.91-1.33) | 0.34 |
| 29+ | 12/109 (11) | 1033/14158 (7) | 1.50 (0.87-2.56) | 0.14 |
|
| ||||
| Death by 120 days Overall |
89/504 (18) | 3235/33892 (10) | 1.25 (1.04-1.50) | 0.03 |
| By gestational age (weeks) 2/ | ||||
| <25 | 40/122 (33) | 1283/3843 (33) | 0.96 (0.75-1.23) | 0.76 |
| 25-28 | 40/273 (15) | 1548/15884 (10) | 1.49 (1.13-1.97) | 0.005 |
| 29+ | 9/109 (8) | 402/14158 (3) | 2.98 (1.58-5.63) | <0.001 |
|
| ||||
| Infants who survived to 120 days3/ | N=412 | N=30466 | ||
|
| ||||
| LOS by discharge/transfer/120 days (all pathogens) Overall |
107/412 (26) | 6646/30466 (22) | 0.88 (0.75-1.02) | 0.08 |
| By gestational age (weeks) 2/ | ||||
| <25 | 36/81 (44) | 1365/2546 (54) | 0.79 (0.61-1.01) | 0.059 |
| 25-28 | 65/231 (28) | 4047/14250 (28) | 0.97 (0.80-1.19) | 0.79 |
| 29+ | 6/100 (6) | 1234/13665 (9) | 0.63 (0.29-1.35) | 0.23 |
|
| ||||
| LOS by discharge/transfer/120 days (non-CoNS pathogens) Overall |
59/412 (14) | 3647/30466 (12) | 0.87 (0.69-1.10) | 0.23 |
| By gestational age (weeks) 2/ | ||||
| <25 | 24/81 (30) | 833/2546 (33) | 0.88 (0.62-1.24) | 0.47 |
| 25-28 | 32/231 (14) | 2188/14250 (15) | 0.90 (0.65-1.24) | 0.52 |
| 29+ | 3/100 (3) | 626/13665 (5) | 0.64 (0.21-1.94) | 0.43 |
RR=relative risk, CI=confidence interval. Overall relative risks and CIs were from modified Poisson regression models fit to each outcome that included study center, gestational age (<25, 25-28, 29+ w), BW (401-750, 751-1000, 1001-1250, 1251-1500), male sex, race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other) and an EOS indicator. RRs and CIs in each GA group were from a model that included the interaction between GA. and EOS group in addition to the covariates isted. P-values are by the Score chi-square (overall) or Wald chi-square (byGA) from these models.
Gestational age was missing for 7 infants without EOS (5 among survivors to 120 days).
Survivors to 120 days were defined as infants who had been discharged to home from the birth hospital by 120 days of life, infants who remained in the birth hospital at 120 days, and infants who had been transferred to another hospital or chronic care facility before 120 days who remained in the transfer hospital or had been discharged to home by 120 days. 194 infants with incomplete status information after transfer before 120 days (unknown final status or discharged to home with missing discharge date) were excluded.
Death or LOS by discharge or 120d occurred in 196 (39%) infants who had EOS and in 9906 (29%) who did not (Table II), unadjusted p<0.001. After adjusting for GA and other covariates, no difference was found in overall risk for death or LOS within 120d for infants with EOS compared with those without EOS [adjusted RR: 0.99 (0.89-1.09), p=0.79]. However, a statistically significant reduction in risk of death or LOS was found for infants born at <25 wk GA who had EOS [adjusted RR: 0.87 (0.76-0.99), p=0.048]. No significant differences in risk of this composite outcome were found among infants of higher GA or in BW strata. No significant differences in risk of death or LOS due to non-CoNS pathogens by 120d were found for infants with EOS compared with those without EOS.
Risk of LOS by 120d among survivors to 120d
Among survivors to 120d, infants with EOS received more days of parenteral nutrition than those without EOS [median (IQR): 22 (13-35) vs. 15 (8-29), p<0.001] and more days of mechanical ventilation [10 (2-37) vs. 2 (0-17), p<0.001]. LOS was diagnosed in 26% of surviving infants who had EOS and 22% of infants who did not (Table II), unadjusted p=0.048. After adjusting for GA and other covariates, overall risk of LOS by discharge or 120d was no longer significantly different for infants with and without EOS [adjusted RR: 0.88 (0.75-1.02), p=0.08]. However, a statistically significant reduction in risk of LOS was found for infants with BW 401-750g who had EOS compared with those who did not [adjusted RR: 0.80 (0.64-0.99), p=0.047]. A similar, although not statistically significant, reduction in risk of LOS was found for infants born at <25 wk GA who had EOS. Risk of LOS due to non-CoNS pathogens was not significantly different for surviving infants with and without EOS.
Impact of EOS pathogen type on outcomes
EOS pathogens were examined for 473 (94%) of the 504 infants with a known single pathogen on early blood culture. Gram negative bacteria were most common (237, 50%) with E. coli the most frequent pathogen (159; 34% overall, 67% of gram-negative infections). Gram-positive bacteria were responsible for 220 (47%) of the 473 infections [CoNS: 66, 14% overall, 30% of gram-positive infections; group B Streptococcus: 62, 13% overall, 28% of gram-positive organisms]. Fungi were infrequent (16, 3%). Risk of death by 120d was highest for infants with non-CoNS gram-positive EOS pathogens [adjusted RR: 1.36 (0.98-1.90), p=0.07] and gram-negative EOS pathogens [adjusted RR: 1.28 (0.99-1.66), p=0.06] compared with infants without EOS. Statistically significant differences between infants in each EOS pathogen group and infants without EOS were not found for death or LOS, or for LOS among survivors (data not shown).
Impact of EOS on LOS pathogens
Most of the 8232 infants who had LOS by 120d had one episode (76%). On first LOS episode, CoNS was the most frequent pathogen (49%), followed by other gram positive pathogens (20%) [Staphylococcus aureus: 8.6% overall; group B Streptococcus: 1.8%], and gram-negative pathogens (16%) [E. coli: 4.5% overall; Klebsiella spp.: 4.1%; Enterobacter spp.: 2.8%]. Fungal infections accounted for 7.5% of LOS and mixed type infections for 6.9%; specific pathogen information was unavailable for 1%. Infants with EOS had fewer non-CoNS gram positive LOS infections than infants without EOS (13% vs. 20%, p=0.05) but other LOS pathogen distributions were not significantly different [CoNS: 49% vs. 49%, p=1.0; gram negative: 18% vs. 16%, p=0.48; fungal: 10% vs. 7%, p=0.25].
Risk of death or LOS by 120d of life—excluding infants without EOS who received antibiotics
Data about early-onset clinical infections was not collected. Due to concerns that some infants in the group without culture-proven EOS may have had a clinical infection, we performed a secondary analysis following exclusion of infants from the “without EOS” group who received antibiotics for 5 or more days starting within the first 72 hours of life (Table III). Information was not collected regarding why these infants received prolonged empirical antibiotic treatment, and thus their exclusion is likely to overestimate the proportion with clinical infection. Overall, 40% without culture-proven EOS were excluded, with the proportion who received antibiotics greatest among infants of lower GA (<25 wk: 67%, 25-28 wk: 47%, 29-32 wk: 27%, 33+ wk: 16%). The 20,357 (60%) of 33,892 infants without culture-proven EOS who were included had later median GA (29 wk vs. 28 wk) and greater median BW (1125 vs. 1040g) than this group as a whole. Results using this refined group of infants as controls were largely similar to those reported in Table II.
Table 3. Mortality and late onset sepsis (LOS) in the first 120 days of life in VLBW infants with early onset sepsis (EOS) compared with other VLBW infants in the NRN born Sept. 1, 1998-Dec. 31, 2009 who survived > 3 days—excluding infants without EOS who received antibiotics.
| Outcome, n (%) | Infants with EOS | Infants without EOS who did not receive AB for 5+ days starting in first 72 h1/ | Adjusted RR (95% CI) for outcome, infants with vs. without EOS 2/ |
Adjusted p-value 2/ |
|---|---|---|---|---|
| Infants who survived > 3 days | N=504 | N=20357 | ||
|
| ||||
| Death or LOS by 120 days (all pathogens) |
||||
| Overall | 196/504 (39) | 4625/20357 (23) | 1.03 (0.93-1.15) | 0.59 |
| By gestational age (weeks) 3/ | ||||
| <25 | 76/122 (62) | 861/1274 (68) | 0.88 (0.76-1.02) | 0.09 |
| 25-28 | 105/273 (38) | 2708/8487 (32) | 1.12 (0.97-1.30) | 0.12 |
| 29+ | 15/109 (14) | 1055/10592 (10) | 1.27 (0.79-2.04) | 0.32 |
|
| ||||
| Death or LOS by 120 days (non-CoNS pathogens) |
||||
| Overall | 148/504 (29) | 3089/20357 (15) | 1.12 (0.98-1.28) | 0.12 |
| By gestational age (weeks) 3/ | ||||
| <25 | 64/122 (52) | 675/1274 (53) | 0.97 (0.81-1.15) | 0.69 |
| 25-28 29+ | 72/273 (26) 12/109 (11) | 1757/8487 (21) 656/10592 (6) | 1.20 (0.99-1.47) 1.68 (0.98-2.88) | 0.065 0.061 |
|
| ||||
| Death by 120 days | ||||
| Overall | 89/504 (18) | 1264/20357 (6) | 1.45 (1.19-1.75) | 0.001 |
| By gestational age (weeks) 3/ | ||||
| <25 | 40/122 (33) | 376/1274 (30) | 1.08 (0.84-1.38) | 0.56 |
| 25-28 | 40/273 (15) | 666/8487 (8) | 1.73 (1.30-2.30) | <0.001 |
| 29+ | 9/109 (8) | 221/10592 (2) | 3.88 (2.04-7.39) | <0.001 |
|
| ||||
| Infants who survived to 120 days4/ | N=412 | N=18979 | ||
|
| ||||
| LOS by discharge/transfer/120 days (all pathogens) |
||||
| Overall | 107/412 (26) | 3353/18979 (18) | 0.90 (0.77-1.05) | 0.17 |
| By gestational age (weeks) 3/ | ||||
| <25 | 36/81 (44) | 484/891 (54) | 0.78 (0.60-1.01) | 0.057 |
| 25-28 | 65/231 (28) | 2038/7778 (26) | 1.01 (0.82-1.24) | 0.93 |
| 29+ | 6/100 (6) | 831/10307 (8) | 0.67 (0.31-1.46) | 0.32 |
|
| ||||
| LOS by discharge/transfer/120 days (non-CoNS pathogens) |
||||
| Overall | 59/412 (14) | 1821/18979 (10) | 0.91 (0.72-1.15) | 0.42 |
| By gestational age (weeks) 3/ | ||||
| <25 | 24/81 (30) | 298/891 (33) | 0.88 (0.61-1.26) | 0.47 |
| 25-28 | 32/231 (14) | 1090/7778 (14) | 0.97 (0.70-1.35) | 0.85 |
| 29+ | 3/100 (3) | 433/10307 (4) | 0.66 (0.22-2.03) | 0.47 |
Information about antibiotics received in the first 72 hours after birth was missing for 25 infants who were excluded. Among those with information, 40% (13510/33867) of infants without EOS who received antibiotics for ≥ 5 days started within the first 72 hours were excluded.
RR=relative risk, CI=confidence interval. Overall relative risks and CIs were from modified Poisson regression models fit to each outcome that included study center, gestational age (<25, 25-28, 29+ w), BW (401-750, 751-1000, 1001-1250, 1251-1500), male sex, race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other) and an EOS indicator. RRs and CIs in each GA group were from a model that included the interaction between GA and EOS group in addition to the covariates listed. P-values are by the Score chi-square (overall) or Wald chi-square (by GA) from these models.
Gestational age was missing for 4 infants without EOS who did not receive antibiotics as described (3 among survivors to 120 days).
Survivors to 120 days were defined as infants who had been discharged to home from the birth hospital by 120 days of life, infants who remained in the birth hospital at 120 days, and infants who had been transferred to another hospital or chronic care facility before 120 days who remained in the transfer hospital or had been discharged to home by 120 days. 117 infants with incomplete status information after transfer before 120 days (unknown final status or discharged to home with missing discharge date) were excluded.
Risk of death or LOS by 120d of life—excluding infants with CoNS EOS who received antibiotics
Outcomes were re-examined after exclusion of the 66 infants with EOS due to CoNS, and results were similar (data not shown). Among survivors, risk of LOS by 120 days was reduced for infants born at < 25 weeks GA who had non-CoNS EOS compared with those without EOS [adjusted RR: 0.76 (0.57-0.99), p=0.047], but not among infants born at later GA.
Risk of death or LOS by 28 days of life
At 28 days after birth, 86% of infants remained hospitalized. Results for 28 day outcomes were similar to those at 120 days, with an increased risk of death for infants with EOS compared with infants without EOS [adjusted RR: 1.37 (1.10-1.71), p=0.01] and no statistically significant differences in risk of death or LOS by 28d [adjusted RR: 1.03 (0.91-1.16), p=0.66] or in LOS by 28d among survivors to 28 days [adjusted RR: 0.90 (0.76-1.07), p=0.24].
Discussion
We found no epidemiologic evidence to support an increased risk of culture-proven LOS following culture-proven EOS in this large prospective registry of VLBW neonates cared for in the NICHD NRN over an 11-year period, despite the longer median hospitalization for those who had EOS. Among the most prematurely born neonates (<25 weeks) with the highest risk of death and prolonged hospitalization, risk of the composite outcome of death or LOS was not increased following EOS, and risk for LOS was not increased for survivors. Interestingly, our results suggest a possible slight reduction in the risk of subsequent LOS after EOS in this most vulnerable group that warrants further study. Although risk of death was increased following EOS for VLBW neonates born at 25 weeks gestation or later, those who survived had no increased risk for LOS compared with neonates who had not had EOS.
Leviton et al reported on the association of select comorbidities in a cohort of extremely low gestational age neonates (<28wk gestation, ELGAN)(13). The authors described an increased risk of LOS following EOS after adjustment for GA (OR: 2.1, 95% CI: 1.3-3.3). Key differences between that and the present study that might explain our differing results included: 1) population (Infants were enrolled based on GA rather than BW and the analyses were restricted to those who survived to 36wk post menstrual age); 2) significantly higher rate of LOS in their EOS-positive patients (42% versus 26% by 120d or discharge among survivors); and 3) definition used for EOS (by 1 week of life versus ≤72 hours). Interestingly, Strunk et al examined a cohort of infants <30wk gestational age with available placental histopathology and showed a reduced risk of LOS following histologic chorioamnionitis exposure after adjustment for GA (OR: 0.74, 95% CI: 0.57-0.95), suggesting a potential immune priming benefit. None of the infants with EOS (n=25) in that study developed LOS (14). In our study, infants with EOS were more likely to have been exposed to histologic chorioamnionitis than those without EOS. Examination of a large cohort (n = 103,376) of VLBW preterm infants <32 weeks with no overlap to the present study also showed no association between EOS and subsequent LOS risk(15).
The apparent lack of similarity between post-EOS risk of LOS in our cohort of preterm infants and the increased risk of infection following sepsis reported in adults and older children may be due in part to the impact of development on the host immune response. Immune deficits that are associated with sepsis in adults and older children may be present at birth in well-appearing preterm infants(7-11). Accumulating evidence suggests that the preterm infant’s immune system responds differently to immune stimulation for a period of time that extends well into the first year of life(16-18). Because adult-like immune function may take years to develop(19), the newborn’s immune system may be incapable of reacting in the same way as older children or adults following sepsis. Evidence supporting the concept of a unique neonatal host immune response to sepsis was presented in a recent investigation that examined whole blood genome-wide expression profiles among pediatric patients with septic shock. That study, which did not include any former preterm neonates, revealed a unique transcriptomic host immune response for neonates compared with infants, toddlers, and school-age children(1).
An absence of immunoparalysis in neonates is one potential explanation for the difference between neonates and adults in risk of repeat infection after an initial episode of sepsis. However, in a recent study, cord blood monocyte major histocompatibility complex (MHC) class 2 surface expression and TNF-α production following endotoxin stimulation ex vivo were significantly reduced in preterm infants with sepsis compared with control preterm infants, suggesting immunoparalysis may occur in preterm infants(20). Infants who survive sepsis might be those with greater immunocompetence, yet a similar selection bias would also be predicted to occur in adult or child sepsis survivors who exhibit an increased risk of subsequent infection. Alternatively, an early immune stimulus may transform the host defense status of the preterm infant from a relative state of tolerance, as required to prevent fetal rejection by the mother’s body, to a level of competence that is better suited to defense against the many microbes in the extrauterine environment. This phenomenon has been demonstrated in vivo in preclinical models and in preterm human neonates who received BCG vaccination shortly after birth. In both examples, a reduced impact of subsequent infection was noted following immune system priming(21-24).
There are several inherent limitations of this retrospective study. We have detailed information on histologic chorioamnionitis exposure for only 22% of the entire cohort. Sepsis was defined by a positive blood culture which may have a low sensitivity, and more sensitive molecular and other laboratory evaluations for sepsis diagnosis were not available. Clinical and laboratory data that would allow us to determine which infants had culture-negative EOS were not collected. We recognize the apparent trade-off between an increased risk of death following EOS among all but the most premature infants with the highest baseline risk of death and no increased risk of LOS after EOS among survivors. Late mortality (>28d after sepsis) was also seen in older children after sepsis and was nearly equal to early mortality (<28d after sepsis)(25).
These data underscore the need for developmental age-specific investigations of the host response to infection and highlight that much remains to be learned about the impact of infection on the subsequent development and function of the preterm infant’s immune system.
Acknowledgments
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Abbreviations
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- VLBW
very low birth weight
- EOS
early-onset sepsis
- LOS
late-onset sepsis
- CoNS
Coagulase negative Staphylococcus
- GA
gestational age
- BW
birth weight
Appendix
The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003-2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006-2011).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – William Oh, MD; Yvette Yatchmink, MD; Robert Burke, MD; Angelita M. Hensman, RN BSN; Suzy Ventura.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Nancy S. Newman, RN.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Barbara Alexander, RN; Kate Bridges, MD; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN; Holly L. Mincey, RN BSN; Jody Hessling, RN.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Katherine A. Foy, RN.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39) – David P. Carlton, MD; Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Linda L. Wright, MD; Elizabeth M. McClure, MEd; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Brenda B. Poindexter, MD MS; James A. Lemons, MD; Dianne E. Herron, RN; Lucy C. Miller, RN BSN CCRC; Leslie Dawn Wilson, BSN CCRC.
RTI International (U10 HD36790) – W. Kenneth Poole, PhD; Dennis Wallace, PhD; Jeanette O’Donnell Auman, BS; Margaret Cunningham, BS; Betty K. Hastings; Elizabeth M. McClure, MEd; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University, California Pacific Medical Center, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70) – David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Marian M. Adams, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Anne Furey, MPH.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Maynard R. Rasmussen MD; Paul R. Wozniak, MD; Kathy Arnell, RNC; Renee Bridge, RN; Clarence Demetrio, RN; Wade Rich, BSHS RRT.
University of Iowa Children’s Hospital (U10 HD53109, M01 RR59) – John A. Widness, MD; Karen J. Johnson, RN BSN.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Charles R. Bauer, MD; Shahnaz Duara, MD; Ruth Everett-Thomas, RN MSN; Amy Mur Worth, RN MS.
University of New Mexico Health Sciences Center (U10 HD27881, U10 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Lu-Ann Papile, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN.
University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44, NCRR UL1 024160) – Dale L. Phelps, MD; Ronnie Guillet, MD PhD; Linda J. Reubens, RN CCRC, Erica Burnell, RN; Mary Rowan, RN; Cassandra A. Horihan, MS.
University of Tennessee Health Science Center (U10 HD21415) – Sheldon B. Korones, MD; Tina Hudson, RN BSN.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Charles R. Rosenfeld, MD; Walid A. Salhab, MD; P. Jeannette Burchfield, RN BSN; Alicia Guzman; Gaynelle Hensley, RN; Melissa H. Leps, RN; Susie Madison, RN; Nancy A. Miller, RN; Lizette E. Torres, RN.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373) – Jon E. Tyson, MD MPH; Kathleen A. Kennedy, MD MPH; Esther G. Akpa, RN BSN; Patty Ann Orekoya, RN BSN; Beverly Foley Harris, RN BSN; Claudia I. Franco, RNC MSN; Anna E. Lis, RN BSN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Patti L. Pierce Tate, RCP; Maegan C. Simmons, RN.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64) – Roger G. Faix, MD; Bradley A. Yoder, MD; Shawna Baker, RN; Jill Burnett, RN; Jennifer J. Jensen, RN BSN; Karen A. Osborne, RN BSN CCRC; Kimberlee Weaver-Lewis, RN BSN.
Wake Forest University Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Nancy J. Peters, RN CCRP.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Rebecca Bara, RN BSN; Debra Driscoll, RN BSN; Geraldine Muran, RN BSN; Elizabeth Billian, RN MBA; Laura Sumner, RN BSN; Kara Sawaya, RN BSN; Kathleen Weingarden, RN BSN.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 RR24139, M01 RR125, M01 RR6022) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Patricia Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Joanne Williams, RN BSN.
Appendix 2
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development provided grant support for the Neonatal Research Network’s Generic Database Study. D.B. receives support from the US Government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and government contract HHSN267200700051C), the Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org), and industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the Data-Coordinating Center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr. Abhik Das (DCC Principal Investigator) and Ms. Nellie Hansen (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding and conflict of interest information is available at www.jpeds.com (Appendix 2).
The authors declare no conflicts of interest.
References
- 1.Wynn JL, Cvijanovich NZ, Allen GL, Thomas NJ, Freishtat RJ, Anas N, et al. The Influence of Developmental Age on the Early Transcriptomic Response of Children with Septic Shock. Molecular Medicine. 2011;17:1146–56. doi: 10.2119/molmed.2011.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, et al. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Annals of Surgery. 1977;186:241–50. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Medicine. 2011;37:525–32. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatric Clinics of North America. 2008;55:647–68. xi. doi: 10.1016/j.pcl.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol. 2009;29:79–88. doi: 10.1038/jp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011;3:90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birle A, Nebe CT, Gessler P. Age-related low expression of HLA-DR molecules on monocytes of term and preterm newborns with and without signs of infection. J Perinatol. 2003;23:294–9. doi: 10.1038/sj.jp.7210906. [DOI] [PubMed] [Google Scholar]
- 11.Genel F, Atlihan F, Ozsu E, Ozbek E. Monocyte HLA-DR expression as predictor of poor outcome in neonates with late onset neonatal sepsis. J Infect. 2010;60:224–8. doi: 10.1016/j.jinf.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 13.Leviton A, Dammann O, Engelke S, Allred E, Kuban KC, O’Shea TM, et al. The clustering of disorders in infants born before the 28th week of gestation. Acta Paediatrica. 2010;99:1795–800. doi: 10.1111/j.1651-2227.2010.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strunk T, Doherty D, Jacques A, Simmer K, Richmond P, Kohan R, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. 2012;129:e134–41. doi: 10.1542/peds.2010-3493. [DOI] [PubMed] [Google Scholar]
- 15.Lin CB, Hornik CP, Clark R, Cotten CM, Benjamin DK, Jr., Cohen-Wolkoweiz M, et al. Very low birth weight neonates who survive early-onset sepsis do not have an increased risk of developing late-onset sepsis. Early Hum Dev. 2012;88:905–9. doi: 10.1016/j.earlhumdev.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, et al. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: Low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–37. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 18.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–41. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azizia M, Lloyd J, Allen M, Klein N, Peebles D. Immune status in very preterm neonates. Pediatrics. 2012;129:e967–74. doi: 10.1542/peds.2011-1579. [DOI] [PubMed] [Google Scholar]
- 21.Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized Trial of BCG Vaccination at Birth to Low-Birth-Weight Children: Beneficial Nonspecific Effects in the Neonatal Period? The Journal of Infectious Diseases. 2011;204:245–52. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 22.Roth A, Jensen H, Garly ML, Djana Q, Martins CL, Sodemann M, et al. Low birth weight infants and Calmette-Guerin bacillus vaccination at birth: community study from Guinea-Bissau. Pediatr Infect Dis J. 2004;23:544–50. doi: 10.1097/01.inf.0000129693.81082.a0. [DOI] [PubMed] [Google Scholar]
- 23.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–8. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuenca AG, Wynn JL, Kelly-Scumpia KM, Scumpia PO, Vila L, Delano MJ, et al. Critical role for CXC ligand 10/CXC receptor 3 signaling in the murine neonatal response to sepsis. Infection and Immunity. 2011;79:2746–54. doi: 10.1128/IAI.01291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123:849–57. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]