Abstract
Toll-like Receptors (TLRs) play a pivotal role in inflammatory processes and individual TLRs have been investigated in nociception. Here, we examine overlapping and diverging roles of spinal TLRs and their associated adaptor proteins in nociceptive processing. Intrathecal (IT) TLR2, TLR3, or TLR4 ligands (-L) evoked persistent (7 day) tactile allodynia (TA) that was abolished in respective TLR deficient mice. Using Tnf−/− mice, we found that IT TLR2 and TLR4 TA was TNF-dependent, while TLR3 was TNF-independent. In toll-interleukin 1 receptor (TIR) domain containing adaptor protein (Tirap−/−) mice (downstream to TLR2 and TLR4), allodynia after IT TLR2-L and TLR4-L was abolished. Unexpectedly, in TIR-domain-containing adapter-inducing interferon-β (Triflps2) mice (downstream of TLR3 and TLR4), TLR3-L allodynia was abrogated, but intrathecal TLR4-L produced a persistent increase (>21 days) in TA. Consistent with a role for interferon (IFN)β (downstream to TRIF) in regulating recovery after IT TLR4-L, prolonged allodynia was noted in Ifnar1−/− mice. Further, IT IFNβ given to Triflps2 mice reduced TLR4 allodynia. Hence, spinal TIRAP and TRIF cascades differentially lead to robust TA by TNF dependent and independent pathways, while activation of TRIF modulated processing through type I IFN receptors. Based on these results, we believe that processes leading to the activation of these spinal TLRs initiate TNF-dependent and -independent cascades, which contribute to the associated persistent pain state. In addition, TRIF pathways are able to modulate the TNF-dependent pain state through IFNβ.
INTRODUCTION
Innate immunity is involved in tissue-level responses to infection and injury. Elements of this process, notably the Toll-like receptors (TLRs) are expressed by glia [3,4,24], and neurons [33,47]. TLRs have been implicated in the nociceptive processing initiated by inflammation and peripheral nerve injury [5,9,12,32,41,55]. These observations, indicating a role for TLRs in these pain models in the absence of an infectious process, are in accord with observations that a variety of endogenous ligands known to activate TLRs have been identified in biologic systems and may serve to act though these constitutively expressed receptors [6,13,25,35]. Several approaches have provided direct support for a role of spinal TLRs in pain processing. Thus, spinal (intrathecal: IT) delivery of TLR4 agonists yields nociception and allodynia [9,10,38,51,54]. Conversely, pharmacological blockade of spinal TLR4 attenuates evolution of a persistent pain state [9]. Additionally, delivery of agents, which reduce glial activation, can inhibit the facilitatory effects of IT TLR agonists [22,39,49,51].
There are thirteen identified TLRs some localized to the cell surface and others on endosomes, which signal through a limited number of adaptor proteins (Figure 1A). The Toll-interleukin 1 receptor (TIR) domain containing adaptor protein, TIRAP, is exclusive to TLR2 and TLR4, and facilitates myeloid differentiation factor 88 (MyD88) activation [20,21]. The MyD88 activation pathway, common to all TLRs except TLR3, leads to activation of NF-κB, yielding production of pro-inflammatory cytokines such as TNF and IL-1 [27]. In contrast, the TIR-domain-containing adapter-inducing interferon-β (TRIF) is shared by TLR3 and TLR4 signaling, and skews to type I interferon production [16,42]. Thus, TLR activation, through either the MyD88 or the TRIF pathways, can lead to a wide-range of effects. Given this complex organization and the expression of TLRs by glia and neurons, the net effect of activating any one of the multiple spinal TLRs cannot be predicted in the absence of specific data on outcomes associated with defined spinal TLR activation.
Figure 1. Schematic of the TLR pathways.
(A) This figure highlights the key TLRs and their relevant pathways in this paper. TLR2, 4, and 5 are found on the cell surface, while TLR3, 7, and 9 are in the cell endosomes. MyD88 is a key adaptor protein for all TLRs except TLR3. TIRAP is exclusive to TLR2 and TLR4 leading to proinflammatory cytokine release. TRIF is restricted to TLR3 and TLR4, resulting predominantly in type I interferon production. (B) This table summarizes the knock-out mice used in the presented studies and the nomenclature used throughout the paper.
Here we investigate the role of spinal TLRs and their associated adaptor proteins in spinal nociceptive processing using both in vitro and in vivo techniques. With primary spinal cell cultures of microglia and astrocytes, we determined the expression levels of TNF and IFNβ following TLR activation. To assess roles of the respective spinal TLRs in initiating a hyperpathic state, eponymous TLR ligands were intrathecally administered and IT TLR2-L (HKLM), TLR3-L (Poly(I:C)) and TLR4-L (LPS) were found to initiate long lasting allodynic states. Using genetically modified mice, we found that TLR2 and TLR4 ligands acted through TNF (as defined by a diminished effect in Tnf−/− mice) while TLR3-L did not. Unexpectedly, in mice that lacked TRIF or type I IFN receptor signaling, a markedly prolonged and enhanced allodynia was noted. Allodynia induced by IT TLR2 or TLR4 ligand was transiently relieved by IT IFNβ. These studies revealed TNF-dependent and -independent spinal pro-allodynic cascades are differentially activated by TRIF and TIRAP signaling, and a potential suppressive role of TRIF signaling through IFNβ.
METHODS
Animals
All animal experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego (under the Guide for Care and Use of Laboratory Animals, National Institutes of Health publication 85-23, Bethesda, MD, USA). Mice were housed up to four per standard cage at room temperature and maintained on a 12-hour light/dark cycle (light on at 07:00h). Testing was performed during the light cycle. Food and water were available ad libitum. C57BL/6 mice (male, 25–30g) were purchased from Harlan (Indianapolis, IN). Tlr2−/−, Tlr3−/−, Tlr4−/−, and Tirap−/− mice were a gift from Dr. S. Akira (Osaka University, Japan) and were bred for 10 generations onto the C57Bl/6 background. Triflps2 mice were a gift from Dr. B. Beutler (UT Southwestern, Texas) and were directly generated on the C57Bl/6 background. Tlr5−/− and Tnf−/− mice were purchased from The Jackson Laboratory. Ifnar1−/− mice were originally obtained from B&K Universal Limited (Hull, United Kingdom) and backcrossed over 10 generations onto the C57Bl/6 background. Figure 1B lists the murine strains used and the standard nomenclature to be used throughout the paper.
Rat Microglia and Astrocyte Primary Cell Culture
Purified cultures of rat spinal microglia and astrocytes were prepared as previously described with some modifications [22]. One- to three-day-old Holtzman Sprague–Dawley rat pups were anesthetized, and the spinal cords were ejected, mechanically triturated, then centrifuged at 215 g for 5 min and re-suspended in DMEM containing 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA), 1% penicillin/streptomycin (P/S; Gibco, Carlsbad, CA, USA), and plated in a flask previously coated with poly l-lysine (Sigma, St. Louis, MO, USA). Flasks were maintained at 37°C in a humidified 5% CO2 incubator for 2 weeks until 80–90% confluent, with media changes every other day. On day 14, microglia were removed by shaking for 2 hours at 37 °C, centrifuged at 215 g for 5 min, and plated onto 24-well plates at 80,000 cells/mL and allowed to adhere for 4 hours. For astrocyte cultures, on day 15 mother cultures were shaken a second time, media discarded and replaced, trypsinized, centrifuged at 215×g for 5 min, re-suspended in DMEM with 10% FBS and 1% P/S, and plated on to 24-well plates at 100,000 cells/mL until they reached 70–80% confluence (2 days). Individual wells were stimulated with 5μL of specific murine TLR agonists available in a complete kit (Invivogen: tlrl-kit1mw; Supplementary Table 1). The doses chosen were within the range recommended by the manufacturer.
TNF ELISA
TNF in the culture supernatant was assayed at both 6- and 20-hours following TLR agonist administration, by ELISA kits per the manufacturers instructions (R&D Biosystems). TNF is expressed as pg/mL of culture media sample.
Quantitative real time-PCR
At 20-hours post TLR agonist addition to the culture media, the media was removed and immediately replaced with 0.5mL of Trizol (Invitrogen), allowed to sit for 2 minutes then flash frozen. The mRNA was then isolated using RNeasy columns (Qiagen). Complementary DNA was prepared using the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen). Quantitative real-time PCR was performed with pre-developed specific primers and probes (Taqman Gene Expression Assay, Applied Biosystems) were used to detect mouse Interferon-beta 1 (IFNβ1) (Assay ID Rn00569434_s1) and GAPDH (Assay ID Rn01775763_g1) (Applied Biosystems). The relative abundance was calculated by comparing delta-CT values [44] and the data were then normalized to GAPDH gene expression and presented as relative gene expression.
Primary Cell Culture Staining
Primary microglia and astrocytes cells in cDMEM were aliquoted (200μL) in to coated 8-chamber cell culture slides at the same density as stated previously and allowed to proliferate for 24-hours (microglia) or 36-hours (astrocytes). Cells were fixed for 5 minutes in 4% PFA then washed with PBS and stained overnight in primary antibodies at 4°C. Astrocytes and microglia were incubated with anti-Vimentin (Zymed, 1:500) and anti-Iba-1 (Abcam, 1:250) antibodies. Secondary antibodies conjugated to Alexa-488 and 594 were used at 1:300. Slides were visualized and images captured on a Leica confocal microscope.
LDH Cytotoxicity Assay
Cytotoxicity following the TLR ligands was assessed by Lactate dehydrogenase (LDH) release. LDH was measured in media samples of microglia and astrocytes following each TLR ligand treatment group at the 20-hour time point using a CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) following the manufacturer’s directions.
Intrathecal (IT) Injection and Drug Administration
Intrathecal needle placement procedure for the IT saline and IT sham treatment is performed as previously described [23,55]. Briefly, mice were induced with 3% isoflurane (with 2% oxygen and 2% room air) in a chamber until a loss of the righting reflex was observed. A 1″ 30-gauge needle attached to a 50μL Hamilton syringe was inserted between the L5 and L6 vertebrae, evoking a tail flick reflex. The following TLR agonists were administered in 5μL diluted in 0.9% sterile saline: HKLM (1×108cells/5μL), Poly(I:C) (1μg/5μL), LPS-EK Ultrapure (1μg/5μL), and FLA-ST (1μg/5μL) (Supplementary Table 2). The doses were chosen from the Invivogen product protocol, as well as the results of the cell culture studies outlined above. Doses ranging from 0.1μg/5μL to 10μg/μL were first tested, and 1μg/μL produced the maximal effect at the lowest dose for all ligands. For the TLR5 ligand (FLA-ST) the 10μg/5μL dose showed the same minimal TA as the 1μg/5μL dose (Supplemental Figure 2A). Thus, the 1μg/5μL dose was chosen to correspond to the other TLR ligand doses. All ligands were diluted in 0.9% sterile saline to a stock solution and then aliquoted to avoid repeated freeze-thaw cycles. IT administration doses were then diluted to the specified concentration from a frozen aliquot.
Following recovery from anesthesia, as evidenced by a vigorous righting reflex and spontaneous ambulation, typically around 1–2 minutes, mice were evaluated for motor coordination and muscle tone. Tactile thresholds were measured using the up-down application of von Frey hairs along the following time course: Baseline (P=Pre-injection), 30-, 60-, 90-, 120-, 180-, and 240-minutes, 24-hours and 7-days after treatment. We previously noted the effects of the use of isoflurane in this procedure and TA [55], thus, while all the above time points were recorded, only the baseline, 4-hour, 24-hour, and 7-day time points are presented here. IFNβ (Chemicon, 100ng/5μL in 0.1% BSA) was administered intrathecally either 1 hour before IT LPS (1μg/5μL) or as a post-treatment, 7 days after IT LPS induced TA. Gabapentin (Toronto Research Chemicals) was administered (100mg/kg) i.p. diluted in 0.9% sterile saline.
Behavioral Tests
Mechanical sensitivity was assessed using the von Frey up-down method. Filaments with values ranging from 2.44 to 4.31 (0.03g to 2.00g) were applied to the paw as previously described [7]. The 50% probability withdrawal threshold (in principle, the calculated force to which an animal reacts to 50% of the presentations) was recorded. Mechanical values for both the paws were measured and averaged to produce a single data point per day of measurement.
Western Blot
Mice were deeply anaesthetized and spinal cords were ejected from the vertebral column using a saline-filled syringe. The lumbar part of the spinal cord was immediately homogenized in extraction buffer [50 mm Tris buffer, pH 8.0, containing 0.5% Triton X-100, 150 mm NaCl, 1 mm EDTA, protease inhibitor cocktail (P-8340; Sigma, 1:100), phosphatase inhibitor cocktail I and II (Sigma, 1:100)] by sonication. The tissue extracts were subjected to denaturing NuPAGE 4–12% Bis-Tris gel electrophoresis and then transferred to nitrocellulose membranes (Micronic Separation Inc. Westborough, MA, USA). Membrane was first blocked with 5% non-fat milk in Tris-buffer (50 mm Tris-HCl, 6 mm NaCl) containing 0.1% Tween 20 for 1 hour at room temperature. The membranes were incubated with antibodies overnight at 4°C (IFNβ 1:1000; Chemicon and β-actin 1: 10,000). After washing, the antibody–protein complexes were probed with appropriate secondary antibodies labeled with horseradish peroxidase for 1 hour at room temperature and detected with chemiluminescent reagents (SuperSignal; Pierce, Rockford, IL, USA). Intensity of immunoreactive bands was quantified using Image Quant software (Molecular Dynamics, Sunnyvale, CA, USA). The intensity of the IFNβ immunopositive bands was normalized relative to that of β-actin. Two exposures for anti-IFNβ of the same blot are shown. The longer exposure is presented to demonstrate that all lanes had a band present. Quantification was performed on the shorter exposure, since it provided a more accurate differential lane expression.
Statistics
Data are presented as group mean ± SEM. Tactile threshold time course curves (plotted as the mean ± SEM vs. time after treatment) were analyzed with a 2-way analysis of variance (ANOVA) with repeated measures over time, followed by Bonferroni post hoc test. The allodynic index is the area under the time course curve after treatment, in which the percentage change from baseline threshold is plotted against time: 100 × ((baseline threshold treatment threshold)/(baseline threshold)). Multiple comparison tests were performed by two-way ANOVA with Bonferonni post hoc tests. Statistical analyses utilized Prism software (GraphPad Software, Inc., San Diego, CA).
RESULTS
Primary astrocyte and microglia cultures
Rat spinal primary microglia and astrocyte cell cultures were generated from neonatal rats and stained with anti-vimentin and anti-Iba-1 to assess purity (Supplementary Figure 1C and D). These primary microglia and astrocyte cultures were stimulated with TLR ligands specific to individual receptors (Supplementary Table 1), supernatants collected at both 6-hours and 20-hours, and the levels of TNF release in the media was assayed by ELISA. Primary microglia cultures (Figure 2B) showed a robust TNF release at the 6-hour time point following the addition of TLR2, 4, and 5 ligands, and at the 20-hour time point following TLR1/2, 2, 4, 5, and 2/6 ligands. Primary astrocyte cultures (Figure 2A) showed a significant level of TNF release at the 6-hour time point following the addition of TLR2, and 5 ligands, and at the 20-hour time point following TLR2, 4, and 5 ligands. The TLR3-L (Poly(I:C)) produced a minimal response as expected since TNF release has been reported to have slower kinetics and to be at a lower level. However, both astrocyte (Figure 2C) and microglia (Figure 2D) showed an increase in IFNβ following TLR3-L (Poly(I:C)) and TLR7/8 (ssRNA40) agonist treatment, which attained statistical significance for (Poly(I:C)) treatment. To control for cytotoxicity, LDH release was measured in the primary cell culture media samples at 20-hours post TLR agonist addition. In both the microglia and astrocyte cultures, only Poly(I:C) and LPS administration resulted in minimal cell death signal (5–10%), while the other TLRs had no apparent effect (Supplementary Figure 1A–B).
Figure 2. TLR activation of rat primary cultured astrocyte and microglia.
Specific ligands of TLRs were added to primary spinal astrocyte (A, C) and microglia (B, D) cell cultures (Supplementary Table 1). Media samples were harvested, and evidence of cell activation was assessed by measurement of TNF in the astrocyte (A) and microglia (B) media samples at 6-hours and 20-hours post TLR agonist addition. IFNβ mRNA was detected using quantitative RT-PCR for astrocyte (C) and microglia (D) cultures 20-hours post TLR agonist addition. Data are expressed as mean ± SEM (n=3 samples/group) and analyzed by 1-way ANOVA, followed by Dunnett’s post hoc test to compare each treatment to the media control (*p<0.05).
Spinal TLR activation and nociceptive thresholds
The following in vivo studies address the role of spinal activation of TLRs on tactile thresholds. TLR2-L (HKLM), TLR4-L (LPS) and TLR5-L (FLA-ST) were selected for further study in vivo based on TNF release and TLR3-L (Poly(I:C)) based on IFNβ mRNA induction in the primary microglia and astrocyte cell cultures (Figure 2). TLR ligands were spinally delivered via IT injection at the L5 level in C57Bl/6 mice (Figure 3). The effect of IT administration of these selected TLR ligands on tactile thresholds was measured by von Frey filament testing using the up-down method. Mice were tested before IT administration (P) and at 0.5, 1, 1.5, 2, 3, 4, 24 hours, and 7 days post injection. We previously noted the effects of the use of isoflurane in this procedure and TA [55] and, thus, while all the above time points were recorded, only the, baseline (P), 4-hour, 24-hour, and 7-day time points are presented here. TLR2-L (HKLM), TLR3-L (Poly(I:C)) and TLR4-L (LPS) produced a robust TA, lasting longer than 7 days (Figure 3A–C). Alternatively, TLR5-L (FLA-ST) produced a short-lived 3-hour TA that was resolved by the 4-hour time point (Figure 3D and Supplementary Figure 2B).
Figure 3. IT administration of TLR ligands in C57Bl/6 mice produced robust TA.
TLR ligands were spinally delivered via IT injection at the L5 level in C57Bl/6 mice. The effect of intrathecal (IT) administration of TLR ligands (Supplemetary Figure 2) on tactile thresholds was measured by von Frey filament testing, using the up-down method (A–D). Data expressed as mean ± SEM (n=5–8 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare each time point to the IT saline group (*p<0.05; **p<0.01). The same IT saline group is represented in all four graphs.
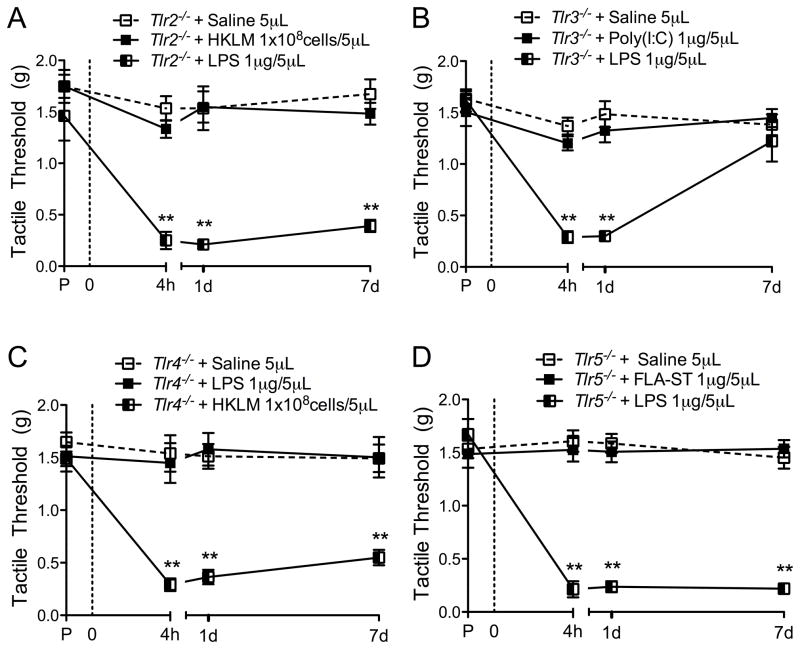
TLR deficient mice and spinal TLR ligands
To assess the specificity of the administered TLR ligands to their receptor, TLR deficient mice were used. This method was employed since there are no specific antagonists available for these receptors, with the exception of TLR4. Each TLR null mouse received the corresponding TLR ligand IT, as well as a different TLR ligand to show that other TLR signaling pathways were not impaired. As expected IT administration of the corresponding TLR ligand did not produce a TA in the respective TLR strain (Figure 4A–D). IT TLR4-L (LPS) was used as a control in Tlr2−/−, Tlr3−/−, and Tlr5−/− mice, as it is specific to TLR4, and produced a robust TA in these mice (Figure 4A–B, D, Supplementary Figure 2C). IT TLR2-L (HKLM) was used as a control for Tlr4−/− mice, as it is specific to TLR2, and produced a robust TA in the Tlr4−/− mice (Figure 4C). These results not only confirm the specificity of the selected TLR ligands, but also the integrity of the gene targeted strain and its signaling pathways.
Figure 4. Specific TLR null mice confirm TLR ligand specificity.
TLR ligands were spinally delivered via IT injection at the L5 level in Tlr2−/− (A), Tlr3−/− (B), Tlr4−/− (C), and Tlr5−/− (D) mice. Saline and TLR ligand matching that of the TLR null mouse were first used to confirm agonist specificity. A different TLR ligand was then administered to show that the each mouse strain was still able to produce a robust TA following TLR activation. The effect of intrathecal (IT) administration of TLR ligands on tactile thresholds was measured by von Frey filament testing, using the up-down method. Data expressed as mean ± SEM (n=5–8 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare the groups over the entire time course (*p<0.05; **p<0.01). Asterisks indicate comparison of corresponding TLR ligand group vs. different TLR ligand group. All comparisons of the IT saline vs. corresponding TLR ligand group, were not significant in these panels.
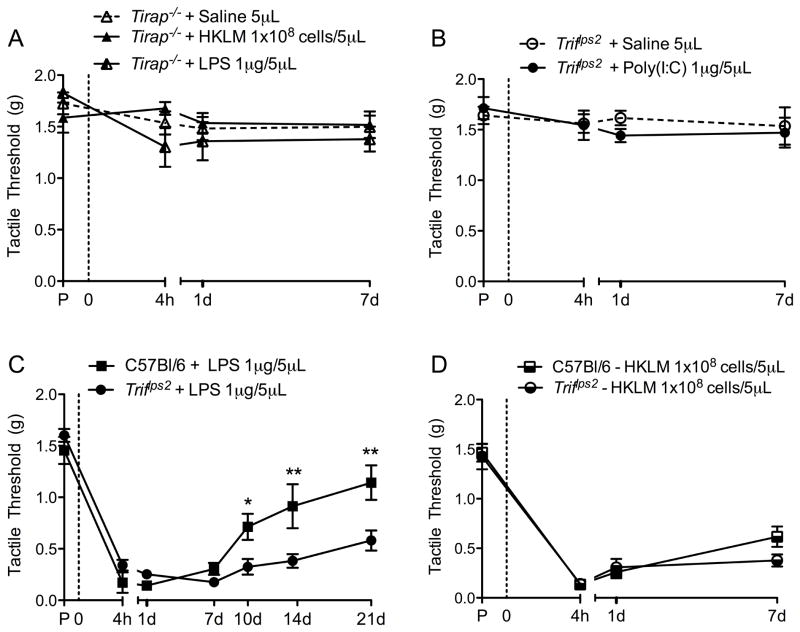
TLR adapter protein deficient mice and spinal TLR ligands
We next assessed the role of signaling intermediates in the TLR pathways: TIRAP, which leads to proinflammatory cytokine release, and TRIF, which leads to type I interferon production (Figure 1A). To investigate signaling mediators, mice deficient in TIRAP and TRIF signaling were subject to IT TLR ligands (Figure 1B). TIRAP is specific to TLR2 and TLR4 signaling, while TRIF is specific to TLR4 and TLR3 signaling. IT LPS had no effect in Tirap−/− mice (Figure 5A), while IT TLR4-L (LPS) in Triflps2 mice produced a robust, long-lasting TA (Figure 5C). This finding suggests a role for the TRIF pathway, and possibly interferon release, in the resolution phase following injury. Additionally, as expected, IT TLR2-L (HKLM) had no effect in Tirap−/− mice (Figure 5A) and IT TLR3-L (Poly(I:C)) had no effect in Triflps2 mice (Figure 5B), but IT TLR2-L (HKLM) did have a robust effect in Triflps2 mice (Figure 5D). This confirms the roles of TRIF and TIRAP as specific adaptor proteins to TLR3 and TLR2, respectively.
Figure 5. Functional loss of specific TLR adaptor proteins suggest TRIF-mediated resolution pathway.
TLR ligands were spinally delivered via IT injection at the L5 level in Tirap−/− (A) and Triflps2 mice (B, C, D). In Tirap−/− mice there was no effect on tactile thresholds of IT HKLM or LPS when compared to IT saline (A). Triflps2 mice showed a robust long-lasting TA following IT LPS (B). IT Poly(I:C) had no effect on tactile thresholds in the Triflps2 mice (C), but IT HKLM produced a robust TA in Triflps2 mice (D). Data expressed as mean ± SEM (n=4–7 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare the Triflps2 group to the C57Bl/6 group (*p<0.05; **p<0.01).
Role of TNF in nociceptive processing
Considerable work with genetically engineered animals and spinally derived anti-TNF agents has pointed to a pervasive role of this cytokine in mediating neuraxial events underlying pain processing [40,49,59]. To assess the role of TNF in the TA elicited by IT TLR ligands administration, Tnf−/− mice were used. Tnf−/− mice received IT injections of the same TLR ligands used previously. Following IT TLR2-L (HKLM) only a modest TA was observed (Figure 6A), while the IT TLR4-L (LPS) produced only a brief TA similar to that of IT saline (Figure 6C). This result suggested that the TLR4 allodynia-inducing pathway is absolutely TNF-dependent, while TLR2 induced TA was only partially dependent on TNF. In contrast, IT TLR3-L (Poly(I:C)) in the Tnf−/− mice continued to produce a robust TA (Figure 6B), suggesting that the pronociceptive effects of the TLR3/TRIF pathway was independent of TNF in producing pain. The brief 3-hour TA effect of the TLR5 agonist was also almost completely abolished in the TNF deficient mice (Figure 6D). Accordingly, when the allodynic indices were calculated, Tnf−/− mice were shown to be less responsive to the TLR2-L (HKLM), TLR4-L (LPS), and TLR5-L (FLA-ST) (Figure 6E), while the TLR3-L (Poly(I:C)) produced a robust effect.
Figure 6. TLR activation in Tnf−/− mice.
TLR ligands were spinally delivered via IT injection at the L5 level in Tnf−/− mice. The effect of intrathecal (IT) administration of TLR ligands on tactile thresholds was measured by von Frey filament testing, using the up-down method. (A–D) Data expressed as mean ± SEM (n=5–8 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare each time point to the IT saline group (*p<0.05; **p<0.01). The same IT saline group is represented in all four graphs. (E) Hyperalgesic indices were calculated for each mouse using their individual baseline threshold and calculating the area under the curve. Data expressed as mean ± SEM. Hyperalgesic index was analyzed via 2-way ANOVA followed by Bonferroni post hoc test to compare each C57Bl/6 treatment group to the same Tnf−/− treatment group (*p<0.05; **p<0.01).
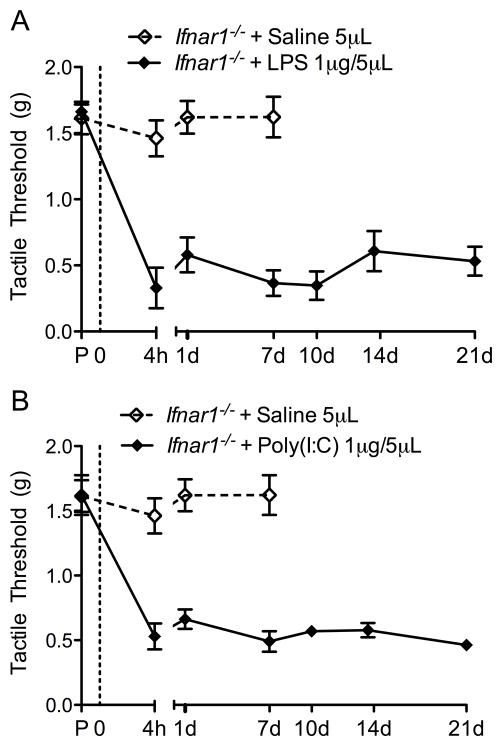
Role of IFN in nociceptive processing
Type I interferons (IFNs) can have both proinflammatory actions (as in the response to viral infections via TLR3, 7, 8 and 9 activation) primarily through macrophage stimulation and can serve to suppress the inflammatory cascade as in models of tumor growth [30]. To assess the role of interferon on TLR induced TA, Ifnar1−/− mice were used. Similar to that of the Triflps2 mice, IT TLR4-L (LPS) produced a robust, long-lasting TA in the Ifnar1−/− mice (Figure 7A). This TA was transiently reversed by 100mg/kg i.p. gabapentin (Supplementary Figure 3). The anti-allodynic effects observed in these studies after systemic gabapentin is consistent with the presence of a facilitated state. In contrast to the Triflps2 mice, the Ifnar1−/− mice developed prolonged allodynia following IT TLR3-L (Poly(I:C)) resembling the pattern of the IT TLR4-L (LPS) treated mice (Figure 7B). Together these results suggested that the TA following TLR3 activation was independent of TNF, but the rapid resolution of the facilitated TA required type I IFN signaling. Confirming the increased presence of interferons following TLR3 signaling, an increase in spinal IFNβ was indeed detected by western blot following TLR3-L (Poly(I:C)) (Supplementary Figure 4).
Figure 7. IT TLR3 and TLR4 agonist produce TA in Ifnar1−/− mice.
Ifnar1−/− mice were treated with IT TLR3-L (Poly(I:C)) and TLR4-L (LPS). (A) IT LPS produced a robust long-lasting TA, very similar to that observed in the Triflps2 mice. (B) IT Poly(I:C) agonist also produced a robust TA, but was slower onset when compared to IT LPS. The same IT saline group is represented in both graphs. Data expressed as mean ± SEM (n=6–7 mice/group). The same C57Bl/6 saline group is represented in this figure. Data analyzed via 1-way ANOVA followed by Bonferroni post hoc test to compare IT saline treatment group to IT LPS and IT Poly(I:C) treatment group. For IT saline vs. IT LPS and IT Saline vs. IT Poly(I:C) p<0.01.
Roles of spinal IFN in regulating spinal TLR mediated TA
To test if type I IFN treatment could relieve pain after IT TLR4-L (LPS) treatment we selected Triflps2 mice, which develop a protracted course of mechanical allodynia after IT LPS treatment (Figure 5C), and have intact type I IFN receptor signaling. In Triflps2 mice at 7-days post IT TLR4-L (LPS) or TLR2-L (HKLM), a post-treatment with IT IFNβ (100ng/5μL) reversed the TA (Figure 8). In two separate TLR-induced TA pathways IFNβ treatment temporarily alleviated the observed allodynia. Additionally, Triflps2 mice were pre-treated with IT IFNβ (100ng/5μL) one hour before IT LPS (Supplemental Figure 5). Pre-treatment with IFNβ transiently blocked the LPS-induced TA. IT vehicle plus IT LPS still produced a robust TA in the Triflps2 mice, and IFNβ plus IT saline had no effect on tactile thresholds. This confirmed the importance of IFNβ on resolving the central pain state following TLR activation.
Figure 8. Post-treatment with IFNβ blocks LPS and HKLM-induced TA in Triflps2 mice.
Triflps2 mice were treated with either IT LPS (1μg/5μL) or IT HKLM (1×108 cells/5μL) 7 days before IT IFNβ (100ng/5μL). Post-treatment with IFNβ 7-days post IT LPS (1μg/5μL) transiently reversed the LPS-induced TA (A) and HKLM-induced TA (B). Data expressed as mean ± SEM (n=3–4 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare each time point after IFNβ post-treatment to the respective 7-day post IT LPS or HKLM group (*p<0.05; **p<0.01).
DISCUSSION
In models of persistent inflammation, and mono-/poly-neuropathy, mice with defects in TLR expression show a prominent attenuation of behaviorally defined hyperpathy [4,8,11,28,36,49]. Although those studies employed globally deficient mice, expression of TLRs on spinal glia [3,4,24] and neurons [33,47] and the ability of TLR agonists to evoke release of cytokines, including TNF, has emphasized the role of spinal TLRs in spinally mediated facilitated pain states. In the face of tissue injury and inflammation there can be a transition from an acute to a persistent facilitated state [2,8]; and spinal TLRs may particularly function in this transition [9]. One characteristic of the “painful” phenotype after tissue and nerve injury is the response to anti-hyperpathic agents such as gabapentin, which we show here to antagonize the TA observed after IT TLR4 agonist. Collectively these data suggest complex roles of TLRs in both the induction and the recovery of facilitated pain states.
Prior reports of the contributions TLRs to spinal facilitated pain states focused on individual receptors, However, there are 13 TLR family members, which signal through a more restricted number of adaptor proteins resulting in the release of neurohumoral factors (Figure 1). We undertook to characterize the signaling cascades in the spinal cord initiated by local TLR activation of membrane bound (TLR2, TLR4, TLR5) and endosomal (TLR3) receptors though the use of intrathecal TLR ligands (Supplemental Table 2). Here, we show that activation of spinal TLR2, TLR3, and TLR4 evokes a long lasting (up to 7 days) decrease in touch evoked hind paw withdrawal thresholds (tactile allodynia). The TLR5 ligand, though also a membrane TLR, produced only a short-lived (<4hr) TA, regardless of dose. These results are consistent with previously reported results in mice [9] and rats [51]. In contrast to the morbidity observed with peripheral TLR agonists [18,29,58], IT TLR agonists did not elicit detectable changes in general behavior, body weight, or motor function.
TLR coupling
As the TLR2, TLR3 and TLR4 deficient mice prevented TA produced only by IT HKLM, Poly(I:C), and LPS, respectively, these effects reflect a specific action mediated by the eponymous spinal receptor and confirm the lack of cross talk between the respective spinal receptors. In particular, the specificity of Poly(I:C) for TLR3 was confirmed in the Tlr3−/− mice. There are other intracellular RNA sensors, such as RIGI and MDA5 that would be present in the Tlr3−/− mice, yet the TA was absent in Tlr3−/− mice. To characterize the signaling cascades initiated by spinal membrane and endosomal TLR activation leading to TA, we examined the role of associated adaptor proteins for the TLRs selected for in vivo studies. As schematically summarized in Figure 9, IT TLR agonists in these mutant mice revealed several novel characteristics of these spinal TLR-effector cascades.
Figure 9. Schematic of the TLR cascades emphasized in the present work.
Endogenous ligands present in the injured and inflamed system activate resident TLRs, localized to glia and/or neurons. Based on the effects of IT TLR ligands, it is hypothesized that TLRs signaling through TIRAP and MyD88 lead to NFκB mediated cytokine (TNF) release and to a TNF dependent allodynia (e.g. TLR2 and TLR4). However, TLR3 leads to a TNF independent allodynia and IFNβ production. Based on the effects of Ifnar−/− mice, the increased IFNβ production regulates the allodynic actions mediated by TIRAP (TLR2/TLR4 activation) and induced by IT TLR3-L. As TLR4 activated both TRIF and TIRAP signaling, the net allodynic effect reflects the facilitation mediated by spinal TNF release and the inhibition initiated by TRIF mediated IFNβ production.
TIRAP signaling in spinal TLR initiated allodynia
Consistent with coupling of TLR2 and TLR4, but not TLR3, through TIRAP, IT TLR2 and TLR4 agonists initiated TA was absent in Tirap−/− mice, while TLR3-L effects were unaltered. TIRAP signals through MAPKs and NF-κB, leading to inflammatory cytokine release [20]. The development of TA by IT TLR4-L injection was absent in the Tirap−/− mice indicating that the TIRAP-MyD88 pathway predominantly elicits pain after IT TLR4-L (LPS) injection. Hence, the TRIF pathway might have slower activation kinetics and, thus, might not be associated with pain induction, but rather pain resolution. The Tlr3−/− mice injected with IT TLR4-L (LPS) had a complete resolution of pain by 7 days, suggesting that the absence of TLR3, may have facilitated TLR4 signaling toward TRIF activation.
Activation of TLR2/TLR4 in a variety of cell systems, including glia in the present study, increases TNF synthesis and release [27]. In the present work, TA after IT TLR2-L and TLR4-L was reduced in Tnf−/− mice, indicating that TLR2/TLR4 allodynia is TNF-dependent (Figure 9). These results are consistent with previous work in which IT TNF inhibitors block effects of IT TLR4 agonists [51]. Further, TNF is upregulated in various chronic inflammatory and nerve injury models [14,50,52,61], but other inflammatory cytokines such as IL-1 and IL-6 have also been associated with spinal pain states. In contrast to effects observed with TLR2 and TLR4 ligands, TLR3 activation in Tnf−/− mice produced a TA, suggesting that the TLR3/TRIF pathway leading to TA is TNF independent. This lack of effect of TNF on TLR3 allodynia was unexpected as the TRIF pathway (downstream to both TLR3 and TLR4), can induce NF-κB activation and cytokine production [43,60]. Myd88−/− mice retain TLR4-L activated phosphorylation of mitogen-activated protein kinase family members (ERK1/2, p38 kinase and Jun kinase), and NF-κB, albeit with delayed kinetics [26]. Conversely, in the absence of TRIF signaling (e.g. Triflps2), TLR4 stimulation continues to activate ERK and IκBα degradation and produce TNF [19].
TRIF signaling in spinal TLR initiated allodynia
The TRIF adapter protein is common to TLR3 and TLR4 cascades, but not TLR2, and functions through IRF3 to induce type I interferon production, specifically IFNβ [16,20,21,42,57]. Consistent with this cascade, IT TLR3-L, but not TLR2-L initiated TA was abolished in Triflps2 mice. However IT TLR3-L initiated TA was unaltered in Tnf−/− mice. Other proinflammatory cytokines, such as IL-6, or IL-1β, down stream to TRIF, likely provided parallel signaling in the Tnf−/− mice that received IT TLR3-L.
An unexpected finding was the exaggerated effect of IT TLR4 ligand signaling in the Triflps2 and Ifnar1−/− mice. IT TLR4 agonism resulted in a robust, long-lasting (>21 days) TA, which was transiently reversed by gabapentin. These results suggested that the 7-day course of TLR4 mediated TA in C57/Bl6 mice was, in part, dependent upon TRIF signaling. Triflps2 mice do not produce IFNβ after TLR stimulation, while Ifnar1−/− mice produce IFNβ, but the IFN receptor is unable to signal. Since Triflps2 mice had functional IFN receptors, we showed the importance of IFNβ in regulating spinal TLR4 initiated TA by reintroducing it in the Triflps2 mouse by IT delivery. While Triflps2 mice continued to respond to IT TLR2-L, IT IFNβ also antagonized that TA. Accordingly, given the TLR4 coupling though Tirap−/− and Triflps2, we believe that unlike TLR2, which activates only the TIRAP-TNF cascade, TLR4 initiates an allodynic state though the TIRAP-TNF pathway, and concurrently activates the TRIF-IFN-β pathway which counter-modulates TIRAP-TNF cascade (Figure 9).
With regard to spinal IFN, the effects of type I interferons are complex. Both IT IFNα and IFNβ inhibit CFA-hypersensitivity [56] and proinflammatory cytokine upregulation [28]. Conversely, IFNα has been reported to enhance excitatory transmission [48]. Consistent with present spinal results, IFNβ suppression of proinflammatory cytokines has been shown in a variety of peripheral and neuraxial inflammatory states [11,36,37,53]. Mechanisms of IFNβ action can include an increase in anti-inflammatory IL-10, reduction of proinflammatory IL-17 and modulation of matrix metalloproteinase [31]. Also, IFNβ stimulates the production of IL-1 receptor antagonist (IL1Ra), which directly binds to the IL-1 receptor as an endogenous regulator. An imbalance between IL-1β and IL-1Ra has been reported in the spinal fluid of patients with rheumatoid arthritis, a condition with chronic inflammation and pain [34].
TLRs and glial activation
Microglia show constitutive expression of virtually all TLRs, while astrocytes predominately express TLR3 and sometimes TLR2 [3,4,24]. We show that with primary microglia and astrocyte cultures, ligands activating a TIRAP cascade released TNF. Conversely, TLR3 coupled though TRIF displayed IFNβ release. In vivo, spinal glial cells play a role in nociceptive processing. Intrathecal inhibitors of glial activation suppress injury-evoked hyperpathias [22,39,49,51]. Upon activation, glia release proinflammatory mediators, including cytokines such as TNF, which activate neighboring glial cells and neurons leading to a facilitation of their response to subsequent afferent traffic and an increase in IFNβ. While we specifically assessed TLR activation on primary glial cells, TLR3 expression by brain neurons [33,46], and TLRs 3, 4, 7 and 9 in dorsal root ganglia (DRG) [1,47] has been reported, suggesting that neurons can directly respond to TLR ligands. Cultured mouse DRGs stimulated with TLR ligands augment expression of proinflammatory chemokines and cytokines, and repression of the TRPV1 receptor [47], demonstrating the complexity of TLR responses in several molecular mechanisms associated with pain responses.
In conclusion, IT TLR agonists show the robust effects of spinal TLR activation on nociceptive processing and the complexity of the downstream signaling initiated by the direct activation of these spinal TLRs. The present work reveals TNF-dependent and -independent facilitatory signaling, as well as an unexpected modulatory feedback through the TRIF pathway of TNF-dependent signaling. While we emphasize here the effects of TLR activation on spinal cytokines, processes leading to activation of NF-κB are also associated with the upregulation of a variety of channels and transcription factors such as ATF3 [17]. TLR4 null mice have indeed been shown to prevent such changes in ATF3 expression [9]. Finally, these effects reflect the downstream coupling initiated by tissue and nerve injury states in the absence of an infectious process, which can lead to the release of molecules shown to activate spinal TLRs [6,13,15,25,35,45]. Elucidation of the events leading to such release, and the subsequent activation of the downstream facilitatory and inhibitory cascades noted here is an important target for future research.
Supplementary Material
Supplementary Table 1: Target and concentration of TLR ligands used in primary cell culture experiments.
Supplementary Table 2: Target and concentration of TLR ligands used in IT injections for in vivo experiments.
Supplementary Figure 1: LDH assay confirms cell viability and fluorescent staining confirms culture purity.
An assay detecting LDH in the media was performed at 20-hours post agonist administration to assess cell death and toxicity of the TLR ligands to the primary cell cultures, astrocytes (A) and microglia (B). Primary astrocyte and microglia cell cultures were stained of with antibodies specific to astrocytes (vimentin) and microglia (Iba-1). Astrocyte culture (C) stained positive for vimentin (green), but not Iba-1 (red). Microglia cultures (D) stained positive for Iba-1 (red), but not vimentin (green).
Supplementary Figure 2: TLR5 ligand administration.
TLR5-L (FLA-ST) was spinally delivered via IT injection at the L5 level in C57Bl/6 mice.
(A) Two doses were tested for the TLR5 ligand and an extended 7-day effect was not observed. (B) The 1μg/5μL FLA-ST dose was chosen for the remaining studies to correspond to the other TLR ligand doses. (C) In Tlr5−/− mice IT Saline and FLA-ST was first used to confirm the TLR ligand specificity. TLR4-L was then administered to show that the Tlr5−/− mouse was still able to produce a robust TA following TLR activation. Data expressed as mean ± SEM (n=3–8 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare the groups over the entire time course (*p<0.05; **p<0.01). Asterisks indicate comparison of corresponding TLR5-L group vs. TLR4-L group. Comparison of the IT saline vs. corresponding TLR5-L group was not significant.
Supplementary Figure 3: Gabapentin reduces LPS produced TA.
At 22 days post IT LPS, allodynic Ifnar1−/− mice were administered 100mg/kg i.p. of gabapentin which completely reversed the TA resulting from the IT LPS at 60 minutes. This effect was eliminated by 24 hours. Data expressed as mean ± SEM (n=4–5 mice/group) and analyzed via 1-way ANOVA, followed by Dunnetts post hoc test to comparing allodynic day 22 Ifnar1−/− mice with the gabapentin post-treatment group (**p<0.01).
Supplementary Figure 4: IFNβ increased following TLR3 ligand.
C57Bl/6 mice received intrathecal injections of TLR2-L (HKLM), TLR3-L (Poly(I:C)), or TLR4-L (LPS) and the lumbar sections of their spinal cords assayed for IFNβ via western blot (A). There is an increase in IFNβ following TLR3-L relative to the IT saline treatments. Data expressed as mean ± SEM (n=3 mice/group) and analyzed via 1-way ANOVA, followed by Dunnetts post hoc test (*p<0.05).
Supplementary Figure 5: Pre-treatment with IFNβ blocks LPS-induced TA in Triflps2 mice.
Triflps2 mice were pre-treated with IT IFNβ (100ng/5μL) one hour before IT LPS (1μg/5μL). Tactile thresholds were measured before IT IFNβ or vehicle pretreatment (baseline). One hour later, IT LPS or IT saline was then injected and tactile thresholds measured 1-, 2-, 4-, 24-hours, and 7-days post injection. Data expressed as mean ± SEM (n=3–4 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare each time point to the pre-treatment IT vehicle + IT LPS group, or IT vehicle post-treatment group (*p<0.01).
Summary.
Spinal TLR activation mediates persistent change leading to robust TA by TNF-dependent and -independent pathways. TRIF leads to modulation of TLR-evoked TA mediated by IFNβ.
Acknowledgments
This work was supported by grants from National Institutes of Health: NS16541 and DA02110 (TLY/MPC) and T32 GM007752-31 (JAS).
Footnotes
Conflicts of Interest Statement
The authors claim no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57(11):1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bas DB, Su J, Sandor K, Agalave NM, Pettersson J, Codeluppi S, Baharpoor A, Nandakumar KS, Holmdahl R, Svensson CI. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum. 2012 doi: 10.1002/art.37686. [DOI] [PubMed] [Google Scholar]
- 3.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43(3):281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 4.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61(11):1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 5.Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009;158(2):896–903. doi: 10.1016/j.neuroscience.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205(11):2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 8.Christianson CA, Corr M, Firestein GS, Mobargha A, Yaksh TL, Svensson CI. Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain. 2010;151(2):394–403. doi: 10.1016/j.pain.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152(12):2881–2891. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30(2):573–582. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobelens PM, Tiebosch IA, Dijkhuizen RM, van der Meide PH, Zwartbol R, Heijnen CJ, Kesecioglu J, van den Bergh WM. Interferon-beta attenuates lung inflammation following experimental subarachnoid hemorrhage. Crit Care. 2010;14(4):R157. doi: 10.1186/cc9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90(1–2):1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 13.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87(6):989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 14.George A, Schmidt C, Weishaupt A, Toyka KV, Sommer C. Serial determination of tumor necrosis factor-alpha content in rat sciatic nerve after chronic constriction injury. Exp Neurol. 1999;160(1):124–132. doi: 10.1006/exnr.1999.7193. [DOI] [PubMed] [Google Scholar]
- 15.Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol. 2010;184(5):2655–2662. doi: 10.4049/jimmunol.0903359. [DOI] [PubMed] [Google Scholar]
- 16.Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, Kamps MP, Raz E, Wagner H, Hacker G, Mann M, Karin M. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439(7073):204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 17.Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component? Gene Expr. 2010;15(1):1–11. doi: 10.3727/105221610x12819686555015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424(6950):743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 20.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420(6913):329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 21.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2(9):835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 22.Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22(10):2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 23.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67(2–3):313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 24.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175(7):4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 25.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279(13):12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13(5):816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 28.Kawanokuchi J, Mizuno T, Kato H, Mitsuma N, Suzumura A. Effects of interferon-beta on microglial functions as inflammatory and antigen presenting cells in the central nervous system. Neuropharmacology. 2004;46(5):734–742. doi: 10.1016/j.neuropharm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17 (Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 30.Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M, Marini FC. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12(5):615–625. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- 31.Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs. 2011;25(6):491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Kim D, Lee S, Lee SJ. Toll-like receptors in peripheral nerve injury and neuropathic pain. Curr Top Microbiol Immunol. 2009;336:169–186. doi: 10.1007/978-3-642-00549-7_10. [DOI] [PubMed] [Google Scholar]
- 33.Lafon M, Megret F, Lafage M, Prehaud C. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29(3):185–194. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 34.Lampa J, Westman M, Kadetoff D, Agreus AN, Le Maitre E, Gillis-Haegerstrand C, Andersson M, Khademi M, Corr M, Christianson CA, Delaney A, Yaksh TL, Kosek E, Svensson CI. Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proc Natl Acad Sci U S A. 2012;109(31):12728–12733. doi: 10.1073/pnas.1118748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28(2):131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda Y, Musoh K, Shichijo M, Tanaka H, Nagai H. Interferon-beta prevents antigen-induced bronchial inflammation and airway hyperreactivity in mice. Pharmacology. 1997;55(1):32–43. doi: 10.1159/000139510. [DOI] [PubMed] [Google Scholar]
- 37.Mannon PJ, Hornung RL, Yang Z, Yi C, Groden C, Friend J, Yao M, Strober W, Fuss IJ. Suppression of inflammation in ulcerative colitis by interferon-beta-1a is accompanied by inhibition of IL-13 production. Gut. 2011;60(4):449–455. doi: 10.1136/gut.2010.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33(11):1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 39.Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol. 2007;560(2–3):142–149. doi: 10.1016/j.ejphar.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23(3):1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234(2):316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7(5):353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 43.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4(2):161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piccinini AM, Midwood KS. Endogenous Control of Immunity against Infection: Tenascin-C Regulates TLR4-Mediated Inflammation via MicroRNA-155. Cell Rep. 2012;2(4):914–926. doi: 10.1016/j.celrep.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79(20):12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, Klinman D, Oppenheim JJ, Howard OM. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol. 2011;186(11):6417–6426. doi: 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin ZF, Hou DY, Fang YQ, Xiao HJ, Wang J, Li KC. Interferon-alpha enhances excitatory transmission in substantia gelatinosa neurons of rat spinal cord. Neuroimmunomodulation. 2012;19(4):235–240. doi: 10.1159/000335167. [DOI] [PubMed] [Google Scholar]
- 49.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104(3):655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 50.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20(2):467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 51.Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, Schaloske RH, Deems RA, Dennis EA, Yaksh TL. Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF. Br J Pharmacol. 2010;160(7):1754–1764. doi: 10.1111/j.1476-5381.2010.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855(1):83–89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- 53.Smeets TJ, Dayer JM, Kraan MC, Versendaal J, Chicheportiche R, Breedveld FC, Tak PP. The effects of interferon-beta treatment of synovial inflammation and expression of metalloproteinases in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43(2):270–274. doi: 10.1002/1529-0131(200002)43:2<270::AID-ANR5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 54.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31(43):15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stokes JA, Corr M, Yaksh TL. Transient tactile allodynia following intrathecal puncture in mouse: contributions of Toll-like receptor signaling. Neurosci Lett. 2011;504(3):215–218. doi: 10.1016/j.neulet.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan PH, Gao YJ, Berta T, Xu ZZ, Ji RR. Short small-interfering RNAs produce interferon-alpha-mediated analgesia. Br J Anaesth. 2012;108(4):662–669. doi: 10.1093/bja/aer492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell Mol Life Sci. 2008;65(19):2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639(2):283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- 59.Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14(4):166–174. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169(12):6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Fu ZJ, Sun T, Zhao XL, Song WG, Jia MR, Wei GF. Expression of NF-kappaB and TNF-alpha in spinal dorsal horn in a rat model of neuropathic pain. Zhonghua Yi Xue Za Zhi. 2010;90(15):1067–1071. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Target and concentration of TLR ligands used in primary cell culture experiments.
Supplementary Table 2: Target and concentration of TLR ligands used in IT injections for in vivo experiments.
Supplementary Figure 1: LDH assay confirms cell viability and fluorescent staining confirms culture purity.
An assay detecting LDH in the media was performed at 20-hours post agonist administration to assess cell death and toxicity of the TLR ligands to the primary cell cultures, astrocytes (A) and microglia (B). Primary astrocyte and microglia cell cultures were stained of with antibodies specific to astrocytes (vimentin) and microglia (Iba-1). Astrocyte culture (C) stained positive for vimentin (green), but not Iba-1 (red). Microglia cultures (D) stained positive for Iba-1 (red), but not vimentin (green).
Supplementary Figure 2: TLR5 ligand administration.
TLR5-L (FLA-ST) was spinally delivered via IT injection at the L5 level in C57Bl/6 mice.
(A) Two doses were tested for the TLR5 ligand and an extended 7-day effect was not observed. (B) The 1μg/5μL FLA-ST dose was chosen for the remaining studies to correspond to the other TLR ligand doses. (C) In Tlr5−/− mice IT Saline and FLA-ST was first used to confirm the TLR ligand specificity. TLR4-L was then administered to show that the Tlr5−/− mouse was still able to produce a robust TA following TLR activation. Data expressed as mean ± SEM (n=3–8 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare the groups over the entire time course (*p<0.05; **p<0.01). Asterisks indicate comparison of corresponding TLR5-L group vs. TLR4-L group. Comparison of the IT saline vs. corresponding TLR5-L group was not significant.
Supplementary Figure 3: Gabapentin reduces LPS produced TA.
At 22 days post IT LPS, allodynic Ifnar1−/− mice were administered 100mg/kg i.p. of gabapentin which completely reversed the TA resulting from the IT LPS at 60 minutes. This effect was eliminated by 24 hours. Data expressed as mean ± SEM (n=4–5 mice/group) and analyzed via 1-way ANOVA, followed by Dunnetts post hoc test to comparing allodynic day 22 Ifnar1−/− mice with the gabapentin post-treatment group (**p<0.01).
Supplementary Figure 4: IFNβ increased following TLR3 ligand.
C57Bl/6 mice received intrathecal injections of TLR2-L (HKLM), TLR3-L (Poly(I:C)), or TLR4-L (LPS) and the lumbar sections of their spinal cords assayed for IFNβ via western blot (A). There is an increase in IFNβ following TLR3-L relative to the IT saline treatments. Data expressed as mean ± SEM (n=3 mice/group) and analyzed via 1-way ANOVA, followed by Dunnetts post hoc test (*p<0.05).
Supplementary Figure 5: Pre-treatment with IFNβ blocks LPS-induced TA in Triflps2 mice.
Triflps2 mice were pre-treated with IT IFNβ (100ng/5μL) one hour before IT LPS (1μg/5μL). Tactile thresholds were measured before IT IFNβ or vehicle pretreatment (baseline). One hour later, IT LPS or IT saline was then injected and tactile thresholds measured 1-, 2-, 4-, 24-hours, and 7-days post injection. Data expressed as mean ± SEM (n=3–4 mice/group) and analyzed via 2-way ANOVA, followed by Bonferroni post hoc test to compare each time point to the pre-treatment IT vehicle + IT LPS group, or IT vehicle post-treatment group (*p<0.01).