Abstract
Inorganic complexes are versatile platforms for the development of potent and selective pharmaceutical agents. Cobalt possesses a diverse array of properties that can be manipulated to yield promising drug candidates. Investigations into the mechanism of cobalt therapeutic agents can provide valuable insight into the physicochemical properties that can be harnessed for drug development. This review presents examples of bioactive cobalt complexes with special attention to their mechanisms of action. Specifically, cobalt complexes that elicit biological effects through protein inhibition, modification of drug activity, and bioreductive activation are discussed. Insights gained from these examples reveal features of cobalt that can be rationally tuned to produce therapeutics with high specificity and improved efficacy for the biomolecule or pathway of interest.
Introduction
The clinical success of inorganic drugs, such as platinum (II) chemotherapeutics and gold-containing antiarthritic agents, has significantly advanced the use of transition metals in medicine in recent years [1,2]. Numerous transition metals (including cobalt) can adopt a wide variety of coordination numbers, geometries, oxidation states, and ligand binding affinities that can be exploited in the development of innovative therapeutic drugs [2,3,4**]. Despite their well-known versatility, cobalt derivatives have not been studied extensively as inorganic pharmaceuticals as compared to other metals. To date, the only cobalt-based therapeutic that has reached clinical trials is Doxovir, a Co(III) Schiff base complex effective against drug-resistant herpes simplex virus 1 [5]. The mechanism of action of Doxovir, however, is not fully understood.
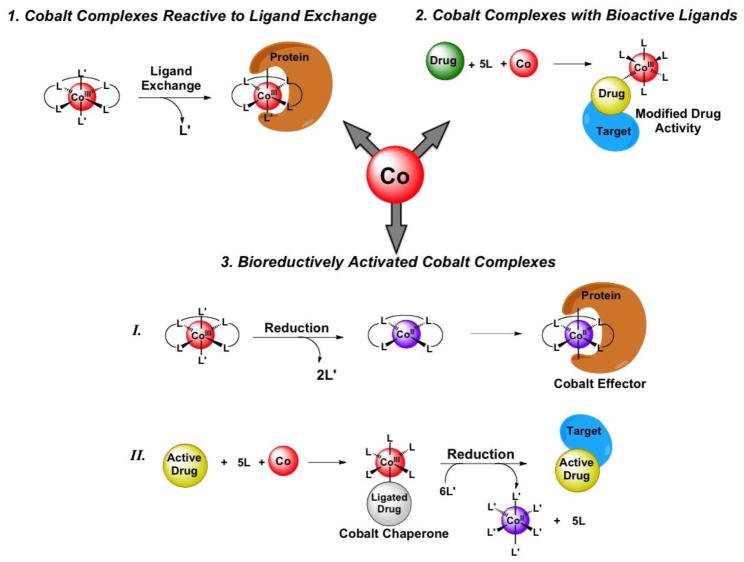
A substantial amount of literature on bioactive cobalt derivatives has been published in the last decade, demonstrating its rich potential in medicinal applications [6,7**]. However, the rationale behind the design and mechanisms of many of these agents has not been clearly elucidated. An understanding of how the unique properties of cobalt complexes interplay with biology to elicit therapeutic effects is clearly necessary for the development of cobalt-based drugs. To this end, we highlight recent articles that address the mechanisms of action of specific cobalt coordination complexes rather than surveying all the bioactive cobalt agents reported. Particular attention has been given to published examples wherein the effects of the physicochemical properties of cobalt are investigated to emphasize and understand the advantages of its use in therapeutic applications (Figure 1).
Figure 1.
Schematic representation of the different modes of action of bioactive cobalt complexes. Three classes of cobalt complexes are discussed: 1) complexes that directly act on biomolecules through ligand exchange, 2) complexes that modify the activity of ligated drugs and 3) complexes that are activated by bioreduction to either (I) yield a cobalt effector species or (II) release a small molecule drug.
Cobalt Complexes Reactive to Ligand Exchange
The promising antiviral activity of Doxovir is attributed to the direct interaction of the Co(III) Schiff base complex with proteins involved in viral penetration [5]. While the exact mechanism of action of Doxovir is unknown, studies have suggested that this class of Co(III) complexes, [Co(acacen)(L)2]+, interacts with proteins by coordinating histidine (His) residues through a dissociative exchange of the labile axial ligands, L (e.g. L = 2-methylimidazole [2MeIm] in Doxovir) (Figure 2) [8,9].
Figure 2.
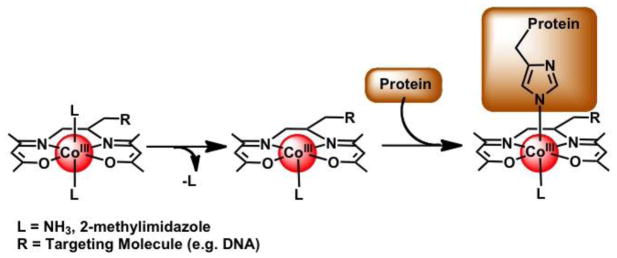
A schematic showing the Co(III) Schiff base complex, [Co(acacen)(L)2]+, that undergoes a dissociative exchange of the labile axial ligands and coordinates His residues [8,9]. Attachment of a targeting molecule, R, on the ancillary acacen chelate allows for selective inhibition of the protein of interest [8,11,12].
Our lab has been investigating the application and mechanism of [Co(acacen)(L)2]+ as selective inhibitors of His-containing proteins wherein the axial ligands are labile ammines (L = NH3) [8–10,11*,12]. Conjugation of targeting molecules such as peptides or oligonucleotides to the ancillary acacen chelate improves the specificity of the cobalt complexes for His residues in the proteins of interest. Such conjugates have been recently shown to inhibit Cys2His2 zinc finger transcription factors with remarkable specificity, namely the Snail and Gli family of transcription factors associated with embryonic development and cancer progression [10,11*,12]. Aberrant signaling by transcription factors has been implicated in the pathogenesis of diseases including cancer and inflammation and thus, such proteins are attractive drug targets [13,14]. In these examples, DNA sequences mimicking the native binding partners of the respective transcription factors were tethered to [Co(acacen)(NH3)2]+ for target selectivity. The conjugates were found to inhibit relevant pathways in in vivo embryo models and cancer cell lines [11*,12].
The mechanism of protein inhibition by [Co(acacen)(NH3)2]+ has been investigated spectroscopically by employing model peptides of the zinc finger proteins (MC Heffern et al., unpublished and [8]). Significant changes in NMR 1H resonances (>1 ppm) of His residues in the peptides were observed upon addition of [Co(acacen)(NH3)2]+. Furthermore, CD, NMR and electronic absorption studies correlated axial Co(III)-His coordination to structural perturbation of the zinc finger motif, likely due to displacement of zinc in the tetrahedral binding site by the octahedral Co(III) complex. These results demonstrated that His is preferentially coordinated to [Co(acacen)(NH3)2]+ and consequent perturbation of protein structure is likely to be responsible for the loss of activity.
In addition to zinc finger transcription factors, His-containing enzymes including thermolysin, α-thrombin and MMP-2 can be inhibited by [Co(acacen)(L)2]+ complexes [9,15,16]. Enzyme inhibition studies revealed that complexes with the most labile axial ligands (L = NH3 or 2MeIm) exhibited the highest degree of inhibition [9]. Interestingly, analogous complexes with His mimics (such as imidazole and N-methylimidazole) as the axial ligands showed significantly lower inhibition [9]. We have recently correlated these observations with ligand exchange dynamics through spectroscopic and computational methods (LM Manus et al., unpublished; LM Matosziuk et al., unpublished). The lability of NH3 and 2MeIm was attributed to a combination of pKa of the N-donor and the steric interactions between the axial ligand and the acacen ancillary ligand. The relationship between enzyme inhibition and axial ligand lability validates the proposed dissociative ligand exchange mechanism for His coordination to Co(III).
This current understanding of Co(III) Schiff base complexes demonstrates their ability to directly affect protein function through ligand exchange. The properties of the complexes can be adjusted depending on the oxidation state and ancillary ligands, affording a versatile scaffold to target biomolecules of therapeutic relevance [4**].
Cobalt Complexes with Bioactive Ligands
Bioactive small molecules that elicit therapeutic action in vivo, such as non-steroidal anti-inflammatory drugs (NSAIDs) [17–19], antibacterial agents [20,21], antiprotozoal agents [22,23], antifungal agents [24,25], and antihelmintic agents [26], have been attached to cobalt complexes to improve or alter their therapeutic efficacy. Although mechanisms for many of these agents are not understood, complexation to cobalt scaffolds is thought to influence the physicochemical properties of the bioactive ligand [2,6,27,28*]. For example, therapeutic agents have been tethered to the naturally occurring Co(III) complex, cobalamin (Vitamin B12) for drug delivery. This concept has been reviewed extensively elsewhere [28*,29]. In brief, peptides, proteins and other small molecules such as Rh-based CO-releasing molecules [30] and cisplatin analogues [31] have been tethered to cobalamin to improve the uptake, biocompatibility and stability of the agents.
The dinuclear hexacarbonyl cobalt cluster Co2(CO)6 is known to modify the bioactivity of small molecules that are coordinated to the cluster via an alkyne bond ([alkyne]-Co2(CO)6). A series of mechanistic studies have evaluated the effect of [alkyne]-Co2(CO)6 on the anticancer activity of the NSAID aspirin (Figure 3A). Aspirin displays modest pro-apoptotic activity through inhibition of cyclooxygenase (COX) enzymes [27,32*]. Coordination of aspirin to [alkyne]-Co2(CO)6 was shown to significantly enhance COX inhibition and the resulting antiproliferation levels were comparable to cisplatin. By systematically altering the alkyne bond, the bioactive ligand and the Co2(CO)6 cluster, Ott et al. and Rubner et al. demonstrated that while the NSAID may be involved in directing the complex to the COX enzymes, all these components were necessary for the remarkable potency [33,34].
Figure 3.
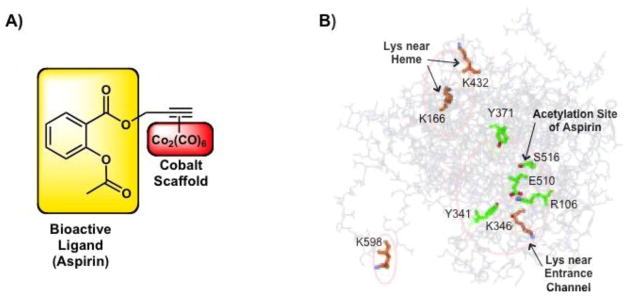
Cobalt complexes with bioactive ligands. A) Structure of the bioactive molecule aspirin complexed to Co2(CO)6 through an alkyne bond [32*,33]. B) Interaction of aspirin-[alkyne]-Co2(CO)6 with the human COX-2 enzyme with residues relevant to catalytic activity (green) and acetylation by cobalt complex (orange) is highlighted [32*]. Aspirin induces anti-inflammatory effects through acetylation of Ser-516 in the catalytic site of the COX enzyme. In contrast, conjugation of aspirin to the cobalt scaffold alters the pharmacology of the NSAID, as lysine residues are acetylated throughout the enzyme. In particular, acetylation of Lys166 and Lys432 near the heme prosthetic group and Lys346 near the entrance channel of the active site is suspected to result in potent COX inhibition and antiproliferative effects. Figure adapted from ref [32*] with permission.
Enhancement of activity of [alkyne]-Co2(CO)6 was initially attributed to improved cell uptake due to increased lipophilicity. However, comparison of the aspirin-[alkyne] ligand with more hydrophobic derivatives showed no conclusive correlation between lipophilicity and efficacy, implicating that there were additional factors that affected bioactivity. Ott et al. identified a potential difference in the mode of action between aspirin and aspirin-[alkyne]-Co2(CO)6 using Trypsin-digest MS studies of the purified COX enzyme (Figure 3B) [32*]. Both aspirin and its alkyne derivative acetylated serines in the active site, while the cobalt complex acetylated lysines throughout the enzyme. Although the exact mechanism by which the cobalt cluster modifies the NSAID mode of action remains unclear, this example highlights the importance of a systematic approach to understanding the activity of cobalt agents in biological systems.
Bioreductive Cobalt Complexes
Co(III) complexes can be used as prodrugs that are capable of undergoing bioreduction, a process whereby intracellular reduction produces a bioactive agent [7**,35**,36**]. Reduction of inert d6 Co(III) complexes yields labile d7 high-spin Co(II) complexes which rapidly undergo ligand substitution [2]. The reduction potentials of Co(III) complexes can be tuned to the eukaryotic cytosolic reduction potential (−0.2 V to −0.4 V) [37] by ligand modification. The significant difference in lability of the accessible oxidation states and the large range of the reduction potential makes cobalt an ideal candidate for use in redox-activated prodrugs [7**]. An appropriately designed complex can undergo bioreductive activation to yield a cobalt species that acts as an effector or release an effector ligand that was deactivated by coordination to the cobalt. In this context, an effector is defined as a molecule that can alter biological activity [38,39].
Cobalt Complexes as Redox-Activated Effectors
Redox activation of Co(III) to generate complexes that act as effectors of biological activity was demonstrated by Tomco et al. who studied Co(LNN′O)x complexes (LNN′O = 2,4-diiodo-6-((pyridine-2-ylmethylamino)methyl)phenol) [40,41*]. These complexes are thought to inhibit proteasomes through ligand exchange with amino acid residues in the active site. Co(III)(LNN′O)2 was found to be far more inert to substitution than Co(II)(LNN′O)2. However, the Co(III) complex exhibited higher inhibition of chymotrypsin-like activity in purified proteasomes as well as improved apoptotic induction in PC-3 cancer cells [40].
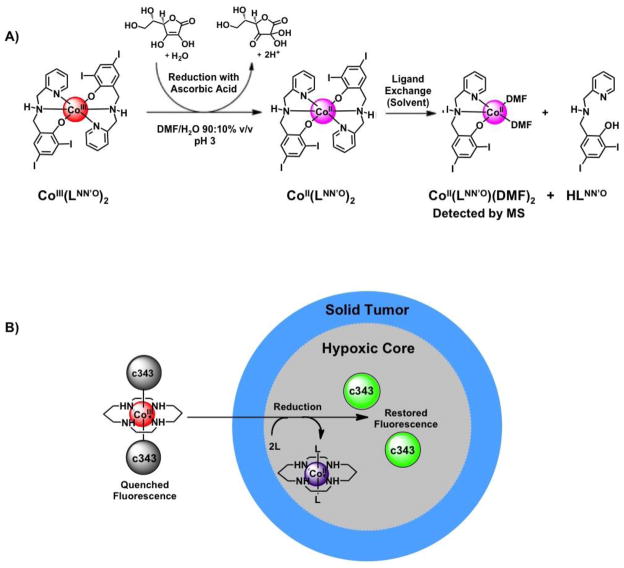
The difference in activity between the Co(III) and Co(II) complexes was attributed to complex stability. The authors postulated that the higher lability of the Co(II) complex results in rapid degradation in biological environments whereas the stability of the inert Co(III) complex offers improved bioavailability. Consequently, the substitutional inertness facilitates intracellular activation of the Co(III) complex following reduction [41*]. The feasibility of this concept was tested by examining the LMCT absorption band of the Co(III) complex. In the presence of a cellular reductant, a decrease in the LMCT band was observed, suggesting a change in oxidation state and ligand binding. The formation of a labile cobalt species upon reduction followed by solvent exchange was observed by mass spectroscopy (Figure 4A), and the experimental results were corroborated with DFT calculations. These findings show that bioreductive activation is a viable mechanism for ligand release and pharmacophore formation in Co(III) prodrugs. The ability to modulate the stability of the agent and to generate an active species with this strategy shows potential for adding a level of control to the efficacy of cobalt therapeutics.
Figure 4.
Cobalt complexes that undergo bioreductive activation. A) Co(III)(LNN′O)2 undergoes reduction to the more labile Co(II) counterpart to yield an active proteasome inhibitor [40,41*]. In the presence of the reducing agent ascorbic acid, one of the LNN′O ligands of Co(III)(LNN′O)2 is lost and replaced by a solvent (DMF) molecule, as identified by mass spectrometry. The activated complex is thought to bind to the active site of the proteasome to elicit inhibition. Scheme adapted from ref [31] with permission. B) The fluorescence of coumarin-343 (c343) is quenched upon coordination to Co(III)/cyclam. When the complex encounters the reducing environment of a hypoxic spheroid core, the complex undergoes reduction, releasing the fluorophores and restoring fluorescence. The process is monitored by fluorescence confocal microscopy [51*].
Cobalt Complexes as Redox-Responsive Drug Carriers
The principle of bioreductive activation can be applied to the selective release of bioactive ligands from Co(III) complexes. Coordination to Co(III) can deactivate antitumor cytotoxins such as nitrogen mustards [42–45], DNA intercalators [38,46,47], and the MMP-inhibitor marimastat [48]. These agents take advantage of the hypoxic nature of the core of solid tumors. Bioreductive conversion of these complexes in hypoxic environments leads to selective release of the cytotoxins for antitumor activity [7**,35**,36**,49].
Fluorophores have been employed as visualization tools to probe the site and timescale of activation of redox-responsive Co(III) complexes in biological systems. The fluorescence of fluorophores such as coumarin-343 is quenched upon coordination to Co(III) but returns upon ligand release following reduction [50]. Kim et al. developed a 3-dimensional system of fluorescently labeled hypoxic cells within an avascular solid tumor model to determine if bioreductively activated Co(III) complexes effectively target the hypoxic regions of the model (Figure 4B) [51*]. Fluorescence return from a Co(III)/cyclam complex of coumarin-343 (c343) was observed in the same region as the fluorescently labeled hypoxic cells located within the core of the tumor models. This suggests that the release of the fluorophore payload is occurring at the correct site, validating the use of bioreductive Co(III) complexes for delivering anticancer agents to the target site [50,51*].
Future Prospects and Conclusions
The cobalt complexes highlighted in this review illustrate the diverse mechanisms by which cobalt complexes can act as potential pharmaceutical agents. Evaluations of the physicochemical properties of different cobalt materials in biological systems reveal intricate functions beyond indiscriminate binding of bio-macromolecules. The examples discussed here include selective protein inhibition, bioactivity enhancement, and bioreductive prodrug activation. However, the versatility of cobalt has been further exemplified in other materials that have not been discussed here, such as the cobalt-based metallacarborane HIV protease inhibitor [52,53] and cobalt nanoparticles [54,55] for magnetic hyperthermia applications. In addition to these strategies, the flexible cobalt platform can be enhanced with further levels of control. For example, the low-energy d-d transitions and MLCT bands of cobalt complexes allows for the incorporation of radiation-[38,46,47] or photo-responsive elements [56–60]. Complexes that respond to such exogenous energy sources will permit spatial and temporal control of therapeutic activity.
The diverse potential of cobalt materials in disease treatment can be further realized by the rational employment of its properties. The overall stability, compatibility and activity of cobalt agents are directly influenced by the reduction potential, overall charge, coordination environment, solubility and hydro- or lipophilicity. These factors can be rationally tuned to effectively target the pathway or biomolecule of interest. Mechanistic insight into the diverse bioactivities of cobalt materials may inform the design of such well-controlled and selective cobalt-based drugs. Understanding the mode of action can thus guide the progression of promising cobalt agents to the clinic.
Highlights.
Cobalt is a versatile transition metal for drug development.
The tunable physicochemical properties can be exploited by understanding the mechanism of bioactive cobalt complexes.
The review highlights examples of mechanistic investigations of cobalt therapeutics.
Acknowledgments
The authors are grateful to Daniel J. Feld and Lauren M. Matosziuk for helpful discussions. M. C. H. would like to acknowledge the National Science Foundation Graduate Research Fellowship. The authors gratefully acknowledge funding from the Center for Cancer Nanotechnology Excellence (CCNE) initiative of the National Institutes of Health’s National Cancer Institute under Award U54CA151880.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Bruijnincx PC, Sadler PJ. New trends for metal complexes with anticancer activity. Curr Opin Chem Biol. 2008;12:197–206. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hambley TW. Developing new metal-based therapeutics: challenges and opportunities. Dalton Trans. 2007:4929–4937. doi: 10.1039/b706075k. [DOI] [PubMed] [Google Scholar]
- 3.Haas KL, Franz KJ. Application of metal coordination chemistry to explore and manipulate cell biology. Chem Rev. 2009;109:4921–4960. doi: 10.1021/cr900134a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Meggers E. Targeting proteins with metal complexes. Chem Commun. 2009:1001–1010. doi: 10.1039/b813568a. This review highlights recent advances on the use of transition metal complexes to alter protein activity. Focus is given to the unique aspects of transition metal complexes that allow for targeted protein inhibition. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz JA, Lium EK, Silverstein SJ. Herpes simplex virus type 1 entry is inhibited by the cobalt chelate complex CTC-96. J Virol. 2001;75:4117–4128. doi: 10.1128/JVI.75.9.4117-4128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang EL, Simmers C, Knight DA. Cobalt Complexes as Antiviral and Antibacterial Agents. Pharmaceuticals. 2010;3:1711–1728. doi: 10.3390/ph3061711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Hall MD, Failes TW, Yamamoto N, Hambley TW. Bioreductive activation and drug chaperoning in cobalt pharmaceuticals. Dalton Trans. 2007:3983–3990. doi: 10.1039/b707121c. This is a detailed review that discusses the history and development of cobalt complexes used as bioreductively activated prodrugs. [DOI] [PubMed] [Google Scholar]
- 8.Louie AY, Meade TJ. A cobalt complex that selectively disrupts the structure and function of zinc fingers. Proc Natl Acad Sci U S A. 1998;95:6663–6668. doi: 10.1073/pnas.95.12.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi T, Bottcher A, Quezada CM, Meade TJ, Gray HB. Inhibition of thermolysin and human alpha-thrombin by cobalt(III) Schiff base complexes. Bioorg Med Chem. 1999;7:815–819. doi: 10.1016/s0968-0896(98)00272-7. [DOI] [PubMed] [Google Scholar]
- 10.Harney AS, Lee J, Manus LM, Wang PJ, Ballweg DM, LaBonne C, Meade TJ. Targeted inhibition of Snail family zinc finger transcription factors by oligonucleotide-Co(III) Schiff base conjugate. Proc Natl Acad Sci U S A. 2009;106:13667–13672. doi: 10.1073/pnas.0906423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Harney AS, Meade TJ, LaBonne C. Targeted inactivation of Snail family EMT regulatory factors by a Co(III)-Ebox conjugate. PLoS One. 2012;7:e32318. doi: 10.1371/journal.pone.0032318. This paper demonstrates the capacity of Co(III) Schiff base-oligonucleotide conjugates to selectively inhibit transcription factors in embryo models and cancer cell lines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurtado RR, Harney AS, Heffern MC, Holbrook RJ, Holmgren RA, Meade TJ. Specific inhibition of the transcription factor Ci by a cobalt(III) Schiff base-DNA conjugate. Mol Pharm. 2012;9:325–333. doi: 10.1021/mp2005577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung C-H, Chan DS, Ma VP, Ma D-L. DNA-Binding Small Molecules as Inhibitors of Transcription Factors. Med Res Rev. 2012 doi: 10.1002/med.21266. ASAP. [DOI] [PubMed] [Google Scholar]
- 14.Berg T. Inhibition of transcription factors with small organic molecules. Curr Opin Chem Biol. 2008;12:464–471. doi: 10.1016/j.cbpa.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Harney AS, Sole LB, Meade TJ. Kinetics and thermodynamics of irreversible inhibition of matrix metalloproteinase 2 by a Co(III) Schiff base complex. J Biol Inorg Chem. 2012;17:853–860. doi: 10.1007/s00775-012-0902-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi T, Bottcher A, Quezada CM, Simon MI, Meade TJ, Gray HB. Selective inhibition of human alpha-thrombin by cobalt(III) Schiff base complexes. J Am Chem Soc. 1998;120:8555–8556. doi: 10.1016/s0968-0896(98)00272-7. [DOI] [PubMed] [Google Scholar]
- 17.Dimiza F, Papadopoulos AN, Tangoulis V, Psycharis V, Raptopoulou CP, Kessissoglou DP, Psomas G. Biological evaluation of non-steroidal anti-inflammatory drugs-cobalt(ii) complexes. Dalton Trans. 2010;39:4517–4528. doi: 10.1039/b927472c. [DOI] [PubMed] [Google Scholar]
- 18.Dimiza F, Papadopoulos AN, Tangoulis V, Psycharis V, Raptopoulou CP, Kessissoglou DP, Psomas G. Biological evaluation of cobalt(II) complexes with non-steroidal anti-inflammatory drug naproxen. J Inorg Biochem. 2012;107:54–64. doi: 10.1016/j.jinorgbio.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Tsiliou S, Kefala L-A, Perdih F, Turel I, Kessissoglou DP, Psomas G. Cobalt(II) complexes with non-steroidal anti-inflammatory drug tolfenamic acid: Structure and biological evaluation. Eur J Med Chem. 2012;48:132–142. doi: 10.1016/j.ejmech.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Adewuyi S, Kareem KT, Atayese AO, Amolegbe SA, Akinremi CA. Chitosan-cobalt(II) and nickel(II) chelates as antibacterial agents. Int J Biol Macromol. 2011;48:301–303. doi: 10.1016/j.ijbiomac.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Patel M, Chhasatia M, Bhatt B. In vitro bacteriostatic and DNA interaction studies of drug-based mixed-ligand complexes of cobalt(II) Med Chem Res. 2011;20:220–230. [Google Scholar]
- 22.Adediji JF, Olayinka ET, Adebayo MA, Babatunde O. Antimalarial mixed ligand metal complexes: synthesis, physicochemical and biological activities. Int J Phys Sci. 2009;4:529–534. [Google Scholar]
- 23.Ajibade PA, Kolawole GA. Cobalt(III) Complexes of Some Antimalarial Drugs: Synthesis, Characterization, and in vitro Antiprotozoal Studies. Synth React Inorg Me. 2010;40:273–278. [Google Scholar]
- 24.Sathyadevi P, Krishnamoorthy P, Alagesan M, Thanigaimani K, Thomas Muthiah P, Dharmaraj N. Synthesis, crystal structure, electrochemistry and studies on protein binding, antioxidant and biocidal activities of Ni(II) and Co(II) hydrazone complexes. Polyhedron. 2012;31:294–306. [Google Scholar]
- 25.Shreaz S, Sheikh RA, Bhatia R, Neelofar K, Imran S, Hashmi AA, Manzoor N, Basir SF, Khan LA. Antifungal activity of α-methyl trans cinnamaldehyde, its ligand and metal complexes: Promising growth and ergosterol inhibitors. BioMetals. 2011;24:923–933. doi: 10.1007/s10534-011-9447-0. [DOI] [PubMed] [Google Scholar]
- 26.Khatiwora E, Mundhe K, Deshpande NR, Kashalkar RV. Anthelmintic activity of transition metal complexes of some benzamides. Der Pharma Chemica. 2012;4:1264–1269. [Google Scholar]
- 27.Ott I, Schmidt K, Kircher B, Schumacher P, Wiglenda T, Gust R. Antitumor-active cobalt-alkyne complexes derived from acetylsalicylic acid: studies on the mode of drug action. J Med Chem. 2005;48:622–629. doi: 10.1021/jm049326z. [DOI] [PubMed] [Google Scholar]
- 28*.Randaccio L, Geremia S, Demitri N, Wuerges J. Vitamin B12: unique metalorganic compounds and the most complex vitamins. Molecules. 2010;15:3228–3259. doi: 10.3390/molecules15053228. This review outlines the biological properties of Vitamin B12 as well as some examples of its bioconjugates that have been used to improve efficacy of tethered agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrus A, Fairchild TJ, Doyle RP. Traveling the vitamin B12 pathway: oral delivery of protein and peptide drugs. Angew Chem Int Ed Engl. 2009;48:1022–1028. doi: 10.1002/anie.200800865. [DOI] [PubMed] [Google Scholar]
- 30.Zobi F, Blacque O, Jacobs RA, Schaub MC, Bogdanova AY. 17 e- rhenium dicarbonyl CO-releasing molecules on a cobalamin scaffold for biological application. Dalton Trans. 2012;41:370–378. doi: 10.1039/c1dt10649j. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Sanchez P, Koenig C, Ferrari S, Alberto R. Vitamin B12 as a carrier for targeted platinum delivery: in vitro cytotoxicity and mechanistic studies. J Biol Inorg Chem. 2011;16:33–44. doi: 10.1007/s00775-010-0697-z. [DOI] [PubMed] [Google Scholar]
- 32*.Ott I, Kircher B, Bagowski CP, Vlecken DH, Ott EB, Will J, Bensdorf K, Sheldrick WS, Gust R. Modulation of the biological properties of aspirin by formation of a bioorganometallic derivative. Angew Chem Int Ed Engl. 2009;48:1160–1163. doi: 10.1002/anie.200803347. This paper describes mechanistic investigations into the possible effects of [alkyne]-Co2(CO)6 on aspirin activity. Along with ref [33] and [34], this reference exemplifies comprehensive structure-activity relationship studies of cobalt-containing therapeutic agents. [DOI] [PubMed] [Google Scholar]
- 33.Ott I, Koch T, Shorafa H, Bai Z, Poeckel D, Steinhilber D, Gust R. Synthesis, cytotoxicity, cellular uptake and influence on eicosanoid metabolism of cobalt-alkyne modified fructoses in comparison to auranofin and the cytotoxic COX inhibitor Co-ASS. Org Biomol Chem. 2005;3:2282–2286. doi: 10.1039/b504294c. [DOI] [PubMed] [Google Scholar]
- 34.Rubner G, Bensdorf K, Wellner A, Kircher B, Bergemann S, Ott I, Gust R. Synthesis and biological activities of transition metal complexes based on acetylsalicylic acid as neo-anticancer agents. J Med Chem. 2010;53:6889–6898. doi: 10.1021/jm101019j. [DOI] [PubMed] [Google Scholar]
- 35**.Graf N, Lippard SJ. Redox activation of metal-based prodrugs as a strategy for drug delivery. Adv Drug Deliv Rev. 2012;64:993–1004. doi: 10.1016/j.addr.2012.01.007. This recent review provides a comprehensive overview of bioreductive transition metal complexes, including cobalt complexes, and applications in prodrug development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Jungwirth U, Kowol CR, Keppler BK, Hartinger CG, Berger W, Heffeter P. Anticancer activity of metal complexes: involvement of redox processes. Antioxid Redox Signal. 2011;15:1085–1127. doi: 10.1089/ars.2010.3663. This review surveys the use of bioreductive transition metal complexes for anticancer applications with particular focus given to their interactions with the cellular redox environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 38.Milbank JBJ, Stevenson RJ, Ware DC, Chang JYC, Tercel M, Ahn GO, Wilson WR, Denny WA. Synthesis and Evaluation of Stable Bidentate Transition Metal Complexes of 1-(Chloromethyl)-5-hydroxy-3-(5,6,7-trimethoxyindol-2-ylcarbonyl)-2,3-dihydro-1H-pyrrolo[3,2-f]quinoline (seco-6-azaCBI-TMI) as Hypoxia Selective Cytotoxins. J Med Chem. 2009;52:6822–6834. doi: 10.1021/jm9008746. [DOI] [PubMed] [Google Scholar]
- 39.Wilson WR, Hay MP. Targeting Hypoxia in Cancer Therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 40.Tomco D, Schmitt S, Ksebati B, Heeg MJ, Dou QP, Verani CN. Effects of tethered ligands and of metal oxidation state on the interactions of cobalt complexes with the 26S proteasome. J Inorg Biochem. 2011;105:1759–1766. doi: 10.1016/j.jinorgbio.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Tomco D, Xavier FR, Allard MM, Verani CuN. Probing chemical reduction in a cobalt(III) complex as a viable route for the inhibition of the 20S proteasome. Inorg Chim Acta. 2012;393:269–275. Following on from reference [40], this paper probes the molecular mechanism by which a Co(LNN′O)x complex undergoes bioreductive activation in the presence of a cellular reductant. [Google Scholar]
- 42.Ware DC, Brothers PJ, Clark GR, Denny WA, Palmer BD, Wilson WR. Synthesis, structures and hypoxia-selective cytotoxicity of cobalt(III) complexes containing tridentate amine and nitrogen mustard ligands. J Chem Soc Dalton Trans. 2000:925–932. [Google Scholar]
- 43.Ware DC, Denny WA, Clark GR. [N,N-Bis(2-chloroethyl)-1,2-ethanediamine-N,N′]bis(3-methyl-2,4-pentanedionato-O,O′)cobalt(III) perchlorate: a potential hypoxia selective anticancer agent. Acta Crystallogr Sec C. 1997;C53:1058–1059. [Google Scholar]
- 44.Ware DC, Palmer HR, Brothers PJ, Rickard CEF, Wilson WR, Denny WA. Bis-tropolonato derivatives of cobalt(III) complexes of bidentate aliphatic nitrogen mustards as potential hypoxia-selective cytotoxins. J Inorg Biochem. 1997;68:215–224. doi: 10.1016/s0162-0134(97)00090-1. [DOI] [PubMed] [Google Scholar]
- 45.Ware DC, Wilson WR, Denny WA, Richard CEF. Design and synthesis of cobalt(III) nitrogen mustard complexes as hypoxia selective cytotoxins. The x-ray crystal structure of bis(3-chloropentane-2,4-dionato)(RS-N,N′-bis(2-chloroethyl)ethylenediamine)cobalt(III) perchlorate, [Co(Clacac)2(bce)]ClO4. J Chem Soc Chem Comm. 1991:1171–1173. [Google Scholar]
- 46.Ahn GO, Botting KJ, VPA, CWD, Tercel MRWW. Radiolytic and cellular reduction of a novel hypoxia-activated cobalt(III) prodrug of a chloromethylbenzindoline DNA minor groove alkylator. Biochem Pharmacol. 2006;71:1683–1694. doi: 10.1016/j.bcp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Ahn GO, Ware DC, Denny WA, Wilson WR. Optimization of the auxiliary ligand shell of cobalt(III)(8-hydroxyquinoline) complexes as model hypoxia-selective radiation-activated prodrugs. Radiat Res. 2004;162:315–325. doi: 10.1667/rr3229. [DOI] [PubMed] [Google Scholar]
- 48.Failes TW, Cullinane C, Diakos CI, Yamamoto N, Lyons GJ, Hambley TW. Studies of a Co(III) complex of the MMP inhibitor marimastat: a potential hypoxia activated prodrug. Chem-Eur J. 2007;13:2974–2982. doi: 10.1002/chem.200601137. [DOI] [PubMed] [Google Scholar]
- 49.Wilson WR, Moselen JW, Cliffe S, Denny WA, Ware DC. Exploiting tumor hypoxia through bioreductive release of diffusible cytotoxins: the cobalt(III)-nitrogen mustard complex SN 24771. Int J Radiat Oncol. 1994;29:323–327. doi: 10.1016/0360-3016(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto N, Danos S, Bonnitcha PD, Failes TW, New EJ, Hambley TW. Cellular uptake and distribution of cobalt complexes of fluorescent ligands. J Biol Inorg Chem. 2008;13:861–871. doi: 10.1007/s00775-008-0374-7. [DOI] [PubMed] [Google Scholar]
- 51*.Kim BJ, Hambley TW, Bryce NS. Visualising the hypoxia selectivity of cobalt(III) prodrugs. Chem Sci. 2011;2:2135–2142. This paper investigates the hypoxia-targeting ability of bioreductively activated Co(III)/cyclam prodrugs in a 3-dimensional solid tumor model using fluorescence. [Google Scholar]
- 52.Kozisek M, Cigler P, Lepsik M, Fanfrlik J, Rezacova P, Brynda J, Pokorna J, Plesek J, Gruner B, Grantz Saskova K, et al. Inorganic polyhedral metallacarborane inhibitors of HIV protease: a new approach to overcoming antiviral resistance. J Med Chem. 2008;51:4839–4843. doi: 10.1021/jm8002334. [DOI] [PubMed] [Google Scholar]
- 53.Řezáčová, Pokorná P, Brynda J, Kožíšek J, Cígler M, Lepsík P, Fanfrlík M, Rezác J, Šasková J, SieglovaáI KG, et al. Design of HIV Protease Inhibitors Based on Inorganic Polyhedral Metallacarboranes. J Med Chem. 2009;52:7132–7141. doi: 10.1021/jm9011388. [DOI] [PubMed] [Google Scholar]
- 54.Seo WS, Lee JH, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat Mater. 2006;5:971–976. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 55.Fortin J-P, Wilhelm C, Servais J, Menager C, Bacri J-C, Gazeau F. Size-Sorted Anionic Iron Oxide Nanomagnets as Colloidal Mediators for Magnetic Hyperthermia. J Am Chem Soc. 2007;129:2628–2635. doi: 10.1021/ja067457e. [DOI] [PubMed] [Google Scholar]
- 56.Saha S, Majumdar R, Dighe RR, Chakravarty AR. Enhanced photodynamic effect of cobalt(III) dipyridophenazine complex on thyrotropin receptor expressing HEK293 cells. Metallomics. 2010;2:754–765. doi: 10.1039/c0mt00028k. [DOI] [PubMed] [Google Scholar]
- 57.Sun Y, Hou YJ, Zhou QX, Chen JR, Zhang BW, Wang XS. A new Co(III)-hypocrellin B complex with enhanced photonuclease activity. J Inorg Biochem. 2011;105:978–984. doi: 10.1016/j.jinorgbio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Roy S, Saha S, Majumdar R, Dighe RR, Jemmis ED, Chakravarty AR. Cobalt(II) complexes of terpyridine bases as photochemotherapeutic agents showing cellular uptake and photocytotoxicity in visible light. Dalton Trans. 2011;40:1233–1242. doi: 10.1039/c0dt00223b. [DOI] [PubMed] [Google Scholar]
- 59.Kawade VA, Kumbhar AA, Kumbhar AS, Nather C, Erxleben A, Sonawane UB, Joshi RR. Mixed ligand cobalt(II) picolinate complexes: synthesis, characterization, DNA binding and photocleavage. Dalton Trans. 2011;40:639–650. doi: 10.1039/c0dt01078b. [DOI] [PubMed] [Google Scholar]
- 60.Nagababu P, Shilpa M, Latha JN, Bhatnagar I, Srinivas PN, Kumar YP, Reddy KL, Satyanarayana S. Synthesis, characterization, DNA binding properties, fluorescence studies and toxic activity of cobalt(III) and ruthenium(II) polypyridyl complexes. J Fluoresc. 2011;21:563–572. doi: 10.1007/s10895-010-0743-9. [DOI] [PubMed] [Google Scholar]