Abstract
Paraoxonase 2 (PON2), a member of a gene family that also includes PON1 and PON3, is expressed in most tissues, including the brain. In mouse brain, PON2 levels are highest in dopaminergic areas (e.g. striatum), and are higher in astrocytes than in neurons. PON2 is primarily located in mitochondria and exerts a potent antioxidant effect, protecting mouse CNS cells against oxidative stress. The aim of this study was to characterize PON2 expression and functions in the brains of male and female mice. Levels of PON2 (protein, mRNA, and lactonase activity) were higher in brain regions and cells of female mice. Astrocytes and neurons from male mice were significantly more sensitive (by 3–4-fold) to oxidative stress-induced toxicity than the same cells from female mice. Glutathione levels did not differ between genders. Importantly, no significant gender differences in susceptibility to the same oxidants were seen in cells from PON2−/− mice. Treatment with estradiol induced a time- and concentration-dependent increase in the levels of PON2 protein and mRNA in male (4.5-fold) and female (1.8-fold) astrocytes, which was dependent on activation of estrogen receptor alpha. In ovariectomized mice, PON2 protein and mRNA were decreased to male levels in brain regions and in liver. Estradiol protected astrocytes from wild-type mice against oxidative stress-induced neurotoxicity, but did not protect cells from PON2−/− mice. These results suggest that PON2 is a novel major intracellular factor that protects CNS cells against oxidative stress, and confers gender-dependent susceptibility to such stress. The lower expression of PON2 in males may have broad ramifications for susceptibility to diseases involving oxidative stress, including neurodegenerative diseases.
Keywords: Paraoxonase 2, oxidative stress, gender difference, estrogen, neuroprotection
Introduction
Paraoxonase 2 (PON2) is a member of a multigene family of enzymes that also includes PON1 and PON3; the three genes share a high degree of identity, and are located adjacent to each other on chromosome 7q21-22 in humans, and on chromosome 6 in mouse [1]. The name of these enzymes derives from the active metabolite of the organophosphorus insecticide parathion, paraoxon, which is a substrate of PON1, and has been applied to the other two PONs, despite their lack of esterase activity. All three PONs are lactonases, and can hydrolyze a number of acyl-homoserine lactones (acyl-HCL), molecules that mediate bacterial quorum-sensing signals and which are important in regulating expression of virulence factors and inducing a host inflammatory response, with PON2 displaying the highest activity [2–5]. Two common polymorphisms have been found in human PON2, Ala147Gly and Ser311Cys [1, 6]; the latter affects lactonase activity, with Cys311 displaying lower activity [7].
While PON1 and PON3 are expressed primarily in liver and are associated with high-density lipoproteins in plasma [8], PON2 is a ubiquitously expressed intracellular enzyme, not present in plasma [6, 9, 10]. PON2 mRNA and/or protein have been detected in several tissues including liver, lung, kidney, heart, pancreas, small intestine, muscle, testis, endothelial cells, tracheal epithelial cells, and macrophages [3, 6, 9–15]. Prompted by limited information suggesting that PON2 is also expressed in the nervous system [1, 6, 9], we have recently characterized its presence and functions in the mouse CNS [15].
PON2 protein, mRNA and lactonase activity were found in all mouse brain regions, with the highest levels in three dopaminergic regions (striatum, substantia nigra, nucleus accumbens). PON1 was detected at very low levels (30–40-fold less than PON2) and did not show any regional differences, while PON3 was not detected in brain [15]. In all regions, PON2 levels were higher in astrocytes than in neurons, and levels in cortical microglia were similar to those of neurons [15]. In both astrocytes and neurons, PON2 levels were highest in mitochondria, a finding that is corroborated by results in other tissues [16]. Mitochondria are a major source of free radical-related oxidative stress [17]; PON2 has been shown to bind to Coenzyme Q10 that associates with Complex III in mitochondria [16], thus reducing the release of superoxide from the inner mitochondrial membrane [18]. It is not surprising, therefore, that in several tissues and cell types, PON2 exerts an antioxidant effect [5, 9, 11–13, 19]. In the CNS, the ability of two oxidants [hydrogen peroxide (H2O2) and 2,3-dimethoxy-1,4-naphthoquinone (DMNQ)] to induce the formation of reactive oxygen species and to decrease viability of astrocytes and neurons, was significantly enhanced in cells from PON2 knockout (PON2−/−) mice [15]. As glutathione (GSH) levels do not differ between wild-type and PON2−/− mice, the observed 5 to 11-fold difference in susceptibility is likely due to the presence or absence of PON2 [15].
In the course of these studies [15], we discovered that in all peripheral tissues (lung, heart, small intestine, liver, kidney), PON2 expression (indicated by protein and mRNA levels, and by lactonase activity) was significantly higher in female than in male mice. Similarly, in the CNS, a 2 to 3-fold difference between genders was seen in all brain regions, with the nucleus accumbens in female mice displaying the highest level of PON2 [15]. Given the antioxidant functions of PON2, these findings raised the possibility that the male’s CNS might be intrinsically more susceptible to oxidative stress. Studies presented in this paper show that this is indeed the case; they also show that estrogens modulate PON2 expression, and that PON2 confers increased resistance of female CNS cells to oxidative stress-mediate toxicity.
Materials and Methods
Materials
Neurobasal-A medium, DMEM medium, fetal bovine serum (FBS), Hank’s balanced salt solution (HBSS), GlutaMAX, gentamycin, and SuperScript® III First-Strand Synthesis System were from Invitrogen (Carlsbad, CA). TaqMan Gene Expression Master Mix was from Applied Biosystems Inc. (Foster City, CA). Anti PON2 antibodies were from Abcam (Cambridge, MA, USA). The kit for measurement of serum estradiol levels was from Cayman (Ann Arbor, MI). The estrogen receptor antagonists PHTPP (4-{2-phenyl-5,7-bis (trifluoromethyl) pyrazolo [1,5-α]pyrimidin-3-yl)} phenol), ICI 182,780 (7α, 17β-[9-(4,4,5,5,5-pentafluoropentyl) sulfinyl] nonyl estra-1,3,5(10)-triene-3,17-diol), and MPP dihydrochloride (1,3-bis (4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride) were purchased from Tocris Bioscience (Ellisville, MO). Dimethylsulfoxide (DMSO), hydrogen peroxide (H2O2), 2,3-dimethoxy-1,4-naphthoquinone (DMNQ), mouse anti-β-actin antibody, reduced glutathione, 5-sulfosalicylic acid, naphthalene dicarboxaldehyde, dihydrocoumarin (3,4-dihydro-2H,1-benzopyran-2-one), actinomycin-D, 17β-estradiol, and 3-(4,5-dimethyltiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) were from Sigma-Aldrich (St. Louis, MO).
Animals
Male and female C57BL/6J mice (wild-type mice) and PON2 knockout (PON2−/−) mice (kindly provided by Drs. A.J. Lusis, D.M. Shih and S. Reddy at UCLA) were used in this study. Animals were 2–3 months old at the time of the study. PON2−/− mice were generated using the embryonic stem cell line XE661 (strain 129/Ola) and a gene-trap vector, as described in detail elsewhere [20]. After germ line transmission, backcrossing was performed with C57BL/6J mice for six generations. Genotyping of mice was done as described by Ng et al. [20]. Mice were housed in a specific pathogen-free facility with ad libitum access to food and water and a 12-h light/dark cycle. In some experiments, time-pregnant Sprague-Dawley rats were purchased from Charles River (Hollister, CA), and newborn pups were used for preparation of cortical astrocytes. All procedures for animal use were in accordance with the National Institute of Health Guide for the Use and Care of Laboratory Animals, and were approved by the University of Washington Institutional Animal Care and Use Committee.
Ovariectomy
Ovariectomized and sham-operated female mice were purchased from Charles River (Wilmington, MA). One month-old mice were anesthesized with ether and an incision was made in the lower abdomen. Ovaries were removed and the abdomen was sutured; sham-operated mice underwent an identical procedure without removal of the ovaries. Animals were sacrificed at 3 months of age; at sacrifice, lack of ovaries was confirmed by visual inspection. In addition to different tissues, blood was also collected for measurement of serum estradiol levels with a commercial kit.
Primary cell cultures
Primary astrocytes were obtained from PND 0.5 male and female mice, as previously described [15]. Gender was determined by inspecting the genital area and measuring the ano-genital distance. After tissue removal, gender identification was confirmed by inspecting internal organs for identification of the uterine horns or the testes. The striatum was dissected, mechanically dissociated and incubated with trypsin, followed by trituration, repeated washing, and filtering. After counting, cells were plated at a density of 107 cells per flask in 75 cm2 tissue culture flasks pre-coated with poly-D-lysine and grown in DMEM containing 10% (v/v) FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C in 5% CO2/95% (v/v) air. After 10 days in culture, cells were plated in 24-well plates for the experiments at a density of 5×104 astrocytes/well. Striatal neurons were also prepared from PND 0.5 mice, as described by Giordano et al. [15, 21]. Briefly, the striatum was collected in HBSS medium containing 0.02% (w/v) bovine serum albumin (BSA) and 10 mM HEPES. The tissue was digested for 25 min in HBSS containing papain (1 mg/ml) and DNAse 40 ug/ml) and centrifuged at 300 × g (max g-force is always indicated) for 5 min at room temperature. The supernatant (containing papain) was removed and the pellet was gently triturated in Neurobasal A Medium supplemented with B27, using a Pasteur pipette to dissociate larger aggregates. Cells were centrifuged at 200 × g at 4°C for 10 min and the cell pellet was gently resuspended. Neurons were then counted, seeded on poly-D-lysine coated 48-well plates at a density of 5 × 104/cm2, and cultured in neurobasal medium supplemented with B27 (minus AO). Neurons were cultured for 8 days before experiments.
Rat astrocytes were obtained from 0.5 day-old pups from either gender, with a protocol essentially identical to that used for mice. For preparation of fetal human astrocytes, tissue was provided by the Birth Defects Research Laboratory of the University of Washington, and the experiments were carried out in accordance with the University of Washington Institutional Review Board. Mixed cultures were prepared from brains of legally aborted human fetuses (12–15 weeks gestation), as described by Satoh and Kim [22], with some modifications. In brief, brains were dissected into small blocks and incubated with trypsin followed by trituration. The resulting cell suspension was plated into poly-L lysine-coated 75-cm2 culture flasks, allowed to adhere for 1 h, washed with phosphate-buffered saline (PBS), and then fed with DMEM/F12 supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin. Cells were kept at 37 °C in 5% CO2/95% air in an incubator, and medium was changed every 3–4 days. After 3–4 weeks, mixed cultures were trypsinized, and plated into poly-L-lysine-coated 75-cm2 culture flasks. This high initial seeding density allowed enrichment of the cultures for astrocytes. Astrocyte purity assessed by GFAP staining was ~90%.
Cell treatments
Estradiol was dissolved in DMSO (the concentration of DMSO in the cultures did not exceed 0.2%). Cells were exposed to estradiol for 24 h or less. Exposure to DMNQ (in DMSO) or H2O2 was for 24 h. Estrogen-receptor antagonists were incubated with cells 30 min before addition of estradiol.
Immunoblots
Immunoblots were carried out as described by Giordano et al. [15, 23]. Protein samples (25 ug) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted using antibodies against PON2 or beta-actin. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes that were incubated with antibodies using the following dilutions: 1:250 (for PON2), and 1:1500 (for beta-actin). After antibody incubations, blots were rinsed in Tris-buffered saline (pH=7.5) and further incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody at the appropriate dilution of 1:750 (for PON2), or incubated with horseradish peroxidase-conjugated anti-mouse secondary antibody at the dilution of 1:2500 (for beta-actin). Intensity of the 43 kDa band, corresponding to the MW of PON2, was measured by densitometry and normalized to beta-actin content. In a number of experiments we also quantified a second, 55 kDa band, recognized by the PON2 antibody (see Results).
RT-PCR
Reverse transcription was performed according to the manufacturer’s established protocol using total RNA and the SuperScript® III First-Strand Synthesis System. For gene expression measurements, 4 uL of cDNA were included in a PCR reaction (25 uL final volume) that also consisted of the appropriate forward (FP) and reverse (RP) primers at 360 nM each, 80 nM TaqMan probe, and TaqMan Gene Expression Master Mix. The PCR primers and the dual-labeled probes (6-carboxy-fluorescein and 6-carboxy-tetramethyl-rhodamine) for all genes were designed using ABI Primer Express v.1.5 software (Applied Biosystems Inc., Foster City, CA). Amplification and detection of PCR amplicons were performed with the ABI PRISM 7900 system (Applied Biosystems) with the following PCR reaction profile: 1 cycle of 95°C for 10 min, 40 cycles of 95°C for 30 sec and 62°C for 1 min. Beta-actin amplification plots derived from serial dilutions of an established reference sample were used to create a linear regression formula in order to calculate expression levels, and beta-actin gene expression levels were utilized as an internal control to normalize the data.
PON2 activity
PON2 lactonase activity was measured as described by Rosenblatt et al., [11], with minor modifications. Briefly, cells were washed and re-suspended in Tris buffer containing 25 mM Tris/HCL, pH 7.6 at 18 °C/1 mM CaCl2. The homogenates were sonicated twice for 10 seconds on ice. An aliquot was saved to measure protein concentration. Enzyme activities were measured using 200 to 300 μg of protein per mL assay mixture. The lactonase activity was measured kinetically using dihydrocoumarin (DHC) as substrate, by monitoring the absorbance at 270 nm in a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA). Though all three PONs have lactonase activity [2], PON1 and PON3 were not detected in brain cells [15, 59], and the DHC hydrolyzing activity is likely due solely to PON2.
Cytotoxicity
Cell viability was quantified using the metabolic dye MTT [-(4,5-dimethyltiazol-2-yl)-2,5 diphenyltetrazolium bromide], as previously described [15, 23]. Cells in 24-well plates were treated with either H2O2 or DMNQ (1–25 uM in neurons, 10–100 uM in astrocytes), dissolved in distilled water and DMSO, respectively, for 24 h at 37 °C. At the end of exposure, medium was removed and cells were incubated with 500 ul/well of Locke’s buffer solution containing 4 ug/ml MTT for 30 min. MTT solution was removed and the precipitated formazan reaction product was dissolved in 0.25 ml DMSO/well. The absorbance was read at 570 nm in a SpectraMax 190 microplate reader, and results expressed as percentage of viable cells relative to controls. Values of IC50 were determined from concentration-response curves using 4–5 concentrations of each compound.
Measurement of reactive oxygen species (ROS)
ROS formation was determined by fluorescence using 2′,7′-dichlorofluorescin diacetate (DCFH2-DA), as previously described [15, 23]. DCFH2-DA is readily taken up by cells and is subsequently de-esterified to DCFH2, which can be oxidized to dichlorofluorescein (DCF) by hydrogen peroxide, peroxynitrite, and other ROS or reactive nitrogen species. Cells were first washed with Locke’s solution and then pre-incubated for 30 min at 37 °C with DCFH2-DA (50 nmol/mg cell protein) in Locke’s solution. DCFH2-DA was added from a stock solution prepared in DMSO which was also added to the blank. Cells were then washed with Locke’s solution to remove extracellular DCFH2-DA before treatments with H2O2 or DMNQ. After treatments (at 37 °C), cells were washed twice with Locke’s buffer and lysed with 0.1 M KH2PO4 and 0.5% (v/v) Triton X-100, pH 7.2 for 5 min. Cell lysates were then scraped from the dishes, and the supernatant was collected. The fluorescence (λEX = 488 nm and λEM = 525 nm) was read immediately, using a SpectraMax Gemini XS spectrofluorometric plate reader. ROS formation was quantified as the amount of fluorescence per mg of protein measured using the bicinchoninic acid protein assay kit, and is expressed as percent of control.
Glutathione levels
Total intracellular GSH levels were measured as described by Giordano et al. [23]. Briefly, cells were homogenized in Locke’s buffer and an aliquot was taken to measure protein concentration by the bicinchoninic acid method. A second aliquot was diluted (1:1) in 10% (w/v) 5-sulfosalicylic acid, centrifuged at 13,000 × g for 5 min at 4 °C, and the supernatant was used for GSH determination. Aliquots from the SSA fraction were added to a black flat-bottomed 96-well plate, and pH was adjusted to 7 with 0.2 M N-ethylmorpholine/0.02 M KOH. Oxidized glutathione was reduced by adding 10 ul of 10 mM tris-(2-carboxyethyl)-phosphine hydrochloride for 15 min at room temperature. The pH was then adjusted to 12.5 by using 0.5 NaOH before derivatizing samples with 10 mM naphthalene dicarboxaldehyde for 30 min. Finally, samples were analyzed on a SpectraMax Gemini XS spectrofluorometric plate reader (λEX = 472 and λEM = 528 nm). Total amount of GSH in the sample is expressed as nanomoles per mg of protein, determined from a standard curve obtained by plotting known amounts of GSH incubated in the same experimental conditions versus fluorescence.
Statistical analysis
Data are expressed as the mean (± SD) of at least three independent experiments. Statistical analysis was performed using one-way or two-way ANOVA followed by a Bonferroni test for multiple comparisons.
Results
We had previously shown that in mouse brain, PON2 was expressed at high levels in the striatum [15]. This may be related to the intrinsically high level of oxidative stress in this region due to dopamine metabolism. In addition, dopamine itself has been shown to induce PON2 expression (at least in kidney) by activating dopamine D2 receptors, which are highest in the striatum [24, 25]. Furthermore, PON2 protein levels were approximately 2.5-fold higher in striatum of female mice compared to males [15]. The striatum was thus the brain region chosen for the present studies.
Fig. 1A shows that striatal astrocytes from both genders had significantly higher levels of PON2 than striatal neurons, confirming our previous observations [15]. In both cell types, PON2 protein levels in female mice were significantly higher than in male mice (Fig. 1A). Similar results can be seen when measuring PON2 mRNA and lactonase activity (Fig. 1B, C). Fig. 1D shows a representative Western blot of PON2 protein in astrocytes and neurons. The antibody recognized two bands, a lower band of 43 kDa, corresponding to the MW of PON2, and a higher 55 kDa band, which has been found by several [5, 15, 19, 20, 26], but not all [9, 12, 13] investigators, and may represent a PON2 isoform, in accordance with the two splice variants of PON2 mRNA [5, 6, 19]. At present, the identity of the upper band has not been clarified; of note, however, is that it is absent in PON2−/− mice [15]. The variability in the levels of the upper 55 kDa is very high, possibly due to instability of this protein, or to the conditions used in the Western blot, which are optimized for the detection of PON2 (the 43 kDa band). Of relevance to the present study is that the abundance of the 55 kDa band appears to differ between genders (Fig. 1E), though to a somewhat lesser extent than the 43 kDa band corresponding to PON2.
Fig 1. PON2 protein, mRNA and activity levels in striatal astrocytes and neurons from female and male mice.
A. Quantification of PON2 (43 kDa band) after normalization by beta-actin. Results are expressed as percent of male neurons. B. mRNA levels of PON2. C. PON2 lactonase activity (dihydrocoumarin hydrolysis). D. Representative blot of PON2 in striatal astrocytes and neurons from mae and female mice, showing the 43 kdA band (PON2) and the upper 55 kDa band. E. Quantification of the 55 kDA band in male and female astrocytes and neurons. Results indicate the mean (± SD) of three separate experiments each done in triplicate. Significantly different from female: *p<0.05; **p<0.01; ***P<0.001.
PON2 protein levels were also measured in cortical astrocytes from rats and in human fetal astrocytes. As shown in Fig. 2, in all three species PON2 was expressed in females at higher levels than in males in rats, mice and humans, suggesting that the observations in mice may be extendable to other species including humans.
Fig 2. PON2 protein level in male and female rat and human astrocytes.
A, B. Representative blots of PON2 in rat vs. mouse and human vs. mouse astrocytes of both genders. C. Quantification of results from three independent experiments. Results are expressed as mean ± SD. Significant difference between genders, *p<0.05; **p<0.01.
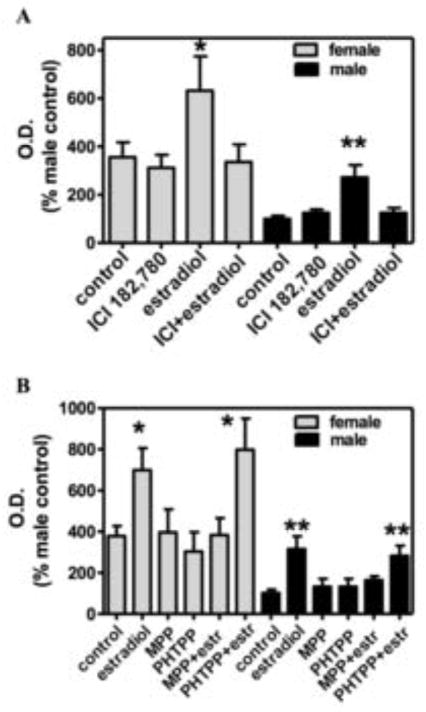
The higher levels of PON2 in cells from female mice may be related to a positive modulatory effect by estrogens. Fig. 3 shows time-course and concentration-response experiments with 17β-estradiol to address this hypothesis. Estradiol (24 h) caused a concentration-dependent increase of PON2 protein in male striatal astrocytes. After 24 h incubation with 200 nM estradiol, levels of PON2 in male astrocytes increased by 3.5-fold, reaching the level normally present in females (Fig. 3A, B). Interestingly, incubation of striatal astrocytes with estradiol could increase female PON2 levels even further (Fig. 3A; B). In order to ascertain the potential involvement of estrogen receptors (ER) in PON2 induction by estradiol, we first measured its effect in the presence of ICI 182,780, a non-selective ER antagonist. As shown in Fig. 4A, this compound completely blocked the effect of estradiol on PON2 expression. To further probe the ER subtype involved in the effect of estradiol, we utilized MPP and PHTPP, which are specific antagonists for ER-alpha and ER-beta, respectively [27, 28]. Fig. 4B shows that the estradiol-induced increase in PON2 protein levels, in both male and female striatal astrocytes, was due to activation of ER-alpha. Additional experiments showed that estradiol also increases levels of PON2 activity (Fig. 5A) and mRNA (Fig. 5B) through activation of ER-alpha. The transcriptional nature of estradiol’s effect on PON2 was further confirmed by experiments with actinomycin D, which completely blocked the estradiol-induced increase in PON2 protein levels (Fig. 5C). In all of these experiments, we also quantified the 55 kDa band recognized by the PON2 antibody. Estradiol (200 nM, 24 h) increased the intensity of this band in male astrocytes by 2-fold (as compared to 3.5-fold for PON2), but had no effect in female astrocytes, and its effect was antagonized by actinomycin D (not shown).
Fig 3. Estradiol increases PON2 expression in striatal astrocytes from male and female mice in a concentration- and time-dependent dependent manner.
A. Time-course. Cells were incubated with 200 nM estradiol for the indicated times and collected after 24 hours. B. Concentration-response. Cells were incubated with the indicated concentrations of estradiol for 24 h. Intensity of bands corresponding to PON2 (43 kDa) were quantified and normalized by intensity of bands corresponding to beta-actin. Results are expressed as percent of untreated male astrocytes, and represent the mean (± SD) of three separate experiments. Significantly different from control of the same gender, *p<0.05; **p<0.01; ***p<0.001.
Fig 4. The effect of estradiol on PON2 is mediated by activation of estrogen receptor-alpha.
Striatal astrocytes from male and female mice were pre-incubated for 30 min. with the ER antagonist ICI 182,780 (1 uM), the selective ER- alpha antagonist MPP (100 nM), or the selective ER-beta antagonist PHTPP (100 nM) before a 24 h incubation with 200 nM estradiol. A. Effect of ICI 182,780. B. Effect of PPP and PHTPP. Results are presented as percent of male untreated astrocytes, and represent the mean (± SD) of three separate experiments. Significantly different from control of the same gender, *p<0.05; **p<0.01.
Fig 5. ER-alpha mediate estradiol-induced increase of PON2 activity and mRNA levels in striatal astrocytes from male and female mice.
Striatal astrocytes were pre-treated for 30 min with MPP (100 nM) prior to a 24 hours exposure to 200 nM estradiol. A. Estradiol-induced increase in PON2 lactonase activity is antagonized by MPP. B. Estradiol-induced increase in PON2 mRNA levels is antagonized by MPP. C. Actinomycin D (1 ug/ml) antagonizes the estradiol-induced increase of PON2 protein. Results represent the mean (± SD) of three separate experiments. Significantly different from same gender control, *p<0.05; **p<0.01; ***p<0.001.
To investigate the effect of estrogens on PON2 expression in vivo, we measured PON2 levels in ovariectomized mice. Plasma levels of estradiol were significantly lower in ovariectomized mice, as expected (Fig. 6A). PON2 protein levels in two brain regions, striatum and cerebral cortex, and in liver, were significantly lower in ovariectomized mice than in naïve or sham-operated female mice, and reached the level present in male mice (Fig. 6B–D). Levels of PON2 mRNA in striatum were similarly decreased (Fig. 6E).
Fig 6. PON2 is decreased in ovariectomized mice.
A. Levels of estradiol in serum of naïve and sham-operated female mice, ovariectomized mice, and male mice. Estradiol levels were measured using an ELISA assay. B–D. PON2 protein levels in striatum (B), cerebral cortex (C), and liver (D) of mice. E. PON2 mRNA levels in striatum of mice. Results are expressed as pg/ml, and represent the mean (± SD) of four different animals/group. Significantly different from sham-operated mice, *p<0.05; *p<0.01.
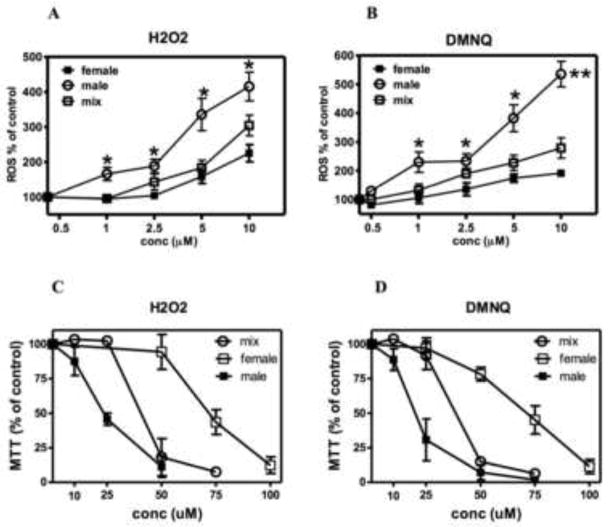
Given that brain cells from PON2−/− mice were significantly more sensitive to oxidative stress and to the ensuing toxicity caused by H2O2 and DMNQ, we hypothesized that astrocytes and neurons from male mice would be more sensitive than those from female mice, because of lower levels of PON2 expression. Fig. 7A and 7B shows that ROS formation induced by H2O2 and by DMNQ was significantly higher in striatal astrocytes from male than female mice, while mixed-gender cultures showed an intermediate response. As a consequence, the toxicity of both compounds was significantly higher in astrocytes from male than female mice (Fig. 7C, D). Similar results were found in striatal neurons (not shown). Table 1 shows the overall IC50s for cytotoxicity of both compounds in striatal astrocytes and neurons.
Fig 7. Oxidative stress and toxicity of H2O2 and DMNQ are higher in striatal astrocytes from male than female mice.
A, B. ROS formation induced in striatal astrocytes from male or female mice or in mixed-gender cultures by different concentrations of H2O2 or DMNQ after 60 min. incubation. C, D. Cell viability (measured by the MTT assay) in striatal astrocytes. Results represent the mean (± SD) of three separate experiments. Significantly different from female, *p<0.05; **p<0.01.
Table 1.
Toxicity of H2O2 and DMNQ in striatal astrocytes and neurons from wild-type male and female mice
| Astrocytes | Neurons | |||||
|---|---|---|---|---|---|---|
| Female | Male | Ratio F/M | Female | Male | Ratio F/M | |
| H2O2 | 72.1 ± 4.6 | 21.2 ± 3.1* | 3.4 | 20.7 ± 2.1 | 4.9 ± 0.8* | 4.2 |
| DMNQ | 70.2 ± 4.4 | 19.1 ± 2.7* | 3.7 | 21.1 ± 3.7 | 6.8 ± 1.0* | 3.1 |
Shown are the values of IC50 (uM) for cytotoxicity, as measured by the MTT assay. Results represent the mean (± SD) of at least three separate determinations carried out with 4–5 concentrations of each compound in duplicate.
Significantly different from females, p<0.01.
Values of experiments carried out in mixed-gender cultures provided the following results (IC50, uM, n=3): H202, astrocytes 41.2 ± 3.4, neurons 11.7 ± 1.3; DMNQ, astrocytes 38.8 ± 2.2, neurons 10.5 ± 1.6.
When comparing neurons and astrocytes of either gender, it is apparent that neurons were more sensitive than astrocytes (by about 3-fold) to the toxicity of the two oxidants (Table 1); this may be due to the differential expression of PON2 (Fig. 1), as well as of glutathione (GSH), a major cellular antioxidant factor (Fig. 8; [21]). In contrast, when comparing genders, it is apparent that both astrocytes and neurons from male mice were significantly more sensitive (by 3–4-fold) than those from females to the toxicity of H2O2 and DMNQ (Table 1). In this case, the differential susceptibility is likely to be related to the differential expression of PON2, as GSH levels did not differ between genders in either astrocytes or neurons (Fig. 8). This hypothesis is further supported by results obtained in striatal astrocytes from PON2−/− mice (Table 2). As expected, the toxicity of both H2O2 and DMNQ was greatly enhanced in cells from PON2−/− mice (compare results of Table 1 and Table 2), confirming our previous observations [15]. However, in the absence of PON2, no gender difference was observed, and astrocytes from male and female mice appeared to be almost equally sensitive to the toxicity of the two oxidants (Table 2).
Fig 8. Levels of GSH in striatal neurons and astrocytes from male and female mice.

GSH levels were measured using the NDA assay, as described in Methods, and are expressed as nmol/mg protein. Results represent the mean (± SD) of three separate determinations. Levels of GSH in neurons are significantly different from those in astrocytes, p<0.01.
Table 2.
Toxicity of H2O2 and DMNQ in striatal astrocytes from PON2−/− male and female mice
| Female | Male | Ratio F/M | |
|---|---|---|---|
| H2O2 | 3.4 ± 0.3 | 2.7 ± 0.4 | 1.3 |
| DMNQ | 5.1 ± 1.5 | 3.5 ± 0.8 | 1.5 |
Shown are the values of IC50 (uM) for cytotoxicity, as measured by the MTT assay. Results represent the mean (± SD) of at least three separate determinations carried out with 4–5 concentrations of each compound in duplicate. The differences between genders are not statistically significant.
Further evidence supporting a central role of PON2 in modulating gender-dependent differences in susceptibility to oxidant toxicity was provided by additional experiments with estradiol. Striatal astrocytes from male mice were treated for 24 h with 200 nM estradiol. This treatment, which increases PON2 expression (Fig. 2), provided protection against the toxicity of H2O2 and DMNQ (Table 3). Protection afforded by estradiol was not surprising, given the known neuroprotective effects of estrogens [29–31] the mechanism(s) of which are nevertheless far from clear [32]. Of great interest are, therefore, the results of similar experiments carried out in striatal astrocytes from PON2−/− mice (Table 3). As expected, these cells were extremely sensitive to the toxicity of both oxidants; however, in the absence of PON2, treatment with estradiol was unable to provide any significant degree of protection (Table 3).
Table 3.
Protective effect of estradiol against H2O2 and DMNQ toxicity in striatal astrocytes from wild-type (PON2+/+) male mice, but not from PON2−/− male mice
| WT (−E) | WT (+E) | KO (−E) | KO (+E) | |
|---|---|---|---|---|
| H2O2 | 19.9 ± 3.3 | 57.2 ± 7.7* | 3.1 ± 0.3 | 3.4 ± 0.5 |
| DMNQ | 19.7 ± 4.2 | 53.1 ± 11.6* | 2.8 ± 0.3 | 3.1 ± 0.6 |
Shown are values of IC50 (uM) in cytotoxicity assays using MTT. Results represent the mean (± SD) of three separate determinations carried out using 4–5 concentrations of each compound in duplicate. Cells from either wild-type (WT) or PON2−/− (KO) mice were treated for 24 with vehicle (−E) or 200 nM estradiol (+E), then after washout, for 24 h with the test compound.
Significantly different from control (−E), p<0.05.
Discussion
Gender is a variable often ignored in most toxicological and neurotoxicological studies [33–35], though scientists would readily acknowledge that major gender differences may exist in the response to toxicants, which can be ascribed to differences in exposure, toxicokinetics and metabolism, and to pharmacodynamic factors [34–36]. The present study suggests that an important intrinsic factor modulating gender-dependent susceptibility to oxidative stress may be PON2, a mitochondrial enzyme with antioxidant properties. In the course of its initial characterization in the CNS of mice, we discovered that females had 2 to 3-fold higher levels of PON2 than males [15]. This finding prompted the present study that adds important new information on gender-dependent expression of PON2 in brain tissue and its potential role in modulating differential susceptibility to certain neurotoxicants.
In vitro and in vivo experiments show that estrogens are important modulators of PON2. When astrocytes and neurons from male mice are exposed to estradiol in vitro, PON2 levels are increased; of interest is also the fact that estradiol can further increase PON2 in CNS cells from female mice. The molecular mechanisms of estradiol’s effect are currently under investigation; nevertheless, the present study indicates that positive modulation of PON2 by estradiol is a transcriptional effect, mediated by activation of ER-alpha, and suggests that the regulation of the PON2 gene may be qualitatively similar in both genders. The importance of estrogens in positively modulating PON2 is confirmed in vivo by the finding that in ovariectomized mice PON2 protein and mRNA levels are decreased to the levels present in male mice.
An important aspect of the present study relates to the functional consequences of differential PON2 expression. In several tissues and cell types, including striatal and cerebellar astrocytes and neurons, PON2 has been shown to exert a potent antioxidant effect [5, 9, 11–13, 15, 19]; as a consequence, the absence of PON2, as in cells derived from PON2−/− mice, greatly increases susceptibility to oxidative stress [15]. Here we show that CNS cells from female mice, which have higher levels of PON2, are more resistant than male mice-derived cells to oxidative stress and ensuing toxicity caused by two oxidants. Though gender-dependent differences in other cell defense mechanisms cannot be excluded, it is noteworthy that levels of GSH, the major intracellular antioxidant factor, do not appear to differ between genders (Fig. 8). Most importantly, there is no gender-dependent difference in susceptibility to the same oxidants in cells from PON2−/− mice, supporting the hypothesis that gender-dependent differences in susceptibility to oxidative stress are due to the level of PON2 expression. Additional support for this hypothesis comes from the experiments with estradiol. In CNS cells from male mice, exposure to estradiol provides protection toward toxicity induced by two oxidants. This is not surprising, as neuroprotective actions of estrogens are well known [29–32]. However, the protective effect of estradiol is absent in cells from PON2−/− mice, suggesting that a major mechanism of estrogen neuroprotection may be represented by induction of PON2.
Although the present experiments were carried out in mice, we provide evidence that similar gender differences in the levels of PON2 expression are also present in brain cells (astrocytes) from rats and humans. This observation, albeit limited, would suggest that results obtained in mice may be extendable to other species, including humans.
The functional consequences of a higher expression of PON2 in females may indeed have several important ramifications. With regard to the CNS, the role of oxidative stress in the etiopathology of Parkinson’s disease is well established [37], and its incidence is 90% higher in males [38, 39]. Even though dopaminergic areas (striatum, substantia nigra, and nucleus accumbens) have the highest level of PON2 in both genders, levels in females are still 2 to 3-fold higher then in males ([15]; Fig. 1). Lower PON2 levels in dopaminergic neurons in males may thus provide fewer defenses against oxidative stress. In this regard, of much interest are the recent findings that activation of dopamine D2 receptors in the kidney positively modulates PON2 expression, leading to a decrease in reactive oxygen species production [25]. In the CNS, the highest levels of dopamine D2 receptors are found in the striatum, nucleus accumbens, substantia nigra and olfactory tubercle [24], areas that also have the highest level of PON2 expression [15; Giordano et al., unpublished results]. If a similar mechanism as observed in kidneys also occurs in the CNS, the loss of dopamine associated with Parkinson’s disease would lead to decreased PON2 levels, thus fostering a spiral of events further aggravating neurodegeneration. Of interest is also the finding that the toxicity of the dopaminergic neurotoxin MPP+ is 6-fold higher toward striatal neurons (mixed genders) from PON2−/− mice than toward those in wild-type mice (IC50 (uM) of MPP+ (MTT assay) was 6.0 ± 0.7 and 1.0 ± 0.1 in wild-type and PON2−/− mice respectively; n=3; p<0.01) [Giordano et al., unpublished observations]. Ongoing studies in our laboratory are exploring gender-difference in MPTP neurotoxicity in relationship to PON2 expression, to expand recent in vitro results showing an enhanced susceptibility of male mesencephalic astrocytes to MPP+ [40]. Additional evidence that males may be more sensitive than females to oxidative stress-mediated CNS toxicity is provided by studies with 6-hydroxydopamine [41, 42], methamphetamine [43, 44], or manganese [45], or by analysis of oxidative changes in brain mitochondria [46]. Furthermore, as PON2 is expressed in most tissues, and levels appear to be higher in females in each tissue examined [15], the reported higher sensitivity of males to oxidative stress in the heart, the kidney, or the liver may be related to the same mechanism [47–52].
Given the widespread distribution of PON2 and its roles in several pathologies, a varied number of investigations may be fostered by our findings. PON2 plays important roles in the atherosclerotic process [16, 20], which is known to be more prominent in males than in females [53, 54]. Strong evidence also indicates that PON2 may represent a major defense mechanism against bacterial infections, by virtue of its ability to metabolize acyl-HCL, which mediate bacterial quorum-sensing signals, important in regulating expression of virulence factors and in inducing a host inflammatory response [2–5]. In this regard, there is limited evidence that males may be more susceptible to infections than females [55, 56]. PON2 also appears to have anti-inflammatory properties; in the gastrointestinal tract, PON2 antagonizes oxidative and inflammatory processes that may affect mucosal integrity [12]. The absence of PON2 in PON2−/− mice exacerbates the macrophage inflammatory response [57]. Furthermore, PON2 acts as a potent anti-inflammatory agent against the inflammatory response caused by administration of pyocyanin (a quorum sensing signal factor) [58]. In support of this hypothesis, we have recently found that neuroinflammation (e.g. increase in levels of pro-inflammatory cytokines such as TNF-alpha and several interleukins) observed in several brain regions of mice upon exposure to diesel exhaust is significantly more pronounced in male than in female mice [Giordano et al., unpublished results].
Conclusions
Gender differences are an important, yet often overlooked aspect of toxicological investigations, and of biomedical research in general. In this study we present evidence that brain cells from male mice are intrinsically more susceptible to oxidative stress because of a lower expression of the mitochondrial antioxidant enzyme PON2. As many adverse health outcomes in the CNS and other organs involve oxidative stress, this finding may explain the gender-dependent differential incidence of several diseases. The lactonase activity of PON2 and its potential anti-inflammatory actions may also pertain to other pathological processes, providing the stimulus for numerous investigations addressing gender effects.
Highlights.
Paraoxonase 2 (PON2) is expressed at higher levels in astrocytes and neurons from female mice
Brain cells from male mice are more susceptible to oxidative stress-induced toxicity
Estradiol modulates PON2 expression in vitro and in vivo
Estradiol-induced neuroprotection is not present in cells from PON2-null mice
Acknowledgments
These studies were supported in part by grants P42ES04696 and P30ES07033 from the National Institute of Environmental Health Sciences, and P30HD02274 from the National Institute of Child Health and Human Development. We thank Dr. Fred Farin and Jasmine Wilkerson from the Functional Genomics and Proteomics Facility in the Dept. of Environmental and Occupational Health Sciences at the University of Washington for measuring PON2 mRNA levels, Dr. Thomas Möller from the Dept. of Neurology, University of Washington, for providing the human astrocytes, and Ms. Khoi Dao for assistance in some experiments.
Abbreviations
- BSA
bovine serum albumin
- CNS
central nervous system
- DCFH2-DA
2′,7′-dichlorofluorescin diacetate
- DMNQ
2,3-dimethoxy-1,4-naphthoquinone
- DMSO
dimethylsulfoxide
- GSH
glutathione (reduced)
- HCL
homoserine lactone
- H2O2
hydrogen peroxide
- MPP
1,3-bis (4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H- pyrazole dihydrochloride
- MTT
3-(4,5-dimethyltiazol-2-yl)-2,5 diphenyltetrazolium bromide
- PHTPP
4-{2-phenyl-5,7-bis (trifluoromethyl) pyrazolo [1,5-α]pyrimidin-3-yl)} phenol
- PON
paraoxonase
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
Footnotes
Conflict of interest
All authors declare that they have no financial interest or any other conflict of interest in connection to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 2.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2 and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Stoltz DA, Ozer EA, Ng CJ, Yu JM, Reddy ST, Lusis AJ, Bourquard N, Parsek MR, Zabner J, Shih DM. Paraoxonase-2 deficiency enhances Psudomonas aeruginosa quorum sensing in murine tracheal epithelia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L852–L860. doi: 10.1152/ajplung.00370.2006. [DOI] [PubMed] [Google Scholar]
- 4.Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, Kramer GL, Haley RW, Draganov DI. Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infect Immun. 2008;76:2512–2519. doi: 10.1128/IAI.01606-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horke S, Witte I, Altenhoffer S, Wilgenbus P, Goldeck M, Forstermann U, Xiao J, Kramer GL, Haines DC, Chowdhary PK, Haley RW, Teiber JF. Paraoxonase-2 is down regulated by the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanyl)-L-homoserine lactone and attenuates oxidative stress induced by pyocyanin. Biochem J. 2010;426:73–83. doi: 10.1042/BJ20091414. [DOI] [PubMed] [Google Scholar]
- 6.Mochizuki H, Scherer SW, Xi T, Nickle DC, Majer M, Huizenga JJ, Tsui LC, Prochazka M. Human PON2 gene at 7q21.3: cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene. 1998;213:149–157. doi: 10.1016/s0378-1119(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 7.Stoltz DA, Ozer EA, Recker TJ, Estin M, Yang X, Shih DM, Lusis AJ, Zabner J. A common mutation in paraoxonase-2 results in impaired lactonase activity. J Biol Chem. 2009;284:35564–35571. doi: 10.1074/jbc.M109.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorenson RC, Aviram M, Bisgaier CL, Billecke S, Hsu C, La Du BN. Human serum paraoxonase/arylesterase’s retained hydrophobic N-terminal leader sequence associates with HDLs by binding phospholipids: apolipoprotein A-I stabilizes activity. Arterioscler Thromb Vasc Biol. 1999;19:2214–2225. doi: 10.1161/01.atv.19.9.2214. [DOI] [PubMed] [Google Scholar]
- 9.Ng CJ, Wadleigh DJ, Gangopadhyyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001;276:44444–44449. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 10.Marsillach J, Mackness B, Mackness M, Riu F, Beltran R, Joven J, Camps J. Immunohistochemical analysis of paraoxonase-1, 2 and 3 expression in normal mouse tissues. Free Rad Biol Med. 2008;45:146–157. doi: 10.1016/j.freeradbiomed.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Rosenblatt M, Draganov D, Watson CE, Bisgaier CL, La Du BN, Aviram M. Mouse macrophage paraoxonase-2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:468–474. doi: 10.1161/01.ATV.0000059385.95664.4D. [DOI] [PubMed] [Google Scholar]
- 12.Levy E, Trudel K, Bendayan M, Seidman E, Delvin E, Elchebly M, Lavoie JC, Precourt LP, Amre D, Sinnett D. Biological role, protein expression, subcellular localization, and oxidative stress response of paraoxonase 2 in the intestine of human and rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1252–G1261. doi: 10.1152/ajpgi.00369.2007. [DOI] [PubMed] [Google Scholar]
- 13.Precourt LP, Seidman E, Delvin E, Amre D, Deslandres C, Dominguez M, Sinnett D, Levy E. Comparative expression analysis reveals differences in the regulation of intestinal paraoxonase family members. Int J Biochem Mol Biol. 2009;41:1628–1637. doi: 10.1016/j.biocel.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Mackness B, Beltran-Debon R, Aragones G, Joven J, Camps J, Mackness M. Human tissue distribution of paraoxonases 1 and 2 mRNA. IUBMB Life. 2010;62:480–482. doi: 10.1002/iub.347. [DOI] [PubMed] [Google Scholar]
- 15.Giordano G, Cole TB, Furlong CE, Costa LG. Paraoxonase 2 (PON2) in the mouse central nervous system: a neuroprotective role? Toxicol. Appl. Pharmacol. 2011;256:369–378. doi: 10.1016/j.taap.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, Clarke C, Vergnes L, Reue K, Teiber JF, Reddy ST. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antiox Redox Signal. 2011;14:341–351. doi: 10.1089/ars.2010.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimers Dis. 2010;20(Suppl 2):S453–S473. doi: 10.3233/JAD-2010-100321. [DOI] [PubMed] [Google Scholar]
- 18.Altenhofer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Forstermann U, Horke S. One enzyme, two functions. PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem. 2010;285:24398–24403. doi: 10.1074/jbc.M110.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horke S, Witte I, Wilgenbus P, Kruger M, Starnd D, Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- 20.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, Fogelman AM, Lusis AJ, Young S, Reddy ST. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins. Antiatherogenic role for paraoxonase-2. J Biol Chem. 2006;281:29491–29500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- 21.Giordano G, Kavanagh TJ, Costa LG. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satoh J, Kim SU. Cytokines and growth factors induce HSP27 phosphorylation in human astrocytes. J Neuropathol Exp Neurol. 1995;54:504–512. doi: 10.1097/00005072-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol Pharmacol. 2006;70:2116–21126. doi: 10.1124/mol.106.027748. [DOI] [PubMed] [Google Scholar]
- 24.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Zhang Y, Cuevas-Gonzalez S, Villar VA, Escano C, Asico L, Yu P, Grandy DK, Felder RA, Armando I, Jose PA. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Rad Biol Med. 2012;53:437–446. doi: 10.1016/j.freeradbiomed.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shamir R, Hartman C, Karry R, Pavlotzky E, Eliakim R, Lachter J, Suissa A, Aviram M. Paraoxonases (PONs) 1, 2 and 3 are expressed in human and mouse gastrointestinal tract and in Caco-2 cell line: selective secretion of PON1 and PON2. Free Rad Biol Med. 2005;39:336–344. doi: 10.1016/j.freeradbiomed.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor α. Endocrinology. 2002;143:941–947. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- 28.Compton DR, Sheng S, Carlson KE, Rebacz NA, Lee IY, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazol [1,5-a] pyrimidines: estrogen receptor ligands possessing estrogen receptor β antagonist activity. J Med Chem. 2004;47:5872–5893. doi: 10.1021/jm049631k. [DOI] [PubMed] [Google Scholar]
- 29.Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta. 2010;1800:1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: implications for neuroprotection. Biochim Biophys Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab. 2011;22:467–473. doi: 10.1016/j.tem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Arnold S, Victor MB, Beyer C. Estrogen and the regulation of mitochondrial structure and function in the brain. J Ster Biochem Mol Biol. 2012;131:2–9. doi: 10.1016/j.jsbmb.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Wald C, Wu C. Of mice and women: the bias in animal models. Science. 2010;327:1571–1572. doi: 10.1126/science.327.5973.1571. [DOI] [PubMed] [Google Scholar]
- 34.Weiss B. Same sex, no sex, and unaware sex in neurotoxicology. Neurotoxicology. 2011;32:509–517. doi: 10.1016/j.neuro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mergler D. Neurotoxic exposure and effects: gender and sex matter! Hänninen lecture 2011. Neurotoxicology. 2012;33:644–651. doi: 10.1016/j.neuro.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Vahter M, Gochfeld M, Casati B, Thiruchelvam M, Falk-Flippeson A, Kavlock R, Marafante E, Cory-Slechta D. Implications of gender differences for human health risk assessment and toxicology. Environ Res. 2007;104:70–84. doi: 10.1016/j.envres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Surmeier DJ, Guzman JN, Sanchez-Padilla J, Goldberg JA. The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxid Redox Signal. 2011;14:1289–1301. doi: 10.1089/ars.2010.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 39.Wirdefeld K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26:S1–S58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 40.Sundar-Boyalla S, Victor MB, Roemgren A, Beyer C, Arnold S. Sex-and brain region specific role of cytochrome c oxidase in 1-methyl-4-phenylpyridinium-mediated astrocyte vulnerability. J Neurosci Res. 2011;89:2068–2082. doi: 10.1002/jnr.22669. [DOI] [PubMed] [Google Scholar]
- 41.Tamas A, Lubics A, Szalontay L, Lengvari I, Reglodi D. Age and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra. Behav Brain Res. 2005;158:221–229. doi: 10.1016/j.bbr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Misiak M, Beyer C, Arnold S. Gender-specific role of mitochondria in the vulnerability of 6-hydroxydopamine-treated mesencephalic neurons. Biochim Biophys Acta. 2010;1797:1178–1188. doi: 10.1016/j.bbabio.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Dluzen DE, McDermott JL, Darvesh AS. Relationships among gender, age, time, and temperature in methamphetamine-induced striatal dopaminergic neurotoxicity. Neuroscience. 2010;167:985–993. doi: 10.1016/j.neuroscience.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 44.Bourque M, Liu B, Dluzen DE, Di Paolo T. Sex differences in methamphetamine toxicity in mice: effect on brain dopamine signaling pathways. Psychoneuroendocrinology. 2011;36:955–969. doi: 10.1016/j.psyneuen.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Gender and manganese exposure interactions on mouse striatal neurons morphology. NeuroToxicology. 2011;32:896–906. doi: 10.1016/j.neuro.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guevara R, Gianotti M, Oliver J, Roca P. Age and sex-related changes in rat brain mitochondrial oxidative status. Exp Gerontol. 2011;46:923–928. doi: 10.1016/j.exger.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Dai G, He L, Chou N, Wan YJ. Acetaminophen metabolism does not contribute to gender difference in its hepatotoxicity in mouse. Toxicol Sci. 2006;92:33–41. doi: 10.1093/toxsci/kfj192. [DOI] [PubMed] [Google Scholar]
- 48.McConnachie LA, Mohar I, Hudson FN, Ware CB, Ladiges WC, Fernandez C, Chatterton-Kirchmeier S, White CC, Pierce RH, Kavanagh TJ. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol Sci. 2007;99:628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- 49.Valle A, Guevara R, Garcia-Palmer FJ, Roca P, Oliver J. Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am J Physiol Cell Physiol. 2007;293:C1302–C1308. doi: 10.1152/ajpcell.00203.2007. [DOI] [PubMed] [Google Scholar]
- 50.Wang F, He Q, Sun Y, Dai X, Yang XP. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension. 2010;55:1172–1178. doi: 10.1161/HYPERTENSIONAHA.110.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li QY, Li M, Feng Y, Guo Q, Gu SY, Liu JL, Zhang RF, Wan HY. Chronic intermittent hypoxia induces thioredoxin system changes in a gender-specific fashion in mice. Am J Med Sci. 2012;343:458–461. doi: 10.1097/MAJ.0b013e318235b03e. [DOI] [PubMed] [Google Scholar]
- 52.Bhatia K, Elmarakby AA, El-Remessey A, Sullivan JC. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R274–R282. doi: 10.1152/ajpregu.00546.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett-Connor E. Sex difference in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 54.Kardys I, Vliegenthart R, Oudkerk M, Hofman A, Witteman JCM. The female advantage in cardiovascular disease: do vascular beds contribute equally? Am. J Epidemiol. 2007;166:403–412. doi: 10.1093/aje/kwm115. [DOI] [PubMed] [Google Scholar]
- 55.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 56.Satyanarayana G, Heysell SK, Scully KW, Houpt ER. Mycobacterial infections in a large Virginia hospital, 2001–2009. BMC Infect Dis. 2011;11:113. doi: 10.1186/1471-2334-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bourquard N, Ng CJ, Reddy ST. Impaired hepatic insulin signaling in PON2-deficient mice: a novel role for the PON2/apoE axis on the macrophage inflammatory response. Biochem J. 2011;436:91–100. doi: 10.1042/BJ20101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schweikert EM, Amort J, Wilgenbus P, Forsterman U, Teiber JF, Horke S. Paraoxonases-2 and -3 are important defense enzymes against Pseudomonas aeruginosa virulence factors due to their anti-oxidative and anti-inflammatory properties. J Lipids. 2012 doi: 10.1155/2012/352857. ID 352857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tait L. MS Thesis. University of Washington; 2011. Modulation of paraoxonase 2 (PON2) in the CNS; pp. 1–45. [Google Scholar]