Abstract
Aims
The goal of this study is to evaluate whether an elevated neutrophil-lymphocyte ratio (NLR) at the time of diagnosis predicts survival of patients with hepatocellular carcinoma (HCC) after liver transplant (LT). We hypothesize that the NLR is predictive of overall survival (OS) and recurrence-free survival (RFS) in patients with HCC who undergo LT.
Methods
This is a retrospective analysis of adult patients undergoing LT for HCC between 2000 and 2008 at our institution. We define an elevated NLR as a ratio of five or greater.
Results
We included 160 patients who underwent LT for HCC in the time period, of whom 28 had an elevated NLR. Seventeen subjects experienced recurrent HCC during the study period. The cumulative survival among subjects with an elevated NLR was significantly lower than among subjects with a normal NLR. On univariate analysis, several factors (including an elevated NLR) predicted decreased overall survival and recurrence-free survival. However, after multivariate analysis, only three factors (including elevated NLR) remained significant as predictors of overall survival. Additionally, multivariate analysis revealed that an elevated NLR was the only significant independent predictor of recurrence-free survival.
Conclusions
Preoperative NLR is a powerful independent predictor of overall survival and recurrence-free survival in patients undergoing LT for HCC. Measurement of NLR could serve as a useful and easily obtained adjunct to the MELD score and Milan criteria when evaluating this patient population and determining which patients will gain the most survival benefit from transplant.
Keywords: hepatocellular carcinoma, liver transplant, neutrophil-lymphocyte ratio
INTRODUCTION
While cases of hepatocellular carcinoma (HCC) are decreasing in developing countries, the incidence of HCC is actually steeply rising in industrialized nations, including the United States1. The vast majority of patients with HCC have underlying cirrhosis, making successful treatment quite complicated2. In patients with HCC in the setting of cirrhosis, liver transplantation (LT) provides both resection of the neoplastic lesion and cure for the end-stage liver disease. However, as is the case with resective therapy for any malignancy, the risk for recurrence from occult metastatic disease remains.
In the early years of LT, there were no clearly defined exclusion criteria for HCC patients. By the 1980s, LT was routinely performed for patients with advanced HCC, including those with lymph node involvement and extrahepatic spread. These practices resulted in dismal outcomes, with recurrence rates as high as 50% and 5 year survival rates of 20-40%3,4. The early experience with LT for HCC was so poor that HCC was actually declared a contraindication for LT in 19895.
In 1996, the Milan criteria were defined by Mazzaferro et al and were shown to predict improved overall survival and recurrence-free survival after LT for HCC6. Subsequently, these criteria have become the basis of the pretransplant evaluation of most patients with HCC. Although the outcomes of LT for HCC have improved dramatically since the adoption of the Milan criteria, the recurrence rate under this system remains between 10 and 20%7. While the Milan criteria (and other, more recent criteria such as the UCSF and Metroticket criteria) provide an accurate description of tumor size and location, they can only approximate tumor biology. It is thought that aggressive tumor biology, which cannot be determined by pre-transplant imaging alone, might be responsible for the relatively high rate of recurrence of HCC after LT.
Recent attention has focused on the systemic inflammatory state as a surrogate for tumor biology in multiple solid tumors. In particular, the ratio of absolute neutrophil count to absolute lymphocyte count (neutrophil-lymphocyte ratio or NLR) has been shown to predict overall and postoperative survival in gastric cancer, non-small-cell lung cancer, and liver metastases of colorectal cancer8-10. Recently, Halazun et al11 and Bertuzzo et al12 have shown that an elevation of the preoperative NLR has a negative impact on survival after LT for HCC. The NLR is particularly useful, as it can be easily measured by routine pre-transplant bloodwork.
The goal of this study was to evaluate whether an elevated NLR at the time of diagnosis predicts overall survival (OS) and recurrence-free survival (RFS) of patients with HCC who undergo LT.
METHODS
Patient Selection
We constructed a database consisting of all adult patients who underwent LT for HCC at Shands Hospital at the University of Florida between January 1st, 2000 and December 31st, 2008. Subsequently, a retrospective analysis was carried out on these subjects. We included all subjects over the age of 18 years, with a UNOS diagnosis of HCC, who underwent LT. Subjects with a prior LT, with evidence of sepsis or exposure to steroids at the time of diagnosis, and those who subsequently were diagnosed with non-HCC malignancy (primarily cholangiocarcinoma) on explant pathology were excluded. Any patients with prior treatment for HCC (such as prior resection) were excluded. The diagnosis of HCC consisted of characteristic findings on dynamic imaging, elevation of alpha fetoprotein (AFP) and/or pre-transplant biopsy findings.
A complete blood count (CBC) is routinely obtained in all patients undergoing pre-transplant evaluation. The NLR at the time of diagnosis was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count on the CBC obtained on the day of (or within 3 days of) the diagnosis of HCC. Similarly, we calculated the NLR on the day of transplant. We defined an elevated NLR as greater than or equal to 5, in accordance with the majority of the published literature10-12.
Immunosuppression and Surveillance
All transplants were performed at Shands Hospital and the University of Florida utilizing standard techniques. Per institution protocol, post-transplant patients receive immunosuppression with steroids (IV bolus induction followed by transition to oral steroids and taper over three months) and a calcineurin inhibitor (most commonly tacrolimus). Mycophenolate mofetil is occasionally added in patients with renal insufficiency. Patients undergo surveillance with serial dynamic/contrasted imaging [either computed tomography (CT) or magnetic resonance imaging (MRI)] at three, six, and 12 months post-transplant and monitoring of AFP every three months for the first post-transplant year.
Statistical Analysis
Differences among the groups of subjects with normal and elevated NLR were evaluated with the chi squared, Fisher’s exact, and Mann-Whitney U tests as appropriate. Kaplan Meier survival analyses were conducted to determine OS and RFS in the two groups. Univariate analyses were conducted to identify potential factors which influenced survival in the subjects. A stepwise Cox regression analysis was then performed to determine which of these preoperative factors impacted OS and RFS. Confidence intervals were constructed at 95%, and a p value of < 0.05 was considered significant. All statistical analyses were conducted with IBM-SPSS version 19 (IBM-SPSS Inc, Chicago, IL).
RESULTS
Of the 182 adult subjects who underwent LT for HCC at our institution during the study period, 22 were excluded (12 due to incomplete data, 3 who had recurrent HCC after resection, 4 whose explants did not reveal HCC, and 3 who were undergoing a second LT). Median follow up was 38 months (range 1 to 116 months). Of the 160 subjects included in the study, death was confirmed in 48 (30%) at the end of the study period. Of these subjects, recurrent HCC was the cause of death in 14 (9 of these subjects had an elevated NLR at the time of diagnosis). Other causes of death included ESLD due to recurrent HCV in 21 subjects (44%), sepsis/multi-organ system failure in 9 subjects (19%), GI bleeding in 3 (6%), and end-stage liver disease secondary to chronic rejection in 1 subject (2%). Non-HCC causes of death were not more frequent in the subjects with elevated NLR. Overall, 17 subjects (10.6%) had recurrence of HCC within the study period, with a median time to recurrence of 10 months (range 1 to 63 months).
The majority of subjects (47%) had cirrhosis secondary to chronic hepatitis C infection. Nine percent of the subjects had cirrhosis related to alcohol consumption alone, 23 percent had cirrhosis related to both alcohol and hepatitis C, eight percent had cirrhosis secondary to hepatitis B, and the remainder (13%) had cirrhosis secondary to autoimmune hepatitis, non-alcoholic fatty liver disease, or cryptogenic cirrhosis. The majority of subjects (69%) received at least one session of locoregional therapy (either bland embolization, chemo-embolization, or radiofrequency ablation) prior to transplantation. Overall, the subjects spent an average of 25 days on the transplant list before undergoing transplantation (range 6-88 days).
The subjects were categorized as having either a “normal NLR” (defined as an NLR of less than 5.0, n= 132) or “elevated NLR” (defined as an NLR of 5.0 or greater, n = 28). Demographic characteristics, alpha fetoprotein (AFP) levels, and Model for End Stage Liver Disease (MELD) scores were similarly distributed between the 132 subjects with a normal NLR and the 28 subjects with an elevated NLR (Table 1). The overall survival among subjects with an elevated NLR (1 year cumulative survival 87% +/- 0.08, 3 year cumulative survival 57% +/- 0.09, 5 year cumulative survival 50% +/- 0.11) was significantly lower than among subjects with a normal NLR (1 year survival 94% +/- 0.04, 3 year survival 78% +/- 0.04, 5 year survival 75% +/- 0.06) (log rank test, P = 0.01, Figure 1) Similarly, there was a significantly lower recurrence-free survival in patients with an elevated NLR (log rank test, P = 0.005, Figure 2). The mean time to recurrence was shorter in the patients with elevated NLR (108 days) than that of patients with normal NLR (224 days) (t-test, P = 0.02). Of the 17 subjects with recurrent HCC, 15 had an elevated NLR (88%). Of the 15 subjects with elevated NLR and recurrent HCC, eight were within Milan criteria (53%).
Table 1.
Comparison of demographic, clinical, and pathologic characteristics of subjects with normal and elevated NLR.
| Variable | Normal NLR n = 132 | Elevated NLR n = 28 | p-value |
|---|---|---|---|
| Male gender | 104 (79%) | 26 (93%) | 0.12 |
| Age in years (mean) | 55.5 | 55.1 | 0.87 |
| Etiology of Cirrhosis | |||
| HCV | 57 | 18 | 0.06 |
| HCV and Alcohol | 33 | 4 | 0.32 |
| Alcohol alone | 13 | 2 | 0.74 |
| HBV | 12 | 2 | 0.98 |
| Other | 17 | 2 | 0.53 |
| Mean total lymphocyte count (thousand per cubic millimeter) | 3.2 | 3.1 | 0.77 |
| Mean biochemical MELD score at diagnosis | 10 | 9 | 0.61 |
| Mean AFP at diagnosis (ng/dL) | 275 | 296 | 0.74 |
| Outside Milan criteria at diagnosis | 19 (14%) | 7 (25%) | 0.06 |
| Mean time on wait list (days) | 23 | 26 | 0.49 |
| Locoregional therapy prior to transplantation | 92 (70%) | 18 (64%) | 0.08 |
| Incidental HCC | 11 | 4 | 0.47 |
| T stage underestimated by pre-operative imaging | 6 | 2 | 0.63 |
| Poorly differentiated tumor on explant | 19 | 2 | 0.37 |
| Any tumor greater than 3cm in diameter | 58 | 8 | 0.15 |
| Mean number of tumors | 1.4 | 1.6 | 0.21 |
| Mean size of largest tumor (in cm) | 2.2 | 2.0 | 0.11 |
| Microvascular invasion | 20 (15%) | 5 (18%) | 0.77 |
Figure 1.
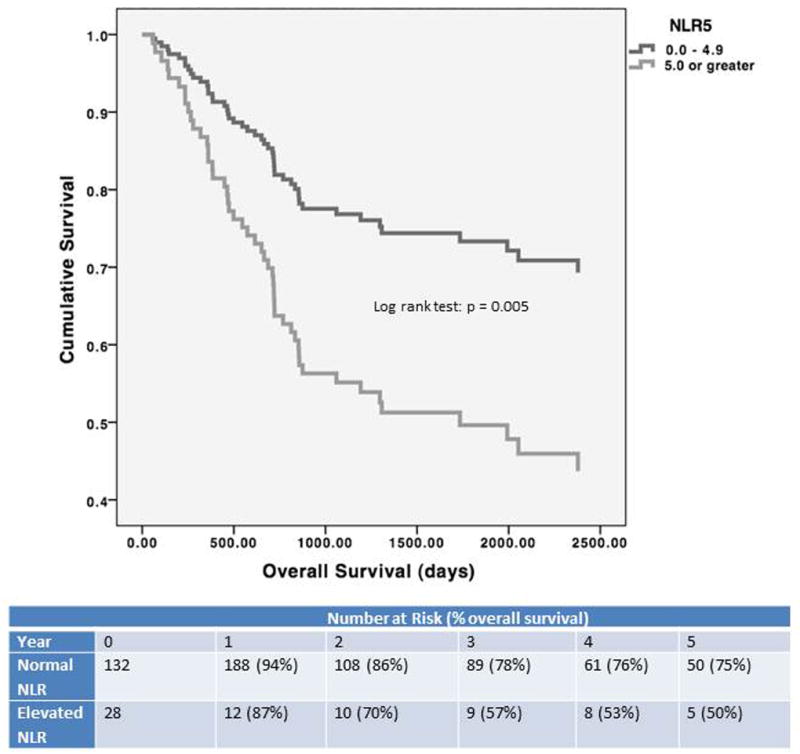
Overall Survival by Neutrophil Lymphocyte Ratio
Figure 2.
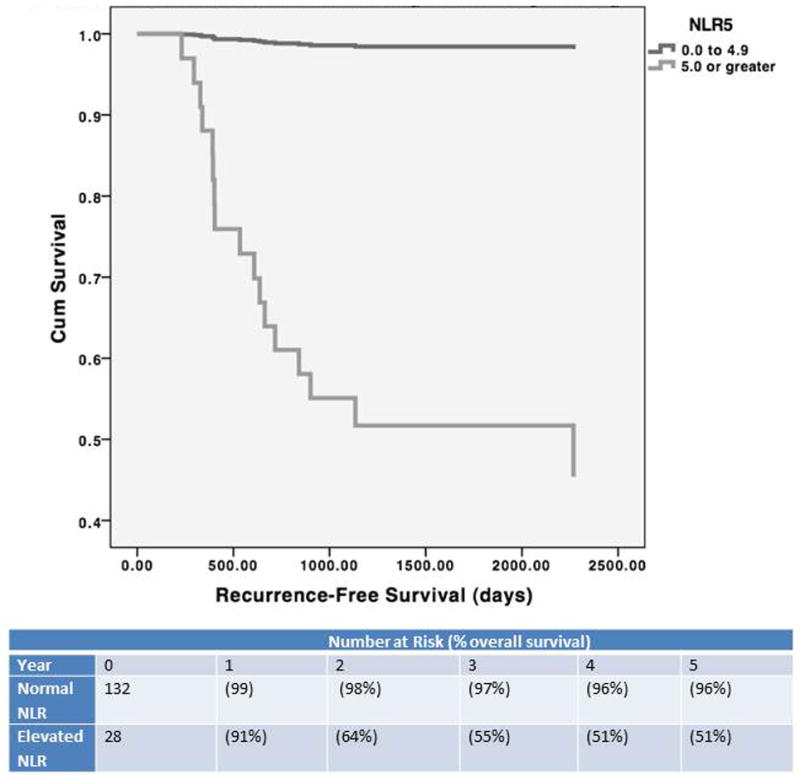
Recurrence-Free Survival by Neutrophil Lymphocyte Ratio
The impact of the NLR at the time of transplantation was similarly analyzed. The overall survival among subjects with an elevated NLR at the time of transplantation was significantly lower than among subjects with a normal NLR at the time of transplantation (log rank test, p = 0.007). Similarly, there was a significantly lower recurrence-free survival in patients with an elevated NLR (log rank test, p = 0.018). Analysis of the data stratified by HCC stage did not alter the overall survival or recurrence-free survival (data not shown).
To determine the adjusted hazard ratio (HR) of death or recurrence for an elevated NLR, we fitted a multivariate Cox regression model using backwards stepwise construction. First, univariate analyses were conducted to determine the effects of the following variables on OS and RFS: age greater than 60, male gender, HCV as etiology of cirrhosis, AFP greater than 400 ng/dL at time of diagnosis, biochemical MELD score (excluding any exception points granted for HCC) greater than 15 at time of diagnosis, time on transplant wait list, use of locoregional therapy before transplantation, fulfillment of Milan criteria at the time of transplantation, largest tumor diameter greater than 3cm, presence of microvascular invasion on explant pathology, poorly-differentiated tumor on explant pathology, underestimation of tumor stage by pre-operative imaging, and incidental diagnosis of HCC on explant pathology (Table 2, Table 3).
Table 2.
Univariate analysis of variables predicting overall survival.
| Variable | P value | HR (CI) |
|---|---|---|
|
| ||
| Gender | ||
| Male (n = 130) | 0.017 | 1.46 (1.06 – 1.87) |
| Female (n = 30) | ||
|
| ||
| Age | ||
| ≥60 (n = 50) | 0.050 | 1.32 (1.17 – 2.01) |
| <60 (n = 110) | ||
|
| ||
| Etiology of Cirrhosis | ||
| HCV (n = 75) | 0.042 | 1.87 (1.31 - 3.76) |
| Non-HCV (n = 85) | ||
|
| ||
| Biochemical MELD score at diagnosis | ||
| < 15 (n = 111) | 0.013 | 3.14 (1.41 - 4.88) |
| ≥ 15 (n = 49) | ||
|
| ||
| AFP at diagnosis (ng/dL) | ||
| ≥ 400 (n = 52) | 0.029 | 2.61 (1.91 – 3.10) |
| < 400 (n = 108) | ||
|
| ||
| Outside Milan criteria at diagnosis | ||
| Yes (n = 26) | 0.571 | 9.76 (0.19 – 16.71) |
| No (n = 134) | ||
|
| ||
| Time on wait list (days) | ||
| < 30 days (n = 96) | 0.610 | 1.18 (0.82 - 1.71) |
| ≥ 30 days (n = 64) | ||
|
| ||
| Locoregional therapy prior to transplantation | ||
| Yes (n = 110) | ||
| No (n = 50) | 0.058 | 1.39 (0.98 - 1.92) |
|
| ||
| Incidental HCC | ||
| Yes (n = 15) | 0.980 | 0.85 (0.08 - 2.77) |
| No (n = 145) | ||
|
| ||
| Tumor stage underestimated by pre-operative imaging | 0.781 | 1.42 (0.84 – 2.14) |
| Yes (n = 8) | ||
| No (n = 152) | ||
|
| ||
| Poorly differentiated tumor on explant | ||
| Yes (n = 21) | 0.767 | 1.08 (0.88 – 1.65) |
| No (n = 139) | ||
|
| ||
| Any tumor greater than 3cm in diameter | ||
| Yes (n = 66) | 0.799 | 1.48 (0.09 – 1.76) |
| No (n = 64) | ||
|
| ||
| NLR at time of diagnosis | ||
| ≥5 (n = 28) | 0.032 | 2.12 (1.41 – 4.62) |
| <5 (n = 132) | ||
|
| ||
| Microvascular invasion | ||
| Yes (n = 25) | 0.047 | 1.66 (1.39 – 3.70) |
| No (n = 135) | ||
Table 3.
Univariate analysis of variables predicting recurrence-free survival.
| Variable | P value | HR (CI) |
|---|---|---|
|
| ||
| Gender | ||
| Male (n = 133) | 0.621 | 1.58 (0.45 – 4.62) |
| Female (n = 27) | ||
|
| ||
| Age | ||
| ≥60 (n = 50) | 0.034 | 1.99 (1.27 – 5.44) |
| <60 (n = 110) | ||
|
| ||
| Etiology of Cirrhosis | ||
| HCV (n = 75) | 0.826 | 1.07 (0.97 – 2.22) |
| Non-HCV (n = 85) | ||
|
| ||
| Biochemical MELD score at diagnosis | ||
| < 15 (n = 111) | 0.771 | 0.62 (0.14 – 0.87) |
| ≥ 15 (n = 49) | ||
|
| ||
| AFP at diagnosis (ng/dL) | ||
| ≥ 400 (n = 52) | 0.458 | 1.52 (0.75 – 1.98) |
| < 400 (n = 108) | ||
|
| ||
| Outside Milan criteria at diagnosis | ||
| Yes (n = 26) | 0.616 | 0.71 (0.44 – 1.06) |
| No (n = 134) | ||
|
| ||
| Time on wait list (days) | ||
| < 30 days (n = 96) | 0.884 | 1.14 (0.83 – 1.56) |
| ≥ 30 days (n = 64) | ||
|
| ||
| Locoregional therapy prior to transplantation | ||
| Yes (n = 110) | 0.067 | 1.51 (0.16 – 3.72) |
| No (n = 50) | ||
|
| ||
| Incidental HCC | ||
| Yes (n = 15) | 0.254 | 1.46 (0.80 – 2.16) |
| No (n = 145) | ||
|
| ||
| Tumor stage underestimated by pre-operative imaging | 0.622 | 1.02 (0.70 – 1.14) |
| Yes (n = 8) | ||
| No (n = 152) | ||
|
| ||
| Poorly differentiated tumor on explant | ||
| Yes (n = 21) | 0.408 | 2.03 (0.89 – 5.37) |
| No (n = 139) | ||
|
| ||
| Any tumor greater than 3cm in diameter | ||
| Yes (n = 66) | 0.516 | 1.28 (0.32 – 1.56) |
| No (n = 64) | ||
|
| ||
| NLR at time of diagnosis | ||
| ≥5 | 0.001 | 6.88 (2.99 – 16.20) |
| <5 | ||
|
| ||
| Microvascular invasion | 0.028 | 2.72 (1.89 – 5.03) |
| Yes (n = 25) | ||
| No (n = 135) | ||
On univariate analysis, the following variables were associated with decreased overall survival: age greater than 60, male gender, HCV as etiology of cirrhosis, AFP > 400 ng/dL at diagnosis, biochemical MELD score greater than 15 at diagnosis, NLR greater than 5 at diagnosis, and the presence of microvascular invasion by explant pathology. Multivariate Cox regression revealed that three predictors were independently associated with overall survival: elevated NLR, presence of microvascular invasion, and AFP > 400 ng/dL at diagnosis. The adjusted HR of death for an elevated NLR was 2.22 (p = 0.021, 95% CI 1.1-1.4).
On univariate analysis, the following variables were associated with decreased recurrence-free survival: age greater than 60, NLR greater than 5 at diagnosis, and the presence of microvascular invasion. Multivariate Cox regression revealed that an elevated NLR was the only predictor which was independently associated with recurrence-free survival. The adjusted HR of recurrence for an elevated NLR was 67 (p = 0.001 95% CI 11-413).
The overall survival of the elevated NLR group was significantly lower than that of the normal NLR group. The five year cumulative survival of the elevated NLR group was only 38%, compared to a five year cumulative survival of 68% in the normal NLR group (log rank test: p = 0.005, Figure 1). Similarly, the recurrence-free survival of the elevated NLR group was significantly lower than that of the normal NLR group (log rank test: p = 0.01, Figure 2), with a five year recurrence-free survival of only 27% in the elevated NLR group, compared to a five year recurrence-free survival of 79% in the normal NLR group.
DISCUSSION
The survival of HCC patients who undergo LT has dramatically improved since the adoption of the Milan criteria. The Milan criteria rely on imaging characteristics such as size and number of lesions, but the relatively high recurrence rate indicates that we continue to lack a reliable surrogate of tumor biology. The NLR is easily calculated and applied in the clinical setting. In this study, we demonstrate that the NLR is a powerful predictor of overall survival and recurrence-free survival in patients undergoing LT for HCC. We also found that nearly 40% of subjects within Milan criteria but with an elevated NLR developed recurrent HCC. An elevated NLR may indicate a more aggressive tumor biology that is not appreciated purely by the size or number of lesions on imaging and places patients at higher risk for tumor recurrence.
Our study confirms the findings of Halazun (11), Bertuzzo (12), and Huang (13) that an elevated NLR is predictive of decreased overall and recurrence-free survival in HCC patients who undergo LT (11,12) or chemo-embolization (13). While these studies have demonstrated the predictive power of the NLR in the days prior to LT or locoregional therapy, we show that this ratio also has prognostic power when determined earlier, such as at the time of initial diagnosis. In addition, we show that the majority of our subjects with an elevated NLR have relative lymphopenia, which might implicate an inadequate host immune response to the tumor.
A limitation of our study is the relatively small number of subjects with an elevated NLR. While previous studies have shown the presence of microvascular invasion and a tumor size greater than 3 cm to predict recurrence-free survival in HCC, we did not find them to be independent predictors. This difference may be due to the small size of the elevated NLR group. In addition, after 2005, some LT patients with HCC were transitioned from tacrolimus to sirolimus, which has been shown in a recent meta-analysis to decrease HCC recurrence in this population (14). This transition has not been standardized at our institution, and therefore we were unable to fully capture this data, which might have had an effect on recurrence rates and survival. Finally, as we did not have complete data on the timing of locoregional therapy, we were unable to analyze the effects of these modalities on the usefulness of the NLR. Nevertheless, our study is an important addition to the growing evidence that systemic inflammatory markers can serve as surrogates for solid tumor biology.
The idea that tumors are associated with a particular inflammatory state dates back to Rudolf Virchow’s work in the 1860s (15). Since that time, translational and basic research has revealed that certain systemic inflammatory markers, such as C-reactive protein, can be predictive of survival in solid tumors (16).
The NLR has been proposed as another systemic marker of inflammation that acts as a surrogate of solid tumor biology. Although the exact pathophysiology of this relationship is not yet clear, there does appear to be an association between tumor lymphocyte invasion and improved prognosis (17), which indicates that the host tumor response is at least in part dependent on lymphocyte infiltration. Therefore, an elevated NLR might be reflective of a relative lymphopenia, which could be responsible for an inadequate host immune response to the tumor. In addition, the activity of specific subsets of lymphocytes appears to be important. Previously, we have shown patients with HCC have elevations in regulatory T cells (Treg) and blunted CD4 effector T cells (Teff) responses that correlate with tumor burden (18). We have also shown that soluble factors in the serum of HCC patients suppress the activity of Teff by downregulating the expression of cell surface CD25 and enhancing Treg-mediated suppression of Teff (19). A recent study demonstrated that LT patients with high levels of adenosine triphosphate produced by CD4 Teff were more likely to have recurrence of HCC (20). In our study, the majority of subjects with an elevated NLR had relative lymphopenia.
Other groups have proposed that an elevated NLR is more reflective of a relative neutrophilia, which has been shown to correlate with more aggressive tumor behavior in uterine cancer (21). Neutrophils can secrete tumor-promoting agents such as vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP-9) (22,23). Recently, Kuang et al demonstrated a correlation between high levels of neutrophilic infiltration and progression of angiogenesis in HCC (24). Regardless of which mechanism is in effect in an individual patient, it seems that the NLR is a reflection of the global immunity status that has prognostic implications for this patient population.
Given the dearth of donor liver availability, preoperative prediction of post-transplant survival has become critical in allocating this precious resource. The current standard of allocation, based on radiographic appearance, remains limited in its ability to accurately identify particularly aggressive tumors, as evidenced by the high percentage of subjects in our study who were within Milan criteria but had elevated NLR, and subsequently developed recurrent HCC. While pre-treatment liver biopsy would provide histological samples for an investigation of tumor biology, the theoretical risk of tumor seeding has rendered this intervention rarely utilized (25). Therefore, a validated pre-treatment prognostic indicator which can be calculated solely with routine blood work is particularly attractive. Our study confirms that the NLR at the time of diagnosis is predictive of overall survival and recurrence-free survival in patients undergoing LT for HCC. Future endeavors will be required to evaluate the NLR in a larger HCC population and to investigate the significance of changes in the NLR over time (both prior to and following various interventions). Subsequently, the NLR might become a simple and non-invasive addition to the current allocation system for liver transplantation.
Acknowledgments
This work was supported in part by National Institutes of Health/National Center for Research Resources award UL1 RR029890 and National Institutes of Health/National Cancer Institute award K24CA139570.
References
- 1.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003 Nov 18;139(10):817–23. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 2.Ishizaki Y, Kawasaki S. The evolution of liver transplantation for hepatocellular carcinoma (past, present, and future) J Gastroenterol. 2008;43:18–26. doi: 10.1007/s00535-007-2141-x. [DOI] [PubMed] [Google Scholar]
- 3.Ringe B, Picklmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270–85. doi: 10.1007/BF01659064. [DOI] [PubMed] [Google Scholar]
- 4.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhosis. Ann Surg. 1993;218:145–51. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991 Sep;214(3):221–8. doi: 10.1097/00000658-199109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. N Engl J Med. 1996 Mar 14;334(11):693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143(2):182–188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 9.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–8. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 11.Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009 Jul;250(1):141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 12.Bertuzzo VR, Cescon M, Ravaioli M, Grazi GL, Ercolani G, Del Gaudio M, Cucchetti A, D’Errico-Grigioni A, Golfieri R, Pinna AD. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011 Jun 15;91(11):1279–85. doi: 10.1097/TP.0b013e3182187cf0. [DOI] [PubMed] [Google Scholar]
- 13.Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011 May;22(5):702–9. doi: 10.1016/j.jvir.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 14.Liang W, Wang D, Ling X, Cao AA, Kong Y, Shang Y, Guo Z, He X. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2011 Sep 30; doi: 10.1002/lt.22441. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet. 2001 Feb 17;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, Yamaoka T, Iwatani Y, Akazawa K, Takenaka K. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005 May 1;103(9):1856–64. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- 17.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998 Feb;27(2):407–14. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 18.Cao M, Cabrera R, Xu Y, Firpi R, Zhu H, Liu C, Nelson DR. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+) CD25 (+) regulatory T cells. Lab Invest. 2007 Jun;87(6):582–90. doi: 10.1038/labinvest.3700540. [DOI] [PubMed] [Google Scholar]
- 19.Cabrera R, Ararat M, Eksioglu EA, Cao M, Xu Y, Wasserfall C, Atkinson MA, Liu C, Nelson DR. Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scand J Immunol. 2010 Oct;72(4):293–301. doi: 10.1111/j.1365-3083.2010.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng JW, Shi YH, Fan J, Huang XW, Qiu SJ, Xiao YS, Wang Z, Dai Z, Tang ZY, Zhou J. An immune function assay predicts post-transplant recurrence in patient with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2011 Oct;137(10):1445–53. doi: 10.1007/s00432-011-1014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavares-Murta BM, Mendonca MA, Duarte NL, da Silva JA, Mutao TS, Garcia CB, Murta EF. Systemic leukocyte alterations are associated with invasive uterine cancer. Int J Gynecol Cancer. 2010 Oct;20(7):1154–9. doi: 10.1111/igc.0b013e3181ef8deb. [DOI] [PubMed] [Google Scholar]
- 22.Gong Y, Koh DR. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2010 Feb;339(2):437–48. doi: 10.1007/s00441-009-0908-5. [DOI] [PubMed] [Google Scholar]
- 23.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000 Oct;2(10):747–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011 May;54(5):948–55. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Wee A. Fine needle aspiration biopsy of hepatocellular carcinoma and hepatocellular nodular lesions: role, controversies and approach to diagnosis. Cytopathology. 2011 Jul 18; doi: 10.1111/j.1365-2303.2011.00882.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


