Abstract
Antidepressants that produce rapid and robust effects, particularly for severely ill patients, represent one of the largest unmet medical needs for the treatment of depression. Currently available drugs that modulate monoamine neurotransmission provide relief for only a subset of patients and this minimal efficacy requires several weeks of chronic treatment. The recent discovery that the glutamatergic agent ketamine produces rapid antidepressant effects within hours has opened a new area of research to explore the molecular mechanisms through which ketamine produces these surprising effects. Clinical and preclinical findings have exposed some of ketamine's unique actions and identified a cell-signaling pathway known as the mammalian target of rapamycin (mTOR). Activation of mTOR and increased synaptogenesis in the prefrontal cortex appear to be crucial in mediating the antidepressant effects of ketamine. Importantly, the synaptic actions of ketamine allow rapid recovery from the insults produced by exposure to repeated stress that cause neuronal atrophy and loss of synaptic connections. In the following review, we explore some of the clinical and preclinical findings that have thrust ketamine to the forefront of rapid antidepressant research and unveiled some of its unique molecular and cellular actions.
Keywords: GABA, glutamate, BDNF, lithium, scopolamine, GSK-3
Introduction
Depression affects the lives of over 30 million adults in the US (1). While currently available antidepressants may provide roughly 60% of these patients with some relief, achieving this clinical response may require 6-12 weeks of chronic pharmacotherapy, and even longer before an effective treatment is identified. Particularly in the case of acutely suicidal patients, this poses a serious concern to patient welfare and presents a critical need for antidepressants that are more efficacious and rapid-acting. Therapeutic drugs currently approved to treat depressive symptoms target the monoaminergic neurotransmitter systems, however, the discovery that the glutamatergic drug ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, produces rapid (within 2 hrs) and long-lasting (~1 week) antidepressant effects in patients that have previously not responded to several courses of typical antidepressants (2, 3) has sparked a flurry of research to understand how ketamine produces surprisingly fast and efficacious actions.
Findings in post-mortem tissue of depressed patients as well as in rodent stress models designed to mimic depressive-like symptoms suggest that depression is characterized by neuronal atrophy in the prefrontal cortex (PFC) and hippocampus. Interestingly, ketamine has the unique ability to rapidly reverse these neuronal deficits, an effect that typical antidepressants lack, suggesting that recovery of synaptic connections is critical for a rapid antidepressant response. Recent evidence points toward a signaling cascade involved in regulating protein translation and synaptic plasticity known as the mammalian target of rapamycin (mTOR) in mediating these rapid and robust effects of ketamine, which may represent a mechanism common to other putative rapid antidepressants.
In the following review, we describe clinical and preclinical neuronal alterations observed in depression as well as discuss research demonstrating the molecular signaling changes produced by ketamine that mediate the rapid reversal of neuronal deficits and antidepressant responses. Finally, we will discuss novel treatment strategies that may allow ketamine-like rapid antidepressant effects by targeting other receptor systems.
Chronic stress produces neuronal atrophy and maladaptive impairments of neuroplasticity
Theories of depression suggest that exposure to chronic stress, and the neuronal changes that follow, produce susceptibility to mood disorders by impairing synaptic number and function (4-6). Studies of post-mortem human tissue report decreases in neuronal size in the dorsolateral PFC (dlPFC) (7), anterior cingulate cortex (8, 9), orbitofrontal cortex (10-12), and hippocampus (13). Alterations in glial density in the PFC and hippocampus have also been observed (7, 9, 12). A recent electron microscopy study demonstrates a significant reduction in the number of synapses in the dlPFC of depressed patients (14) providing direct evidence for alterations in synaptic structure.
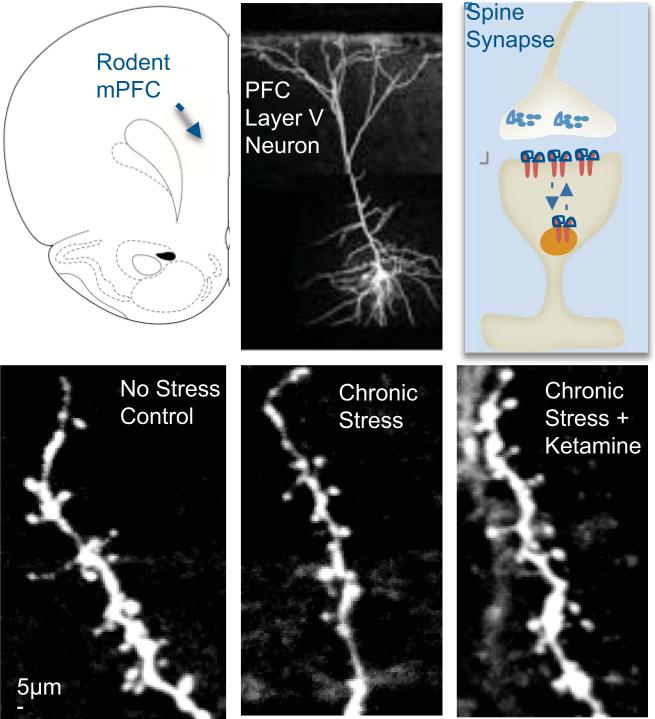
Rodent models using chronic stress exposure to produce depressive-like behavioral states also provide clear evidence of stress-induced neuronal atrophy. Stress or chronic glucocorticoid treatment decreases the number and length of dendritic branches of CA3 pyramidal cells of the hippocampus (15-17). Similar atrophy of glutamatergic pyramidal neuron apical dendrites and decreased spine number are also observed in layers II/III and V of the rodent medial PFC (mPFC) following stress or glucocorticoid treatment (18-20) (21, 22 and Figure 1). Similarly, the loss of dendritic spines following chronic stress is accompanied by a loss of key synaptic proteins such as post-synaptic density 95 (PSD-95), the GluR1 subunit of -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and the presynaptic protein Synapsin I (23). Other brain regions involved in emotional behavior also demonstrate stress-induced changes. For example, dendritic hypertrophy is observed in the amygdala following chronic immobilization stress (24) and a recent report demonstrates that the manifestation of stress-induced anhedonic behavior is mediated by decreased synaptic excitation in the nucleus accumbens (25).
Figure 1. Pyramidal neurons in the PFC with spine synapses and influence of stress and ketamine treatments.
Top panel: shown on the left is a schematic displaying the rat mPFC. In the middle is an image of a neurobiotin-labeled layer V pyramidal neuron from rat mPFC; on the right is a diagram of a spine, with pre- and postsynaptic elements. Bottom panel: the influence of chronic stress exposure (21 d) without or with ketamine administration, compared to non-stressed controls, on apical dendrite spines in layer V pyramidal neurons of rat mPFC. (Adapted from 23).
In addition to neuronal atrophy, chronic stress impairs normal plasticity and some forms of learning and memory. The hippocampus, a brain region that is important for declarative memory, is particularly vulnerable to stress and stress hormones because of the high levels of glucocorticoid receptors in this region. For example, reduced hippocampal volume and reduced neurogenesis are observed following chronic stress (reviewed in 26), which also reduces hippocampal long-term potentiation (LTP), a cellular model of memory (27-29). These alterations in synaptic plasticity are accompanied by impairments of memory in rodent models (30-34). The neuronal and behavioral deficits produced by stress strongly implicate impaired plasticity in the etiology of depression. Recent evidence indicates that mTOR, an important kinase that regulates long-term, protein synthesis-dependent forms of synaptic plasticity may be a key mediator capable of reversing these neuronal and behavioral deficits induced by stress exposure.
mTOR regulates protein translation and synaptic plasticity
mTOR is a ubiquitously expressed serine-threonine protein kinase that integrates signals from neuronal activity, growth factors, energy and nutrient levels to regulate rates of protein translation and synaptic plasticity, as well as other cellular functions. mTOR exists in two complexes known as mTORC1 and mTORC2, which are bound to distinct accessory proteins Raptor and Rictor, respectively, and have different substrates (for an extensive review of mTOR signaling see 35). Downstream of mTORC1 lie two major substrates, p70S6 kinase (S6K) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1). Activation of S6K by mTOR regulates a number of downstream targets, including the S6 ribosomal subunit, leading to induction of mRNA translation. Phosphorylation of 4E-BP1 by mTOR results in its separation from an inhibitory complex with eukaryotic initiation factor 4E (eIF4E), which allows complete formation of the eIF4F complex and cap-dependent translation.
mTOR activity is regulated by a number of upstream mechanisms that converge on the tuberous sclerosis complex 1/2 (TSC1/2) consisting of hamartin (TSC1) and tuberin (TSC2) proteins. This complex has GTPase activating protein (GAP) activity toward the GTPase Ras homologue enriched in brain (RHEB), shifting RHEB toward the GDP-bound form and reduced mTOR signaling. Upstream phosphorylation of TSC1/2 by extracellular signal-related kinase (ERK) and Protein Kinase B (PKB/Akt) inhibit the TSC1/2 complex and therefore activate mTOR. Conversely, activating phosphorylation by glycogen synthase kinase 3 (GSK-3) leads to increased GAP activity of TSC1/2 and subsequent suppression of mTOR signaling. Thus, mTOR has multiple points of upstream regulation that allow it to integrate signals from a variety of stimuli (Figure 2).
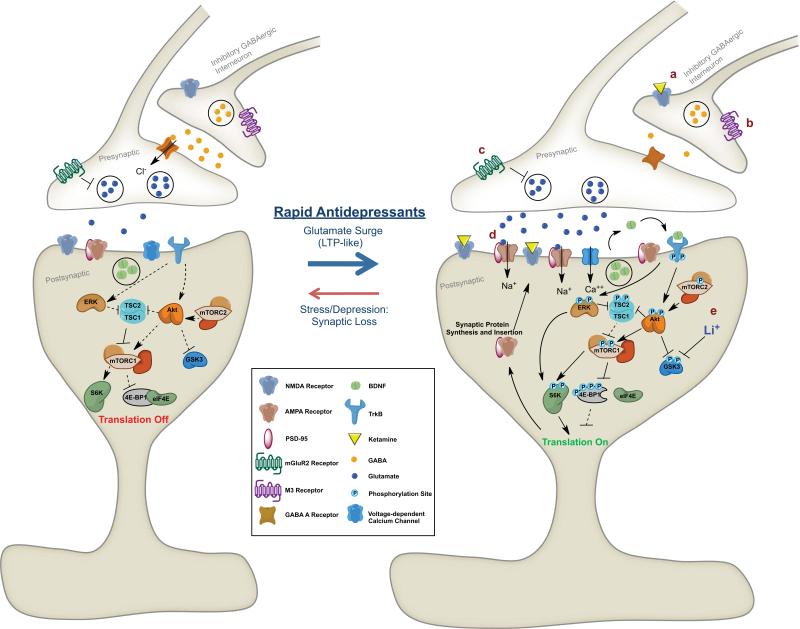
Figure 2. Increases in mTOR signaling and synapse formation in response to treatment with rapid-acting antidepressants.
Excitatory synapse before (Left) and after (Right) treatment with rapid-acting antidepressants. Decreases in inhibitory GABAergic signaling from interneurons induced by (a) NMDA receptor blockade or (b) M3 muscarinic receptor blockade lead to increased glutamate release from pyramidal neurons. Blockade of pre-terminal mGluR2 receptors (c) also leads to increased glutamate release. Activation of postsynaptic AMPA receptors by increased glutamate transmission or (d) direct acting Ampakines leads to depolarization, activation of voltage-dependent calcium channels and release of BDNF. BDNF binds TrkB receptors, leading to transphosphorylation and downstream activation of the ERK and Akt pathways and suppression of GSK-3, which can be augmented by (e) Lithium. These signaling events activate mTOR, leading to downstream phosphorylation of mTOR substrates, S6K and 4E-BP1. mTOR signaling activation leads to increase protein translation and synaptogenesis, mediating rapid antidepressant effects. See text for additional details of pathways leading to regulation of mTOR signaling, including the TSC1/2 complex.
Protein translation, especially translation mediated by mTOR signaling plays an important role in long-term synaptic plasticity. mTOR is activated by signals from growth factors such as Brain-Derived Neurotrophic Factor (BDNF) to regulate protein translation and plasticity locally in the synapse (36). BDNF binds TrkB receptors to activate Ras-MAP Kinase and phophoinositide 3-kinase (PI3K)-Akt pathways, which converge on TSC1/2 to increase mTOR signaling. Treatment of hippocampal slices with the mTORC1 inhibitor rapamycin prevents late-phase, protein synthesis-dependent LTP (37). Tsc1 heterozygous (+/-) deletion mutant mice exhibit enhanced hippocampal LTP, owing to increased activation of mTOR, and have deficits in spatial and context discrimination learning (most likely as a result of aberrant synaptic activity) that can be reversed by inhibiting mTOR with rapamycin (38).
Aberrant mTOR signaling is observed in neurological and psychiatric disorders
A large body of evidence implicates mTOR dysregulation in the etiology of various neurological and psychiatric disorders (39). For example, mTOR signaling is increased in Alzheimer's disease (40) and is involved in learning deficits observed in tuberous sclerosis (38). mTOR signaling is altered in patients with Fragile X syndrome (41), an autism spectrum disorder that is caused by the silencing of the FMR1 gene (42). The product of FMR1 is the mRNA binding protein Fragile X Mental Retardation Protein (FMRP), which controls the expression of hundreds of mRNAs (43) and can be regulated by the downstream effector of mTOR, S6K (44). In a mouse model of Fragile X syndrome, mTOR signaling is increased in the hippocampus and may be an important mediator of this disorder (45). A recent study reports that mTOR signaling proteins are decreased in postmortem PFC of depressed subjects (46).
An interesting feature shared by these disorders is that they are due, in part, to impairments of synaptic plasticity. Given it's important role in regulating synaptic protein translation and LTP, mTOR is perfectly positioned to a mediate rapid reversal of synaptic deficits. Indeed, mTOR activation can regulate rapid translation of both PSD-95 (47) and GluR1 (48), proteins critical for synaptic function that are decreased by chronic stress (23). Novel drugs that can regulate mTOR activity could have utility in the treatment of some of these psychiatric and neurological disorders associated with aberrant mTOR signaling, especially depression.
NMDA receptor antagonists activate mTOR signaling, produce mTOR-dependent behavioral responses, and rapidly reverse the effects of stress
Ketamine produces rapid and acute antidepressant-like effects in the rodent forced swim and learned helplessness tests (49-51). Similarly, in non-stressed, naïve rats, systemic treatment with ketamine rapidly increases levels of synaptic proteins (i.e., GluR1, PSD95) and the number and function of excitatory glutamatergic synapses in the PFC (51). Biochemical studies have discovered that ketamine rapidly activates signaling through the mTOR pathway, as well as downstream substrates of mTOR including, S6K and 4E-BP1 (51). The NR2B selective compound, Ro 25-6981 produces similar activation of mTOR and its substrates (51). Ketamine also increases signaling through ERK and Akt (51), two upstream mTOR regulators that are activated by neurotrophic factors such as BDNF. Importantly, signaling through the mTOR pathway is required for the effects of ketamine as pretreatment with the mTOR inhibitor rapamycin completely abolishes the rapid antidepressant-like activity of ketamine and induction of spine-synapses (51). Rapamycin specifically inhibits mTORC1 (52) indicating a primary role for this complex, although mTORC2 may play a role since ketamine induces phosphorylation of Akt at Ser473 (51), a substrate for mTORC2 (53).
Interestingly, there is a lack of evidence suggesting that some NMDA receptor antagonists, such as PCP and memantine, produce rapid antidepressant effects in humans. The reason for this discrepancy requires further study, but may be due to the doses tested or off-target effects of these compounds. It is also noteworthy that high, subchronic doses of systemic rapamycin, but not acute treatment, produces antidepressant-like effects in rodents (54), which may be due to mTORC2 inhibition by prolonged, but not acute rapamycin (55) or other non-specific pharmacological targets. At present, only systemic ketamine administration has been tested, and future local infusion studies are necessary to determine the specific brain regions that mediate the rapid effects of ketamine.
More recently, studies have focused on the use of rodent chronic stress models that require several weeks of treatment with typical antidepressants to reverse behavioral deficits, recapitulating the therapeutic lag observed in depressed patients. Chronic stress exposure results in anhedonia, a core symptom of depression that is measured by preference for a sweetened solution. In the chronic unpredictable stress (CUS) model, ketamine, as well as the NR2B selective antagonist, Ro 25-6981 produces rapid antidepressant-like behavioral responses within 24 hours of a single treatment, compared to 3 weeks administration of a typical antidepressant (23).
In addition to producing mTOR-dependent antidepressant-like behavioral responses, ketamine reverses the neuronal atrophy produced by stress. Rats exposed to CUS demonstrate reduced levels of synaptic proteins and number and function of glutamatergic synapses in layer V pyramidal cells in the mPFC (21). Ketamine rapidly reverses these synaptic deficits within 24 hours in an mTOR-dependent manner (23). Together, these data demonstrate that NMDA receptor antagonists have the unique ability to rapidly activate mTOR signaling and reverse the impairment of synapses produced by chronic stress exposure.
In contrast to NMDA receptor antagonists, typical antidepressants (e.g., SSRIs or tricyclics) that require several weeks to produce antidepressant effects fail to activate mTOR signaling (51), indicating that these agents produce antidepressant effects through a different mechanism. There are reports that typical antidepressants can influence synaptic plasticity and related proteins. For example, chronic fluoxetine administration is reported to increase dendritic spine density in the retrosplenial granular and prelimbic cortical regions (56). Chronic treatment with typical antidepressants can also reverse the neuronal atrophy and decrease in spine density in the hippocampus and PFC produced by chronic stress (57). Interestingly, other reports found no reversal of stress-induced neuronal atrophy in the hippocampus by typical antidepressants but did find prevention by the atypical antidepressant tianeptine (58). In ovariectomized female rats, chronic fluoxetine increases synaptic levels of PSD-95 and GluR1, as well as phospho-synapsin (59). However, these changes in AMPA receptor subunit expression may be due to trafficking of receptors to the synaptic membrane induced by typical antidepressants and not via protein translation (60, 61). These data highlight ketamine's unique ability to rapidly activate mTOR signaling, increase synaptic protein synthesis and synaptogenesis, and rapidly reverse the synaptic deficits caused by chronic stress.
Several studies provide insight into the mechanisms through which NMDA receptor antagonists activate mTOR signaling and increase synaptogenesis. Low, sub-anesthetic doses of ketamine preferentially decrease the firing rate of fast-spiking GABAergic interneurons, decreasing inhibitory tone, and increasing extracellular levels of glutamate in the rat PFC (62). Although acute stress is also reported to increase extracellular glutamate in the PFC (63) and treatment with typical antidepressants prevents glutamate release induced by acute stress (reviewed in 64), the effects of stress may be more long-lasting and widespread than ketamine, which produces rapid but transient effects. Additionally, synaptic and extrasynaptic NMDA receptors can have different effects on signaling pathways. For example, activation of synaptic NMDA receptors can activate ERK whereas extrasynaptic NMDA receptors suppress ERK signaling (for review see 65). Thus, it is also possible that the detrimental effects of prolonged glutamate elevation produced by chronic stress are mediated by extrasynaptic NMDA receptors, whereas ketamine's primary effects may be mediated by synaptic NMDA receptors, although this requires further study.
Activation of AMPA receptors is required for the antidepressant-like effects of ketamine (50) and blocking AMPA receptors prevents the ketamine-induced increase in mTOR signaling (51). Studies in cultured cells demonstrate that activation of AMPA receptors causes post-synaptic depolarization that opens voltage-dependent calcium channels (VDCCs) leading to calcium influx and release of neurotrophins, including BDNF (66) (Figure 2).
BDNF is required for the actions of ketamine
Reduced BDNF expression and signaling have long been linked to actions of stress and conversely induction of this factor has been implicated in the response to antidepressants. Electroconvulsive shock and chronic antidepressants increase BDNF expression in the hippocampus and PFC (67). Furthermore, infusion of BDNF is sufficient to produce antidepressant-like effects in rodents (68, 69).
Recent studies have demonstrated the importance of BDNF signaling in the rapid antidepressant effects of ketamine. A human single-nucleotide polymorphism (SNP) that substitutes a methionine for valine (Vall66Met) in the gene coding for BDNF produces impaired trafficking and activity-dependent secretion of BDNF (70). Val66Met is associated with memory impairments and hippocampal dysfunction in humans (70). Homozygous mice carrying the Met allele exhibit impaired BDNF secretion and increased anxiety-related behaviors (71), as well as increased susceptibility to stress and altered response to typical antidepressants (72). Interestingly, ketamine fails to produce rapid synaptogenic and behavioral responses in Met mice (73). Importantly, a new study reports that the therapeutic response to ketamine is significantly decreased in depressed patients carrying the Val66Met SNP (74). Ketamine also rapidly increases BDNF translation and ketamine fails to produce antidepressant-like effects in BDNF conditional deletion mutant mice (75). Together, these studies suggest an important role for BDNF signaling in the rapid antidepressant effects of ketamine. Studies are needed to determine if BDNF is sufficient to produce a rapid antidepressant-like response in rodent CUS models.
GSK-3 and inhibition of long-term depression
GSK-3 is important for regulating gene expression and synaptic plasticity and is thought to play an important role in depression, as well as other psychiatric illnesses such as schizophrenia (76). Genetic analyses suggest that SNPs in the GSK-3β gene are associated with occurrence of depression and structural brain changes (77, 78). GSK-3 acts on numerous downstream effectors including TSC1/2. Phosphorylation of TSC2 by GSK-3 enhances the suppression of mTOR signaling in cultured cells (79) and GSK-3 suppresses mTOR signaling in hippocampal tissue (80). GSK-3 can be controlled by several upstream regulators (e.g., Akt and protein phosphatases), which in turn are controlled by NMDA receptor activation. Phosphorylation by Akt suppresses the activity of GSK-3 and leads to activation of mTOR. A recent study provides evidence that suppression of GSK-3 is required for the rapid antidepressant-like effects of ketamine. Treatment of mice with ketamine rapidly increases phosphorylation of both α and β isoforms of GSK-3, leading to suppressed GSK-3 activity (81). Mice carrying a mutant form of GSK-3 that prevents phosphorylation are resistant to the antidepressant-like effects of ketamine (81). The mechanisms through which ketamine inhibits GSK-3 could occur via AMPA receptor activation, BDNF release and activation of Akt signaling leading to decreased GSK-3 activity and activation of mTOR.
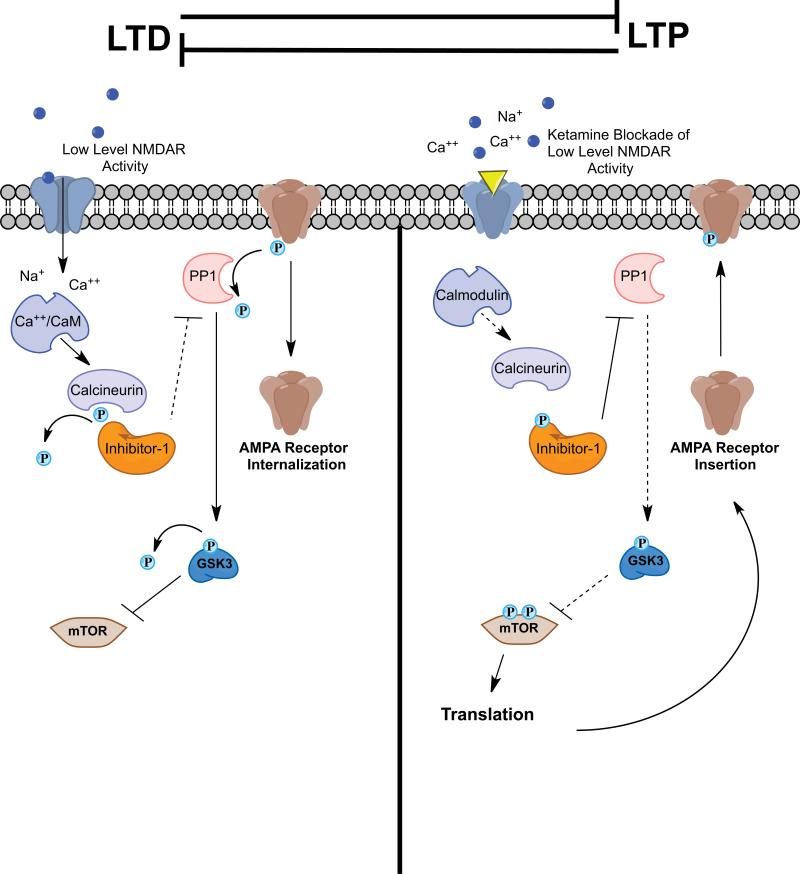
In addition, inhibition of GSK-3 could occur via blockade of postsynaptic NMDA receptors that mediate LTD-like effects. LTP and LTD are cellular models for the two major forms of plasticity in the mammalian nervous system (Figure 3). Maintaining the proper balance of these neuronal plasticity processes is important for regulation of neuronal function, and disruption of this delicate balance may contribute to psychiatric disorders. Ketamine increases the number of mature, mushroom-type spines in the PFC, an indication of increased synaptic stabilization and function, and enhances post-synaptic excitatory responses to serotonin and hypocretin (51). These effects of ketamine are suggestive of an LTP-like mechanism. Conversely, signaling through GSK-3 contributes to LTD mediated by NMDA receptors (NMDA-LTD). During NMDA-LTD, low levels of NMDA receptor stimulation lead to calcium influx and binding of calcium to calmodulin. Calcium/calmodulin activates the phosphatase calcineurin (also known as PP2B) leading to dephosphorylation of Inhibitor-1 (I-1), which suppresses I-1 inhibition of PP1 (i.e., increased PP1 activity) (82). Dephosphorylation of GSK-3 by PP1 may lead to GSK-3 activation and subsequent induction of LTD. Blockade of NMDA receptors with low doses of ketamine may prevent low-level NMDA receptor signaling and LTD, leading to synaptic bias toward LTP and could contribute to enhanced formation and maturation of excitatory spine synapses (Figure 3).
Figure 3. Signaling mechanisms for the regulation of LTD and LTP: potential site of action for ketamine.
LTP and LTD are distinct and opposing processes. During some forms of LTD, low levels of NMDA receptor activation lead to calcium influx and binding to calmodulin. Calcium/Calmodulin activates calcineurin (PP2B), which dephosphorylates and inactivates Inhibitor-1 leading to loss of PP1 suppression. PP1 dephosphorylates AMPA receptor subunits and can lead to receptor internalization. PP1 also dephosphorylates GSK-3 leading to its activation and suppression of mTOR. Blockade of NMDA receptors by ketamine may prevent low-level NMDA receptor activity and subsequent inhibition of mTOR, ultimately leading to activation of mTOR signaling and a synaptic bias toward LTP.
Novel targets for rapid-acting antidepressants
There is an unmet need for novel antidepressant agents that produce more efficacious and rapid antidepressant actions. Recent studies have demonstrated that putative rapid-acting antidepressants may share ketamine's ability to increase mTOR signaling and rapid reversal of the effects of stress. Some of these mechanisms are discussed below and shown in Figure 2.
Combination/Continuation Therapies
Ketamine represents a major advance for the treatment of depression, but the psychotomimetic effects and abuse potential limit its widespread use. Therapies that allow the use of lower and more tolerable doses of ketamine combined with a safer antidepressant or a single dose of ketamine followed by another agent may have promise for improvements. One example of a putative combination therapy may be a low-dose ketamine plus lithium. Lithium inhibits GSK-3 activity and in combination with low-dose ketamine may act synergistically to increase mTOR signaling and produce rapid antidepressant effects. Lithium can take several weeks of treatment to be clinically effective, but when combined with ketamine rapidly decreases immobility time in the forced swim test (FST) in mice, similar to a higher dose of ketamine (83). Given the widespread clinical use of lithium, a ketamine/lithium combination treatment strategy could be quickly evaluated in depressed patients.
As of yet, studies have failed to demonstrate successful prevention of relapse following ketamine and subsequent chronic continuation therapy with safer and better-tolerated agents. For example, treatment with riluzole, a compound that can alter glutamatergic signaling by blocking NMDA receptors (84, 85), facilitating glutamate uptake (86) and increasing membrane AMPA receptor expression (87), failed to delay relapse when administered following a single dose of ketamine (88, 89). Further studies are needed to evaluate additional drugs that could potentially prevent relapse when given following ketamine. It is worth noting, however, that ketamine continues to be effective after repeated treatments (3 × per week for two weeks) in depressed patients (90), indicating that safer agents could prove effective for long-term care.
AMPA Receptors
Based on the evidence that the actions of ketamine require glutamate-induced AMPA receptor activation, it is possible that agents that directly activate or modulate AMPA receptors could produce rapid, ketamine-like antidepressant actions. Ampakines are a unique class of drugs that positively modulate AMPA receptors by altering AMPA receptor kinetics (91). Recently, Ampakines have been developed as nootropic agents due to their ability to positively modulate synaptic plasticity and enhance memory (92, 93). These compounds likely produce their effects, in part by enhancing neurotrophin signaling. For example, the Ampakine CX614, in cultured neurons, increases BDNF release, TrkB receptor activation, and stimulation of mTOR signaling and local protein synthesis (66). Recent studies have demonstrated that Ampakines produce antidepressant-like effects more rapidly than fluoxetine in a rat model of submissive behavior (94). Given these characteristics, Ampakines are good candidates for novel rapid-acting antidepressants, although studies will be required to determine the clinical efficacy and safety of such agents.
mGluR2/3 Receptors
The antidepressant actions of NMDA receptor antagonists may occur via increases in extracellular glutamate (62), which can also be regulated by glutamate terminal autoreceptors. mGluR2 and mGluR3 receptors are Group II metabotropic glutamate receptors that are expressed in limbic brain regions associated with depression including the hippocampus and PFC (95-97). mGluR2 receptors are located in the pre-terminal region of presynaptic neurons (98), while mGluR3 receptors are located post-synaptically and on glia (97). These receptors function through a Gi-coupled mechanism to negatively regulate adenylyl cyclase activity, and presynaptic mGluR2 receptors inhibit the release of glutamate and other neurotransmitters. Selective mGluR2/3 receptor antagonists are reported to produce antidepressant-like effects (99). Similar to the actions of ketamine, blockade of mGluR2/3 receptors with the selective compound LY341495 increases glutamate outflow in limbic regions and the PFC (100, 101). In addition, blockade of post-synaptic AMPA receptors blocks the antidepressant-like effects of mGluR2/3 antagonists as observed with ketamine (99). Recent data also demonstrate that LY341495 increases signaling through the mTOR pathway and increases the expression of the synaptic proteins GluR1, PSD-95 and Synapsin I (102). Indeed, the antidepressant-like behavioral actions of mGluR2/3 antagonists require mTOR signaling (102, 103). Given these similarities to ketamine, compounds that block mGluR2/3 receptors are strong candidates for novel rapid-acting antidepressants. A recent study demonstrates that an mGluR2-selective potentiator also produces antidepressant-like effects in rodents (104), and studies are needed to determine if this is a characteristic of this particular agent or of a class of mGluR2-selective agonists.
Muscarinic Receptors
In the early 1970s, the discovery that the acetylcholinesterase inhibitor, physostigmine, produces symptoms of depression led to the hypothesis that hyperactivity of the cholinergic system contributes to the etiology of depression (105). Early preclinical evaluation of the non-selective muscarinic receptor antagonist, scopolamine, identified antidepressant-like effects in mice, however, these effects were prematurely regarded as non-specific and unrelated to antidepressant activity (106). Recent clinical work demonstrates that intravenous infusion of scopolamine produces rapid antidepressant effects within 3 to 5 days following treatment, with anecdotal reports of improvement after only 1 day (107, 108). Since scopolamine non-selectively blocks all muscarinic receptor subtypes (M1-M5), further studies are necessary to determine which subtype(s) mediate the rapid antidepressant effects. There is evidence that muscarinic receptors modulate glutamate neurotransmission in the cortico-striatal circuit (109). Scopolamine increases the release of glutamate in the striatum (110) and increases excitatory neurotransmission in the medial enthorhinal cortex (111). In the rat visual cortex M3 receptors are located on GABAergic interneurons and antagonists of these receptors lead to decreased inhibitory signaling (112). There is also evidence that postsynaptic M1 receptor agonists produce LTD and decrease postsynaptic glutamate activity in the hippocampus and PFC (113, 114). It is possible that blockade of these M1 and M3 receptor-mediated actions by scopolamine could lead to increased excitatory signaling, glutamate release and subsequent mTOR activation.
Summary and Conclusions
The discovery that ketamine produces rapid and efficacious antidepressant responses in treatment-resistant patients has had a profound impact on research to understand and develop additional rapid-acting agents. Unlike typical antidepressants, ketamine has the unique ability to rapidly reverse the neuronal deficits and impaired plasticity produced by chronic stress. The mTOR pathway plays a critical role in these effects by activating synaptic translational machinery and increasing mature excitatory synapse number and function in the PFC. Future studies aimed at identifying novel targets that increase mTOR signaling could lead to the generation of new antidepressant agents with rapid onset and hold promise for developing more efficacious treatments for depression. Caution must be used when developing compounds that activate mTOR given the role of these cell growth and translation pathways in cancer biology (35). Further studies are also needed to determine the mechanisms underlying the loss of synaptic connections and neuronal atrophy caused by chronic stress (e.g., inhibition of mTOR function). In addition, the neuronal circuits and connections to and from the PFC that underlie the antidepressant actions of ketamine will require further studies. Characterizing the activity of ketamine in other brain regions sensitive to chronic stress will also enhance our understanding of the actions of rapid-acting antidepressants. Finally, it may be possible to develop strategies for preventing, as well treating stress-related mood disorders, such as behaviors that reduce the damaging effects of stress (e.g., stress reduction and management) and approaches that enhance synaptic flexibility and function (e.g., diet, exercise, enriched environments). Combined pharmacological and behavioral therapies that target growth and stabilization of the appropriate cortical and limbic synaptic connections will provide benefits for improved and sustained mental health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Duman reports having received honorarium fees from Lilly, Pfizer, Bristol Myers Squibb, Johnson & Johnson, Forest, and Lundbeck, consulting fees Taisho, and research support from Lilly, Lundbeck, Johnson & Johnson, and Forest. Mr. Dwyer reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 3.Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 8.Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53:1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 9.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 10.Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005;7:358–369. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 11.Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 13.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang H-J, Voleti B, Hajszan T, Rajkowska G, Stockmeier C, Licznerski P, et al. Decreased expression of synapse related genes and loss of synapses in major depression. Nat Med. 2012 doi: 10.1038/nm.2886. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 17.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 18.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 19.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 21.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 27.Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- 28.Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 29.Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 30.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 31.Krugers HJ, Douma BR, Andringa G, Bohus B, Korf J, Luiten PG. Exposure to chronic psychosocial stress and corticosterone in the rat: effects on spatial discrimination learning and hippocampal protein kinase Cgamma immunoreactivity. Hippocampus. 1997;7:427–436. doi: 10.1002/(SICI)1098-1063(1997)7:4<427::AID-HIPO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 33.Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biol Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 35.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, et al. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 41.Hoeffer CA, Sanchez E, Hagerman RJ, Mu Y, Nguyen DV, Wong H, et al. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 2012;11:332–341. doi: 10.1111/j.1601-183X.2012.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 43.Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 44.Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, et al. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- 48.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 50.Maeng S, Zarate CA, Jr., Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 51.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 53.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 54.Cleary C, Linde JA, Hiscock KM, Hadas I, Belmaker RH, Agam G, et al. Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res Bull. 2008;76:469–473. doi: 10.1016/j.brainresbull.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 56.Ampuero E, Rubio FJ, Falcon R, Sandoval M, Diaz-Veliz G, Gonzalez RE, et al. Chronic fluoxetine treatment induces structural plasticity and selective changes in glutamate receptor subunits in the rat cerebral cortex. Neuroscience. 2010;169:98–108. doi: 10.1016/j.neuroscience.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 57.Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 58.McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magarinos AM, McKittrick C. Prevention of stress-induced morphological and cognitive consequences. Eur Neuropsychopharmacol. 1997;7(Suppl 3):S323–328. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 59.O'Leary OF, Wu X, Castren E. Chronic fluoxetine treatment increases expression of synaptic proteins in the hippocampus of the ovariectomized rat: role of BDNF signalling. Psychoneuroendocrinology. 2009;34:367–381. doi: 10.1016/j.psyneuen.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Martinez-Turrillas R, Frechilla D, Del Rio J. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43:1230–1237. doi: 10.1016/s0028-3908(02)00299-x. [DOI] [PubMed] [Google Scholar]
- 61.Martinez-Turrillas R, Del Rio J, Frechilla D. Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology. 2005;49:1178–1188. doi: 10.1016/j.neuropharm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- 64.Musazzi L, Racagni G, Popoli M. Stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem Int. 2011;59:138–149. doi: 10.1016/j.neuint.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 70.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 71.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Antidepressant Efficacy of Ketamine in Depressed Patients. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inkster B, Nichols TE, Saemann PG, Auer DP, Holsboer F, Muglia P, et al. Association of GSK3beta polymorphisms with brain structural changes in major depressive disorder. Arch Gen Psychiatry. 2009;66:721–728. doi: 10.1001/archgenpsychiatry.2009.70. [DOI] [PubMed] [Google Scholar]
- 78.Saus E, Soria V, Escaramis G, Crespo JM, Valero J, Gutierrez-Zotes A, et al. A haplotype of glycogen synthase kinase 3beta is associated with early onset of unipolar major depression. Genes Brain Behav. 2010;9:799–807. doi: 10.1111/j.1601-183X.2010.00617.x. [DOI] [PubMed] [Google Scholar]
- 79.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 80.Ma T, Tzavaras N, Tsokas P, Landau EM, Blitzer RD. Synaptic stimulation of mTOR is mediated by Wnt signaling and regulation of glycogen synthetase kinase-3. J Neurosci. 2011;31:17537–17546. doi: 10.1523/JNEUROSCI.4761-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 83.Ghasemi M, Raza M, Dehpour AR. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacol. 2010;24:585–594. doi: 10.1177/0269881109104845. [DOI] [PubMed] [Google Scholar]
- 84.Debono MW, Le Guern J, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993;235:283–289. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- 85.Hubert JP, Delumeau JC, Glowinski J, Premont J, Doble A. Antagonism by riluzole of entry of calcium evoked by NMDA and veratridine in rat cultured granule cells: evidence for a dual mechanism of action. Br J Pharmacol. 1994;113:261–267. doi: 10.1111/j.1476-5381.1994.tb16203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 87.Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- 88.Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 91.Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 92.Granger R, Staubli U, Davis M, Perez Y, Nilsson L, Rogers GA, et al. A drug that facilitates glutamatergic transmission reduces exploratory activity and improves performance in a learning-dependent task. Synapse. 1993;15:326–329. doi: 10.1002/syn.890150409. [DOI] [PubMed] [Google Scholar]
- 93.Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, et al. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- 94.Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, et al. Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur J Pharmacol. 2002;440:27–35. doi: 10.1016/s0014-2999(02)01338-9. [DOI] [PubMed] [Google Scholar]
- 95.Ohishi H, Ogawa-Meguro R, Shigemoto R, Kaneko T, Nakanishi S, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron. 1994;13:55–66. doi: 10.1016/0896-6273(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 96.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- 97.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 98.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92–98. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 100.Hascup ER, Hascup KN, Stephens M, Pomerleau F, Huettl P, Gratton A, et al. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem. 2010;115:1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 102.Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol. 2012;15:429–434. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011;61:1419–1423. doi: 10.1016/j.neuropharm.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 104.Fell MJ, Witkin JM, Falcone JF, Katner JS, Perry KW, Hart J, et al. N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methy l-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J Pharmacol Exp Ther. 2011;336:165–177. doi: 10.1124/jpet.110.172957. [DOI] [PubMed] [Google Scholar]
- 105.Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 106.Browne RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58:331–334. doi: 10.1016/0014-2999(79)90483-7. [DOI] [PubMed] [Google Scholar]
- 107.Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niittykoski M, Ruotsalainen S, Haapalinna A, Larson J, Sirvio J. Activation of muscarinic M3-like receptors and beta-adrenoceptors, but not M2-like muscarinic receptors or alpha-adrenoceptors, directly modulates corticostriatal neurotransmission in vitro. Neuroscience. 1999;90:95–105. doi: 10.1016/s0306-4522(98)00447-3. [DOI] [PubMed] [Google Scholar]
- 110.Rawls SM, McGinty JF. Muscarinic receptors regulate extracellular glutamate levels in the rat striatum: an in vivo microdialysis study. J Pharmacol Exp Ther. 1998;286:91–98. [PubMed] [Google Scholar]
- 111.Hamam BN, Sinai M, Poirier G, Chapman CA. Cholinergic suppression of excitatory synaptic responses in layer II of the medial entorhinal cortex. Hippocampus. 2007;17:103–113. doi: 10.1002/hipo.20249. [DOI] [PubMed] [Google Scholar]
- 112.Amar M, Lucas-Meunier E, Baux G, Fossier P. Blockade of different muscarinic receptor subtypes changes the equilibrium between excitation and inhibition in rat visual cortex. Neuroscience. 2010;169:1610–1620. doi: 10.1016/j.neuroscience.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 113.Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci. 2009;29:9888–9902. doi: 10.1523/JNEUROSCI.1366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jo J, Son GH, Winters BL, Kim MJ, Whitcomb DJ, Dickinson BA, et al. Muscarinic receptors induce LTD of NMDAR EPSCs via a mechanism involving hippocalcin, AP2 and PSD-95. Nat Neurosci. 2010;13:1216–1224. doi: 10.1038/nn.2636. [DOI] [PubMed] [Google Scholar]