Abstract
Septic shock is one of leading causes of morbidity and mortality in hospital patients. However, genetic factors predisposing to septic shock are not fully understood. Our previous work showed that MCP-induced protein 1 (MCPIP1) was induced by lipopolysaccharides (LPS), which then negatively regulates LPS-induced inflammatory signaling in vitro. Here we report that although MCPIP1 was induced by various toll-like receptor (TLR) ligands in macrophages, MCPIP1-deficient mice are extremely susceptible to TLR4 ligand (LPS)-induced septic shock and death, but not to the TLR2, 3, 5 and 9 ligands-induced septic shock. Consistently, LPS induced tumor necrosis factor α (TNFα) production in MCPIP1-deficient mice was 20-fold greater than that in their wild-type littermates. Further analysis revealed that MCPIP1-deficient mice developed severe acute lung injury after LPS injection and JNK signaling was highly activated in MCPIP1-deificient lungs after LPS stimulation. Finally, macrophage-specific MCPIP1 transgenic mice were partially protected from LPS-induced septic shock, suggesting that inflammatory cytokines from sources other than macrophages may significantly contribute to the pathogenesis of LPS-induced septic shock. Taken together, these results suggest that MCPIP1 selectively suppresses TLR4 signaling pathway and protects mice from LPS-induced septic shock.
Keywords: MCPIP1, Toll-like receptor, LPS, Septic shock, TNF
1. Introduction
The innate immune system is the first line of defense against invading pathogens through an evolutionarily conserved system of pattern recognition [1]. Innate immune cells, including macrophages and dendritic cells, express a series of receptors known as Toll-like receptors (TLRs), which bind to highly conserved sequences expressed by microorganisms [2, 3]. In humans, more than 10 TLRs have been identified. TLR2 heterodimerizes with TLR1 or TLR6, leading to the recognition of Gram-positive bacterial components, such as lipoprotein [4]. TLR4, the first human TLR cloned [5, 6], is engaged by lipopolysaccharide (LPS) found on Gram-negative bacteria, whereas TLR3, TLR5, TLR7, TLR8 and TLR9 recognize other bacterial and/or viral components, including dsRNA (TLR3), flagellin (TLR5), ssRNA (TLR7 and TLR8), and CpG DNA (TLR9) [7]. All TLRs activate NF-κB and MAPKs, but the immediate signaling molecules used by a particular TLR vary [8]. For example, cells deficient in the adaptor protein MyD88 are completely refractory to signaling through near all TLRs, with the notable exceptions of TLR3 and TLR4 [9, 10]. TLR3 is completely dependent on TRIF but not MyD88, whereas TLR4 has two signaling pathways: one is MyD88 dependent, and the other is TRIF dependent. The engagement of TLR by these ligands results in a potent inflammatory response characterized by the release of proinflammatory cytokines, including TNFα, IL-1β, IL-6, IL-12, and IL-18. Activation of the innate immune system is important for subsequent activation of lymphocytes and other cell types and clearance of infectious organisms. However, exuberant production of proinflammatory cytokines leads to severe immunopathology such as endotoxic shock [11]. To prevent deleterious TLR activation, a number of signaling mechanisms are evoked. These mechanisms include the down-regulation of surface TLR expression, transcriptional induction of negative regulators such as IL-1 receptor-associated kinase (IRAK-M), suppressor of cytokine signaling 1 (SOCS1), and SH2-containing inositol phosphatase (SHIP), and production of anti-inflammatory cytokines, mainly IL-10 and TGF-β [12].
MCP-induced protein 1 (MCPIP1, also known as ZC3H12A) is a recently identified gene in human peripheral blood monocytes treated with MCP-1 [13, 14]. The gene undergoes rapid and potent transcription induction upon stimulation with LPS and other proinflammatory molecules, such as TNFα, MCP-1, IL-1β [14–16]. Further studies showed that MCPIP1 plays an important role in both physiological and pathological processes related to inflammation [17–22]. In the experiments on cultured cells, MCPIP1 was proved to be a negative regulator of LPS-induced macrophage activation [14]. In recent reports on mice, MCPIP1 deficiency leads to a complex phenotype including severe anemia, autoimmune response and severe inflammatory response and most mice died within 12 weeks of birth [17, 18]. These results suggest that MCPIP1 may be a resolution molecule that critically control inflammation and immunity and would be a potential therapeutic target for treatment of human inflammatory diseases such as atherosclerosis and septic shock.
In this study, we examined the regulatory role of MCPIP1 in various TLR ligands-induced septic shock using MCPIP1-deficient mice and macrophage-specific MCPIP1 transgenic mice. Though MCPIP1 was significantly induced by various TLR ligands in macrophages, MCPIP1-deficient mice were extremely sensitive to LPS (TLR4 ligand)-induced septic shock, but not sensitive to the ligands for TLR2, 3, 5 and 9-induced septic shock. Consistently, LPS induced TNFα production in MCPIP1-deficient mice was 20-fold greater than that in their wild-type littermates. Moreover, macrophage-specific MCPIP1 transgenic mice were partially protected from LPS-induced septic shock, suggesting that inflammatory cytokines from the sources other than macrophages may significantly contribute to the pathogenesis of LPS-induced septic shock. These results suggest that MCPIP1 may selectively repress TLR4 signaling and protect mice from LPS-induced septic shock.
2. Materials and Methods
2.1 Mice
MCPIP1-deficient (MCPIP1−/−) mice and their wild-type (MCPIP1+/+) littermates on a C57BL/6 background were generated as described previously [17]. Two lines of macrophage-specific MCPIP1 transgenic mice were generated and used in this study (see Fig.5). All mice were housed in the Laboratory Research Animal Center at the University of Missouri Kansas City. All mice were maintained in sterilized filter-top cages and fed autoclaved food and water under specific pathogen-free conditions. All mice used were between the ages of 6 and 8 weeks, unless indicated otherwise. Experimental procedures were approved by the Animal Care and Use Committee of University of Missouri Kansas City.
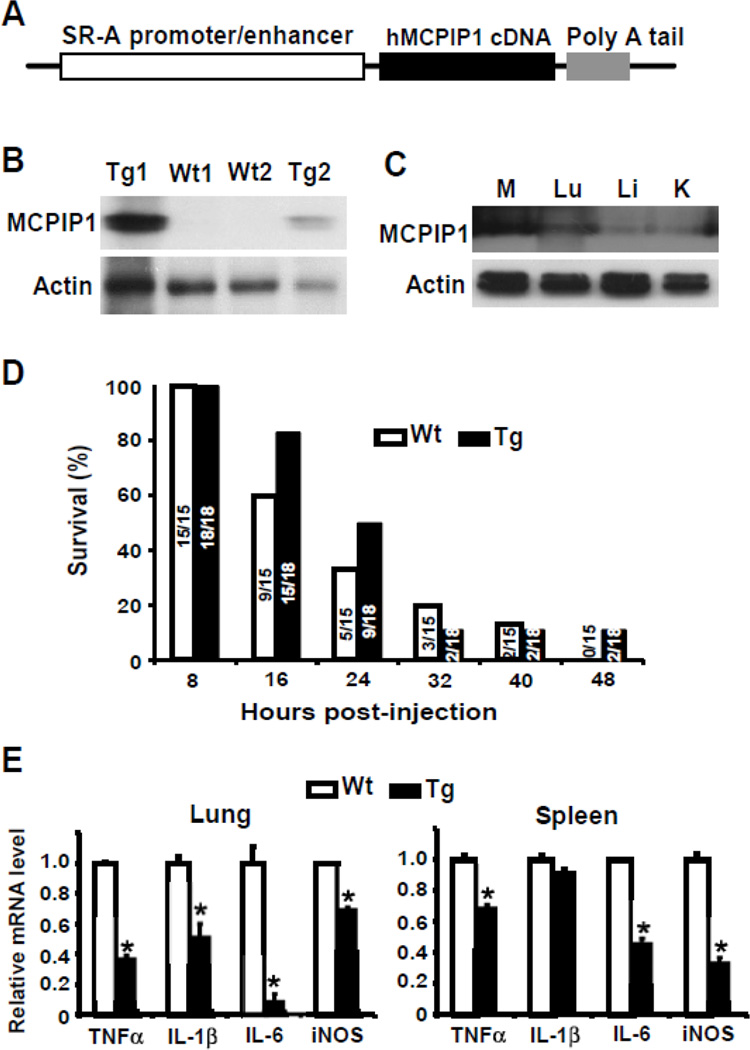
Figure 5. Overexpression of MCPIP1 in macrophages partially protects mice from LPS-induced septic shock.
A) A scheme of construct for generation macrophage-specific MCPIP1 transgenic mice. B) Two transgenic lines and two wild-type lines were identified by genotyping and the expression of MCPIP1 in the macrophages was examined with western blot. C) The MCPIP1 expression in the macrophages, lungs, livers, and kidneys of transgenic mice was also analyzed with western blot. D) 8–10 week-old Tg or WT mice were intraperitoneally injected with 40 mg/kg of LPS and the survival rate was examined every 8 hours for 48 hours. The number on the Bar means absolute survival mouse number/total mouse number. E) The spleen and lungs were harvested from LPS-injected mice and the expression levels of inflammatory cytokines as indicated were measured by QPCR. Data represented mean±SD, n=3–5.
2.2 Cell culture and Reagents
Raw264.7 cells were obtained from the American Type Culture Collection (ATCC) and grown as a monolayer in DMEM (Invitrogen) containing 10% FBS, 2 mM L-glutamine, with 100 U/ml penicillin and streptomycin in 5.0% CO2. The ligands for TLR1–9 were purchased from Invivogen. Protein isolation and Western blot were essentially performed as described previously [17].
2.3 TLR ligands-induced death and cytokine production in mice
Age-matched MCPIP1+/+ and MCPIP1−/− mice were injected intraperitoneally with various ligands for TLRs: Pam3CSK4 (2.5 mg/kg body weight), Pam3CSK4 plus D-galactosamine (1 g/kg bodyweight), poly (I:C) (20 mg/kg body weight), LPS (5 mg/kg body weight), FLA-ST (1 mg/kg body weight), CpG DNA(5 mg/kg body weight). Survival was monitored every 2–8 hours for 72 hours. Spleen RNA was isolated from LPS and poly(I:C)-challenged mice after 75 min and the expression of inflammatory cytokines was measured by QPCR. Blood was obtained from LPS-challenged mice at 2 hours and the serum concentration of TNFα and IL-6 was measured by ELISA. Two lines of macrophage-specific MCPIP1 transgenic mice and wild-type littermates were injected intraperitoneally with LPS (40 mg/kg body weight). Survival was monitored every two hours for 72 hours. The expression of inflammatory cytokines in spleen and lungs was measured by QPCR.
2.4 Histological analysis
Lungs were obtained from LPS-challenged mice at 2 hours. Tissue samples were fixed in 10% neutral buffered formalin for 1 day, subsequently routinely processed, and embedded in paraffin. Sections of 5 µm thickness were stained with hematoxylin and eosin (H&E).
2.5 Statistics
Data were expressed as mean±SD. For comparison between two groups, the unpaired Student’s test was used. For multiple comparisons, analysis of variance followed by unpaired Student’s test was used. A value of p<0.05 was considered significant.
3. Results
3.1 MCPIP1 was remarkably induced by various TLR ligands in Raw264.7 cells
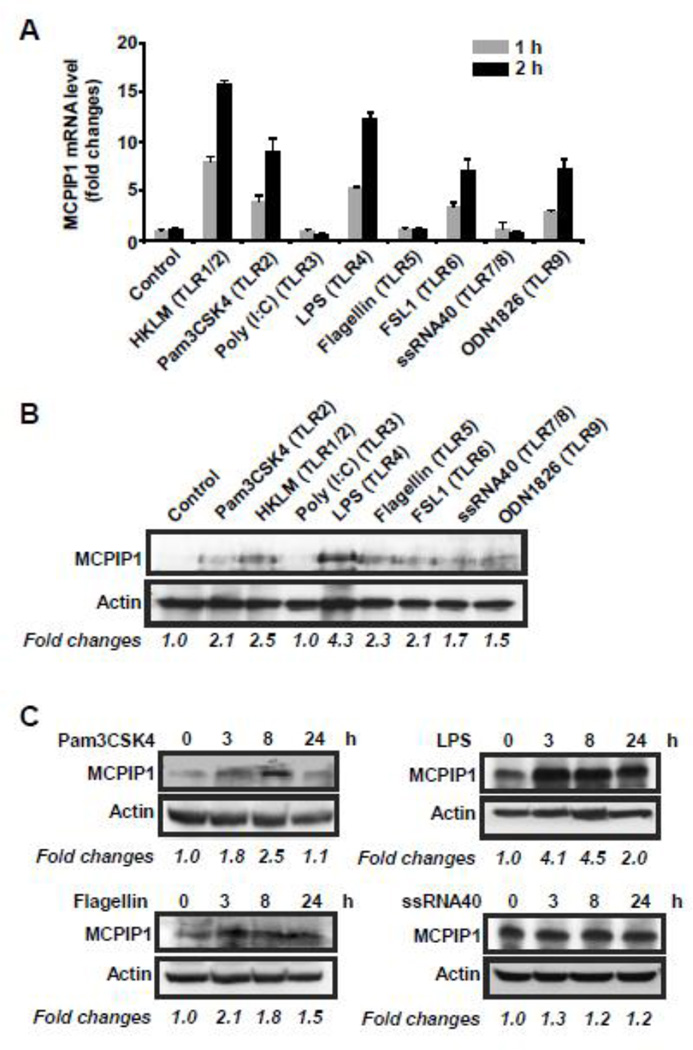
Previously we showed that MCPIP1 was significantly induced by LPS and other inflammatory cytokines in macrophages [14]. To examine whether MCPIP1 is also induced by other TLR ligands, Raw264.7 cells, a murine macrophage cell line, were stimulated with or without various TLR ligands for 1 and 2 hours. QPCR was performed to measure the expression level of MCPIP1 mRNA. As showed in Fig.1A, MCPIP1 expression was significantly induced by the ligands for TLR1/2, 4, 6 and 9 in a time-dependent manner, but not affected by the ligands for TLR3, 5 and 7/8. To verify these results by Western blot, Raw264.7 cells were stimulated with the ligands for TLRs for 8 hours. The cell lysates were isolated and MCPIP1 protein level was detected by Western blot with MCPIP1 antibody (Santa Cruz Biotech.). As shown in Fig.1B, MCPIP1 protein was up-regulated not only by TLR1/2, 4, 6, and 9, but also by TLR5 and TLR7/8. To further confirm these results, Raw264.7 cells were stimulated with the ligands for TLR2, TLR4, TLR5 and TLR7/8 for 0, 3, 8 and 24 hours. As shown in Fig.1C, MCPIP1 protein level was increased by the ligands for TLR2 (Pam3CSK4), TLR4 (LPS) and TLR5 (Flagellin), but not by the ligand for TLR7/8 (ssRNA40) in a time dependent manner. Taken together, these results suggest that MCPIP1 was induced by various TLR ligands and may play a role in the regulation of inflammatory response to various TLR-ligands in innate immune cells.
Figure 1. Activation of TLRs induced MCPIP1 expression in Raw264.7 cells.
A) Raw264.7 cells were stimulated with different ligands for TLRs such as 2×107 cells/ml of HKLM (TLR1/2), 0.2 µg/ml of Pam3CSK4 (TLR2), 5 µg/ml of Poly (I:C) (TLR3), 1 µg/ml of LPS (TLR4), 0.2 µg/ml of Flagellin (TLR5), 0.2 µg/ml of FSL1 (TLR6), 0.5 µg/ml of ssRNA40 (TLR7/8) or 2 µg/ml of ODN1826 (TLR9). 1 and 2 hours later the cells were collected and the MCPIP1 mRNA expression level was examined by QPCR. Data were presented as mean±SD, n=4. B) Raw264.7 cells were stimulated with different ligands for TLRs as above. 8 hours later the cells were collected and the MCPIP1 protein level was examined by Western blot with a MCPIP1 antibody from Santa Cruz Biotech. C) Raw264.7 cells were stimulated with 0.2 µg of Pam3CSK4, 1 µg/ml of LPS, 0.2 µg/ml of Flagellin or 0.5 µg/ml of ssRNA40 (TLR7/8) for different times as indicated. The cells were collected and the MCPIP1 protein level was examined by Western blot as above. The bands of Western blot were quantified by Gel-Pro Analyzer software and presented as fold changes at the bottom of each image.
3.2 MCPIP1 deficient mice are extremely sensitive to LPS-induced septic shock
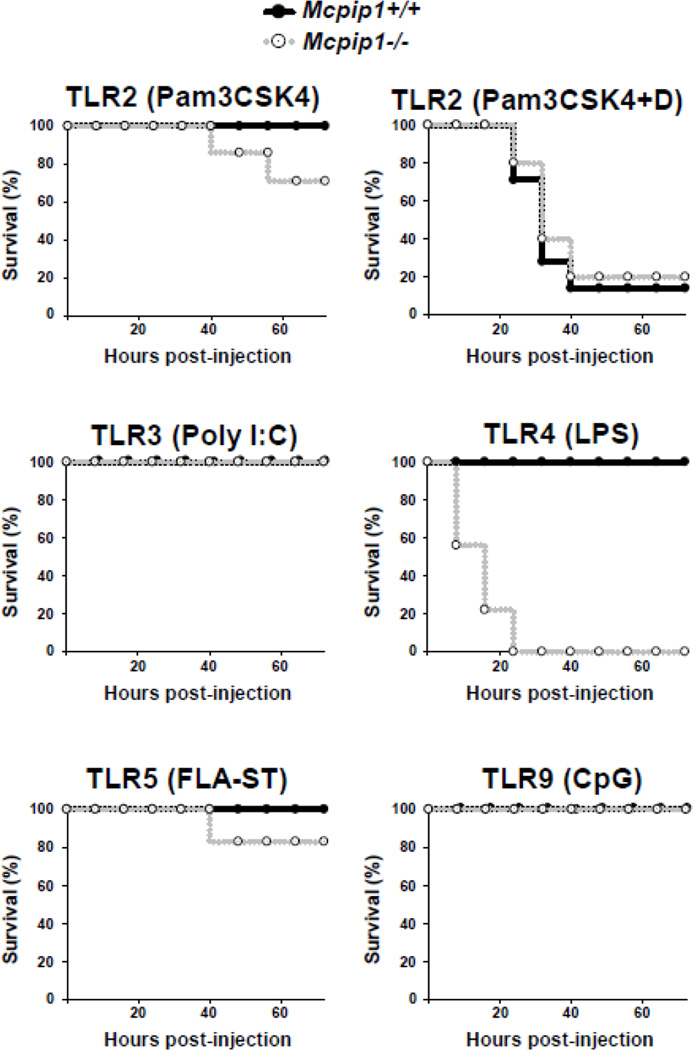
To further investigate whether MCPIP1 is also essential for controlling TLR signaling in vivo, we challenged MCPIP1-deficient mice and their wild-type littermates with sub-lethal dose of ligands for TLR2, 3, 4, 5 and 9, respectively. The mice were closely monitored for 72 hours. As shown in Fig.2, after intraperitoneally injection with 2.5 mg/per kg body weight of Pam3CSK4 (TLR2 ligands), the survival rate of MCPIP1+/+ mice was 100% within 72 hours, however, the survival rate of MCPIP1−/− mice was 66% after 72 hours of treatment. As reported that D-galactosamine can increase the toxicity of TLR2 ligands, we further treated the mice with 2.5 mg/kg body weight of Pam3CSK4 plus 1 g/kg body weight of D-galactosamine. Both MCPIP1−/− mice and their wilt-type littermates developed septic shock and died with similar curves within 72 hour after treatment. These results suggest that MCPIP1 is not effective in preventing TLR2-induced septic shock. However, we observed that MCPIP1−/− mice were extremely sensitive to TLR4 (LPS)-induced death. As shown in Fig.2, after treatment with low dose of LPS (TLR4 ligand, 5 mg/kg, i.p), the survival rate of MCPIP1+/+ mice was 100% within 72 hours, however, all MCPIP1−/− mice were died after 72 hours of treatment. Actually, even injection with 0.5 mg/kg body weight of LPS also causes death of all MCPIP1−/− but none of MCPIP1+/+ mice (data not shown). Both MCPIP1−/− mice and wild-type littermates were 100% survived within 72 hours of treatment with poly(I:C) (TLR3 ligand, 20 mg/kg body weight) or CpG DNA (TLR9 ligand, 5 mg/kg body weight). After treatment with 1 mg/kg of FLA-ST (TLR5 ligand), the survival rate of MCPIP1+/+ mice was 100% within 72 hours, whereas the survival rate of MCPIP1−/− mice were 80% after 72 hours of treatment. Though the ligands for TLR3, 6 and 9 are less lethal, MCPIP1−/− mice were not more sensitive to these ligands-induced septic shock or death. Taken together, these results indicate that MCPIP1 selectively suppresses TLR4-induced inflammation and protects mice from LPS-induced septic shock.
Figure 2. MCPIP1 null mice were extremely sensitive to LPS-induced death.
6–8 weeks old, male and female MCPIP1−/− mice and wild type littermate were intraperitoneally injected with the different ligands for TLRs (Pam3GSK4, 2.5 mg/kg body weight, Pam3GSK4 plus D-galactosamine 1 g/kg), Poly(I:C), 20 mg/kg, FLA-ST 1 mg/kg, LPS 5 mg/kg or CpG, 5 mg/kg). The survival rate of the mice was examined every 2–8 hours for 72 hours. N=6–10.
3.3 LPS, but not poly I:C, induced higher production of inflammatory cytokines in MCPIP1−/− mice than that in wild-type controls
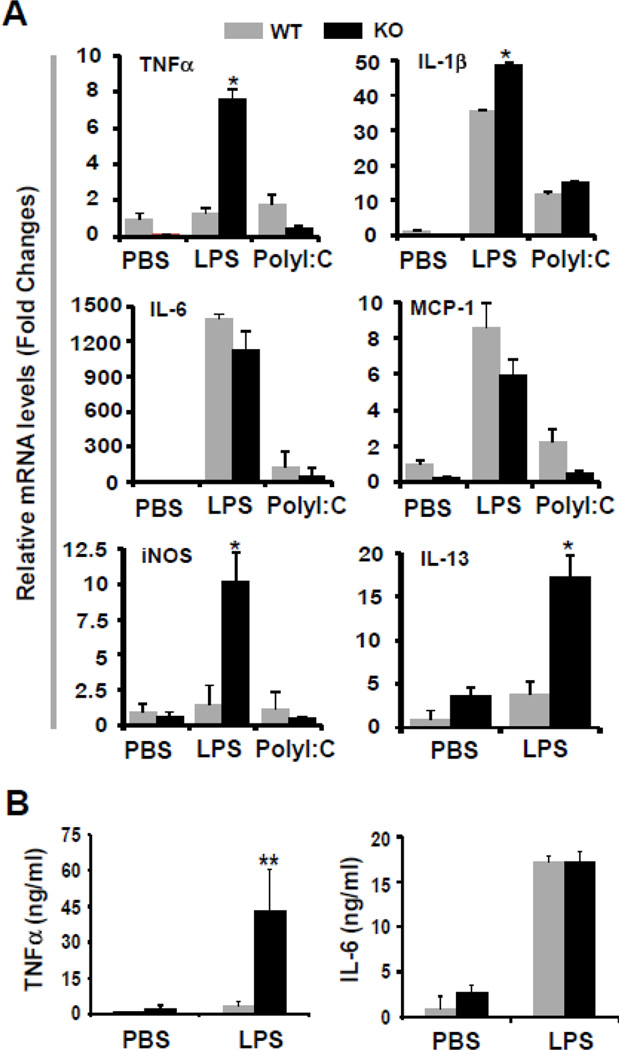
The production of proinflammatory cytokines, such as TNFα, IL-1β, IL-6, IL-12, and IL-13, play a key role in sepsis and septic shock during bacterial infection. To understand the mechanisms underlying that MCPIP1-deficient mice are sensitive to LPS-induced septic shock, we examined the expression of inflammatory cytokines in the spleen from LPS- or poly I:C-treated mice. Adult MCPIP1+/+ and MCPIP1−/− mice were intraperitoneally injected with LPS (5 mg/kg body weight) or PolyI:C (20 mg/kg body weight). After 75 min, the mice were euthanized and the blood and tissues were collected for analysis. The mRNA levels of inflammatory cytokines and iNOS from spleen were measued by QPCR. As shown in Fig.3A, the expression of inflammatory cytokine TNFα, IL-1β and IL-13 and iNOS was significantly increased in the spleen from LPS-treated MCPIP1−/− mice compared with wild-type controls. However, we did not observed the significant difference of the mRNA levels of IL-6 and MCP-1 between MCPIP1−/− mice and wild-type controls. Next, we confirmed that TNFα, but not IL-6 in the blood from LPS-treated MCPIP1−/− mice was increased by 20 folds compared with that from LPS-treated MCPIP1+/+ mice. These results suggest that highly production of TNFα may account for the increase in sensitivity of LPS-induced death in MCPIP1-deficient mice.
Figure 3. LPS, but not polyI:C induced higher production of inflammatory cytokines in MCPIP1−/− mice than that in wild-type control.
6–8 weeks old, both male and female MCPIP1−/− mice and wild type littermate were intraperitoneally injected with 5 mg/kg of LPS or 5 mg/kg of poly(I:C). After 75 minutes, the mice were euthanized and the TNF-α, IL-β, IL-6, MCP-1, iNOS and IL-13 mRNA levels in spleen were examined QPCR (A). The serum TNF-α and IL-6 concentration was also examined by ELISA (B).
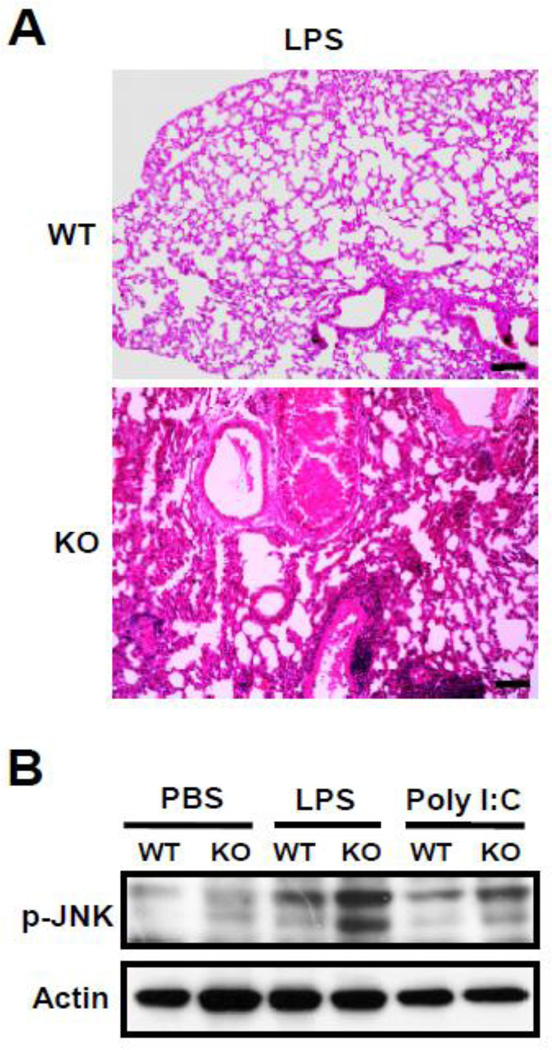
3.4 MCPIP1 deficient mice developed severe acute lung injury and increased JNK phosphorylation in the lungs post-LPS treatment
To further understand how LPS-induced death of MCPIP1−/− mice, we examined the tissue sections by HE staining. As shown in Fig.4A, the lungs from LPS-treated MCPIP1−/− mice developed severe acute inflammation including inflammatory cell infiltration, mucus secretion into bronchioles and edema, which suggests that LPS-treated MCPIP1−/− mice may die from acute respiratory distress syndrome. To further understand why MCPIP1-deficient lungs developed severe acute inflammation post-LPS treatment, we examined LPS-induced inflammatory signaling activation. As shown in Fig.4B, LPS-induced phosphorylation of JNK was significantly increased in the lungs from MCPIP1−/− mice compared that from MCPIP1+/+ mice, which is consistent with our previous finding that MCPIP1 negatively regulates JNK signaling.
Figure 4. LPS-induced acute lung injury and JNK phosphorylation in MCPIP1−/− mice.
A) 6–8 week-old MCPIP1−/− and wild-type mice were injected with 5 mg/kg of LPS (i.p.). After 2 hours, the mice were euthanized and the lungs were harvest for tissue histological analysis. B) 6–8 week-old MCPIP1−/− and wild-type mice were injected with 5 mg/kg of LPS (i.p.) or 20 mg/kg of polyI:C. After 75 min, the mice were euthanized and the lungs were harvest for Western blot analysis with a specific antibody against phosphor-JNK. Data represent three independent experiments.
3.5 Overexpression of MCPIP1 in macrophages partially protects mice from LPS-induced septic shock
Macrophages are the primary source of TNFα. To further investigate whether overexpression of MCPIP1 in macrophages protect mice from LPS-induced septic shock, we generated macrophage-specific MCPIP1 transgenic mice. The human scavenger receptor (SR-A) promoter/enhancer was used to drive macrophage-specific expression of human MCPIP1 in mice. The plasmid pAL1, containing SR-A promoter/ enhancer, was kindly provided by Dr. Christopher Glass (UCSD, La Jolla, CA) [23]. Full-length human MCPIP1 cDNA was cloned into the EcoR V site of the plasmid (Fig.5A). The transgenic DNA injection was performed at the University of Michigan Transgenic Core. The founder lines (on C57Bl/6 background) have been identified by PCR analysis of genomic DNA (data not shown). We have confirmed the specific expression of human MCPIP1 protein in macrophages from two lines by Western blotting with a human MCPIP1 specific antibody (Fig.5B&C). The transgenic mice had normal growth pattern and did not show significant hematological or immune defects. The transgenic mice and wild-type controls were challenged with lethal dose of LPS (40 mg/per kg body weight). As shown in Fig.5D, the macrophage-specific MCPIP1 transgenic mice were partially protected from LPS-induced septic shock. As expected, the expression of inflammatory cytokines, including TNFα, IL-1β, IL-6 and iNOS, was decreased in the lungs and spleen from the transgenic mice compared with that in wild-type mice. These results verified the inhibitory effect of MCPIP1 on the expression of inflammatory cytokines in macrophages. However, overexpression of MCPIP1 in macrophages failed to completely rescue LPS-induced death, suggesting that inflammatory cytokines from the sources other than macrophages may significantly contribute to the pathogenesis of LPS-induced septic shock.
4. Discussion
TLR signaling is a critical activator of immune defense during infection. Effective termination of TLR signaling during innate and adaptive immune responses is essential to prevent potentially detrimental systemic effects, including septic shock and autoimmunity [24–26]. We previously identified MCPIP1 as a critical regulator of LPS-induced macrophage activation in a negatively-feedback loop both in vivo and in vitro [14, 17]. In this study, we compared the effect of MCPIP1 on inflammatory response to different TLR ligands in vivo. The major finding in this study was that although MCPIP1 was induced by various TLR ligands, MCPIP1-null mice were extremely sensitive to LPS-induced death, but not to the septic shock induced by the ligands for TLR2, 3, 5, 7 and 9. The susceptibility of MCPIP1-null mice to LPS-induced death may be mostly accounted by the increase of TNFα production upon LPS stimulation, which is probably related to the increased JNK phosphorylation. These results suggest that MCPIP1 may selectively suppress LPS/TLR4-initiated inflammatory signaling and defect of MCPIP1 function may predispose to the development of gram-negative bacteria-induced septic shock.
Upon binding to TLR ligands, all of TLRs except TLR3 recruit the adaptor molecule MyD88 through the TIR domain, mediating the so-called MyD88-dependent pathway. MyD88 then recruits serine-threonine kinases IL-1R-associated kinase (IRAK) 1. Upon phosphorylation of IRAK1, the IRAK1-TRAF6 complex dissociates from the receptor complex to interact with and activate TGF-β-activated kinase 1 (TAK1). The activation of TAK1 eventually leads to the activation of NF-κB and c-Jun NH2-terminal kinase (JNK), resulting in induction of inflammatory cytokines and chemokines such as TNFα, IL-1β, IL-6 and IL-8. Besides MyD88-dependent pathway, TLR3 and TLR4 also initiate a TRIF-dependent pathway [27, 28]. Why MCPIP1-null mice are selectively sensitive to LPS-induced death is not currently understood. As MCPIP1 null mice developed systemic inflammatory syndromes including increase and activation of various inflammatory cells, heightened production of inflammatory cytokines and multi-organ inflammation, LPS-induced death may be resulted by the over-response of some groups of inflammatory cells to LPS/TLR4 signaling. Moreover, our previous works suggest that MCPIP1 acts as a deubiquitinase and represses inflammatory signaling through deubiquitinating TRAF family. Since TRAFs serve as critical signal transducers not only in TLR4 signaling but also other TLRs signaling, targeting TRAFs would not be the reason that MCPIP1-null mice selectively sensitive to LPS-induced death. However, whether MCPIP1 selectively target a specific signal molecule that is important for TLR4 signaling need be further determined.
Macrophages are primary producer of TNF-α, and suppressing TNF-α has been found to be a major function of MCPIP1 in our present research. Given that TNF-α is a critical drive of septic shock, we reasoned that macrophage-specific MCPIP1 transgenic mice would be protected from LPS-induced septic shock. However, the macrophage-specific MCPIP1 transgenic mice were only partially protected from LPS-induced death. These results suggest that the inflammatory cytokines from other sources or some other mechanisms than TNF-α linked MCPIP1 to LPS induced septic shock. The exact mechanisms need to be further investigated.
Septic shock initiated by Gram-negative bacteria or Gram-positive bacteria is a common cause of death in intensive care unit, which usually showed some different clinical manifestation [29]. The major factor from Gram-negative bacteria to cause septic shock is LPS, which initiates inflammatory response through TLR4. However, the major factors from Gram-positive bacteria to cause septic shock are peptidoglycan (PGN) and lipoteichoic acid (LTA) [30, 31]. PGN is a main component of Gram positive but is also present in Gram-negative bacterial cell walls. LTA is only presented on Gram-positive bacteria. Both of them are known to trigger TLR2 signaling cascade. MCPIP1 selectively suppresses LPS/TLR4-signaling and may be a suitable therapeutic target for Gram-negative bacteria-triggered septic shock. Interestingly, we recently identified another member of MCPIP1 protein family Zc3h12d as a potent inhibitor of TLR2 signaling in vitro [32]. Whether Zc3h12d also selectively suppresses TLR2 signaling in vivo need be further determined.
In summary, in this study we found that MCPIP1-deficient mice are extremely susceptible to TLR4 ligand (LPS)-induced septic shock and death, but not to the TLR2, 3, 5 and 9 ligands-induced septic shock. These results suggest that MCPIP1 may selectively repress TLR4-activated inflammatory signaling and controls inflammatory cytokine production and defection of MCPIP1 function may predispose to gram-negative bacteria-induced septic shock.
Highlights.
We studied the role of MCPIP1 in toll-like receptor signaling in vivo. > MCPIP1 selectively suppressed Toll-like receptor 4 signaling and protects mice from LPS-induced septic shock. > MCPIP1 may be a suitable therapeutic target for Gram-negative bacteria-triggered septic shock.
Acknowledgement
This work was partially supported by National Institute of Health Grants (HL098794 to M.F., CA163808 and CA137126 to J.L., HL097218 and HL076206 to G.L., HL068878 and HL089544 to Y.E.C.). Y.E.C. is an Established Investigator of the American Heart Association (0840025N).
Abbreviations
- MCPIP1
MCP-induced protein 1
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- NF-κB
nuclear factor-κB
- IL-1β
interleukin 1β
- MCP-1
monocyte chemotactic protein-1
- LPS
lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janeway CA CA, Medzhitov R. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Kaisho T, Akira S. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Akira S. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira-Nascimento L, Massari P, Wetzler LM. Front. Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 6.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 7.Kumar H, Kawai T, Akira S. Int. Rev. Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 8.Pennini ME, Perkins DJ, Salazar AM, Lipsky M, Vogel SN. J Immunol. 2013;190:307–316. doi: 10.4049/jimmunol.1201644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. J. Biol. Chem. 2003;278:16713–16719. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- 10.Watters TM, Kenny EF, O’Neill LA. Immunol. Cell Biol. 2007;85:411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Bojorquez LN, Dehesa AZ, Reyes-Teran G. Arch. Med. Res. 2004;35:465–479. doi: 10.1016/j.arcmed.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Liew FY, Xu D, Brint EK, O’Neill LAJ. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 13.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. Circ Res. 2006;98:1177–1185. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang J, Wang J, Azfer A, Song W, Tromp G, Kolattukudy PE, Fu M. J Biol Chem. 2008;283:6337–6346. doi: 10.1074/jbc.M707861200. [DOI] [PubMed] [Google Scholar]
- 15.Liang J, Song W, Tromp G, Kolattukudy PE, Fu M. PLoS One. 2008;3:e2880. doi: 10.1371/journal.pone.0002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skalniak L, Mizgalska D, Zarebski A, Wyrzykowska P, Koj A, Jura J. FEBS J. 2009;276:5892–5905. doi: 10.1111/j.1742-4658.2009.07273.x. [DOI] [PubMed] [Google Scholar]
- 17.Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q, Kolattukudy PE, Fu M. J Exp Med. 2010;207:2959–2973. doi: 10.1084/jem.20092641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- 19.Jura J, Skalniak L, Koj A. Biochim Biophys Acta. 2012;1823:1905–1913. doi: 10.1016/j.bbamcr.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Mizgalska D, Wegrzyn P, Murzyn K, Kasza A, Koj A, Jura J, Jarzab B, Jura J. FEBS J. 2009;276:7386–7399. doi: 10.1111/j.1742-4658.2009.07452.x. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Cao W, Liu H, Zhang W, Liu X, Cai Z, Guo J, Wang X, Hui Z, Zhang H, Wang J, Wang L. PLoS One. 2012;7:e49841. doi: 10.1371/journal.pone.0049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Fu S, Peng W, Rao Z. Protein Cell. 2012;3:903–910. doi: 10.1007/s13238-012-2075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvai A, Palinski W, Wu H, Moulton KS, Kalla K, Glass CK. Proc Natl Acad Sci U S A. 1995;92:5391–5395. doi: 10.1073/pnas.92.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szatmary Z. Gen Physiol Biophys. 2012;31:357–366. doi: 10.4149/gpb_2012_048. [DOI] [PubMed] [Google Scholar]
- 25.Pennini ME, Perkins DJ, Salazar AM, Lipsky M, Vogel SN. J Immunol. 2013;190:307–316. doi: 10.4049/jimmunol.1201644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song DH, Lee JO. Immunol Rev. 2012;250:216–229. doi: 10.1111/j.1600-065X.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 27.Ve T, Gay NJ, Mansell A, Kobe B, Kellie S. Curr Drug Targets. 2012;13:1360–1374. doi: 10.2174/138945012803530260. [DOI] [PubMed] [Google Scholar]
- 28.Layoun A, Huang H, Calvé A, Santos MM. Am J Pathol. 2012;180:2340–2350. doi: 10.1016/j.ajpath.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 29.Martin GS, Mannino DM, Eaton S, Moss M. N Engl J Med. 2000;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 30.Edelson BT, Unanue ER. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 31.Lembo A, et al. Infect. Immun. 2003;71:6058–6062. doi: 10.1128/IAI.71.10.6058-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S, Qi D, Liang J, Miao R, Minagawa K, Quinn T, Matsui T, Fan D, Liu J, Fu M. Cell. Signal. 2012;24:569–576. doi: 10.1016/j.cellsig.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]