Abstract
Defining the molecular mechanisms that underlie development and maintenance of neuronal phenotypic diversity in the CNS is a fundamental challenge in developmental neurobiology. The vast majority of olfactory bulb (OB) interneurons are GABAergic and this neurotransmitter phenotype is specified in migrating neuroblasts by transcription of either or both glutamic acid decarboxylase 1 (Gad1) and Gad2. A subset of OB interneurons also co-express dopamine, but transcriptional repression of tyrosine hydroxylase (Th) suppresses the dopaminergic phenotype until these neurons terminally differentiate. In mature OB interneurons, GABA and dopamine levels are modulated by odorant-induced synaptic activity-dependent regulation of Gad1 and Th transcription. The molecular mechanisms that specify and maintain the GABAergic and dopaminergic phenotypes in the OB are not clearly delineated. In this report, we review previous studies and present novel findings that provide insight into the contribution of epigenetic regulatory mechanisms for controlling expression of these neurotransmitter phenotypes in the OB. We show that HDAC enzymes suppress the dopaminergic phenotype in migrating neuroblasts by repressing Th transcription. In the mature interneurons, both Th and Gad1 transcription levels are modulated by synaptic activity-dependent recruitment of acetylated histone H3 on both the Th and Gad1 proximal promoters. We also show that HDAC2 has the opposite transcriptional response to odorant-induced synaptic activity when compared to Th and Gad1. These findings suggest that HDAC2 mediates, in part, the activity-dependent chromatin remodeling of the Th and Gad1 proximal promoters in mature OB interneurons.
Keywords: dopamine, GABA, tyrosine hydroxylase, glutamic acid decarboxylase, olfactory bulb, adult neurogenesis
1. Introduction
The central nervous system is characterized by its large phenotypic diversity as defined by the partially overlapping expression patterns of neurotransmitters, neuroactive peptides, transmembrane receptors, ion channels and calcium binding proteins. Unique combinations of these molecular factors create distinct functional properties that are essential for the circuits in which specific neurons participate. Elucidating the regulatory mechanisms controlling the expression of genes that encode these molecular features is fundamental not only for understanding the normal development of these circuits, but also for gaining insight into the etiology of nervous system diseases.
The main olfactory bulb (OB) is a well-established brain region to investigate molecular mechanisms that control development of neuronal phenotypic diversity. The OB is the initial processing center for odorant sensory information transduced by olfactory receptor neurons in the olfactory epithelium. Odorant information is relayed from olfactory receptor neurons to several cortical regions via mitral/tufted cells in the OB. Both olfactory receptor neurons and mitral/tufted cells have a glutamatergic neurotransmitter phenotype. Transmission of odorant information by these neurons is modulated by inhibitory GABAergic interneurons in the glomerular and granule cell layers of the OB (Figure 1A).
Figure 1.
Laminar organization and circuit diagram for the olfactory bulb. A, a cartoon summary of the key synaptic connections in the main olfactory bulb based on studies in mice. Olfactory receptor neuronal axons terminate in the glomerular layer where they form axo- and dendro-dendritic connections with both the mitral and tufted cells and inhibitory periglomerular layer interneurons. The mitral/tufted cells are the primary output cells and their axons project to other cortical regions. The transmission of odorant sensory information for higher level processing in the cortex is modulated by inhibitory neurons in the granule cell layer. B, a cartoon of the mouse forebrain showing the position of the individual laminae of the main olfactory bulb (OB), rostral migratory stream (RMS) and subventricular zone (SVZ).
A distinguishing feature of the OB is that the interneurons are continuously generated throughout life. Most embryonic interneuron progenitors originate in the lateral ganglionic eminences, whereas post-natal and adult progenitors are generated within the subventricular zone (SVZ) of the lateral ventricles. In both the embryo and adult, progenitors migrate tangentially towards the OB through the rostral migratory stream (RMS) (Figure 1B). Once in the OB, progenitors migrate radially to their final positions, terminally differentiate and either successfully integrate in the circuitry or undergo apoptosis. Progenitor production in the mouse peaks approximately at post-natal day 4 and continues throughout adulthood at a decreased rate (Hinds, 1968). Disruption in either production or migration of neural progenitors in adult mice impairs olfactory perceptual learning (Moreno et al., 2009), long-term odorant learning and memory (Lazarini et al., 2009; Sultan et al., 2010), odorant based fear conditioning (Valley et al., 2009) and social behaviors (Feierstein et al., 2010).
Studies in the mouse OB indicate that nearly all interneurons express the GABAergic phenotype (Kiyokage et al., 2010; Panzanelli et al., 2007; Parrish-Aungst et al., 2007), but are not otherwise homogenous. A subset of periglomerular interneurons co-express the neurotransmitter dopamine, whereas other subsets are characterized by the co-expression of either neuro-active peptides or calcium binding proteins (Kosaka et al., 1998; Parrish-Aungst et al., 2007; Toida, 2008). Previous studies have suggested that combinatorial expression patterns of transcription factors are critical for specifying the many different OB interneuron phenotypes (Allen et al., 2007; Brill et al., 2008).
In addition to combinatorial codes of transcription factors, epigenetic regulatory mechanisms are required to coordinate the complex patterns of gene transcription necessary for the maturation of specific OB interneuron phenotypes. Chromatin remodeling and DNA methylation are well established epigenetic mechanisms active in regulating neural stem cell proliferation and progenitor migration in the SVZ and RMS (Fasano et al., 2009; Ferron et al., 2011; Lim et al., 2009; Molofsky et al., 2003; Sheikh et al., 2012; Wu et al., 2010). By contrast, their role in regulating gene transcription during OB interneuron maturation is less understood. Here we review previous studies and provide novel evidence for epigenetic regulation of induction and maintenance of gene transcription necessary to express the GABA and dopaminergic neurotransmitter phenotypes in OB interneurons.
2. Materials and methods
2.1 Animal procedures
All procedures were carried out under protocols approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee and conformed to NIH guidelines. Mice were housed in humidity-controlled cages at 22oC under a 12:12 hour light:dark cycle and provided with food and water ad libitum. Th-GFP transgenic mice expressing an enhanced green fluorescent protein (GFP) reporter driven by a 9kb portion of the Th promoter on a C57BL/6J background were obtained from Dr. Kazuto Kobayashi (Matsushita et al., 2002). For mice subjected to unilateral naris closure, one nostril of the mice (aged 6-8 weeks) was surgically closed using a spark-gap cautery under pentobarbital anesthesia. Details of the naris occlusion procedure have been previously published (Baker et al., 1993; Liu et al., 1999). Slice cultures were prepared from 2-day old Th-GFP mice and all studies examining the effect of HDAC inhibitors on either endogenous Th gene or Th-GFP transgene have been described previously (Akiba et al., 2010).
2.2 Gene expression analysis by quantitative PCR
RNA was isolated from adult wild-type C57BL/6J mouse OBs using an RNA miniprep kit (Qiagen) following the manufacturer’s protocol. Reverse transcription and first strand cDNA synthesis reactions were conducted using SuperScript II first strand synthesis kit (Life Technologies). Quantitative PCR (qPCR) reactions for Th, HDAC2 and β-Actin were performed using SYBR Green Master Mix (Life Technologies) with the following primer sets: Th 5′-CACTCCCTGTCAGAGGAGCC-3′and 5′-ATGAAGGGCAGGAGGAATGC-3′; HDAC2 5′- CAACAGATCGCGTGATGACCGTCT-3′ and 5′-GACAGCATAGTATTTTCCCTTTCCAGCA-3′; β- Actin 5′-AGATGACCCAGATCATGTTTGAGACC-3′ and 5′-GGAGTCCATCACAATGCCTGTGGT-3′. Gad1 gene expression was measured using Taqman Gene Expression Assay primer set Mm00725661.s1 (Applied Biosystems) with the TaqMan Universal PCR Master Mix (Applied Biosystems). All qPCR reactions were carried out on a 7500 Fast Real-time PCR System (Applied Biosystems). Expression levels for all genes were measured in triplicate from two mice and normalized to β-Actin expression levels. Expression levels are reported as the mean with error bars representing the standard deviation. Data were analyzed using two-tailed Student T-tests for each gene, and differences were considered significant at p<0.01.
2.3 Chromatin immunoprecipitation (ChIP) experiments
Olfactory bulbs were dissected and fixed for 20 minutes in PBS and 1% formaldehyde, after which the tissue was rinsed in PBS and then placed in lysis buffer (20mM Tris pH 8.1, 150mM NaCl, 0.5% Triton X-100 and 0.1% SDS). Tissue in the lysate suspension was crushed with a Dounce homogenizer before sonication with a Misonix 3000 sonicator (Misonix Inc). Following sonication, cellular debris was removed via centrifugation and lysate was then pre-cleared with Protein A/G Sepharose beads (Santa Cruz). The lysate was then divided into two equivalent samples before adding 1μg of either rabbit pan-acetyl Histone H3 (Millipore 06-599) or, as a negative control, rabbit IgG (Millipore PP64). Antibody/lysate solutions were incubated with gentle rocking overnight at 4°C, before Protein A/G Sepharose was added to precipitate antibody-protein-DNA complexes. The Protein A/G Sepharose beads were then removed from the lysate via centrifugation and washed twice with lysis buffer, twice with wash buffer (20mM Tris pH 8.1, 150mM NaCl, 0.5% Triton X-100 and 0.1% SDS, 2mM EDTA), once with LiCl buffer (0.25M LiCl, 10mM Tris pH 8.1, 1mM EDTA, 1% NP-40, 1% deoxycholate), twice with TE (10mM Tris-EDTA pH 8.0, 1mM EDTA), and then placed in elution buffer (0.1M NaHCO3 pH 8.0, 1%SDS, 0.33M NaCl). The eluted samples were incubated overnight in 5M NaCl at 65°C before the DNA was isolated using Qiaquick PCR clean-up spin kits (Qiagen). DNA concentrations of both immunoprecipitated and input samples were estimated using the Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies) and the manufacturer’s protocol. The immunoprecipitated DNA containing the proximal promoter regions of either Th or Gad1 was measured by qPCR on a 7500 Fast Real-time PCR System (Applied Biosystems) with the following primer sets: Th proximal promoter, 5′-CGTGTCTAGGGCGGAGGGTGA-3′ and 5′-GCCAGGCAGGCGGCCTCTTA-3′; Gad1 proximal promoter, 5′- TTCCCTTGCCCTCACCCAACATCG-3′ and 5′-ACGGGAGAGCCTGAAGAAGGGAGA-3′. For each mouse, qPCR analysis of all samples was measured in triplicate. Data are reported as the mean of three separate ChIP experiments, each using a different mouse, with error bars representing the standard deviation. Data were analyzed using two-tailed Student T-tests, and differences were considered significant if p<0.01.
2.4 Immunohistochemical analysis
Mice were anesthetized with an overdose of pentobarbital (100 mg/kg) and perfused transcardially with phosphate buffered 4% formaldehyde, generated from paraformaldehyde, post-fixed for 1-2 hours at room temperature or overnight at 4oC and then cryoprotected in 30% sucrose. Sections were cut on a sliding microtome at 30μm thickness, then blocked with 1% bovine serum albumin in PBS and incubated overnight with primary antisera. Antibodies and the dilutions used were: rabbit pan-acetyl Histone H3 (Millipore 06-599) at 1:500; rabbit HDAC2 (Epitomics 1603-1) at 1:500; mouse betatubulin (Millipore/Covance MMS-435P) at 1:2,000; rabbit anti-TH (produced in our laboratory as described in Joh et al., 1973) at 1:25,000. Antigens were visualized either by fluorescence with donkey anti-rabbit AlexFluor 594 (Life Technologies) at 1:400 dilution or colorimetric detection using diamionbenzadine. In situ hybridizations were performed as previously described (Saino-Saito et al., 2004). Sections were imaged on both a Nikon 80i Eclipse fluorescence microscope and a Zeiss Meta 510 scanning laser confocal microscope.
3. Results and Discussion
3.1 Regulation of the GABAergic phenotype
The GABAergic phenotype is established early in the development of OB interneurons. Canonical biosynthesis occurs through the single-step conversion of glutamate to GABA by either Glutamic Acid Decarboxylase 1 (GAD1) or GAD2 enzymes. In situ hybridization studies and analysis of transgenic reporters in rodents indicate that neuroblasts in the SVZ and RMS transcribe either or both Gad1 and Gad2 genes (De Marchis et al., 2004; Plachez and Puche, 2012; Wang et al., 2003). Although the Gad genes are transcribed, GAD2 protein is not detected and GAD1 protein, if present, is at low levels in migrating progenitors (De Marchis et al., 2004; Plachez and Puche, 2012; Wang et al., 2003). This lack of GAD protein is paradoxical since GABA is both produced and released by progenitors as they migrate through the SVZ and RMS (Bolteus and Bordey, 2004; Liu et al., 2005). Resolution of this paradox comes from findings that show progenitors can generate GABA by an alternative biosynthetic pathway involving putrescine (Sequerra et al., 2007). In mature OB interneurons, by contrast, GAD1/2 translation is derepressed and GABA is generated by the canonical biosynthetic pathways
Terminal differentiation of OB interneurons requires integration into neuronal circuitry. Synaptic activity levels within OB circuits, however, are strongly modulated by odorant-induced activation of olfactory receptor neurons. Odor deprivation, both short (1-2 month) and long-term (12 months), enhances the sensitivity of mitral/tufted cells to odorant re-exposure, suggesting that inhibition by OB interneurons is depressed by decreased olfactory stimulation (Wilson and Sullivan, 1995). Consistent with these findings, a recent report showed that chemically-induced suppression of synaptic activity in the OB reduces the amplitude of inhibitory post-synaptic currents and decreases GAD1 expression levels in the glomerular layer (Lau and Murthy, 2012). Together with studies showing GAD1 protein levels are reduced in response to odorant-deprivation (Parrish-Aungst et al., 2011), these findings indicate that synaptic activity-dependent control of GAD1 expression levels and GABA biosynthesis is an important modulator of inhibitory transmission in the OB.
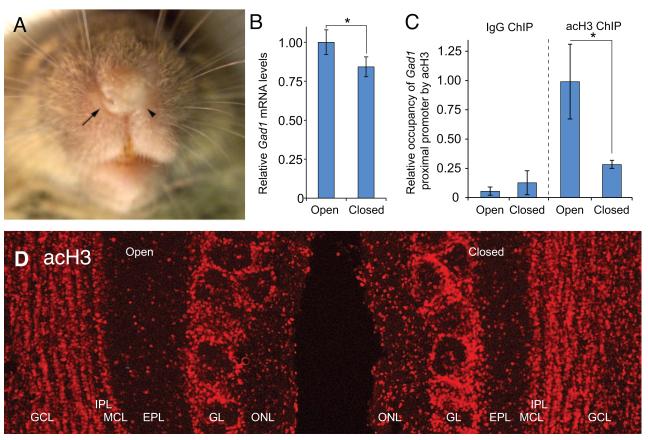
The molecular mechanisms that regulate Gad1 expression in the OB are not established, but epigenetic mechanisms are integral to the control of Gad1 transcription in other brain regions (Dong et al., 2005; Guidotti et al., 2011; Kundakovic et al., 2009; Ramirez and Gutierrez, 2001). Recent studies in our laboratory using mice subjected to unilateral naris occlusion have shown that reduced Gad1 transcription levels in the OB resulting from odor deprivation are associated with a reduced occupancy of acetylated histone H3 on the Gad1 promoter (Figure 2A-C). Acetylated histone H3 is a marker of chromatin environments that are supportive of transcription and these findings indicate that the local chromatin environment of the Gad1 promoter in the OB is regulated by odorant-induced synaptic activity. The reduction of acetylated-H3 on the Gad1 promoter is not a consequence of a general reduction in H3 acetylation caused by odor deprivation since immunofluorescence analysis of the OB in mice with unilateral naris occlusion display no significant decrease in the intensity of staining and no alteration in the distribution of acetylated-H3 (Figure 2D).
Figure 2.
Odorant-mediated regulation of Gad1 transcription in the olfactory bulb. A, a mouse subjected to odorant deprivation by unilateral naris occlusion. The open and closed nares are indicated by the arrow and arrowhead, respectively. B, qRT-PCR analysis reveals Gad1 mRNA levels are reduced in the olfactory bulb that is ipsi-lateral to the closed nares (closed) when compared to the bulb corresponding to the unobstructed contra-lateral nares (open). C, chromatin immunoprecipitation (ChIP) shows that occupancy of pan- acetylated Histone H3 (acH3) on the Gad1 proximal promoter is dramatically reduced in the closed bulb relative to the open bulb. For comparison, negative control ChIP experiments with antibodies to rabbit IgG are also shown. D, immunofluorescence analysis for acH3 in the olfactory bulbs of mice subjected to unilateral naris occlusion shows that the reduced stimulation produced by odor deprivation does not diminish total levels of acH3 in the bulb ipsi-lateral to the naris closure (closed). Rather, there appears to be a slight increase in the acH3 intensity in this bulb. Thus, the changes in acH3 occupancy on the Gad1 proximal promoter observed in C are the result of mechanisms that specifically target the Gad1 promoter.
Taken together, the current evidence indicates that a modified GABAergic phenotype is established early in migrating progenitors. The mature phenotype is not fully expressed until translational repression mechanisms are suppressed as part of the terminal differentiation process. Epigenetic mechanisms modulate GAD1 levels in mature OB neurons by synaptic activity-dependent chromatin remodeling of the Gad1 gene promoter.
3.2 Regulation of the dopaminergic phenotype
In the glomerular layer, a subset of OB interneurons co-express dopamine and GABA. These dopaminergic neurons are readily identified by the expression of tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine biosynthesis. Pre-synaptic release of dopamine by glomerular layer interneurons inhibits both excitation of mitral/tufted cells by olfactory receptor neurons and excitation of interneurons by mitral/tufted cells (Davila et al., 2003; Ennis et al., 2001; Hsia et al., 1999). Recent reports suggest that OB dopaminergic neurons also modulate inter-glomerular connectivity (Kiyokage et al., 2010). Together, these studies suggest that OB dopaminergic neurons are important for both maximizing the dynamic range for odorant detection threshold and improving odorant discrimination.
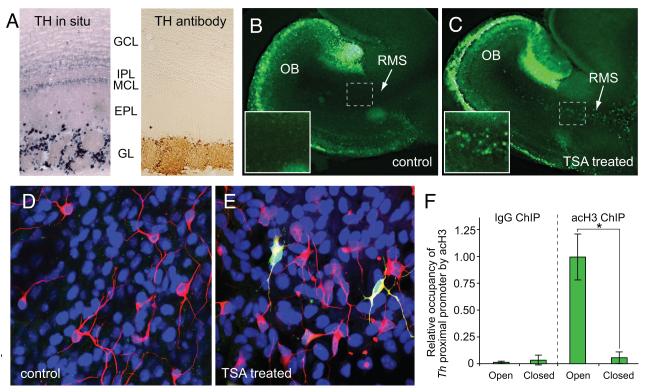
Similar to GAD1, translational regulatory mechanisms spatially restrict TH protein expression to only a subset of cells that transcribe Th (Saino-Saito et al., 2004). Both in situ hybridization and in vivo analysis of Th-GFP reporter gene expression show that Th is transcribed in both the superficial granule cell and glomerular layers, but TH protein expression is limited to only the glomerular layer (Figure 3A). Th-expressing superficial granule cells are reported to co-express NeuN, a marker of mature neurons, suggesting that these cells are terminally differentiated (Kohwi et al., 2005). Belluzzi and colleagues, however, have shown that these cells have electrophysiological properties that are intermediate to migrating progenitors and mature dopaminergic neurons in the glomerular layer, suggesting that they are a reservoir of immature dopaminergic neurons that have not yet completed their migration to the glomerular layer (Pignatelli et al., 2009).
Figure 3.
Histone deacetylase activity suppresses Th transcription in the rostral migratory stream (RMS) and olfactory bulb (OB). A, in situ hybridization in the adult OB shows strong levels of Th transcription in the glomerular layer (GL) as well as weak expression levels in the mitral and superficial granule cell layers (MCL and GCL, respectively). There is scattered transcription in the external plexiform layer (EPL), but no expression in the internal plexiform layer (IPL). In contrast to the in situ hybridization studies, immunohistochemical analysis with antibodies to TH protein reveal that protein expression is limited to only the glomerular layer. B and C, GFP expression in neonatal forebrain slice cultures from Th-GFP mice after 24 hour treatment with either vehicle (control) or 1.2 μM trichostatin A (TSA), respectively. The presence of the HDAC inhibitor, TSA, permits GFP expression within migrating progenitors in the RMS. The slices in B and C were cultured with depolarizing conditions (25mM KCl), which induces GFP expression in the glomerular layer. The presence of TSA, however, substantially increases GFP expression levels in the glomerular layer relative to the control slices. Insets show higher magnifications images of boxed areas. D and E, primary neural progenitor cultures from Th-GFP mice 24 hours after treatment with either vehicle (control) or 1.2 μM trichostatin A (TSA), respectively. The neuronal progenitor marker, β-tubulin III (red) is expressed by several cells in both sets of cultures. By contrast, only TSA-treated cultures show Th-GFP co-expressed in a subset of neuronal progenitors (yellow cells). F, in mice subjected to unilateral naris occlusion, chromatin immunoprecipitation (ChIP) experiments show that occupancy of pan-acetylated Histone H3 (acH3) on the Th proximal promoter is drastically reduced in the bulb ipsi-lateral to nares closure (closed) relative to the bulbs that are contra-lateral (open). For comparison, negative control ChIP experiments with antibodies to rabbit IgG are also shown.
Studies with cultured primary OB neurons indicate that membrane depolarization and activation of L-type calcium channels is sufficient to induce Th transcription (Cigola et al., 1998; McMillian et al., 1994; Puche and Shipley, 1999). Progenitors in either SVZ or RMS, however, do not transcribe the endogenous Th gene (Saino-Saito et al., 2004), despite evidence that they are both depolarized and have L-type calcium channels activated by ambient glutamate in the SVZ and RMS (Darcy and Isaacson, 2009). The molecular mechanisms that mediate this developmental stage-specific repression of Th transcription are not fully established, but we have previously shown that histone deacetylase (HDAC) enzymes are required (Akiba et al., 2010). Treatment with Class I and II HDAC inhibitors (including Trichostatin A, Scriptaid, valproic acid and sodium butyrate) either in forebrain slice cultures or in vivo was sufficient to induce Th promoter activity in the RMS (Figure 3B,C). These studies also found that HDAC inhibitors induced Th transcription in SVZ- derived primary neural progenitor cultures (Figure 3D,E). Together, these findings indicate that transcription factors necessary to initiate Th transcription are present in the migrating neuroblasts, but HDAC activity prevents these proteins from initiating Th transcription.
Our previous studies also found that HDAC inhibitors enhanced Th expression levels in the glomerular layer, suggesting that HDAC-mediated mechanisms continue to regulate Th transcription in newly differentiated and mature dopaminergic neurons. Th expression in the OB glomerular layer is dependent on odorant-mediated synaptic activity (Baker et al., 1983; Saino-Saito et al., 2004). Using mice subjected to unilateral naris occlusion, we have recently found that this activity-dependent transcription is associated with differential occupancy of acetylated H3 on the Th promoter (Figure 3F). This finding reveals that activity-dependent control of Th transcription and dopamine production in mature OB interneurons is mediated, in part, by chromatin remodeling of the Th promoter. Thus, in both neuroblasts and mature neurons epigenetic mechanisms mediated by HDAC enzymes are critical for regulating expression of the OB dopaminergic phenotype.
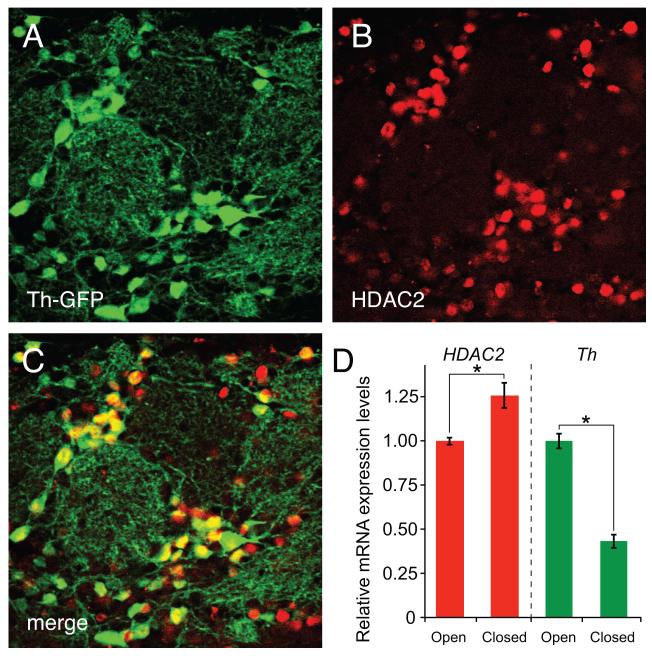
The HDAC enzymes responsible for activity-dependent chromatin remodeling of the Th and Gad promoters have not been established, but recent studies in our laboratory suggest that HDAC2 may play a pivotal role. Previous studies have demonstrated that HDAC2 is preferentially expressed in the terminally differentiated neurons and is critical for the maturation of adult OB interneurons (Jawerka et al., 2010; MacDonald and Roskams, 2008). In agreement with these previous studies, we have found that nearly all dopaminergic OB interneurons in adult Th-GFP transgenic mice (Matsushita et al., 2002) co-express HDAC2 (Figure 4A-C). Interestingly, in mice subjected to unilateral naris occlusion, we have also found that odorant-induced synaptic activity levels differentially modify HDAC2 and Th mRNA levels in the OB (Figure 4D), suggesting that HDAC2 is a negative regulator of the dopaminergic phenotype in mature OB interneurons.
Figure 4.
HDAC2 expression in olfactory bulb dopaminergic neurons. A-C, in the OB of adult Th-GFP mice, immunofluorescence analysis reveals that nearly all GFP-expressing dopaminergic neurons (green) also co-express HDAC2 (red). Regions with Th-GFP and HDAC2 co-expression appear as yellow. D, in mice subjected to unilateral naris occlusion, qRT-PCR analysis shows Th and HDAC2 transcription levels have opposite responses to synaptic activity. In bulbs contra-lateral to naris closure (open), where odorant-mediated synaptic activity is highest, Th expression is greatest and HDAC2 is depressed. By contrast, Th expression is depressed and HDAC2 is maximal in the olfactory bulb ipsi-lateral to naris closure (closed) where synaptic activity is lowest.
3.3 Similarities in epigenetic regulation of Gad1 and Th in the OB
Since Th is preferentially co-expressed with Gad1 in the OB (Parrish-Aungst et al., 2007), a reasonable speculation is that transcription of both Th and Gad1 are regulated by a similar activity-dependent chromatin-remodeling mechanism involving HDAC2. This shared mechanism could be mediated by a common set of transcription factor proteins that bind both the Th and Gad1 promoter regions and recruit HDAC2. Several putative transcription factor binding sites are present in both the Th and Gad1 promoter regions, with some sites found in both genes (Kessler et al., 2003; Kobayashi et al., 2003; Schimmel et al., 1999; Szabo et al., 1996; Yanagawa et al., 1997; Yang et al., 1998). Although a shared regulatory mechanism in the OB has yet to be demonstrated, possible co-regulation by the neural restrictive silencer element (NRSE) is a high priority candidate for further investigation. The NRSE can recruit multi-protein complexes that contain HDAC2 to repress neuronal gene expression (Ballas et al., 2001; Roopra et al., 2000) and NRSEs are present in the proximal promoter regions of both Th and Gad1 (Katarova et al., 1998; Kessler et al., 2003; Kim et al., 2003; Szabo et al., 1996). The ability of membrane depolarization to abrogate NRSE-mediated repression of some neuronal genes is also consistent with a potential role in regulating the synaptic activity-dependent transcription of Th and Gad1 in the OB (Ballas et al., 2005).
Epigenetic regulation of neuronal gene transcription typically involves both covalent modifications to histones and DNA methylation (reviewed in Hsieh and Gage, 2005). The findings discussed in this report provide insight into the role that histone modifications have in regulating the GABAergic and dopaminergic phenotypes in the OB, but the role of DNA methylation in the chromatin remodeling of either Th and Gad1 promoter remains to be established. Methylation of the Gad1 promoter in cortical interneurons reduces transcription and this methylation is modulated by both H3 acetylation levels and activation of metabotropic glutamate receptors (Dong et al., 2007; Matrisciano et al., 2011). Since progenitors of both cortical and OB interneurons are derived from the embryonic ganglionic eminences, similar mechanisms may also mediate methylation of Gad1 in OB GABAergic interneurons. By contrast, the role of DNA methylation in regulating Th transcription within the brain is largely unexplored, although differences in Th promoter methylation levels between neural and non-neural cell lines have been reported (Aranyi et al., 2005; Kilbourne et al., 1991; Yang et al., 2011).
Th and Gad1 transcription appear coordinated in mature OB interneurons, but expression of these genes in migrating neuroblasts is clearly not. The molecular basis for this differential transcriptional regulation in progenitors is not understood, but an important component may be the developmental stage-specific expression of epigenetic regulatory proteins. In olfactory receptor neuron development, HDAC 1/2, methyl CpG binding protein 2 (MeCP2), methyl binding domain (MBD) 2, and DNA methyl transferase (DNMT) 3a/b proteins are co-expressed in developmental stage-specific patterns (MacDonald et al., 2005; MacDonald and Roskams, 2008; Macdonald et al., 2010). These stage-specific combinatorial co-expression patterns are thought to be critical for differentially expressing genes at distinct stages of maturation. Further studies are necessary to establish whether similar combinatorial co-expression patterns of epigenetic regulatory proteins also control the development and maintenance of neurotransmitter phenotypes in the OB.
3.4 Developmental role for regulating timing of neurotransmitter phenotype expression
The restriction of both the mature GABAergic and dopaminergic phenotypes to terminally differentiated neurons in the OB may be related to the role that these neurotransmitters have as non-synaptic modulators of neurogenesis in the SVZ and RMS. Progenitor proliferation and migration rates in these regions are modulated by neurotransmitters generated locally (GABA and glutamate) and distantly in other brain regions (dopamine and serotonin) (reviewed in Bovetti et al., 2011; Young et al., 2011). GABA acts as a negative regulator of both proliferation and migration (Platel et al., 2008a). By contrast, glutamate released by astrocyte-like cells in the SVZ stimulates proliferation and decreases migration (Castiglione et al., 2008; Di Giorgi Gerevini et al., 2004; Gandhi et al., 2008; Platel et al., 2008b). Together, GABA and glutamate function as local modulators of neurogenesis. In addition to these local signals, serotonin and dopamine released by terminals projecting from the Raphe nuclei and midbrain, respectively, stimulate neurogenesis in the SVZ (Baker et al., 2004; Banasr et al., 2004; Brezun and Daszuta, 1999; Freundlieb et al., 2006; Hoglinger et al., 2004; O’Keeffe et al., 2009b). In the case of dopamine, the loss of midbrain dopaminergic inputs either through deafferentation or chemical lesions reduces SVZ progenitor proliferation rates and suggest that OB interneuron neurogenesis is influenced by the behavioral and movement control functions of the midbrain (O’Keeffe et al., 2009a).
Regulation of progenitor proliferation and migration in the SVZ by distant brain regions indicates a need to tightly coordinate local biosynthesis of neurotransmitters. Given the large number of neuroblasts generated daily in the SVZ (Lois and Alvarez-Buylla, 1994), unrestrained production and release of neurotransmitters by neuroblasts could overwhelm regulatory signals from distant regions. Dopamine production by neuroblasts, for example, would likely increase progenitor proliferation rates, but diminish or screen out the input from midbrain neurons. The functional consequences of either a sustained increase in progenitor proliferation or a loss of midbrain input, however, are difficult to predict. By contrast, increased ambient concentrations of GABA in the SVZ and RMS could depress proliferation and migration to the point where the numbers of progenitors are insufficient to replace aging OB interneurons. Reductions in either the production or migration of neural progenitors in adult mice has been shown to impair some olfactory- based cognitive functions (reviewed in Lazarini and Lledo, 2011).
4. Conclusions
Epigenetic mechanisms are integral for establishing and maintaining dopaminergic and GABAergic phenotypes in OB interneurons. HDAC enzymes repress Th transcription and suppress the dopaminergic phenotype in migrating neuroblasts until progenitors terminally differentiate in the OB. In the mature interneurons, both Th and Gad1 transcription levels are modulated by odorant-induced synaptic activity that results in the differential recruitment of acetylated histone H3 on both the Th and Gad1 proximal promoters. The inverse transcription response of HDAC2 to odorant-induced synaptic activity, as compared to Th and Gad1, suggests that HDAC2 mediates, in part, the activity-dependent chromatin remodeling of the Th and Gad1 proximal promoters.
Although epigenetic regulatory mechanisms have a clear role in establishing and maintaining neurotransmitter phenotypes in the OB, there are several important features of this regulation that require further exploration. The role of promoter DNA methylation, in particular, is largely unexplored in OB neurogenesis and is likely an important regulatory mechanism for chromatin remodeling that is coordinated with covalent modifications of histones. Elucidation of these mechanisms will also require establishing the developmental stage-specific roles of epigenetic regulatory proteins during OB neurogenesis. As discussed above, studies in olfactory receptor neuron development indicate that complex combinatorial co-expression patterns of these proteins delineate specific transitions in the neuronal maturation process (Macdonald et al., 2010). In the OB dopamine phenotype, for example, further studies are necessary to establish whether HDAC1 and HDAC2 have distinct developmental roles in regulating Th transcription. These two enzymes have complementary expression patterns during development of OB interneurons: HDAC2 is preferentially expressed in mature neurons and HDAC1 is expressed in neuroblasts and glia (MacDonald and Roskams, 2008). HDAC1 may be responsible for keeping Th expression repressed when migrating neuroblasts are depolarized, whereas HDAC2 may be required for mediating synaptic activity-dependent regulation of Th transcription in mature neurons. Together, the data discussed here provide insight into the role that epigenetic mechanisms have for coordinating both developmental signals and synaptic activity levels to establish and maintain neurotransmitter phenotypes in the OB.
Highlights.
HDAC activity represses dopamine phenotype in migrating olfactory bulb progenitors
Th and Gad1 levels in mature olfactory bulb neurons are synaptic activity dependent
Synaptic activity remodels Th and Gad1 proximal promoter chromatin environments
HDAC2 expression in mature olfactory bulb neurons is reduced by synaptic activity
Table 1.
Gene and protein expression for GABAergic and dopaminergic rate-limiting biosynthetic enzymes (Gad1/2 and Th, respectively) in relationship to HDAC1/2 protein levels during OB interneuron development. Expression levels are indicated as strong (+++), modest (++), low (+), weak (+/−) and not expressed (−). Odorant-induced synaptic activity dependent expression is indicated by "AD." For the GAD and TH proteins, protein expression levels do not necessarily directly correspond to levels of the respective neurotransmitters present in the cell. For GAD, in particular, there are alternative biosynthetic pathways for GABA biosynthesis in progenitor cells (see section 3.1 for details).
| Cell Type | Gad1 | Gad2 | TH | HDAC1 | HDAC2 | |||
|---|---|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | Protein | Protein | |
| Migrating Neuroblast |
+++ | +/− | +++ | − | − | − | +++ | +/− |
| Immature OB Neuron |
+++ | +/− | +++ | − | + | − | ++ | ++ |
| Terminally Differentiated OB Neuron |
+++ | +++ (AD) |
+++ | +++ | +++ | +++ (AD) |
+/− | +++ |
| References | De Marchis et al., 2004; Planchez and Puche, 2012; Wang et al., 2003 | Baker et al., 1983; Saino-Saito et al., 2004 | Jawerka et al., 2010; MacDonald and Roskams, 2008 | |||||
Acknowledgements
We thank the Ratan and Langley Laboratories (Burke Medical Research Institute/Weill Cornell Medical College) for providing antibodies. This work was supported by National Institutes of Health grant R01 DC008955.
Abbreviations
- acH3
acetylated Histone H3
- ChIP
chromatin immunoprecipitation
- GABA
gamma aminobutyric acid
- GAD
glutamic acid decarboxylase
- HDAC
histone deacetylase
- NRSE
neural restrictive silencer elements
- OB
olfactory bulb
- qPCR
quantitative polymerase chain reaction
- RMS
rostral migratory stream
- SVZ
subventricular zone
- TH
tyrosine hydroxylase
- TSA
Trichostatin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiba Y, Cave JW, Akiba N, Langley B, Ratan RR, Baker H. Histone deacetylase inhibitors de-repress tyrosine hydroxylase expression in the olfactory bulb and rostral migratory stream. Biochemical and biophysical research communications. 2010;393:673–677. doi: 10.1016/j.bbrc.2010.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen ZJ, 2nd, Waclaw RR, Colbert MC, Campbell K. Molecular identity of olfactory bulb interneurons: transcriptional codes of periglomerular neuron subtypes. Journal of molecular histology. 2007;38:517–525. doi: 10.1007/s10735-007-9115-4. [DOI] [PubMed] [Google Scholar]
- Aranyi T, Faucheux BA, Khalfallah O, Vodjdani G, Biguet NF, Mallet J, Meloni R. The tissue-specific methylation of the human tyrosine hydroxylase gene reveals new regulatory elements in the first exon. Journal of neurochemistry. 2005;94:129–139. doi: 10.1111/j.1471-4159.2005.03173.x. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. doi: 10.1016/0006-8993(93)91023-l. [DOI] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. The European journal of neuroscience. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, Federoff HJ, Rose DW, Rosenfeld MG, Brehm P, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. doi: 10.1016/s0896-6273(01)00371-3. [DOI] [PubMed] [Google Scholar]
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S, Gribaudo S, Puche AC, De Marchis S, Fasolo A. From progenitors to integrated neurons: role of neurotransmitters in adult olfactory neurogenesis. Journal of chemical neuroanatomy. 2011;42:304–316. doi: 10.1016/j.jchemneu.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Brill MS, Snapyan M, Wohlfrom H, Ninkovic J, Jawerka M, Mastick GS, Ashery-Padan R, Saghatelyan A, Berninger B, Gotz M. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6439–6452. doi: 10.1523/JNEUROSCI.0700-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione M, Calafiore M, Costa L, Sortino MA, Nicoletti F, Copani A. Group I metabotropic glutamate receptors control proliferation, survival and differentiation of cultured neural progenitor cells isolated from the subventricular zone of adult mice. Neuropharmacology. 2008;55:560–567. doi: 10.1016/j.neuropharm.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cigola E, Volpe BT, Lee JW, Franzen L, Baker H. Tyrosine hydroxylase expression in primary cultures of olfactory bulb: role of L-type calcium channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:7638–7649. doi: 10.1523/JNEUROSCI.18-19-07638.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy DP, Isaacson JS. L-type calcium channels govern calcium signaling in migrating newborn neurons in the postnatal olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2510–2518. doi: 10.1523/JNEUROSCI.5333-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila NG, Blakemore LJ, Trombley PQ. Dopamine modulates synaptic transmission between rat olfactory bulb neurons in culture. Journal of neurophysiology. 2003;90:395–404. doi: 10.1152/jn.01058.2002. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Temoney S, Erdelyi F, Bovetti S, Bovolin P, Szabo G, Puche AC. GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. The European journal of neuroscience. 2004;20:1307–1317. doi: 10.1111/j.1460-9568.2004.03584.x. [DOI] [PubMed] [Google Scholar]
- Di Giorgi Gerevini VD, Caruso A, Cappuccio I, Ricci Vitiani L, Romeo S, Della Rocca C, Gradini R, Melchiorri D, Nicoletti F. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain research. Developmental brain research. 2004;150:17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12578–12583. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. Journal of neurophysiology. 2001;86:2986–2997. doi: 10.1152/jn.2001.86.6.2986. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Phoenix TN, Kokovay E, Lowry N, Elkabetz Y, Dimos JT, Lemischka IR, Studer L, Temple S. Bmi-1 cooperates with Foxg1 to maintain neural stem cell self-renewal in the forebrain. Genes & development. 2009;23:561–574. doi: 10.1101/gad.1743709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein CE, Lazarini F, Wagner S, Gabellec MM, de Chaumont F, Olivo-Marin JC, Boussin FD, Lledo PM, Gheusi G. Disruption of Adult Neurogenesis in the Olfactory Bulb Affects Social Interaction but not Maternal Behavior. Frontiers in behavioral neuroscience. 2010;4:176. doi: 10.3389/fnbeh.2010.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron SR, Charalambous M, Radford E, McEwen K, Wildner H, Hind E, Morante-Redolat JM, Laborda J, Guillemot F, Bauer SR, Farinas I, Ferguson-Smith AC. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475:381–385. doi: 10.1038/nature10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlieb N, Francois C, Tande D, Oertel WH, Hirsch EC, Hoglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Luk KC, Rymar VV, Sadikot AF. Group I mGluR5 metabotropic glutamate receptors regulate proliferation of neuronal progenitors in specific forebrain developmental domains. Journal of neurochemistry. 2008;104:155–172. doi: 10.1111/j.1471-4159.2007.04955.x. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011;60:1007–1016. doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. The Journal of comparative neurology. 1968;134:287–304. doi: 10.1002/cne.901340304. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nature neuroscience. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. Journal of neurophysiology. 1999;82:1082–1085. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Current opinion in cell biology. 2005;17:664–671. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Jawerka M, Colak D, Dimou L, Spiller C, Lagger S, Montgomery RL, Olson EN, Wurst W, Gottlicher M, Gotz M. The specific role of histone deacetylase 2 in adult neurogenesis. Neuron glia biology. 2010;6:93–107. doi: 10.1017/S1740925X10000049. [DOI] [PubMed] [Google Scholar]
- Joh TH, Geghman C, Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proceedings of the National Academy of Sciences of the United States of America. 1973;70:2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katarova Z, Mugnaini E, Sekerkova G, Mann JR, Aszodi A, Bosze Z, Greenspan R, Szabo G. Regulation of cell-type specific expression of lacZ by the 5′-flanking region of mouse GAD67 gene in the central nervous system of transgenic mice. The European journal of neuroscience. 1998;10:989–999. doi: 10.1046/j.1460-9568.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- Kessler MA, Yang M, Gollomp KL, Jin H, Iacovitti L. The human tyrosine hydroxylase gene promoter. Brain research. Molecular brain research. 2003;112:8–23. doi: 10.1016/s0169-328x(02)00694-0. [DOI] [PubMed] [Google Scholar]
- Kilbourne EJ, Osaka H, Sabban EL. Hypomethylation of the rat tyrosine hydroxylase gene correlates with its expression in several cell types. Brain research. Developmental brain research. 1991;58:143–146. doi: 10.1016/0165-3806(91)90247-g. [DOI] [PubMed] [Google Scholar]
- Kim TE, Park MJ, Choi EJ, Lee HS, Lee SH, Yoon SH, Oh CK, Lee BJ, Kim SU, Lee YS, Lee MA. Cloning and cell type-specific regulation of the human tyrosine hydroxylase gene promoter. Biochemical and biophysical research communications. 2003;312:1123–1131. doi: 10.1016/j.bbrc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Pan YZ, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, Shipley MT. Molecular identity of periglomerular and short axon cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:1185–1196. doi: 10.1523/JNEUROSCI.3497-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ebihara S, Ishii K, Kobayashi T, Nishijima M, Endo S, Takaku A, Sakagami H, Kondo H, Tashiro F, Miyazaki J, Obata K, Tamura S, Yanagawa Y. Structural and functional characterization of mouse glutamate decarboxylase 67 gene promoter. Biochimica et biophysica acta. 2003;1628:156–168. doi: 10.1016/s0167-4781(03)00138-6. [DOI] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neuroscience research. 1998;30:101–110. doi: 10.1016/s0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Molecular pharmacology. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Murthy VN. Activity-Dependent Regulation of Inhibition via GAD67. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends in neurosciences. 2011;34:20–30. doi: 10.1016/j.tins.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Mouthon MA, Gheusi G, de Chaumont F, Olivo-Marin JC, Lamarque S, Abrous DN, Boussin FD, Lledo PM. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PloS one. 2009;4:e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Cigola E, Tinti C, Jin BK, Conti B, Volpe BT, Baker H. Unique regulation of immediate early gene and tyrosine hydroxylase expression in the odor-deprived mouse olfactory bulb. J. Biol. Chem. 1999;274:3042–3047. doi: 10.1074/jbc.274.5.3042. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nature neuroscience. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- MacDonald JL, Gin CS, Roskams AJ. Stage-specific induction of DNA methyltransferases in olfactory receptor neuron development. Developmental biology. 2005;288:461–473. doi: 10.1016/j.ydbio.2005.09.048. [DOI] [PubMed] [Google Scholar]
- MacDonald JL, Roskams AJ. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:2256–2267. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
- Macdonald JL, Verster A, Berndt A, Roskams AJ. MBD2 and MeCP2 regulate distinct transitions in the stage-specific differentiation of olfactory receptor neurons. Molecular and cellular neurosciences. 2010;44:55–67. doi: 10.1016/j.mcn.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Dong E, Gavin DP, Nicoletti F, Guidotti A. Activation of group II metabotropic glutamate receptors promotes DNA demethylation in the mouse brain. Molecular pharmacology. 2011;80:174–182. doi: 10.1124/mol.110.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita N, Okada H, Yasoshima Y, Takahashi K, Kiuchi K, Kobayashi K. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. Journal of neurochemistry. 2002;82:295–304. doi: 10.1046/j.1471-4159.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- McMillian MK, Mullis SB, Wu GC, Hudson PM, Pennypacker KR, Hong JS. Regulation of tyrosine hydroxylase in olfactory bulb cultures: selective inhibition of depolarization-induced increase by endogenous opioids. Brain research. 1994;658:105–111. doi: 10.1016/s0006-8993(09)90015-4. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe GC, Barker RA, Caldwell MA. Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle. 2009a;8:2888–2894. doi: 10.4161/cc.8.18.9512. [DOI] [PubMed] [Google Scholar]
- O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proceedings of the National Academy of Sciences of the United States of America. 2009b;106:8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzanelli P, Fritschy JM, Yanagawa Y, Obata K, Sassoe-Pognetto M. GABAergic phenotype of periglomerular cells in the rodent olfactory bulb. The Journal of comparative neurology. 2007;502:990–1002. doi: 10.1002/cne.21356. [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst S, Kiyokage E, Szabo G, Yanagawa Y, Shipley MT, Puche AC. Sensory experience selectively regulates transmitter synthesis enzymes in interglomerular circuits. Brain research. 2011;1382:70–76. doi: 10.1016/j.brainres.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. The Journal of comparative neurology. 2007;501:825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Pignatelli A, Ackman JB, Vigetti D, Beltrami AP, Zucchini S, Belluzzi O. A potential reservoir of immature dopaminergic replacement neurons in the adult mammalian olfactory bulb. Pflugers Archiv : European journal of physiology. 2009;457:899–915. doi: 10.1007/s00424-008-0535-0. [DOI] [PubMed] [Google Scholar]
- Plachez C, Puche AC. Early specification of GAD67 subventricular derived olfactory interneurons. Journal of molecular histology. 2012;43:215–221. doi: 10.1007/s10735-012-9394-2. [DOI] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. The Journal of physiology. 2008a;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Heintz T, Young S, Gordon V, Bordey A. Tonic activation of GLUK5 kainate receptors decreases neuroblast migration in whole-mounts of the subventricular zone. The Journal of physiology. 2008b;586:3783–3793. doi: 10.1113/jphysiol.2008.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puche AC, Shipley MT. Odor-induced, activity-dependent transneuronal gene induction in vitro: mediation by NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1359–1370. doi: 10.1523/JNEUROSCI.19-04-01359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M, Gutierrez R. Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain research. 2001;917:139–146. doi: 10.1016/s0006-8993(01)02794-9. [DOI] [PubMed] [Google Scholar]
- Roopra A, Sharling L, Wood IC, Briggs T, Bachfischer U, Paquette AJ, Buckley NJ. Transcriptional repression by neuron-restrictive silencer factor is mediated via the Sin3-histone deacetylase complex. Molecular and cellular biology. 2000;20:2147–2157. doi: 10.1128/mcb.20.6.2147-2157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino-Saito S, Sasaki H, Volpe BT, Kobayashi K, Berlin R, Baker H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. The Journal of comparative neurology. 2004;479:389–398. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- Schimmel JJ, Crews L, Roffler-Tarlov S, Chikaraishi DM. 4.5 kb of the rat tyrosine hydroxylase 5′ flanking sequence directs tissue specific expression during development and contains consensus sites for multiple transcription factors. Brain research. Molecular brain research. 1999;74:1–14. doi: 10.1016/s0169-328x(99)00234-x. [DOI] [PubMed] [Google Scholar]
- Sequerra EB, Gardino P, Hedin-Pereira C, de Mello FG. Putrescine as an important source of GABA in the postnatal rat subventricular zone. Neuroscience. 2007;146:489–493. doi: 10.1016/j.neuroscience.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Sheikh BN, Dixon MP, Thomas T, Voss AK. Querkopf is a key marker of self-renewal and multipotency of adult neural stem cells. Journal of cell science. 2012;125:295–309. doi: 10.1242/jcs.077271. [DOI] [PubMed] [Google Scholar]
- Sultan S, Mandairon N, Kermen F, Garcia S, Sacquet J, Didier A. Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:2355–2363. doi: 10.1096/fj.09-151456. [DOI] [PubMed] [Google Scholar]
- Szabo G, Katarova Z, Kortvely E, Greenspan RJ, Urban Z. Structure and the promoter region of the mouse gene encoding the 67-kD form of glutamic acid decarboxylase. DNA and cell biology. 1996;15:1081–1091. doi: 10.1089/dna.1996.15.1081. [DOI] [PubMed] [Google Scholar]
- Toida K. Synaptic organization of the olfactory bulb based on chemical coding of neurons. Anatomical science international. 2008;83:207–217. doi: 10.1111/j.1447-073X.2008.00247.x. [DOI] [PubMed] [Google Scholar]
- Valley MT, Mullen TR, Schultz LC, Sagdullaev BT, Firestein S. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Frontiers in neuroscience. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. The Journal of physiology. 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:5574–5581. doi: 10.1523/JNEUROSCI.15-08-05574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, Li E, Zhang Y, Sun YE. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y, Kobayashi T, Kamei T, Ishii K, Nishijima M, Takaku A, Tamura S. Structure and alternative promoters of the mouse glutamic acid decarboxylase 67 gene. The Biochemical journal. 1997;326(Pt 2):573–578. doi: 10.1042/bj3260573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kim HS, Seo H, Kim KS. Identification and characterization of potential cis-regulatory elements governing transcriptional activation of the rat tyrosine hydroxylase gene. Journal of neurochemistry. 1998;71:1358–1368. doi: 10.1046/j.1471-4159.1998.71041358.x. [DOI] [PubMed] [Google Scholar]
- Yang JW, Choi EY, Park MJ, Lee MA. Expression of tyrosine hydroxylase is epigenetically regulated in neural stem cells. Biochemical and biophysical research communications. 2011;414:712–718. doi: 10.1016/j.bbrc.2011.09.141. [DOI] [PubMed] [Google Scholar]
- Young SZ, Taylor MM, Bordey A. Neurotransmitters couple brain activity to subventricular zone neurogenesis. The European journal of neuroscience. 2011;33:1123–1132. doi: 10.1111/j.1460-9568.2011.07611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]