Abstract
Nitration of arachidonic acid (AA) to nitroarachidonic acid (AANO2) leads to anti-inflammatory intracellular activities during macrophage activation. However, less is known about the capacity of AANO2 to regulate the production of reactive oxygen species (ROS) under pro-inflammatory conditions. One of the immediate responses upon macrophage activation involves the production of superoxide radical (O2·−), due to the NADPH dependent univalent reduction of oxygen to O2·− by the phagocytic NADPH-oxidase isoform (NOX2), being the activity of NOX2 the main source of O2·− in monocytes/macrophages. Since NOX2 and AA pathways are connected, we propose that AANO2can modulate macrophage activation by inhibiting O2·− formation by NOX2. When macrophages were activated in the presence of AANO2, a significant inhibition of NOX2 activity was observed as evaluated by cytochrome c reduction, luminol chemiluminescence, Amplex Red fluorescence and flow cytometry; this process also occurs in physiological mimic conditions within the phagosomes. AANO2 decreased O2·− production in a dose-(IC50= 4.1 ± 1.8 μM AANO2) and time-dependent manner. The observed inhibition was not due to a decreased phosphorylation of the cytosolic subunits (e.g. p40phox and p47phox), as analyzed by immunoprecipitation and western blot. However, a reduction of the migration to the membrane of p47phox was obtained suggesting that the protective actions involve the prevention of the correct assembly of the active enzyme in the membrane. Finally, the observed in vitro effects were confirmed in an in vivo inflammatory model, where subcutaneous injection of AANO2 was able to decrease NOX2 activity in macrophages from thioglycolate treated mice.
Keywords: NOX2, nitro-fatty acids, inflammation
Introduction
Arachidonic acid (AA) is a precursor of inflammatory signaling mediators by enzymatic and non-enzymatic oxidative pathways. Inflammation is characterized by the production of cytokines, AA-derived eicosanoids and reactive nitrogen (RNS) and oxygen-species (ROS), i.e. through the activation of inducible nitric oxide synthase (NOS2) and NADPH oxidase (NOX), respectively [1–3]. The products of these enzymes, nitric oxide (·NO) and superoxide radical (O2·−), are the precursors of a variety of oxidizing and nitrating agents (e.g. peroxynitrite) which under inflammatory conditions lead to an increase of oxidized as well as nitrated proteins and lipids [4–10]. Indeed, higher levels of ·NO and O2·−, in addition to its dismutation product hydrogen peroxide (H2O2), have been implicated in many physiological and pathophysiological processes [11–14].
It has been well established that AA signaling cascades and RNS pathways are intrinsically related [15]. In fact, nitration of AA could divert the fatty acid from its normal metabolic pathway affecting prostaglandin endoperoxide H synthase activity [16–21] with unknown cell signaling consequences, i.e. affectation of platelet aggregation [22]. In activated macrophages, nitroarachidonic acid (AANO2) exert protective anti-inflammatory actions diminishing NOS2 expression as well as secretion of pro-inflammatory cytokines [4]. We have recently reported that AANO2 is an almost irreversibly inhibitor of the inducible isoform of the prostaglandin endoperoxide H synthase (PGHS-2) [23], which in addition to the down regulation of NOS2 under inflammatory stimulus should contribute to the physiological shut down of inflammatory responses in macrophages. However, less is known about the capacity of AANO2 to regulate the production of ROS under pro-inflammatory conditions. Indeed, one of the open questions in the field is related to the mechanisms involved in the observed anti-inflammatory actions of AANO2 at both molecular and cellular levels.
Macrophages act at the first line of defense by detecting infectious agents through membrane receptors, initiating signaling pathways leading to a wide variety of cellular responses [12,24]. One of the immediate responses involves the production of O2·−, due to the NADPH dependent univalent reduction of oxygen to O2·−, by the phagocytic NADPH oxidase isoform (NOX2) [11,12,24]. NOX2 is an enzyme complex comprised of a membrane–bound flavocytochrome b558 (gp91phox and p22phox), three cytosolic subunits (p47phox, p40phox and p67phox) and GTPase Rac2 [25–29]. In resting macrophages, the NOX complex is unassembled. Upon activation, the cytosolic components associate with gp91phox and p22phox forming the active enzyme complex which at this time generates O2· [28,30–33]; the migration of cytosolic subunits to the membrane involves conformational changes in p47phox secondary to its extensive phosphorylation by protein kinase C (PKC) [34,35]. It has been suggested a strong relationship between the AA pathway and NOX2 assembly/activation [36,37]. This connection, in addition to the previously reported anti-inflammatory actions of AANO2 in macrophages, leads us to propose that AANO2 can modulate macrophage activation by inhibiting O2·− formation by NOX2. In this study, we evaluate the effects of AANO2 on NOX2 activity in activated macrophages; the mechanisms involved in the observed actions were also analyzed.
Materials and Methods
Reagents
Nitroarachidonic acid was synthesized and quantitated as previously described [4]. Amplex Red, Fc-oxyBURST particles and the Alexa-Fluor 488 and Alexa-Fluor 594 conjugated anti-rabbit antibodies were from Invitrogen-Molecular Probes (Los Angeles, CA). Rabbit polyclonal anti-mouse phospho-p40phox and rabbit polyclonal anti-actin antibodies were from Santa Cruz Biotechnology (Heidelberg, Germany). Rabbit anti-mouse p47phox was from MilliPore Biotechnologies Inc. HRP conjugated anti-rabbit antibody was from Sigma Chemicals (St. Louis, MO). C57BL/6 gp91phox−/− mice were obtained from The Jackson Laboratory (Bar Harbor, Maine) [38,39]. Animals were provided with food and water ad libitum. All animal studies were approved by the local authorities (CHEA, Comisión Honoraria de Experimentación Animal).
NADPH oxidase activity in macrophages
J774A.1 murine macrophage cells (ATCC, USA) were maintained by passage in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% heat-inactivated fetal calf serum (FCS). Rats spleen macrophages were obtained as previously described by Nakamura et al [40] with modifications. Briefly, spleen was homogenized and remaining erythrocytes lysed by hypotonic shock. Mixed cells from spleen were harvested, maintained in DMEM medium and plated in a multiwell plate. J774A.1 cells or primary macrophages were treated with AA or AANO2, washed and activated at 37°C by exposing to either phorbol esters (PMA, 3 μg/ml) or opsonized Zymosan (40 mg/ml) The control conditions correspond to absence of stimulus. NOX2 activity was determined by a) O2·− formation by the superoxide dismutase (SOD) inhibitable-cytochrome c (cyt c) reduction assay [41], b) O2·−-dependent luminol chemiluminescence [42,43] and c) H2O2 coming from O2·− spontaneous dismutation by Amplex Red fluorescence in the presence of HRP [44]. Opsonization of zymosan was performed by incubation with fresh human serum at 37°C for 20 min [45]. Luminol chemiluminescence and Amplex Red Fluorescence assays were followed at 37°C in a luminescence (Lumistar, BMG Labtechnologies) and a fluorescence plate reader (λex= 515 nm, λem= 590 nm) (Fluostar, BMG Labtechnologies) respectively.
Macrophages extraction from C57BL/6 gp91phox−/− mice
Superoxide production in macrophages extracted from gp91phox−/− mice was evaluated after macrophages extraction from leg’s bone marrow of the gp91phox−/− mice and wild type (WT) mice. Mice were sacrificed at 6–12 week old and femur as well as tibia extracted in sterile PBS. Both epiphyses were removed and the bones were flushed with a syringe filled with RPMI medium to extrude bone marrow. For cell differentiation to macrophages, the obtained cells were exposed to L929-cell conditioned medium (LCCM) for 7 days as source of granulocyte/macrophage colony stimulating factor [46,47]. Finally, the obtained macrophages from the gp91phox−/− and WT mice were treated with AANO2 (10 μM) and O2·− production after PMA activation performed as previously by following cyt c reduction [41].
Flow cytometry analysis of Fc-oxyBURST oxidation
To evaluate O2·− released during FcγR-mediated phagocytosis, 5,7-DHCF-conjugated bovine serum albumin-IgG immune complexes (Fc-oxyBURST, Molecular Probes) was used [48]. J774A.1 macrophages were treated with methanol (vehicle), DPI (50 μM), AA (10 μM) or AANO2 (10 μM) for 1hr at 37°C, washed with PBS and then incubated with Fc-oxyBURST (50 μg/ml) for an hour. Cells were harvested, washed, resuspended in dPBS, and then kept in the dark until analysis. Fluorescence due to Fc-oxyBURST oxidation was measured in a FACS Calibur (BD Biosciences) (λex= 488 nm, λem= 530 nm) [48].
Preparation of subcellular fractions
J774A.1 macrophages were incubated with AA (10 μM) or AANO2 (10 μM) by 15 min at 37°C and activated with PMA (3 μg/ml) for 15 min. The cells were harvested, washed and resuspended in subcellular fraction buffer (250 mM Sucrose, 20 mM HEPES pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA), containing proteases inhibitors cocktail. Cells were lysed by sonication and the resultant lysate centrifuged at 1,000 g for 10 min generating the nuclear fraction (pellet) followed by a 1 hr centrifugation at 100,000 g resulting in the membrane (pellet) and cytosolic (supernatant) fractions [49]. Proteins were quantified with the Bicinchoninic acid protein assay, and fractions conserved at −80°C until used.
Western blot
Phosphorylation and translocation of cytosolic subunits were analyzed by western blot [49] using rabbit anti-mouse phospho-p40phox polyclonal antibody (Santa Cruz) or rabbit anti-mouse p47phox polyclonal antibody (Millipore Biotechnologies Inc.), respectively. To analyze the translocation of the cytosolic subunits to the membrane, both the membrane and cytosolic fractions were used to determine the presence of p47phox. Controls performed were done using rabbit polyclonal anti-actin and rabbit polyclonal anti-gp91phox antibodies (Santa Cruz). HRP conjugated anti-rabbit antibody (Sigma Chemicals, USA) was used as a secondary antibody and proteins detected by chemiluminescence using SuperSignal Chemiluminescent Substrates (Pierce, USA).
Assessment of macrophages viability
Cell viability was analyzed using the MTT assay (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) measuring the blue stain at 570 nm [50], and the propidium iodide (PI) assay analyzed by flow cytometry (λex= 488 nm, λem= 617 nm, FACS Calibur, BD Biosciences) [51]. In all cases, vehicle (methanol) effects were evaluated without observing any effect on cell viability.
Macrophage phagocytosis
To study macrophage phagocytosis, we used FITC-conjugated beads [52]. Cells cultures were incubated with AANO2 for 15 min at 37°C, washed and treated with the fluorescent beads at 37°C for 3hr. Cells were harvested, washed, and resuspended in dPBS buffer. Intracellular fluorescence was measured by flow cytometry (λex= 490nm, λem= 520 nm) [52]. The effects of AANO2 were compared to the phagocytic inhibitor cytochalasin A (50 μM) [53].
Confocal Microscopy assays
The differential localization of p47phox was analyzed by immunoflurescence confocal microscopy [54]. Macrophages were incubated for 15 min at 37°C with vehicle, AA (10 μM) or AANO2 (10 μM) and activated with FITC-conjugated zymosan for 20 min at 37°C. Cells were fixed, permeabilized and blocked, and then incubated with an anti-p47phox antibody and an Alexa Fluor-594 goat anti-mouse IgG antibody. Samples were analyzed with a Confocal Microscopy TCS SP5 II (Leica Microsystems).
Thioglycolate induced inflammation
Male C57BL/6 mice (18–30 g) were separated in three groups, and injected subcutaneously with vehicle, AANO2 or AA once a day for three days (10 μM/day). At day 0, mice were injected i.p. with thioglycolate (10%v/v) and after four days mice were sacrificed. Peritoneal macrophages were extracted by injecting cold DMEM culture medium [55] followed by evaluation of NADPH oxidase activity after cell activation with PMA. Control groups without thioglycolate for vehicle, AA and AANO2 were included.
Statistical analysis
Experiments were performed at least three times on independent days. Data showed correspond to the mean ± standard deviation, unless otherwise noted. All data are given in mean and a p < 0.05 was considered significant. Means were compared by the Student’s t test and ANOVA test.
Results
AANO2 decreased superoxide radical formation by NOX2 in activated macrophages
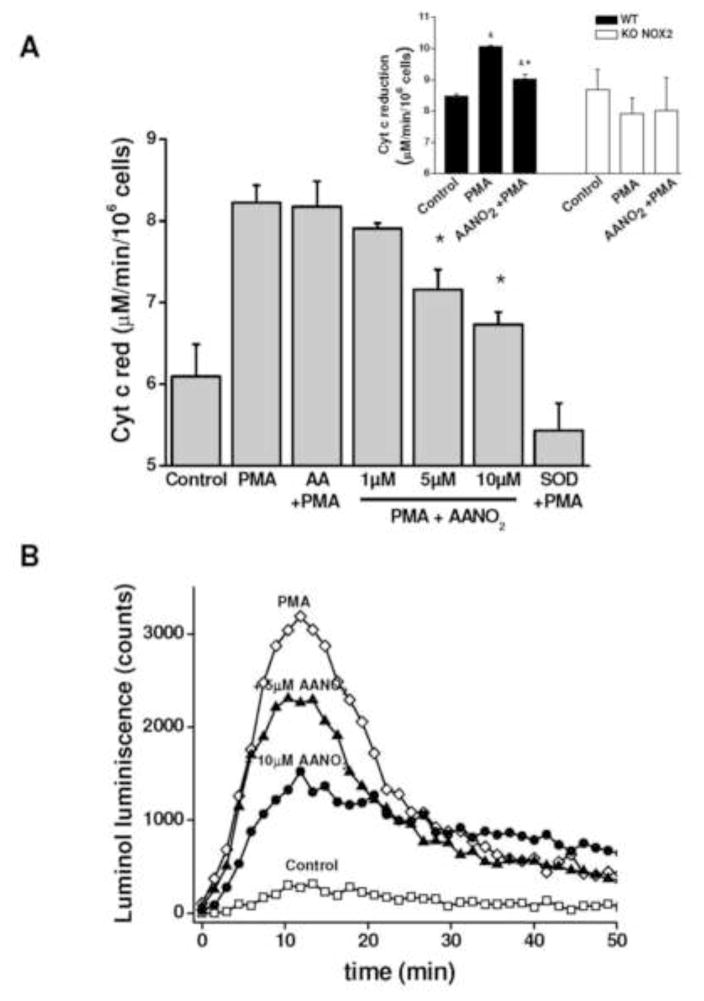
We first evaluated the effect of AANO2 on O2·− production in J774A.1 macrophages (Figure 1). The activation of macrophages with PMA induced an increase in O2·− formation as evidenced by cyt c reduction (Figure 1A), luminol luminescence (Figures 1B) and Amplex Red fluorescence (Figure 1C). Addition of SOD inhibited cyt c reduction almost completely (Figure 1A). Pre-incubation of macrophages with low micromolar levels of AANO2 induced a dose-dependent inhibition of O2·− production (Figures 1 and 3B), with no effects observed when activated with AA treated cells (Figure 1). Accordingly, no effects on Amplex Red oxidation were observed in the presence of AANO2 without PMA (supporting information, Figure 1S). Similarly, AANO2 also caused an inhibition on O2·− detection in PMA-stimulated macrophages obtained from C57BL/6 wt animals. The specificity of AANO2– dependent inhibition of O2·− detection was further substantiated with the use of gp91phox−/− mice. Indeed, we were unable to observe cyt c reduction in macrophages from gp91phox−/− mice upon PMA stimulation either in the absence or presence of AANO2 (inset Figure 1A).
Figure 1. AANO2 inhibits O2·− production in activated macrophages.
The production of O2·− was analyzed in macrophages by cytochrome c reduction (A), Luminol chemiluminescence (B), and Amplex Red fluorescence in the presence of HRP (C). (A) J774A.1 macrophages (2×105 cells) were treated with AANO2 (1, 5 and 10 μM) or AA (10 μM) for 15 minutes in dPBS buffer at 37°C, washed and activated for an additional hour with PMA (3 μg/ml) in the presence of 20 μM cyt c. The supernatant was collected and the levels of reduced cyt c due to O2·− were measured at 550 nm (ε= 21 mM−1. cm−1) [41]. A control with SOD (600 U/ml) was included. Results shown correspond to the mean ± SD, n=3. * indicates statistical differences compared to the PMA condition, p < 0.05. Inset: Macrophages from bone marrow of wt and gp91phox−/− mice were treated with 10 μM AANO2 for 15 minutes in dPBS buffer at 37°C, washed and activated for an additional hour with PMA (3 μg/ml) in the presence of 20 μM cyt c. Superoxide production was evaluated by the cyt c reduction assay. * indicates statistical differences compared to the control condition, p < 0.05; & indicates statistical differences compared to the PMA condition, p < 0.05 (B) J774A.1 macrophages (2×105 cells) were treated with AANO2 (5 and 10 μM, -▲- and -●- respectively) and PMA (-◇-) as in (A). Luminol (100 μM) was added and the formation of O2·− was analyzed following oxidized luminol luminescence for 1hr. A control condition without PMA was also included (-□-). (C) Macrophages (-□-) treated with 5 μM (-▲-) and 10 μM (-●-) AANO2 in dPBS buffer at 37°C, were activated with PMA (3 μg/ml, -◇-). In this case, the formation of O2·− derived-H2O2 was evaluated by following the fluorescence of Amplex Red (50 μM) with 4 μg/ml HRP (λex= 515 nm, λem= 590 nm) in a fluorescence plate reader. Data shown in figures (B) and (C) are representative of at least four independent experiments.
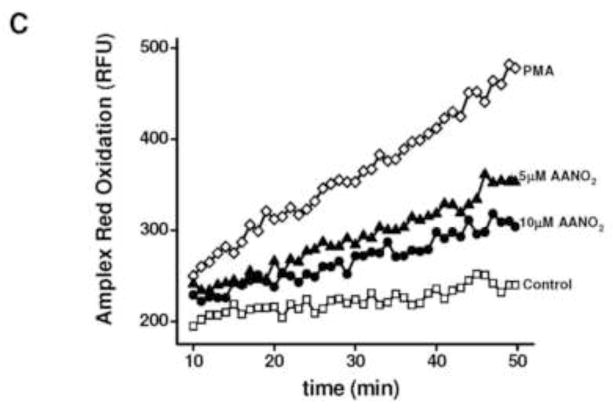
Figure 3. Time- and concentration-dependent inhibition of NOX2 by AANO2.
(A) J774A.1 macrophages (2×105 cells) were activated with PMA (3 μg/ml) after incubation with 10 μM AANO2 for different pre-incubation times (15–120 min). NOX2 activity was analyzed by luminol chemiluminiscence as before. Controls without activation, DPI addition (50 μM), and incubation with AANO2 in the absence of PMA were included. (B) J774A.1 macrophages (2×105 cells) were incubated with increasing doses of AANO2 (0–10 μM) for 15 minutes and then activated with PMA (3 μg/ml); NOX2 activity was analyzed by Amplex Red fluorescence as previously. In both (A) and (B), results shown correspond to the mean ± SD, n=3 representative of at least 4 independent experiments.
Amplex red inhibition exerted by AANO2 was similar when cells activation was performed with the classic phagocytic pathway activator opsonized zymosan [45,56] or using macrophages from rat spleen (supporting information, Figure 2S) in addition to diphenyliodonium (DPI) inhibition (Figure 2 and supporting information, Figure 1S). The inhibitory effect of AANO2 on NOX2 activity was time- and concentration-dependent (Figure 3A and 3B, respectively and supporting information, Figure 3S); NOX2 inhibition by the nitro-fatty acid was maximal between 15 and 30 min (Figure 3A) with an inhibitory concentration 50 (IC50) of 4.1 ± 1.8 μM AANO2 (Figure 3B).
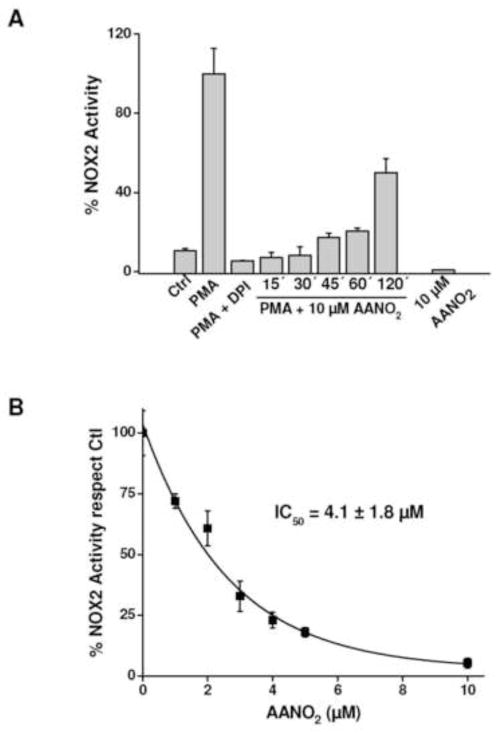
Figure 2. Flow cytometry analysis of NOX2 inhibition by AANO2.
J774-A1 macrophages (2×106 cells) were treated with AANO2 (0–10 μM) or DPI (50 μM), a nonspecific NOX inhibitor. Cells were washed and treated with Fc-oxyBURST (Fc-oxyB, 50 μg/ml) for 1h, and fluorescence of DCF determined by flow cytometry. Representative histograms are shown in A and quantitative analysis of the obtained histograms was done (B). M1 region corresponds to the cell population which exhibits high fluorescence due to H2DCF oxidation to DCF by ROS. Results shown correspond to the mean ± SD, n=3. & indicate statistical differences compared to the control condition, p < 0.05; * indicate statistical differences compared to the non-inhibitor condition, p < 00.05.
NOX2 inhibition by AANO2 occurs in phagosomes
Phorbol esters, i.e. PMA activate phosphorylation of NOX2 cytosolic components with their subsequent migration to the membrane to form the active enzyme [57]. Thus we investigated if the inhibition of NOX2 exerted by AANO2 occurred in physiological mimic conditions within the phagosomes using the Fc-oxyBURST (Figure 2). After binding to membrane Fc-receptors, the Fc-oxyBURST is internalized and the H2DCF oxidized by ROS in the phagosome to the fluorescent dichlorofluorescein (DCF) [48] which can be followed by flow cytometry (Figure 2A), a process which was inhibited by DPI (Figure 2B). When phagocytosis was activated in AANO2 treated macrophages, the extent of Fc-oxyBURST oxidation was lower at both 2 and 10 μM AANO2 (Figure 2B). As expected, AA was unable to decrease Fc-oxyBURST derived fluorescence (Figure 2B). Phagocytic capacity remained unaffected in the presence of AANO2 when analyzed by flow cytometry using fluorescent beads (supporting information, Figure 4S).
AANO2 inhibition does not affect macrophage viability
A decrease in the number of viable cells after treatment with AANO2 could explain the reduction of the observed O2·− production. However, by using the propide iodide as well as the MTT assays [50,51], AANO2 did not have any effect on macrophages viability at the experimental conditions used for NOX2 activity studies (supporting information, Figure 5S).
AANO2 does not affect phosphorylation cascades while prevents active complex assembly
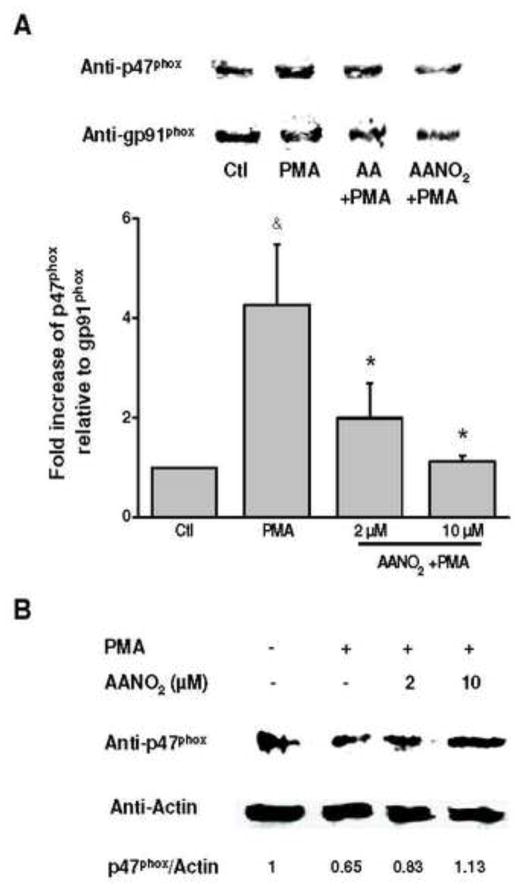
Phosphorylation cascades precede the assembly of the active enzyme in the membrane [33,34,36,58,59]. Phosphorylation of cytosolic components (phospho-p40phox) increased in PMA-activated macrophages, compared to non-activated cells, at similar levels to those observed in cells incubated with AANO2 (supporting information, Figure 6AS). Using an anti-phosphoserine antibody, phosphorylated proteins were inmunoprecipitated and western blot analysis showed similar results for phopho-p47phox (supporting information, Figure 6BS). All together, our results demonstrate that AANO2 effects were not exerted at the phosphorylation cascades necessaries for NOX2 activation. Migration of cytosolic subunits to the membrane to achieve the active form of the enzyme was tested in figure 4 by analyzing the presence of p47phox in both the cytosolic and membrane fraction of activated macrophages. After activation with PMA in the absence or presence of AA, p47phox levels were higher in the membrane fraction and lower in the cytosolic fraction compared to the non-activated cells (Figure 4). In contrast, AANO2 induced a decrease in the migration of p47phox to the membrane as observed by both lower levels of p47phox in the membrane fraction and a concomitant higher concentration of the subunit in the cytosol (Figure 4). These results were confirmed by confocal microscopy studies (Figure 5): macrophages were activated in the absence or presence of AANO2 or AA with FITC-conjugated zymosan (green fluorescence) and followed the migration of the cytosolic subunits using an anti-p47phox antibody (red fluorescence). In activated cells incubated with 10 μM of AANO2, a reduced co-localization of the green and red fluorescence was observed compared to the AA condition (Figure 5).
Figure 4. Translocation of cytosolic p47phox to the membrane is prevented by AANO2.
The effects of AANO2 on the migration of cytosolic subunits to the membrane were evaluated in activated macrophages. Macrophages (3×106 cells) were treated with AANO2 (2 and 10 μM) for 15 minutes, washed and PMA-activated as previously. Cells were harvested and membrane (A) as well cytosolic (B) fractions were obtained by differential centrifugation. The presence of p47phox was determined by western blot. In parallel, the levels of gp91phox (A) as well as actin (B) were determined as reporters of membrane and cytosolic constitutive proteins, respectively. In (A) the bars represent the relative presence of p47phox respect to gp91phox, and results shown correspond to the mean ± SD, n=3. & indicates statistical difference compared to the control condition, p < 0.05; * indicates statistical difference compared to the PMA-activated condition, p < 0.05.
Figure 5. p47phox does not migrate to the membrane in the presence of AANO2.
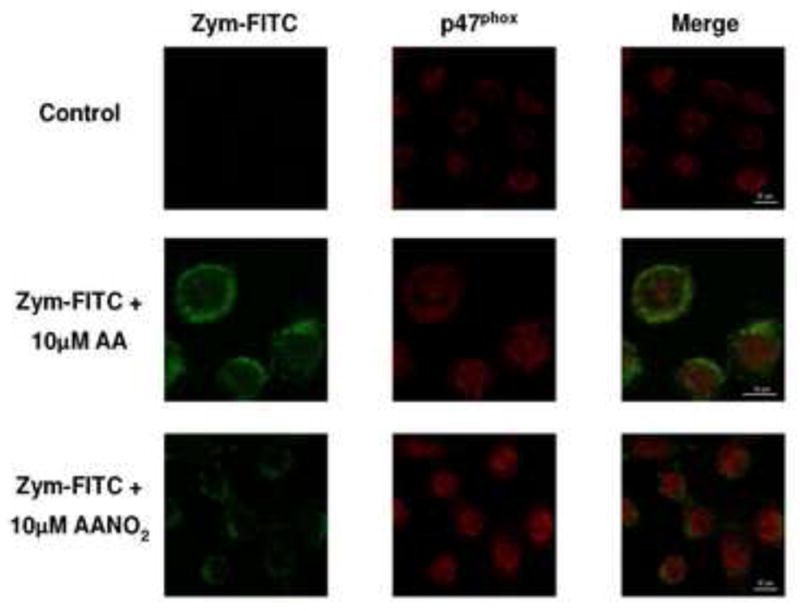
J774A.1 macrophages were incubated with AA (10 μM) or AANO2 (10 μM) as before. Cells were washed and activated with FITC-conjugated zymosan at 37°C. Then, permeated cells were incubated with rabbit anti-mouse p47phox antibody followed by the addition of a secondary Alexa Fluor 594 goat anti-mouse IgG antibody. The intracellular localization of the FITC-zymosan (green fluorescence) and p47phox (red fluorescence) was observed by confocal microscopy.
AANO2 inhibits NOX2 activity in vivo
Finally, the capacity of AANO2 to modulate the enzyme in an inflammatory model was tested (Table 1). Thioglycolate was injected to mice that were treated with vehicle, AA or AANO2 for three consecutive days (one subcutaneous injection of the compounds per day) and peritoneal macrophages were obtained at day 4. Macrophages- NOX2 activity was then analyzed with Amplex Red as previously (Table 1). Macrophages obtained from mice treated with AANO2 showed a decreased formation of O2·− after activation with PMA compared to those from vehicle- or AA- treated animals (Table 1 and data not shown). Amplex Red fluorescence was inhibited in all experiments by the addition of DPI prior to activation with PMA (Table 1).
Table 1. Inhibition of NOX2 by AANO2 in an in vivo model of inflammation.
Mice were treated with i/p thioglycolate and subcutaneous injected with AANO2 and peritoneal macrophages were obtained at day 4 as explained in the experimental section. The formation of O2·− derived-H2O2 in PMA activated-macrophages was evaluated by Amplex Red as previously. DPI addition was performed to isolated macrophages 30 min before PMA addition. Data shown correspond to the mean ± SD, n=4 representative of at least 3 independent experiments.
| Condition | NOX2 activity (%) |
|---|---|
| Vehicle treated mice | 25.9 ± 12.8 |
| AANO2 treated mice | 5.7 ± 3.9a |
| Vehicle mice + PMA | 100 ± 22.6 |
| AANO2 mice + PMA | 21.7 ± 10.3b |
| Vehicle mice + PMA + DPI | 27.1 ± 6.4b |
| AANO2 mice + PMA + DPI | 5.9 ± 1.9b |
indicates statistical difference compared to the Vehicle treated mice, p < 0.05.
indicates statistical difference compared to the Vehicle mice +PMA, p< 0.05.
Discussion
We demonstrate the inhibition of phagocytic NOX2 activity by AANO2 both in activated macrophages and in vivo. NOX2 activity was analyzed by both direct formation of O2·− or its dismutation product H2O2 using different analytical approaches (Figures 1 to 3). The involvement of NOX2 on O2·− production was confirmed by the inhibitory effect of SOD on cyt c reduction and the lack of O2·− detection in macrophages from gp91phox−/− mice (Figure 1). The AANO2 effects on O2·− generation were observed both in a macrophage cell line (J774A.1) as well as in primary macrophages (from C57BL/6 mice and rat spleen).
We then investigated if the inhibition of NOX2 exerted by AANO2 occurred under physiologically-relevant conditions within the phagosomes using the Fc-oxyBURST (Figure 2). The Fc-oxyBURST consists in a bovine serum albumin (BSA) linked to dichlorodihydrofluorescein (H2DCF) and complexed with anti-BSA IgG antibodies, which after internalization is oxidized leading to the formation of the fluorescent dichlorofluorescein (DCF) in the phagosome [48] which can be followed by flow cytometry (Figure 2A); lower levels of Fc-oxyBURST oxidation in the presence of AANO2 were observed compared to activated macrophages supporting inhibition of O2·− generation by the nitroalkene (Figure 2). Phagocytic capacity of macrophages when analyzed with fluorescent beads was inhibited by cytochalasin [60] but was unaffected by AANO2 (supporting information, Figure 4S). One potential explanation for the observed effects on O2·− production could be an effect of AANO2 on cell viability. Thus, we performed experiments with the propide iodide as well as the MTT assays [50,51] without observing any effect after incubation with AANO2 (supporting information, Figure 5S). Overall, our results suggest that the capacity of AANO2 to decrease O2·− formation in the phagosome is by inhibiting NOX2 activity/assembly.
Modulation of the correct assembly of the active enzyme at the cell membrane could be a mechanism which can explain the observed AANO2 enzyme inhibition. Phosphorylation cascades precede the assembly of the active enzyme in the membrane involving phosphorylation of p47phox, and the subsequent phosphorylation of p67phox and p40phox [33,34,36,58,59]. Then, these phosphorylated proteins are triggered to migrate to the cell membrane together with Rac2 thus interacting with the membrane subunits (gp91phox and p22phox) forming the active complex [24,58,61–63]. When macrophages were stimulated with PMA in the presence of AANO2, the levels of phosphor-p40phox were similar to those observed in activated cells without the nitro-fatty acid (supporting information, Figure 6AS), being the role as a positive or negative regulator of phospho-p40phox in the activation of NOX2 controversial [58,62]. Indeed, there are reports suggesting that p40phox is a positive regulator of NOX2 as an adaptor protein linking the cytoplasmic complex subunits (p40phox-p67phox-p47phox) to phagosomes as well as regulating p67phox translocation to the membrane [62,64]. Also, the role of p40phox could be dependent of the phosphorylation status of p47phox [62]. Thus we decided to study the phosphorylation of p47phox, which is phosphorylated in a Serine residue during enzyme activation [59]. Again, no differences were observed discarding phosphorylation of cytosolic subunits as the mechanism for AANO2-mediated NOX2 inhibition. Downstream phosphorylation, cytosolic subunits migrate to the membrane where they associate with gp91phox and p22phox achieving the active form of the enzyme [36,56,64]. Thus, if AANO2 is able to affect the migration of the cytosolic subunits to the membrane the final result is the inhibition of NOX2 activity. This hypothesis was tested in figure 4 by analyzing the presence of p47phox in both the cytosolic and membrane fraction of activated macrophages. Western blot and microscopy results indicate that AANO2 modulated the translocation of p47phox avoiding the formation of the active enzyme complex, thus inhibiting NOX2 dependent O2·− formation.
The effects of AANO2 on NOX2 activity showed a first order decay, with a concentration exerting a reduction of 50% initial activity of 4.1 ± 1.8 μM AANO2 (Figure 3A). Also, pre-incubation time had influence on the nitro-fatty acid action decreasing the inhibitory effect after 30 min suggesting a potential reversible mechanism involved in the observed action. In fact, nitroalkenes have electrophilic reactivity towards reactive nucleophilic amino acids in proteins, e.g. cysteine or histidine residues forming covalent reversible adducts responsible for many of the reported protective actions for nitro-fatty acids [65,66]. Under our experimental conditions we cannot discard that AANO2 is covalently reacting with either the cytosolic or the membrane subunits, preventing the correct assembly of the active enzyme.
Thioglycolate injection into the peritoneum is a well recognized model due to its capacity to elicit an inflammatory response after 4 days, increasing the recruitment and number of macrophages [55]. Mice were treated subcutaneous once a day for four days after thioglycolate injection with vehicle, AA or AANO2 and, after sacrificed macrophages were isolated, activated with PMA and analyzed for Amplex Red oxidation (Table 1). AANO2 was able to decrease macrophage activation supporting a novel nitro-fatty acid anti-inflammatory role.
The data presented in this work are indicative that AANO2 inhibits phagocytic NADPH oxidase in activated macrophages through a mechanism that involves the prevention of the migration of the cytosolic subunits to the membrane, affecting the correct assembly of the active form of the enzyme. The capacity of AANO2 to inhibit NOX2 may generate a less active macrophage [67–69]. In a chronic inflammatory processes, where nitration of AA may occur due to an increase of the oxidative/nitrosative stress [70–74], AANO2 may inhibit key inducible enzymes (e.g. NOX2, NOS2 or PGHS-2) aiding in the resolution of inflammation. In fact, the decrease of macrophage activation in thioglycolate treated mice suggests that AANO2 can exert beneficial anti-inflammatory effects when administered at pharmacological doses.
Supplementary Material
Nitro-arachidonic acid exerts protective activities during macrophage activation.
We propose that Nitro-arachidonic acid modulates phagocytic NADPH oxidase activity/assembly.
Nitro-arachidonic acid prevents the migration of cytosolic subunits to the membrane.
Protective effects were also observed in an in vivo inflammatory model.
Acknowledgments
This work was supported by grants from Fondo Maria Viñas-ANII (FMV_2009_2913) to AT; ICGEB (Italy) to HR, Universidad de la República (CSIC, Uruguay) to HR and RR, and from the National Institutes of Health, 1R01AI095173-01 (NIH, USA) to RR. LGP was partially supported by a fellowship from Sistema Nacional de Becas-ANII.
Abbreviations
- AA
arachidonic acid
- AANO2
nitroarachidonic acid
- O2·−
superoxide radical
- NOX2
phagocytic NADPH oxidase
- PMA
phorbol myristate acetate
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- NOS2
inducible nitric oxide synthase
- H2O2
hydrogen peroxide
- cyt c
cytochrome c
- SOD
superoxide dismutase
- DPI
diphenyliodonium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vachier I, Chanez P, Bonnans C, Godard P, Bousquet J, Chavis C. Endogenous anti-inflammatory mediators from arachidonate in human neutrophils. Biochem Biophys Res Commun. 2002;290(1):219–224. doi: 10.1006/bbrc.2001.6155. [DOI] [PubMed] [Google Scholar]
- 2.Cross AR, Parkinson JF, Jones OT. The superoxide-generating oxidase of leucocytes. NADPH-dependent reduction of flavin and cytochrome b in solubilized preparations. Biochem J. 1984;223(2):337–344. doi: 10.1042/bj2230337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakinuma K, Kaneda M, Chiba T, Ohnishi T. Electron spin resonance studies on a flavoprotein in neutrophil plasma membranes. Redox potentials of the flavin and its participation in NADPH oxidase. J Biol Chem. 1986;261(20):9426–9432. [PubMed] [Google Scholar]
- 4.Trostchansky A, Souza JM, Ferreira A, Ferrari M, Blanco F, Trujillo M, Castro D, Cerecetto H, Baker PR, O’Donnell VB, Rubbo H. Synthesis, isomer characterization, and anti-inflammatory properties of nitroarachidonate. Biochemistry. 2007;46(15):4645–4653. doi: 10.1021/bi602652j. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 7.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell VB, Eiserich JP, Bloodsworth A, Chumley PH, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive species. Methods Enzymol. 1999;301:454–470. doi: 10.1016/s0076-6879(99)01109-x. [DOI] [PubMed] [Google Scholar]
- 9.Trostchansky A, Ferrer-Sueta G, Batthyany C, Botti H, Batinic-Haberle I, Radi R, Rubbo H. Peroxynitrite-mediated LDL oxidation is inhibited by manganese porphyris in the presence of uric acid. Free Rad Biol Med. 2003;35(10):1293–1300. doi: 10.1016/j.freeradbiomed.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269(42):26066–26075. [PubMed] [Google Scholar]
- 11.Cathcart MK. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(1):23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez MN, Trujillo M, Radi R. Peroxynitrite formation from biochemical and cellular fluxes of nitric oxide and superoxide. Methods Enzymol. 2002;359:353–366. doi: 10.1016/s0076-6879(02)59198-9. [DOI] [PubMed] [Google Scholar]
- 13.Mabley JG, Liaudet L, Pacher P, Southan GJ, Groves JT, Salzman AL, Szabo C. Part II: beneficial effects of the peroxynitrite decomposition catalyst FP15 in murine models of arthritis and colitis. Mol Med. 2002;8(10):581–590. [PMC free article] [PubMed] [Google Scholar]
- 14.Mapp PI, Klocke R, Walsh DA, Chana JK, Stevens CR, Gallagher PJ, Blake DR. Localization of 3-nitrotyrosine to rheumatoid and normal synovium. Arthritis Rheum. 2001;44(7):1534–1539. doi: 10.1002/1529-0131(200107)44:7<1534::AID-ART276>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Salvemini D, Seibert K, Masferrer JL, Settle SL, Currie MG, Needleman P. Nitric Oxide Activates the Cyclooxygenase Pathway in Inflammation. Am J Ther. 1995;2(9):616–619. doi: 10.1097/00045391-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Balazy M, Iesaki T, Park JL, Jiang H, Kaminski PM, Wolin MS. Vicinal nitrohydroxyeicosatrienoic acids: vasodilator lipids formed by reaction of nitrogen dioxide with arachidonic acid. J Pharmacol Exp Ther. 2001;299(2):611–619. [PubMed] [Google Scholar]
- 17.Di Rosa M, Ialenti A, Ianaro A, Sautebin L. Interaction between nitric oxide and cyclooxygenase pathways. Prostaglandins Leukot Essent Fatty Acids. 1996;54(4):229–238. doi: 10.1016/s0952-3278(96)90053-8. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. Faseb J. 1999;13(10):1121–1136. doi: 10.1096/fasebj.13.10.1121. 1123: Goodwin DC et al. Reactions of prostaglandin en...[PMID: 10065376]Related Articles, Links. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard-Fillion B, Souza JM, Friel T, Jiang GC, Vrana K, Sharov V, Barron L, Schoneich C, Quijano C, Alvarez B, Radi R, Przedborski S, Fernando GS, Horwitz J, Ischiropoulos H. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J Biol Chem. 2001;276(49):46017–46023. doi: 10.1074/jbc.M105564200. [DOI] [PubMed] [Google Scholar]
- 20.Marnett LJ. Recent developments in cyclooxygenase inhibition. Prostaglandins Other Lipid Mediat. 2002;68–69:153–164. doi: 10.1016/s0090-6980(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 21.Rouzer CA, Marnett LJ. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem Rev. 2003;103(6):2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 22.Salvemini D, Currie MG, Mollace V. Nitric oxide-mediated cyclooxygenase activation. A key event in the antiplatelet effects of nitrovasodilators. J Clin Invest. 1996;97(11):2562–2568. doi: 10.1172/JCI118704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trostchansky A, Bonilla L, Thomas CP, O’Donnell VB, Marnett LJ, Radi R, Rubbo H. Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. J Biol Chem. 2011;286(15):12891–12900. doi: 10.1074/jbc.M110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal Peroxynitrite as a Macrophage-derived Cytotoxin against Internalized Trypanosoma cruzi: CONSEQUENCES FOR OXIDATIVE KILLING AND ROLE OF MICROBIAL PEROXIREDOXINS IN INFECTIVITY. J Biol Chem. 2011;286(8):6627–6640. doi: 10.1074/jbc.M110.167247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinauer MC, Pierce EA, Bruns GA, Curnutte JT, Orkin SH. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990;86(5):1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78(5):1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 27.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59(9):1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juliet PA, Hayashi T, Iguchi A, Ignarro LJ. Concomitant production of nitric oxide and superoxide in human macrophages. Biochem Biophys Res Commun. 2003;310(2):367–370. doi: 10.1016/j.bbrc.2003.08.133. [DOI] [PubMed] [Google Scholar]
- 29.Wientjes FB, Segal AW, Hartwig JH. Immunoelectron microscopy shows a clustered distribution of NADPH oxidase components in the human neutrophil plasma membrane. J Leukoc Biol. 1997;61(3):303–312. doi: 10.1002/jlb.61.3.303. [DOI] [PubMed] [Google Scholar]
- 30.Doussiere J, Vignais PV. Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils. Factors controlling the inhibitory potency of diphenylene iodonium in a cell-free system of oxidase activation. European Journal of Biochemistry. 1992;208(1):61–71. doi: 10.1111/j.1432-1033.1992.tb17159.x. [DOI] [PubMed] [Google Scholar]
- 31.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox Containing NADPH Oxidase Selectively Expressed in Endothelial Cells Is a Major Source of Oxygen Radical Generation in the Arterial Wall. Circ Res. 2000;87(1):26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 32.Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation. 1999;100(14):1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 33.Ambruso DR, Cusack N, Thurman G. NADPH oxidase activity of neutrophil specific granules: requirements for cytosolic components and evidence of assembly during cell activation. Mol Genet Metab. 2004;81(4):313–321. doi: 10.1016/j.ymgme.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Park HS, Lee SM, Lee JH, Kim YS, Bae YS, Park JW. Phosphorylation of the leucocyte NADPH oxidase subunit p47(phox) by casein kinase 2: conformation-dependent phosphorylation and modulation of oxidase activity. Biochem J. 2001;358(Pt 3):783–790. doi: 10.1042/0264-6021:3580783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waite KA, Wallin R, Qualliotine-Mann D, McPhail LC. Phosphatidic acid-mediated phosphorylation of the NADPH oxidase component p47-phox. Evidence that phosphatidic acid may activate a novel protein kinase. J Biol Chem. 1997;272(24):15569–15578. doi: 10.1074/jbc.272.24.15569. [DOI] [PubMed] [Google Scholar]
- 36.Shiose A, Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J Biol Chem. 2000;275(18):13793–13801. doi: 10.1074/jbc.275.18.13793. [DOI] [PubMed] [Google Scholar]
- 37.Chang LC, Lin RH, Huang LJ, Chang CS, Kuo SC, Wang JP. Inhibition of superoxide anion generation by CHS-111 via blockade of the p21-activated kinase, protein kinase B/Akt and protein kinase C signaling pathways in rat neutrophils. Eur J Pharmacol. 2009;615(1–3):207–217. doi: 10.1016/j.ejphar.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 38.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9(2):202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 39.Ehrt S, Schnappinger D, Bekiranov S, Drenkow J, Shi S, Gingeras TR, Gaasterland T, Schoolnik G, Nathan C. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med. 2001;194(8):1123–1140. doi: 10.1084/jem.194.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura K, Yamaji T, Crocker PR, Suzuki A, Hashimoto Y. Lymph node macrophages, but not spleen macrophages, express high levels of unmasked sialoadhesin: implication for the adhesive properties of macrophages in vivo. Glycobiology. 2002;12(3):209–216. doi: 10.1093/glycob/12.3.209. [DOI] [PubMed] [Google Scholar]
- 41.Iio M, Ono Y, Kai S, Fukumoto M. Effects of flavonoids on xanthine oxidation as well as on cytochrome c reduction by milk xanthine oxidase. J Nutr Sci Vitaminol (Tokyo) 1986;32(6):635–642. doi: 10.3177/jnsv.32.635. [DOI] [PubMed] [Google Scholar]
- 42.Radi R, Rubbo H, Thomson L, Prodanov E. Luminol chemiluminescence using xanthine and hypoxanthine as xanthine oxidase substrates. Free Radic Biol Med. 1990;8(2):121–126. doi: 10.1016/0891-5849(90)90084-v. [DOI] [PubMed] [Google Scholar]
- 43.Heinle H, el Dessouki J. Luminol-enhanced chemiluminescence after reaction of hydroperoxides with opsonized zymosan. J Biolumin Chemilumin. 1995;10(2):71–76. doi: 10.1002/bio.1170100202. [DOI] [PubMed] [Google Scholar]
- 44.Votyakova TV, Reynolds IJ. Detection of hydrogen peroxide with Amplex Red: interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys. 2004;431(1):138–144. doi: 10.1016/j.abb.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Wolf HM, Mannhalter JW, Salzmann HC, Gottlicher J, Ahmad R, Eibl MM. Phagocytosis of serum-opsonized zymosan down-regulates the expression of CR3 and FcRI in the membrane of human monocytes. J Immunol. 1988;141(10):3537–3543. [PubMed] [Google Scholar]
- 46.Marim FM, Silveira TN, Lima DS, Jr, Zamboni DS. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One. 5(12):e15263. doi: 10.1371/journal.pone.0015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi KD, Vodyanik M, Slukvin II. Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat Protoc. 6(3):296–313. doi: 10.1038/nprot.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaur I, Simons ER, Castro VA, Mark Ott C, Pierson DL. Changes in neutrophil functions in astronauts. Brain Behav Immun. 2004;18(5):443–450. doi: 10.1016/j.bbi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Birnie GD. Subcellular components: preparation and fractionation. 12. London Baltimore: Butterworth; University Park Press; 1972. p. 320. [Google Scholar]
- 50.Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56(3):279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu K, Kobayashi M, Tahara J, Shiratori K. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology. 2005;128(7):2105–2118. doi: 10.1053/j.gastro.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Fisher E, Scharnagl H, Hoffmann MM, Kusterer K, Wittmann D, Wieland H, Gross W, Marz W. Mutations in the Apolipoprotein (apo) B-100 Receptor-binding region: Detection of apo B-100 (Arg3500->Trp) Associated with Two New Haplotypes and Evidence That apo B-100 (Glu3405->Gln) Diminishes Receptor-mediated Uptake of LDL. Clin Chem. 1999;45(7):1026–1038. [PubMed] [Google Scholar]
- 54.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277(22):19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14(Unit 14):11. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 57.Cannon GJ, Swanson JA. The macrophage capacity for phagocytosis. J Cell Sci. 1992;101(Pt 4):907–913. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- 58.Lopes LR, Dagher MC, Gutierrez A, Young B, Bouin AP, Fuchs A, Babior BM. Phosphorylated p40PHOX as a negative regulator of NADPH oxidase. Biochemistry. 2004;43(12):3723–3730. doi: 10.1021/bi035636s. [DOI] [PubMed] [Google Scholar]
- 59.Chen Q, Powell DW, Rane MJ, Singh S, Butt W, Klein JB, McLeish KR. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol. 2003;170(10):5302–5308. doi: 10.4049/jimmunol.170.10.5302. [DOI] [PubMed] [Google Scholar]
- 60.Hoffmann EK, Rasmussen L, Zeuthen E. Cytochalasin B: aspects of phagocytosis in nutrient uptake in Tetrahymena. J Cell Sci. 1974;15(2):403–406. doi: 10.1242/jcs.15.2.403. [DOI] [PubMed] [Google Scholar]
- 61.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94(26):14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ueyama T, Nakakita J, Nakamura T, Kobayashi T, Kobayashi T, Son J, Sakuma M, Sakaguchi H, Leto TL, Saito N. Cooperation of p40(phox) with p47(phox) for Nox2-based NADPH oxidase activation during Fcgamma receptor (FcgammaR)-mediated phagocytosis: mechanism for acquisition of p40(phox) phosphatidylinositol 3-phosphate (PI(3)P) binding. J Biol Chem. 2011;286(47):40693–40705. doi: 10.1074/jbc.M111.237289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLaughlin NJ, Banerjee A, Khan SY, Lieber JL, Kelher MR, Gamboni-Robertson F, Sheppard FR, Moore EE, Mierau GW, Elzi DJ, Silliman CC. Platelet-activating factor-mediated endosome formation causes membrane translocation of p67phox and p40phox that requires recruitment and activation of p38 MAPK, Rab5a, and phosphatidylinositol 3-kinase in human neutrophils. J Immunol. 2008;180(12):8192–8203. doi: 10.4049/jimmunol.180.12.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueyama T, Tatsuno T, Kawasaki T, Tsujibe S, Shirai Y, Sumimoto H, Leto TL, Saito N. A regulated adaptor function of p40phox: distinct p67phox membrane targeting by p40phox and by p47phox. Mol Biol Cell. 2007;18(2):441–454. doi: 10.1091/mbc.E06-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA. Reversible Post-translational Modification of Proteins by Nitrated Fatty Acids in Vivo. J Biol Chem. 2006;281(29):20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schopfer FJ, Batthyany C, Baker PR, Bonacci G, Cole MP, Rudolph V, Groeger AL, Rudolph TK, Nadtochiy S, Brookes PS, Freeman BA. Detection and quantification of protein adduction by electrophilic fatty acids: mitochondrial generation of fatty acid nitroalkene derivatives. Free Radic Biol Med. 2009;46(9):1250–1259. doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Babior BM. NADPH Oxidase: An Update. Blood. 1999;93(5):1464–1476. [PubMed] [Google Scholar]
- 68.Pizzolla A, Hultqvist M, Nilson B, Grimm MJ, Eneljung T, Jonsson IM, Verdrengh M, Kelkka T, Gjertsson I, Segal BH, Holmdahl R. Reactive Oxygen Species Produced by the NADPH Oxidase 2 Complex in Monocytes Protect Mice from Bacterial Infections. J Immunol. 2012 doi: 10.4049/jimmunol.1103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sareila O, Kelkka T, Pizzolla A, Hultqvist M, Holmdahl R. NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal. 2011;15(8):2197–2208. doi: 10.1089/ars.2010.3635. [DOI] [PubMed] [Google Scholar]
- 70.O’Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol. 1999;12(1):83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 71.Rubbo H, Radi R. Protein and lipid nitration: role in redox signaling and injury. Biochim Biophys Acta. 2008;1780(11):1318–1324. doi: 10.1016/j.bbagen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102(7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trostchansky A, Rubbo H. Lipid nitration and formation of lipid-protein adducts: biological insights. Amino Acids. 2007;32(4):517–522. doi: 10.1007/s00726-006-0426-7. [DOI] [PubMed] [Google Scholar]
- 74.Trostchansky A, Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic Biol Med. 2008;44(11):1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.