Abstract
Objectives
To assess whether digital X-ray radiogrammetry (DXR) analysis of standard clinical hand or wrist radiographs obtained at emergency hospitals can predict hip fracture risk.
Methods
A total of 45,538 radiographs depicting the left hand were gathered from three emergency hospitals in Stockholm, Sweden. Radiographs with insufficiently included metacarpal bone, fractures in measurement regions, foreign material or unacceptable positioning were manually excluded. A total of 18,824 radiographs from 15,072 patients were analysed with DXR, yielding a calculated BMD equivalent (DXR-BMD). Patients were matched with the national death and inpatient registers. Inclusion criteria were age ≥ 40 years, no prior hip fracture and observation time > 7 days. Hip fractures were identified via ICD-10 codes. Age-adjusted hazard ratio per standard deviation (HR/SD) was calculated using Cox regression.
Results
8,257 patients (65.6 % female, 34.4 % male) met the inclusion criteria. One hundred twenty-two patients suffered a hip fracture after their radiograph. The fracture group had a significantly lower DXR-BMD than the non-fracture group when adjusted for age. The HR/SD for hip fracture was 2.52 and 2.08 in women and men respectively. The area under the curve was 0.89 in women and 0.84 in men.
Conclusions
DXR analysis of wrist and hand radiographs obtained at emergency hospitals predicts hip fracture risk in women and men.
Key Points
• Digital X-ray radiogrammetry of emergency hand/wrist radiographs predicts hip fracture risk.
• Digital X-ray radiogrammetry (DXR) predicts hip fracture risk in both women and men.
• Osteoporosis can potentially be identified in patients with suspected wrist fractures.
• DXR can potentially be used for selective osteoporosis screening.
Keywords: Osteoporosis, Hip fracture, Digital X-ray radiogrammetry, Cohort, BMD
Introduction
According to epidemiological studies, about one third of women over 50 years of age will experience a fragility fracture [1, 2]. The number of fractures can be reduced by adequate measures, such as lifestyle adaptations [3–6] and pharmaceutical interventions [7–10]. To be cost effective and avoid unnecessary and potentially harmful pharmaceutical treatment, it is imperative that those at risk are properly identified and targeted.
Previous studies have shown a correlation between bone mineral density (BMD) and the risk of fracture [11–15]. Consequently, BMD measurement has a prominent position in fracture risk assessment and diagnosis of osteoporosis. Dual-energy X-ray absorptiometry (DXA) of the hip or spine is considered the gold standard [12, 15, 16]. Unfortunately, most patients with a high risk of osteoporosis are not scanned by DXA, even when the clinician suspects osteoporosis. This is due to the relatively high costs [17, 18] and low availability of equipment [19–22].
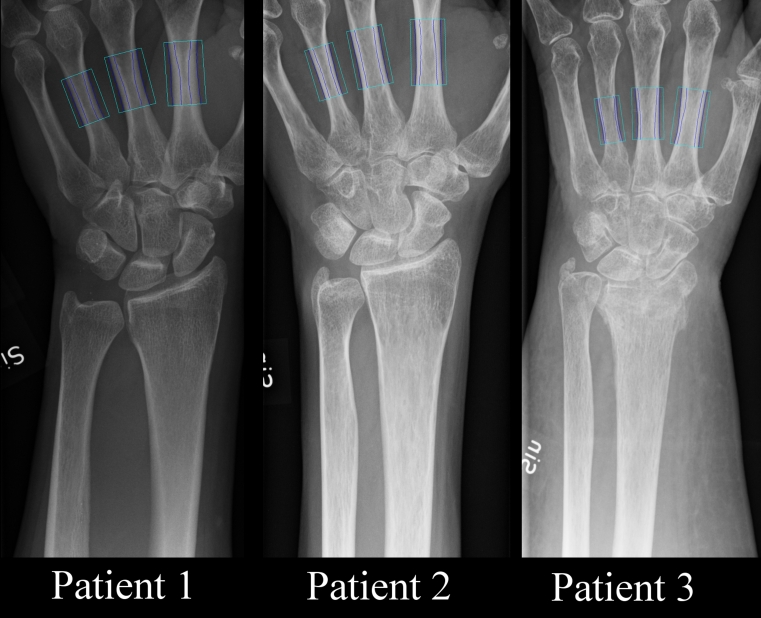
Digital X-ray radiogrammetry (DXR) is a peripheral measurement method based on a combination of an average geometrical measure of the cortical thickness of metacarpals II-IV and structural analysis of cortical bone porosity [23, 24]. The analysis is based on a standard radiograph of the hand (Fig. 1) and gives a computed BMD equivalent measurement. This makes DXR an interesting and potentially cost-effective candidate for the evaluation of osteoporosis-related fracture risk.
Fig. 1.
Wrist radiographs used for DXR analysis of patients with varying DXR T-scores. Measurement regions are indicated over metacarpals II-IV. Patient 1: a 50-year-old woman. DXR T-score 1.18. DXR-BMD 0.64 g/cm2. Patient 2: a 48-year-old woman. DXR T-score −1.90. DXR-BMD 0.49 g/cm2. Patient 3: a 75-year-old woman. DXR T-score −3.70. DXR-BMD 0.41 g/cm2
The objective of this study was to assess whether DXR analysis can predict hip fracture risk by using standard clinical hand or wrist radiographs obtained at emergency hospitals.
Materials and methods
This cohort study includes data from 1 January 2000 to 31 December 2008. The data were retrieved in 2009 and 2010. The ethical committee in Stockholm approved the study.
Radiograph selection
Database queries based on examination codes were used to extract all left hand and wrist radiographs from the digital archives of three major emergency hospitals in the Stockholm region. Clinical indications for left hand or wrist radiographs included suspicion of fracture, luxation, foreign body or arthritis.
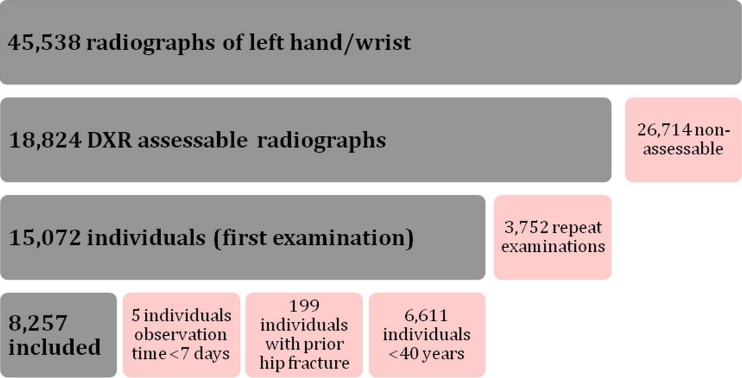
A total of 45,538 radiographs were extracted from the digital archives. All measurements and radiographs were manually reviewed by two of the investigators, who were blinded to all clinical information, and assessed according to suitability for further DXR analysis. Inclusion criteria were radiographs of the left hand or wrist depicting sufficient metacarpal bone for DXR analysis. Exclusion criteria were fractures in the measurement regions, foreign material such as fixation pins and plaster, or unacceptable positioning of the metacarpals. In all, 18,824 examinations from 15,072 patients were considered suitable for DXR analysis. In patients with repeated examinations, only the chronologically first evaluable examination was used. The image and patient selection process is illustrated in a flow chart (Fig. 2).
Fig. 2.
Flow chart of how radiographs and patients were selected (gray = inclusion, red = exclusion)
Patient selection
All patients whose radiographs were included for DXR analysis were identified in the National Patient Register provided by the National Board of Health and Welfare. Patients who subsequently had a hip fracture were identified via ICD-10 codes (S72.0, S72.1, S72.2). Inclusion criteria were age >40 years, no hip fracture prior to acquisition of the radiograph and observation time >7 days. To minimise the risk of erroneous registrations, only those coded for both diagnosis and adequate intervention (either upper femur fracture surgery or hip replacement, i.e. ICD-10 surgical codes NFJ and NFB) were registered as hip fracture. In order not to exclude patients with a hip fracture who were too critically ill for surgery, patients who died within 3 days after a registered fracture were also included. Date of fracture diagnosis, date of death or study end date (31 December 2008) was used as censoring time. The National Cause of Death Register provided date of death.
Digital X-ray radiogrammetry and BMD
Digital X-ray radiogrammetry (DXR) (Onescreen, Sectra Imtec AB, Linköping, Sweden) is a development of the traditional technique of radiogrammetry. On a standard projection radiograph, measurement regions are automatically placed around the narrowest parts of metacarpals II-IV. A BMD equivalent measurement (DXR-BMD) is then computed. The calculation is defined as DXR-BMD = cxVPAx(1-p) where c is a density constant empirically determined so that DXR-BMD on average is equal to the mid-distal forearm region of the Hologic QDR-2000 densitometer (Hologic, Bedford, MA, USA), VPA is cortical bone volume per area and p is porosity. When comparing an individual’s DXR-BMD to the mean DXR-BMD of a young, healthy, normal reference population, a DXR T-score can be derived. When compared to a healthy reference population of the same age, a DXR Z-score is obtained.
Any digital or CR radiography equipment that is applicable for acquiring hand X-ray images can be used to acquire images for DXR-BMD analysis. The DXR analysis process is automated and operator independent. However, there are some requirements about positioning and exposure settings, e.g., when acquiring radiographs intended for DXR analysis. Some requirements are generic (posterior-anterior X-ray image of one hand, palm flat to detector table/image plate surface, focus centred on metacarpal III) and some are specific per modality type and model (image postprocessing settings, focus distance, exposure settings, location on detector).
The DXR technology has been described in more detail previously [23, 24], and normative reference tables have been published [25, 26].
Statistical analysis
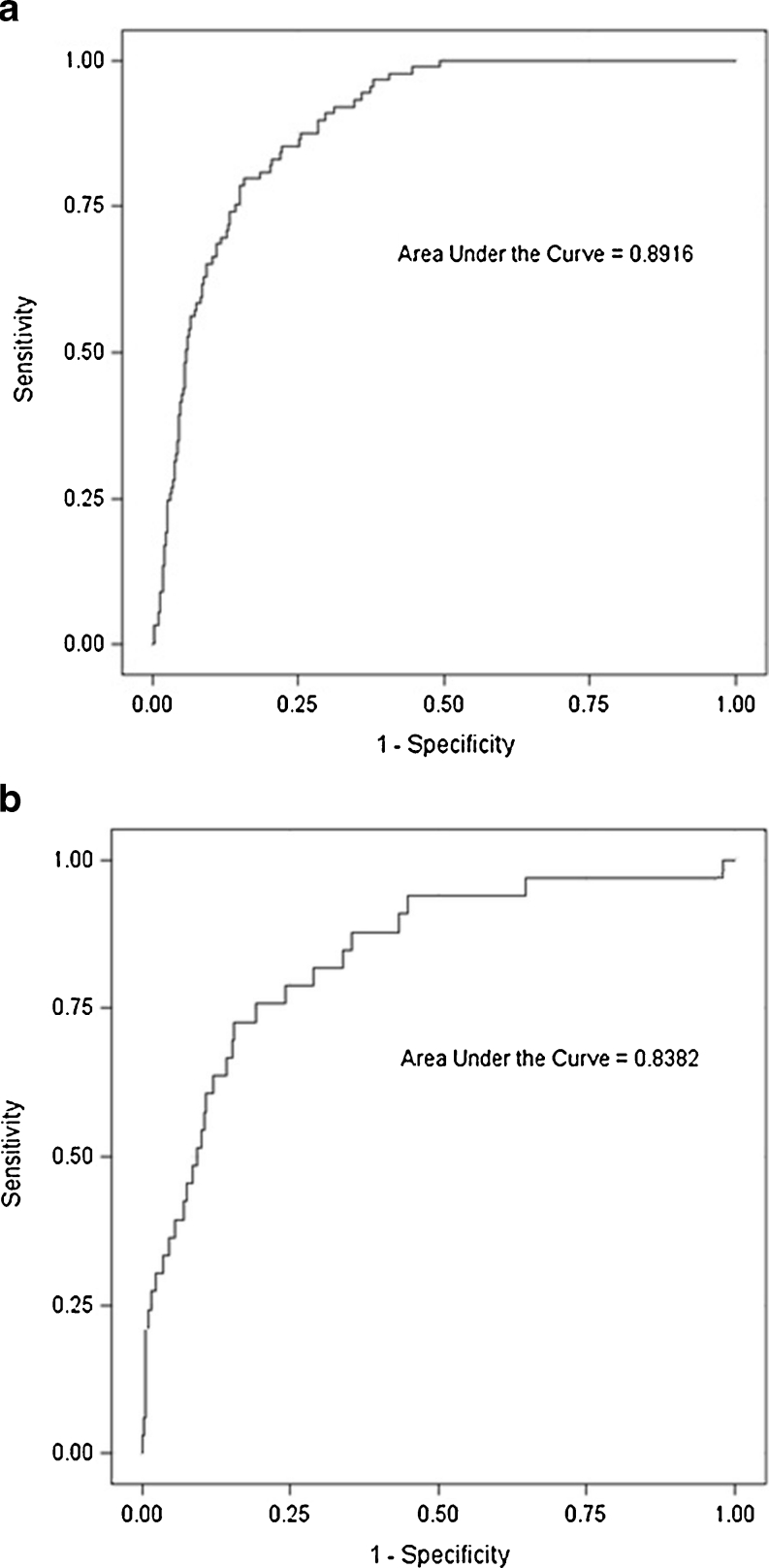
Group comparisons were made using Student’s t-test for continuous normally distributed data. Receiver-operator characteristics (ROC) were plotted (Fig. 3) to evaluate the predictive value of the DXR T-score to assess fracture risk. In these graphs the sensitivity of a parameter, in this case DXR T-score, to predict future fractures is plotted as a function of the proportion of false positives (1-specificity). To compensate for age-related fracture risk that is not related to DXR-BMD (e.g. the increased tendency to fall), the ROCs were adjusted for age. To enable comparison among different studies, the area under the plotted age-adjusted ROC curve, the AUC and the age-adjusted hazard ratio per standard deviation change in DXR T-score (HR/SD) were calculated. The HR/SD was calculated using Cox regression and the risk of sustaining a hip fracture at DXR T-scores >−1 was defined as risk = 1. SAS® 9.2 (SAS Institute, Cary, NC) was used for the statistical analysis.
Fig. 3.
a Age-adjusted ROC curve for women. b Age-adjusted ROC curve for men
Results
The inclusion criteria were met by 8,257 patients (65.6 % women; 34.4 % men). The average age was 59.6 years (SD 12) (60.5 years in women; 57.8 years in men). The average follow-up period was 3 years 3 months with a total observation time of 27,072 person-years. The number of patients, average DXR-BMD, DXR T-score, number of fractures and annual fracture rate are provided per 5-year age group in Table 1 and per DXR T-score in Table 2.
Table 1.
Distribution of numbers, bone mineral density (DXR-BMD), DXR T-score and fracture rate by 5-year age groups
| Age | Number | Mean DXR-BMD (standard deviation) | Mean DXR T-score (standard deviation) | Fractures | Mean annual fracture rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| 40–44 | 523 | 472 | 0.59 (0.05) | 0.66 (0.06) | 0.19 (1.14) | −0.20 (1.01) | 0 | 1 | 0.00 | 0.07 |
| 45–49 | 598 | 424 | 0.58 (0.05) | 0.66 (0.06) | 0.01 (1.08) | −0.27 (1.02) | 1 | 2 | 0.05 | 0.14 |
| 50–54 | 747 | 404 | 0.56 (0.05) | 0.65 (0.06) | −0.43 (1.15) | −0.41 (1.02) | 1 | 3 | 0.04 | 0.23 |
| 55–59 | 982 | 426 | 0.55 (0.05) | 0.64 (0.06) | −0.78 (1.14) | −0.53 (0.99) | 2 | 4 | 0.06 | 0.27 |
| 60–64 | 847 | 360 | 0.52 (0.06) | 0.63 (0.06) | −1.33 (1.18) | −0.69 (0.97) | 8 | 5 | 0.29 | 0.45 |
| 65–69 | 558 | 239 | 0.50 (0.06) | 0.60 (0.06) | −1.80 (1.27) | −1.11 (1.01) | 8 | 0 | 0.44 | 0.00 |
| 70–74 | 403 | 192 | 0.47 (0.06) | 0.59 (0.07) | −2.35 (1.21) | −1.39 (1.10) | 9 | 2 | 0.67 | 0.35 |
| 75–79 | 338 | 154 | 0.44 (0.06) | 0.58 (0.07) | −2.93 (1.18) | −1.54 (1.11) | 17 | 4 | 1.47 | 0.92 |
| 80–84 | 260 | 104 | 0.43 (0.06) | 0.56 (0.07) | −3.24 (1.16) | −1.91 (1.17) | 18 | 5 | 2.19 | 1.63 |
| 85–89 | 116 | 48 | 0.41 (0.05) | 0.53 (0.06) | −3.74 (1.00) | −2.37 (0.98) | 18 | 4 | 6.90 | 3.78 |
| 90–94 | 38 | 12 | 0.39 (0.05) | 0.48 (0.07) | −4.10 (1.05) | −3.06 (1.16) | 7 | 2 | 9.83 | 14.63 |
| 95–99 | 10 | 2 | 0.37 (0.04) | 0.49 (0.05) | −4.49 (0.85) | −2.93 (0.88) | 0 | 1 | 0.00 | 98.98 |
| Total | 5420 | 2837 | 0.53 (0.08) | 0.63 (0.07) | −1.20 (1.60) | −0.69 (1.16) | 89 | 33 | 0.49 | 0.37 |
Table 2.
Distribution of numbers, observation time and fracture rate by various DXR T-scores
| DXR T-score | Number | Mean age | SD age | Fractures | Observation years | Annual fracture rate (%) |
|---|---|---|---|---|---|---|
| Female | ||||||
| <−6 | 5 | 74.9 | 12.0 | 1 | 18 | 5.71 |
| −6 < −5 | 44 | 83.5 | 8.8 | 5 | 121 | 4.12 |
| −5 < −4 | 235 | 78.7 | 8.9 | 23 | 698 | 3.30 |
| −4 < −3 | 516 | 74.2 | 9.4 | 40 | 1,573 | 2.54 |
| −3 < −2 | 803 | 66.9 | 9.6 | 10 | 2,634 | 0.38 |
| −2 < −1 | 1,172 | 61.0 | 9.3 | 6 | 3,834 | 0.16 |
| −1 < 0 | 1,376 | 55.9 | 8.3 | 4 | 4,733 | 0.08 |
| 0 < 1 | 870 | 52.1 | 7.5 | 0 | 3,006 | 0.00 |
| 1 < 2 | 336 | 49.9 | 7.1 | 0 | 1,238 | 0.00 |
| 2 < 3 | 57 | 47.4 | 11.0 | 0 | 211 | 0.00 |
| 3 < 4 | 4 | 46.7 | 7.5 | 0 | 10 | 0.00 |
| 4 < 5 | 2 | 51.5 | 11.5 | 0 | 8 | 0.00 |
| Total | 5,420 | 60.5 | 12.0 | 89 | 18,083 | 0.49 |
| <−2.5 | 1,165 | 73.6 | 10.2 | 72 | 3,581 | 2.01 |
| Male | ||||||
| <−6 | 1 | 81.0 | – | 1 | 5 | 19.50 |
| −6 < −5 | – | – | – | – | – | – |
| −5 < −4 | 16 | 74.6 | 11.3 | 0 | 42 | 0.00 |
| −4 < −3 | 81 | 74.1 | 12.8 | 8 | 217 | 3.68 |
| −3 < −2 | 264 | 67.7 | 12.9 | 9 | 768 | 1.17 |
| −2 < −1 | 679 | 61.0 | 12.3 | 9 | 2,070 | 0.43 |
| −1 < 0 | 1,011 | 55.8 | 10.6 | 4 | 3,296 | 0.12 |
| 0 < 1 | 610 | 53.1 | 9.1 | 1 | 1,959 | 0.05 |
| 1 < 2 | 157 | 49.9 | 8.2 | 1 | 562 | 0.18 |
| 2 < 3 | 18 | 46.7 | 5.2 | 0 | 69 | 0.00 |
| 3 < 4 | – | – | – | – | – | – |
| 4 < 5 | – | – | – | – | – | – |
| Total | 2,837 | 57.8 | 12.2 | 33 | 8,989 | 0.37 |
| <−2.5 | 199 | 72.1 | 12.5 | 12 | 553 | 2.17 |
During the observation period, 122 patients (89 women and 33 men) suffered from a hip fracture. In both men and women, the patients who suffered from a hip fracture were significantly older at the time of X-ray examination and had a significantly lower DXR-BMD, DXR T-score and DXR Z-score than those who did not (Table 3). Out of the 122 patients who suffered a hip fracture, 84 (72 women and 12 men, 69 %) had a DXR T-score of ≤−2.5, resulting in a sensitivity of 81 % and a specificity of 79 % in women and 36 % and 93 % respectively in men (Table 4).
Table 3.
Differences between fracture and non-fracture groups by sex. One standard deviation is given within parentheses. All differences were statistically significant at the P < 0.001 level
| Number | Age at exam (years) | DXR-BMD (g/cm2) | DXR T-score | DXR Z-score | |
|---|---|---|---|---|---|
| Female | |||||
| Fracture | 89 | 78 (10) | 0.419 (0.05) | −3.5 (1.1) | −0.565 (0.988) |
| Non-fracture | 5,331 | 60 (12) | 0.528 (0.08) | −1.2 (1.6) | 0.009 (0.997) |
| Male | |||||
| Fracture | 33 | 70 (16) | 0.538 (0.09) | −2.2 (1.4) | −0.900 (1.110) |
| Non-fracture | 2,804 | 58 (12) | 0.630 (0.07) | −0.7 (1.1) | 0.030 (0.994) |
Table 4.
The sensitivity and specificity of DXR to predict hip fracture at DXR T-score ≤−2.5, with 95 % confidence intervals and the corresponding annual fracture rate
| Age (years) | Sensitivity (95 % CI) | Specificity (95 % CI) | Annual fracture rate (%) | |
|---|---|---|---|---|
| Women | >40 | 81 (73–89) | 79 (78–80) | 2.01 |
| 55–85 | 76 (66–86) | 72 (70–73) | 1.62 | |
| Men | >40 | 36 (20–53) | 93 (92–94) | 2.17 |
| 55–85 | 25 (6–44) | 90 (89–92) | 1.18 |
The age-adjusted AUC for the DXR T-score to predict hip fracture was 0.89 in women and 0.84 in men (Fig. 3). In the 55–85-year age group, the corresponding AUCs were 0.83 and 0.80, respectively.
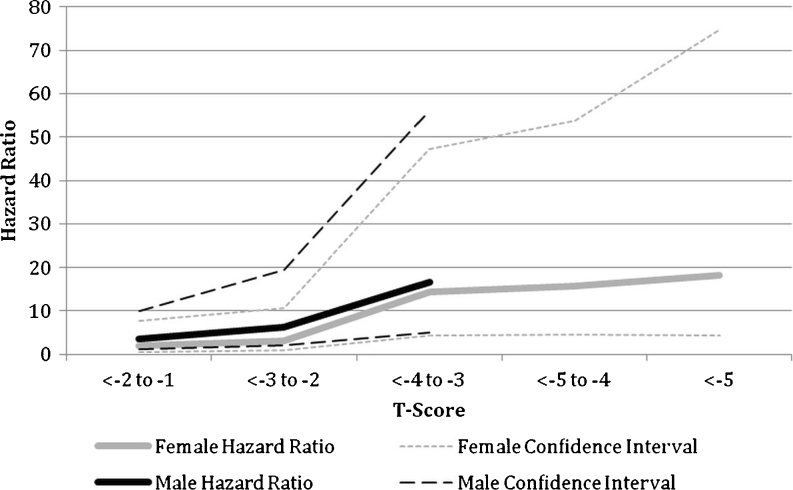
Age-adjusted hazard ratios for hip fracture at different DXR T-scores are shown in Fig. 4 and Table 5. The hazard ratio per standard deviation change in DXR T-score (HR/SD) for hip fracture was 2.52 and 2.08 in the female and male group, respectively. In the 55–85-year age group, the corresponding values were 2.33 and 2.00 for women and men, respectively.
Fig. 4.
Hazard ratio by various DXR T-scores for female and male subjects. No data shown for men at DXR T-score <−4 because of insufficient numbers of subjects
Table 5.
Age-adjusted hazard ratios for hip fracture for different DXR T-scores by sex. DXR T-score >−1.0 was defined as HR = 1
| DXR T-score | Hazard ratio | 95 % Confidence interval | Number | Fractures | Observation years | |
|---|---|---|---|---|---|---|
| Female | ||||||
| <−2 to −1 | 2.1 | 0.56 | 7.7 | 1,172 | 6 | 3,834 |
| <−3 to −2 | 3.1 | 0.87 | 10.8 | 803 | 10 | 2,634 |
| <−4 to −3 | 14.4 | 4.42 | 47.2 | 516 | 40 | 1,573 |
| <−5 to −4 | 15.7 | 4.59 | 53.8 | 235 | 23 | 698 |
| <−5 | 18.3 | 4.45 | 74.8 | 49 | 6 | 139 |
| Male | ||||||
| <−2 to −1 | 3.5 | 1.20 | 10.1 | 679 | 9 | 2,070 |
| <−3 to −2 | 6.3 | 2.01 | 19.6 | 264 | 9 | 768 |
| <−4 to −3 | 16.7 | 4.99 | 56.1 | 81 | 8 | 217 |
| <−5 to −4 | 0.00 | 0.00 | – | 16 | 0 | 42 |
| <−5 | 42.3 | 4.30 | 416.4 | 1 | 1 | 5 |
Discussion
In this study, DXR-BMD of archived radiographs of the wrist or hand were used to evaluate the association with hip fractures occurring after the radiograph had been obtained. In order to evaluate whether DXR of radiographs of the wrist and hand obtained at emergency hospitals can be used for selected screening for osteoporosis, so-called area under the curve (AUC) calculations were made. Those describe the relationship between the sensitivity and specificity. Ideally such curves are close to 1, where only those who will suffer from a hip fracture are discriminated from those who will not. In this study, the age-adjusted AUC for the DXR T-score to predict hip fracture was 0.89 in women and 0.84 in men. These values are similar to, or higher than, those previously published for central DXA [14]. Bousson et al. compared quantitative computed tomography (QCT) and hip DXA in a female study population with a similar age distribution to our cohort and obtained AUCs almost as large as ours (0.84 for QCT and 0.80 for DXA) [27]. In the EPIDOS cohort, AUC calculations for hip fracture risk based on central DXA measurements ranged from 0.68 to 0.72 depending on the region measured [15]. However, the EPIDOS population was older and the age distribution smaller (average age 80.5, SD 3.8), which results in smaller AUCs. Cummings’ study, based on a younger population (average age 73.2) than the EPIDOS cohort, provided a slightly greater AUC, with a maximum of 0.78 for women undergoing DXA of Ward’s triangle (AUC was 0.76 when measured at the femoral neck) [28].
A meta-analysis from 1996 estimated that the increased risk of hip fracture with a decrease of 1.0 in T-score is 2.6 for hip BMD and 1.9 for lumbar spine BMD in women [29]. Later studies have shown similar results [13, 30]. When using DXR, Bouxsein et al. reported a hazard ratio per decrease of 1.0 in DXR T-score of 1.8 for hip fractures in a case-cohort study of a sample from the Study of Osteoporotic Fracture (SOF) cohort, i.e., community-dwelling US women aged >65 years [31]. In their prospective study DXR compared well to other peripheral BMD measurements, but was inferior to central BMD measurements when predicting hip fracture risk. In a DXR analysis of archived radiographs from a subgroup of the Copenhagen City Heart Study cohort, consisting of women >55 years with self-reported joint or bone pain, Bach-Mortensen et al. reported a HR/SD of 1.4 for hip fractures (P = 0.052) [32].
In our study the HR/SD for DXR in women aged 55–85 was 2.33 and in all women 2.52. Thus, DXR provided predictive values comparable to those previously published for central BMD measurements [29] and considerably higher than those previously reported for DXR [31, 32]. However, our study differs from the previous studies by Bouxsein’s and Bach-Mortensen’s groups with regard to the study population. Their studies included patients recruited from selected cohorts (SOF cohort and Copenhagen City Heart Study cohort, respectively). Our study reflects all patients undergoing standard X-ray examination of the left hand at three emergency hospitals in Stockholm. These groups might differ in terms of fracture and disease prevalence. The hazard ratio was also high in the male group; 2.08 in all men and 2.00 in men aged 55–85. To our knowledge, DXR’s predictability has not previously been studied in men.
Due to the inclusion of all left hand/wrist radiographs obtained at three emergency hospitals, the patients included were most likely those with a suspected wrist fracture, i.e. trauma, and patients undergoing routine control for rheumatoid arthritis. Distal forearm fractures have been shown to be associated with other fragility fractures and have therefore been suggested as a possible predictor in fracture risk assessment [12, 33–35]. The correlation between wrist fracture and other fragility fractures appears to be stronger in men [36]. Rheumatoid arthritis has been shown to increase the risk of fracture significantly [30, 37, 38]. Hence, the patients in our study were most likely at higher risk of fragility fractures than a normal population. This might also explain the improved performance of DXR observed in our study compared to previous reports [31, 32].
Defining clinical thresholds
There are no previous recommendations on when patients should receive treatment based on DXR measurements. According to the National Osteoporosis Foundation clinician’s guide to prevention and treatment of osteoporosis, health-care providers should consider FDA-approved medical therapies in postmenopausal women and men aged 50 years and older at T-score ≤−2.5 at the femoral neck or spine or in the case of low bone mass (T-score between −1.0 and −2.5 at the femoral neck or spine) and a 10-year probability of a hip fracture ≥3 % [39]. In clinical fracture outcome trials, a T-score of −2.5 is one of the most commonly used inclusion criteria, for example in the FREEDOM trial [40]. A combination of low BMD and prevalent vertebral fracture has also been used for inclusion. In the HORIZON trial subjects could be included if they had femoral neck BMD with a T-score of ≤−2.5 or if they had a T-score of ≤−1.5 plus at least two prevalent vertebral fractures [41]. When calculating the annual hip fracture rate in women receiving a placebo during those two clinical trials, the rate was 0.37 % and 0.76 % respectively. If the threshold DXR T-score ≤−2.5 is applied for all women in our material, the sensitivity to find hip fractures would be 0.81 and the specificity 0.79. However, in guidelines treatment is normally recommended for the 55–85-year age group. Used in that age group, the threshold DXR T-score ≤−2.5 would result in a sensitivity of 0.76 and a specificity of 0.72, with an annual fracture rate of 1.6 %. That rate is clearly higher than those observed in most pivotal fracture trials. This higher fracture incidence illustrates the difference in fracture incidence between clinical cohorts and that of clinical trials with selected subjects. It can therefore be discussed whether treatment should be given at higher DXR T-scores than <−2.5 in order to give treatment at the same fracture risk level as those shown to be efficient in the clinical trials. However, if the threshold were based on annual hip fracture rates similar to those observed in the placebo group of the HORIZON trial (0.76 %) [41], it would result in a severely reduced specificity. Women aged 55 to 85 would then receive treatment already at DXR T-scores of ≤−0.59, resulting in a sensitivity of 0.97, but with the poor specificity of 0.23. This would lead to substantially higher numbers needed to treat in order to avoid one hip fracture.
Defining the threshold level for men is more challenging. At DXR T-score <−2.5 the sensitivity for all men >40 years is only 0.36 and specificity 0.93, with an annual fracture rate of 2.0 %. Applying a threshold of DXR T-score <−2.5 would therefore imply severe undertreatment, excluding most men at risk. In men the threshold value of osteopenia seems more reasonable when applied to the 55–85-year age group, resulting in a sensitivity of 0.52 and specificity of 0.76, with an annual hip fracture rate of 0.81 %. However, there were only 20 hip fractures in that age group, so the threshold for treatment in men when using DXR cannot be robustly estimated.
Study limitations
The radiographs used in this study were not intentionally intended for DXR analysis. Only 41 % of the retrieved radiographs were considered suitable for analysis with DXR. This percentage might appear small, but one has to consider that all radiographs with plaster, external fixation or fractures that affected the metacarpals (i.e. measurement regions) were excluded. When retrieving radiographs from the digital archives, examinations of fingers or carpal bones were also included as they carry the same examination code as a radiograph of the hand. Such radiographs could not be included as they do not fully depict the metacarpal bones.
In some included cases, the radiographs considered acceptable for DXR analysis were actually slightly suboptimal because they were not obtained according to the DXR protocol. This source of error might affect DXR’s predictive value in this study.
According to population registers from Statistics Sweden, 78 % of the population in Stockholm was born in Sweden, 15 % in other Caucasian countries, 2.6 % in Asian countries, 2.2 % in African countries and 1.7 % in Hispanic countries. Due to the retrospective data collection of radiographs only, there was no information on patient race, body mass index or menopause age. Neither were there records on prior or subsequent treatment for osteoporosis or other diseases. This could influence the predictive value found in this study. If a great proportion of our material had treatment with cytostatics or corticosteroids, this would lead to an overestimation of the predictive value, but on the other hand osteoporosis treatment would lead to an underestimation.
The DXR analysis is intended for use on the non-dominant hand. As there was no patient record regarding hand dominance, the left hand was analysed in all patients. Approximately 10 % of the population has a left hand dominance [42]. This is a source of error, probably resulting in slightly higher DXR-BMD in left-dominant individuals, which could lead to a slight underestimation of DXR’s ability to predict future hip fractures.
The use of The Swedish National Patient Register and National Cause of Death Register minimised the risk of loss to follow-up. This is a major strength of this study due to the high quality of the registers with 99 % completion [43]. Unfortunately, we were not able to identify whether any of the patients had been exposed to high-energy trauma that could have caused their hip fracture. This is a limitation of our study as non-fragility fractures might have been included in the fracture group. However, this effect is believed to be minimal, as patients with hip fractures were on average significantly older than those without fracture and had lower DXR-BMD. Furthermore, included non-fragility, i.e. high-energy trauma, hip fractures would most likely affect DXR’s predictive ability unfavorably, resulting in an underestimation of the method’s predictive value.
In conclusion, hip fractures can be predicted in both women and men by DXR analysis of clinical wrist and hand radiographs obtained at emergency hospitals. DXR may therefore be useful to identify patients with increased hip fracture risk already at the emergency department and might provide an alternative where access to central BMD measurements is limited. However, further studies are warranted to determine DXR’s ability in a normal population.
Acknowledgements
Financial support was provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
Johan Kälvesten and Jakob Algulin are employees of Sectra Imtec AB, Linköping, Sweden.
The authors wish to thank Ali Waqar for the data retrieval of all digital radiographs used in this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Glossary
Abbreviations and acronyms
- DXR
Digital X-ray radiogrammetry
- QCT
Quantitative computed tomography
- DXA
Dual-energy X-ray absorptiometry
- BMD
Bone mineral density
- T-score
Standard deviations above or below the mean for a healthy young adult of the same sex and ethnicity as the patient
- Z-score
The number of standard deviations above or below the mean for the patient’s age, sex and ethnicity
- HR
Hazard ratio
- SD
Standard deviation
- ROC
Receiver-operating characteristic
- AUC
Area under the curve
- ICD-10
International Statistical Classification of Diseases and Related Health Problems, 10th Revision
Footnotes
Institution from which the work originated
Karolinska Institutet Department of Clinical Science, Intervention and Technology, Division of Medical Imaging and Technology, Stockholm, Sweden
References
- 1.Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16:S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22:781–788. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 3.Body JJ, Bergmann P, Boonen S, et al. Non-pharmacological management of osteoporosis: a consensus of the Belgian Bone Club. Osteoporos Int. 2011;22:2769–2788. doi: 10.1007/s00198-011-1545-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rikkonen T, Salovaara K, Sirola J, et al. Physical activity slows femoral bone loss but promotes wrist fractures in postmenopausal women: a 15-year follow-up of the OSTPRE study. J Bone Miner Res. 2010;25:2332–2340. doi: 10.1002/jbmr.143. [DOI] [PubMed] [Google Scholar]
- 5.Bonaiuti D, Shea B, Iovine R et al (2002) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. doi:10.1002/14651858.CD000333 [DOI] [PubMed]
- 6.Gillespie Lesley D, Robertson MC, Gillespie William J et al (2009) Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007146.pub2 [DOI] [PubMed]
- 7.O’Donnell S, Cranney A, Wells George A, Adachi J, Reginster J-Y (2006) Strontium ranelate for preventing and treating postmenopausal osteoporosis. Cochrane Database Syst Rev. doi:10.1002/14651858.CD005326.pub3 [DOI] [PubMed]
- 8.Wells George A, Cranney A, Peterson J et al (2008) Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. doi:10.1002/14651858.CD004523.pub3 [DOI] [PubMed]
- 9.Wells George A, Cranney A, Peterson J et al (2008) Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. doi:10.1002/14651858.CD003376.pub3 [DOI] [PMC free article] [PubMed]
- 10.Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med. 2010;363:2027–2035. doi: 10.1056/NEJMct1004903. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry. JAMA. 2002;288:1889–1897. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 12.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–774. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 13.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 14.Kanis J, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 15.Schott AM, Cormier C, Hans D, et al. How hip and whole-body bone mineral density predict hip fracture in elderly women: the EPIDOS prospective study. Osteoporos Int. 1998;8:247–254. doi: 10.1007/s001980050061. [DOI] [PubMed] [Google Scholar]
- 16.Ismail AA, Pye SR, Cockerill WC, et al. Incidence of limb fracture across Europe: results from the European Prospective Osteoporosis Study (EPOS) Osteoporos Int. 2002;13:565–571. doi: 10.1007/s001980200074. [DOI] [PubMed] [Google Scholar]
- 17.Hayes BL, Curtis JR, Laster A, et al. Osteoporosis care in the United States after declines in reimbursements for DXA. J Clin Densitom. 2010;13:352–360. doi: 10.1016/j.jocd.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewiecki E, Baim S, Siris E. Osteoporosis care at risk in the United States. Osteoporos Int. 2008;19:1505–1509. doi: 10.1007/s00198-008-0716-x. [DOI] [PubMed] [Google Scholar]
- 19.Cadarette SM, Gignac MAM, Jaglal SB, Beaton DE, Hawker GA. Access to osteoporosis treatment is critically linked to access to dual-energy X-ray absorptiometry testing. Med Care. 2007;45:896–901. doi: 10.1097/MLR.0b013e318054689f. [DOI] [PubMed] [Google Scholar]
- 20.Curtis J, Laster A, Becker D, et al. The geographic availability and associated utilization of dual-energy X-ray absorptiometry (DXA) testing among older persons in the United States. Osteoporos Int. 2009;20:1553–1561. doi: 10.1007/s00198-008-0821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DÌaz Curiel M, Carbonell C, Quesada Lopez JM. Osteoporosis in Spain: map of resources for diagnosing. J Clin Densitom. 2008;11:561–567. doi: 10.1016/j.jocd.2008.05.096. [DOI] [PubMed] [Google Scholar]
- 22.Nayak S, Roberts M, Greenspan S. Factors associated with osteoporosis screening and recommendations for osteoporosis screening in older adults. J Gen Intern Med. 2009;24:585–591. doi: 10.1007/s11606-009-0936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosholm A, Hyldstrup L, Bæksgaard L, Grunkin M, Thodberg HH. Estimation of bone mineral density by digital X-ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int. 2001;12:961–969. doi: 10.1007/s001980170026. [DOI] [PubMed] [Google Scholar]
- 24.Jørgensen JT, Andersen PB, Rosholm A, Bjarnason NH. Digital X-ray radiogrammetry: a new appendicular bone densitometric method with high precision. Clin Physiol. 2000;20:330–335. doi: 10.1046/j.1365-2281.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 25.Black DM, Palermo L, Sørensen T, et al. A normative reference database study for pronosco X-posure system(TM) J Clin Densitom. 2001;4:5–12. doi: 10.1385/JCD:4:1:05. [DOI] [PubMed] [Google Scholar]
- 26.Hyldstrup L, Nielsen SP. Metacarpal index by digital X-ray radiogrammetry: normative reference values and comparison with dual X-ray absorptiometry. J Clin Densitom. 2001;4:299–306. doi: 10.1385/JCD:4:4:299. [DOI] [PubMed] [Google Scholar]
- 27.Bousson VD, Adams J, Engelke K, et al. In vivo discrimination of hip fracture with quantitative computed tomography: results from the prospective European Femur Fracture Study (EFFECT) J Bone Miner Res. 2011;26:881–893. doi: 10.1002/jbmr.270. [DOI] [PubMed] [Google Scholar]
- 28.Cummings SR, Browner W, Black DM, et al. Bone density at various sites for prediction of hip fractures. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 29.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009;71:392–397. doi: 10.1016/j.ejrad.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 31.Bouxsein ML, Palermo L, Yeung C, Black DM. Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int. 2002;13:358–365. doi: 10.1007/s001980200040. [DOI] [PubMed] [Google Scholar]
- 32.Bach-Mortensen P, Hyldstrup L, Appleyard M, Hindsø K, Gebuhr P, Sonne-Holm S. Digital X-ray radiogrammetry identifies women at risk of osteoporotic fracture: results from a prospective study. Calcif Tissue Int. 2006;79:1–6. doi: 10.1007/s00223-005-0260-z. [DOI] [PubMed] [Google Scholar]
- 33.Lauritzen JB, Schwarz P, McNair P, Lund B, Transbøl I. Radial and humeral fractures as predictors of subsequent hip, radial or humeral fractures in women, and their seasonal variation. Osteoporos Int. 1993;3:133–137. doi: 10.1007/BF01623274. [DOI] [PubMed] [Google Scholar]
- 34.Mallmin H, Ljunghall S, Persson I, Naessén T, Krusemo U-B, Bergström R. Fracture of the distal forearm as a forecaster of subsequent hip fracture: a population-based cohort study with 24 years of follow-up. Calcif Tissue Int. 1993;52:269–272. doi: 10.1007/BF00296650. [DOI] [PubMed] [Google Scholar]
- 35.Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8:53–60. doi: 10.1007/s001980050048. [DOI] [PubMed] [Google Scholar]
- 36.Haentjens P, Johnell O, Kanis JA, et al. Evidence from data searches and life-table analyses for gender-related differences in absolute risk of hip fracture after Colles’ or spine fracture: Colles’ fracture as an early and sensitive marker of skeletal fragility in white men. J Bone Miner Res. 2004;19:1933–1944. doi: 10.1359/jbmr.040917. [DOI] [PubMed] [Google Scholar]
- 37.Wright NC, Lisse JR, Walitt BT, Eaton CB, Chen Z. Arthritis increases the risk for fractures—results from the women’s health initiative. J Rheumatol. 2011;38:1680–1688. doi: 10.3899/jrheum.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, Ghozlani I. Prevalence and risk factors of vertebral fractures in women with rheumatoid arthritis using vertebral fracture assessment. Rheumatology. 2010;49:1303–1310. doi: 10.1093/rheumatology/keq084. [DOI] [PubMed] [Google Scholar]
- 39.National Osteoporosis Foundation . Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. [Google Scholar]
- 40.Cummings SR, Martin JS, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 41.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 42.Hardyck C, Petrinovich LF. Left-handedness. Psychol Bull. 1977;84:385–404. doi: 10.1037/0033-2909.84.3.385. [DOI] [PubMed] [Google Scholar]
- 43.Socialstyrelsen . Kvalitet och innehåll i patientregistret—Utskrivningar från slutenvården 1964–2007 och besök i specialiserad öppenvård (exklusive primärvårdsbesök) 1997–2007. Stockholm: Socialstyrelsen; 2009. [Google Scholar]