Abstract
This study aims to confirm feasibility of near-infrared (NIR) fluorescence imaging for sentinel lymph node (SLN) biopsy in vulvar cancer and to compare the tracer indocyanine green (ICG) bound to human serum albumin (HSA) versus ICG alone. Patients received 99mTc-nanocolloid and patent blue for SLN detection. Subsequently, patients randomly received ICG:HSA or ICG alone. In 24 patients, 35 SLNs were intraoperatively detected. All SLNs detected were radioactive and NIR fluorescent and 27 (77%) were blue. No significant difference was found between ICG:HSA and ICG alone. This trial confirms the feasibility of NIR fluorescence imaging for SLN mapping in vulvar cancer.
Keywords: Near-Infrared Fluorescence Imaging, Vulvar Cancer, Sentinel Lymph Node Mapping, Image-Guided Surgery, Indocyanine Green
INTRODUCTION
Lymph node status is the most significant prognostic factor for survival in women with vulvar cancer.1,2 Therefore, lymphadenectomy plays a major role in the surgical treatment and staging of vulvar cancer. However, approximately 70% of patients undergo unnecessary lymphadenectomy, which is associated with high morbidity and prolonged hospitalization.3–5 The introduction of sentinel lymph node (SLN) biopsy has provided a less invasive technique for nodal staging.6–8 SLN biopsy in early-stage vulvar cancer is considered accurate and safe without compromising groin recurrence or survival rates.3,9
Currently, radioactive colloids, blue dyes, or a combination of both are used for SLN detection and offer high identification rates and low false-negative rates.9,10 Recently, near-infrared (NIR) fluorescence optical imaging for SLN detection has been introduced. This technique uses the clinically available NIR fluorescent tracer indocyanine green (ICG).11 The use of NIR light (700–900 nm) has several characteristics that can be advantageous in SLN biopsy as it offers relatively high tissue penetration (several millimeters) compared to blue dye and detection of low concentrations of tracer.12–14 Furthermore, NIR fluorescence imaging outperformed blue dye for SLN detection in multiple clinical studies.14–16 In vulvar cancer, two pilot studies demonstrated feasibility of NIR fluorescence for SLN biopsy.17,18 Hutteman et al. used ICG adsorbed to human serum albumin (HSA, complex ICG:HSA) and Crane et al. used ICG alone for SLN mapping in vulvar cancer patients.17,18 In vitro studies demonstrated that adsorption of ICG to HSA, by simple mixing, increases its fluorescence intensity (by threefold) and hydrodynamic diameter, which possibly results in better retention in the SLN.19 However, lymphatic vessels contain high concentrations of HSA and other proteins, which would make adsorption of ICG to HSA before injection redundant. Therefore, clinical assessment and comparison of these two lymphatic tracers is essential.
The aim of this double-blind randomized trial was to confirm feasibility of NIR fluorescence imaging for SLN biopsy in vulvar cancer and to assess whether ICG alone could render the same fluorescence intensity in the SLNs as ICG:HSA.
MATERIALS AND METHODS
Tracer Preparation
ICG (25-mg vials, Pulsion Medical Systems, Munich, Germany) was resuspended in 10 cc of sterile water. To obtain 500 μM, 7.8 mL of the 3.2-mM ICG solution was diluted in 42.8 mL of sterile water or 42.8 ml of Cealb (20% human serum albumin, Sanquin, Amsterdam, The Netherlands) for the preparation of ICG alone or ICG:HSA, respectively. A dose of 500 μM was chosen based on previous dose optimization studies.17,20
Clinical Trial
This double-blind, randomized, non-inferiority trial comparing ICG:HSA with ICG alone was approved by the Medical Ethics Committee of the Leiden University Medical Center and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975. Inclusion criteria were a clinically FIGO stage I vulvar cancer with an unifocal squamous cell carcinoma measuring less than 4cm in diameter, not encroaching the vagina, anus, or urethra and with negative inguinofemoral nodes as determined by palpation and ultrasonography.5 However, four patients with a tumor >4cm were scheduled to undergo SLN biopsy of the inguinofemoral nodes outside this protocol, because of other co-morbidity or age > 80. Exclusion criteria were pregnancy, lactation, or an allergy to iodine or ICG. All patients gave informed consent and were anonymized. Randomization was performed by the Department of Clinical Pharmacy by block randomization.
Patients received the standard-of-care SLN procedure.3 For our institution, this implied peritumoral injections of 60–100 MBq 99mtechnetium-nanocolloid the day before, or the morning prior to surgery. Prior to surgery, 1 mL of patent blue V (Guerbet, France) was injected at 4 sites peritumorally intracutaneously or around the excision scar, in cases of earlier excision biopsy. Subsequently, 1.6 mL total of ICG:HSA or ICG alone was injected as 4 injections at the same location as the blue dye injections. SLN mapping was performed using the Mini-Fluorescence-Assisted Resection and Exploration (Mini-FLARE™) image-guided surgery system as described previously.17,21 The NIR fluorescence signal was measured percutaneously prior to skin incision, and continuously during the surgical procedure. Relative brightness of the SLNs was determined by measuring signal-to-background ratios (SBR). Both the surgeon and the assessor of the Mini-FLARE™ data were blinded to the treatment allocation.
Excised SLNs were routinely analyzed by histopathological frozen section analysis. SLNs were fixed in formalin and embedded in paraffin for hematoxylin, eosin, and immunohistopathological staining for AE1/AE3 at multiple levels, with an interval of 250 μm, according to the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) study protocol.3 Direct groin dissection was performed in cases of tumor positive frozen sections of the SLN or when the SLN could not be identified intraoperatively.
Power Calculation and Statistical Analysis
The power calculation is based on previous data, in which a SBR of 12.8 ± 4.5 was observed during SLN detection.17 These data revealed that 24 patients are needed to achieve at least 90% power to detect non-inferiority using a one-sided, 2-sample t test (α = 0.025) with a margin of equivalence of 6.4 while assuming no difference between the SBR of ICG:HSA and ICG alone. For statistical analysis, SPSS statistical software (Version 16.0, Chicago, IL) was used. To compare the SBR and the number of SLNs identified between ICG:HSA and ICG alone, a 1-sided, 2-sample t test was performed. P < 0.05 was considered significant.
RESULTS
Patient characteristics
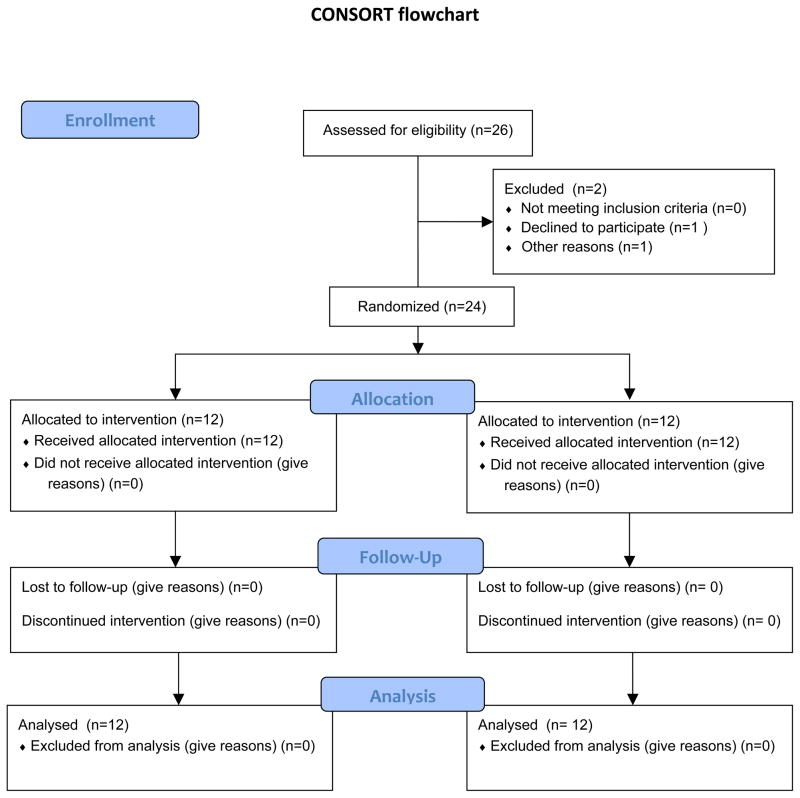
Twenty-four consecutive patients with vulvar cancer undergoing SLN biopsy were included in this study (Fig. 1). Patient and tumor characteristics and previous treatment are presented in Table 1 and were equally distributed over the treatment groups. Three patients underwent previous groin surgery due to varicose veins and SLN biopsy related to a previous vulvar cancer. The two treatment groups included each a total of 12 patients.
Figure 1.
Patient enrollment
Table 1.
Patient and Tumor Characteristics
| Characteristic | ICG:HSA (N = 12) | ICG alone (N = 12) | P |
|---|---|---|---|
| Age (median, range) | 63 (36 – 83) | 73 (47 – 87) | .10 |
| Body mass index (median, range) | 28 (21 – 35) | 30 (24 – 40) | .37 |
| Average tumor size (mm) ± SD | 22 ± 15 | 22 ± 17 | .98 |
| Average tumor infiltration depth (mm) ± SD | 3.6 ± 4.1 | 6.2 ± 8.3 | .37 |
| Previous groin surgery | 1 (8%) | 2 (17%) | .54 |
ICG:HSA, indocyanine green (ICG) adsorbed to human serum albumin (HSA)
Sentinel lymph node detection
Preoperative lymphoscintigraphy identified at least one SLN in each patient. The SLN was located unilaterally in 14 patients and bilateral SLNs were identified in 10 patients. In 8 patients (33%) lymphatic vessels were percutaneously visible using NIR fluorescence, which could assist in the location of the incision (Fig. 2). Average time between skin incision and detection of the first SLN was 10 ± 8 min. Intraoperatively, on average 1.5 ± 1.2 SLNs per patient were identified. Of the 35 SLNs identified 35 (100%) were radioactive, 35 (100%) were fluorescent and 27 (77%) were blue. In all patients, the NIR fluorescence signal in the SLN was detected before patent blue. In 19 out of the 24 patients at least one SLN was detected during surgery. In 25 out of the 34 groins in which a SLN was detected by lymphoscintigraphy at least one SLN was detected. In two patients, in whom no SLN could be located intraoperatively, a fluorescent node could be detected in the resection specimen of the inguinofemoral lymphadenectomy following the SLN procedure. Histological analysis showed lymph node metastases in 7 out of 24 patients of whom 4 patients had macrometastases (> 2 mm) and 3 patients had micrometastases (≤ 2 mm). In all patients with lymph node metastases at least 1 of the tumor positive lymph nodes was appointed SLN. No adverse reactions associated with the use of ICG or ICG:HSA occurred.
Figure 2. NIR Fluorescence-Based SLN Mapping.
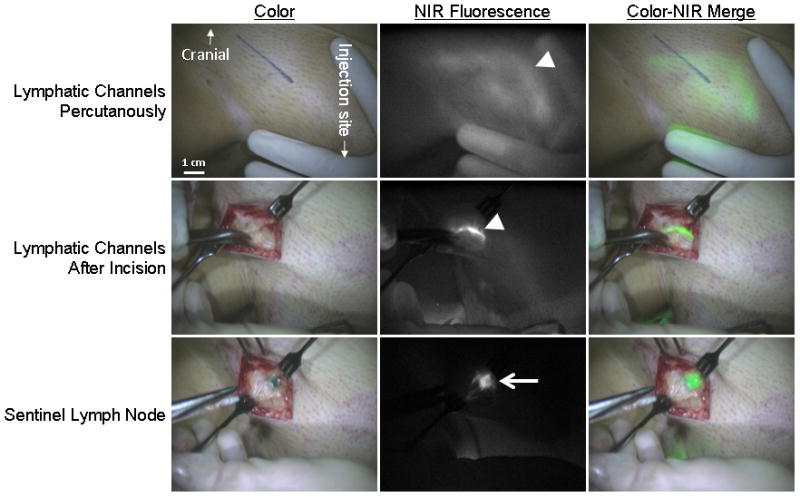
NIR fluorescence SLN mapping in a woman with early-stage vulvar cancer. Upper panel: Percutaneous visualization of the lymphatic vessels (arrowhead). Marker line presents planned incision before NIR fluorescence imaging. Middle panel: Identification of the lymphatic vessel (arrowhead) after incision. Lower panel: Identification of the radioactive, NIR fluorescent and blue SLN (arrow). Camera exposure times were 250 msec (upper panel), 60 msec (middle panel), and 10 msec (lower panel). Scale bars = 1 cm.
Comparison between treatment groups
The average SBR of ICG:HSA (10.3 ± 2.5) and ICG alone (11.2 ± 6.0) were not significantly different (P = 0.65) (Table 2). No significant difference was observed in the average number of in vivo identified fluorescent SLNs per groin between ICG:HSA and ICG alone (1.9 ± 1.4 vs. 1.0 ± 0.7, P = 0.06). Similarly, there was no significant difference in intraoperative detection rate (P = 0.27).
Table 2.
SLN Identification Results
| Characteristic | Total (24 subjects) | ICG:HSA (12 subjects) | ICG alone (12 subjects) | P | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | % | N | % | N | % | ||
|
| |||||||
| SLNs detected by lymphoscintigraphy | .41 | ||||||
| Unilateral | 14 | 42 | 6 | 50 | 8 | 67 | |
| Bilateral | 10 | 58 | 6 | 50 | 4 | 33 | |
|
| |||||||
| Intraoperative detection rate* | |||||||
| Per patient | 19 | 79 | 10 | 83 | 9 | 75 | .61 |
| Per groin† (34 groins) | 25 | 74 | 15 | 83 | 10 | 63 | .17 |
|
| |||||||
| Average number of intraoperative identified SLNs per patient ± SD | 1.5 ± 1.2 | 1.9 ± 1.4 | 1.0 ± 0.7 | .06 | |||
|
| |||||||
| Number of SLNs identified | 35 | 23 | 12 | ||||
|
| |||||||
| Method of SLN detection | |||||||
| Radioactive | 35 | 100 | 23 | 100 | 12 | 100 | |
| Fluorescence | 35 | 100 | 23 | 100 | 12 | 100 | |
| Blue dye | 27 | 77 | 16 | 70 | 11 | 92 | .09 |
|
| |||||||
| Signal-to-background ratio | 10.7 ± 4.4 | 10.3 ± | 2.5 | 11.2 ± 6.0 | .65 | ||
|
| |||||||
| Average time between injection and skin incision ± SD (min) | 17 ± 6 | 18 ± 6 | 16 ± 6 | .39 | |||
|
| |||||||
| Average time between skin incision and first SLN detection ± SD (min) | 10 ± 9 | 10 ± 9 | 11 ± 8 | .75 | |||
|
| |||||||
| Histology | .50 | ||||||
| negative | 17 | 71 | 8 | 67 | 9 | 75 | |
| ITC/micrometastasis | 3 | 13 | 1 | 8 | 2 | 17 | |
| macrometastasis | 4 | 16 | 3 | 25 | 1 | 8 | |
ICG:HSA, indocyanine green (ICG) adsorbed to human serum albumin (HSA); SLN, sentinel lymph node; ITC, isolated tumor cells
Detection rate combining NIR fluorescence imaging, the gamma probe, and blue dye staining
Groins with SLN localization by preoperative lymphoscintigraphy (N=18 ICG:HSA, N=16 ICG alone)
COMMENT
The present study confirmed feasibility of NIR fluorescence for optical guidance for intraoperative SLN biopsy using ICG in vulvar cancer patients. All SLNs that were detected by radio guidance could also be detected by NIR fluorescence, however not all SLNs were detected by blue dye staining. This double-blind randomized trial did not show any advantages of ICG:HSA over ICG alone in SBR and average number of intraoperative detected fluorescent SLNs.
NIR fluorescence was of added value during SLN detection, as all SLNs detected could be identified by the direct optical guidance of NIR fluorescence, but only 77 % by blue dye staining. Moreover, the NIR fluorescence signal in the SLN could be detected before patent blue in all cases. Blue dye staining and NIR fluorescence both provide real-time optical guidance. Tissue penetration of NIR fluorescent light is significantly higher than penetration of visible light, which enables deeper and earlier visualization of signal by NIR fluorescence. This can assist to determine the location of the incision and provide improved optical guidance during SLN localization. Furthermore, since ICG is diluted to levels invisible to the human eye after injection, no discoloration of the surgical field occurs. It can therefore be questioned whether blue dye can be omitted when NIR fluorescence is used.22 Moreover, compared to radioactive lymphatic tracers, NIR fluorescence is not hampered by high background signals of the injection site by the gamma probe, which can interfere SLN detection using the gamma probe. However, due to the limited penetration depth of NIR fluorescence imaging (several mm), radioactive SLN tracers remain necessary for preoperative surgical planning and to detect deeper located SLNs. Therefore, a combination of a radiocolloid tracer for preoperative planning and guidance of deeper located nodes and a NIR fluorescence tracer for real-time optical guidance is advocated.23,24
This study is one out of three simultaneously initiated clinical studies to directly compare the lymphatic tracers ICG:HSA and ICG alone.15,25 In concordance with these studies, no significant difference in SBR and average number of identified SLNs between the lymphatic tracers ICG:HSA and ICG alone was found in vulvar cancer. Since lymph fluids consist of a high protein levels,26 a potential explanation for the lack of difference between ICG:HSA and ICG alone is that ICG rapidly binds to these endogenous proteins when drained in the lymphatic system, eliminating the need for premixing ICG and HSA. Together, the three clinical studies demonstrate no difference between ICG:HSA and ICG alone for the SLN identification in a large heterogeneous group of patients, with different anatomical locations (breast cancer, cervical cancer, and vulvar cancer) and different times from injection to imaging.15,25
All SLNs identified intraoperatively could be detected by both radio guidance and NIR fluorescence. Although a high concordance between detection by NIR fluorescence and radio guidance was found, the intraoperative detection rate (19 out of 24 patients) in this study is relatively low compared to other studies.10 A possible explanation could be that 3 patients underwent previous groin surgery. Moreover, in two patients fluorescent SLNs could be detected in the lymphadenectomy specimen ex vivo, which if detected intraoperatively would have increased the intraoperative detection rate considerably.
In conclusion, this double-blind, randomized trial showed no advantage of using ICG:HSA in comparison to ICG alone and shows the added value of NIR fluorescence for SLN biopsy in vulvar cancer.
Acknowledgments
The authors thank Margriet J.G. Löwik and Dorien M.A. Berends-van der Meer for their assistance during the patient inclusion process and Lindsey Gendall for editing. This work was supported in part by NIH grants R01-CA-115296 and R21-CA-130297 and the Dutch Cancer Society grant UL2010-4732. This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine, project MUSIS (grant 03O-202). Joost van der Vorst is an MD-medical research trainee funded by The Netherlands Organisation for Health Research and Development (grant 92003593).
SOURCES OF FINANCIAL SUPPORT
This work was supported in part by NIH grants R01-CA-115296 and R21-CA-130297 and the Dutch Cancer Society grant UL2010-4732. This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine, project MUSIS (grant 03O-202). Joost van der Vorst is an MD-medical research trainee funded by The Netherlands Organisation for Health Research and Development (grant 92003593).
Footnotes
DISCLOSURE STATEMENT
Boudewijn E. Schaafsma, M.D.: None
Floris P.R. Verbeek, M.Sc. : None
Alexander A.W. Peters, M.D., Ph.D.: None
Joost R. van der Vorst, M.D.: None
Cornelis D. de Kroon, M.D., Ph.D.: None
Mariette I. E. van Poelgeest, M.D., Ph.D.: None
J. Baptist M.Z. Trimbos, M.D., Ph.D.: None
Cornelis J.H. van de Velde, M.D., Ph.D.: None
John V. Frangioni, M.D., Ph.D.: FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. It has been licensed to the FLARE™ Foundation, a non-profit organization focused on promoting the dissemination of medical imaging technology for research and clinical use. Dr. Frangioni is the founder and chairman of the FLARE™ Foundation. The Beth Israel Deaconess Medical Center will receive royalties for sale of FLARE™ Technology. Dr. Frangioni has elected to surrender post-market royalties to which he would otherwise be entitled as inventor, and has elected to donate pre-market proceeds to the FLARE™ Foundation.
Alexander L. Vahrmeijer, M.D., Ph.D.: None
Katja N. Gaarenstroom, M.D., Ph.D.: None
CONTRIBUTION TO AUTHORSHIP
Boudewijn E. Schaafsma, M.D.: Designed the clinical trial with Dr. Frangioni, Verbeek, Gaarenstroom, and Vahrmeijer. Recruited patients and obtained informed consent. Assisted with the execution of the clinical trial. Controlled the Mini-FLARE™ system during surgery. Analyzed all results. Wrote draft version of manuscript. Approved final version of manuscript.
Floris P.R. Verbeek, M.Sc.: Designed the clinical trial with Dr. Frangioni, Schaafsma, Gaarenstroom and Vahrmeijer. Recruited patients and obtained informed consent. Assisted with the execution of the clinical trial. Controlled the Mini-FLARE™ system during surgery. Analyzed all results. Wrote draft version of manuscript. Approved final version of manuscript.
Alexander A.W. Peters, M.D., Ph.D.: Performed multiple surgeries. Edited draft manuscript. Approved final version of manuscript.
Joost R. van der Vorst, M.D.: Controlled the Mini-FLARE™ system during surgery. Assisted with the execution of the clinical trial. Recruited patients and obtained informed consent. Edited draft manuscript. Approved final version of manuscript.
Cornelis D. de Kroon, M.D., Ph.D.: Performed multiple surgeries. Edited draft manuscript. Approved final version of manuscript.
Mariette I. E. van Poelgeest, M.D., Ph.D.: Performed multiple surgeries. Edited draft manuscript.
Approved final version of manuscript.
J. Baptist M.Z. Trimbos, M.D., Ph.D.: Performed multiple surgeries. Edited draft manuscript. Approved final version of manuscript.
Cornelis J.H. van de Velde, M.D., Ph.D.: Surgeon and principal investigator of the study. Assisted in designing the study concept. Edited draft manuscript. Approved final version of manuscript.
John V. Frangioni, M.D., Ph.D.: Inventor of the Mini-FLARE™ imaging system and chief biomedical engineer responsible for the design of all subsystems. Designed the clinical trial with Verbeek, Schaafsma, and Vahrmeijer. Edited draft manuscript. Approved final version of manuscript.
Alexander L. Vahrmeijer, M.D., Ph.D.: Surgeon and principal investigator of the study. Designed the clinical trial with Verbeek, Schaafsma, Gaarenstroom and Frangioni. Edited draft manuscript. Approved final version of manuscript.
Katja N. Gaarenstroom M.D., Ph.D.: Designed the clinical trial with Dr. Frangioni,
Schaafsma, Verbeek and Vahrmeijer. Performed multiple surgeries. Edited draft manuscript. Approved final version of manuscript.
TRIAL REGISTRATION
The Netherlands Trial Register NTR2871
http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2871
DETAILS OF ETHICS APPROVAL
The Medical Ethics Committee of the Leiden University Medical Center approved this clinical study. Approval was obtained 28 februari 2011 for study protocol 09.001 addendum 7 “Vulvar Cancer” (reference P09.001/SH/ib)
References
- 1.Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Maisonneuve P, Pecorelli S, Odicino F, Heintz AP. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S7–27. doi: 10.1016/S0020-7292(06)60028-3. [DOI] [PubMed] [Google Scholar]
- 2.Burger MP, Hollema H, Emanuels AG, Krans M, Pras E, Bouma J. The importance of the groin node status for the survival of T1 and T2 vulval carcinoma patients. Gynecol Oncol. 1995;57:327–334. doi: 10.1006/gyno.1995.1151. [DOI] [PubMed] [Google Scholar]
- 3.Van Der Zee AG, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, Maggioni A, Gaarenstroom KN, Baldwin PJ, Van Dorst EB, Van d V, Hermans RH, van der Putten H, Drouin P, Schneider A, Sluiter WJ. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 4.Johann S, Klaeser B, Krause T, Mueller MD. Comparison of outcome and recurrence-free survival after sentinel lymph node biopsy and lymphadenectomy in vulvar cancer. Gynecol Oncol. 2008;110:324–328. doi: 10.1016/j.ygyno.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Oonk MH, van Hemel BM, Hollema H, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, Maggioni A, Gaarenstroom KN, Baldwin PJ, Van Dorst EB, Van d V, Hermans RH, van der Putten HW, Drouin P, Runnebaum IB, Sluiter WJ, Van Der Zee AG. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol. 2010;11:646–652. doi: 10.1016/S1470-2045(10)70104-2. [DOI] [PubMed] [Google Scholar]
- 6.Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 7.Dhar KK, Woolas RP. Lymphatic mapping and sentinel node biopsy in early vulvar cancer. BJOG. 2005;112:696–702. doi: 10.1111/j.1471-0528.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 8.Levenback C, Burke TW, Gershenson DM, Morris M, Malpica A, Ross MI. Intraoperative lymphatic mapping for vulvar cancer. Obstet Gynecol. 1994;84:163–167. [PubMed] [Google Scholar]
- 9.Devaja O, Mehra G, Coutts M, Adamson S, Montalto SA, Donaldson J, Papadopoulos AJ. A prospective study of sentinel lymph node detection in vulval carcinoma: is it time for a change in clinical practice? Int J Gynecol Cancer. 2011;21:559–564. doi: 10.1097/IGC.0b013e3182119d8d. [DOI] [PubMed] [Google Scholar]
- 10.Van Oostrum NH, Makar AP, Van Den Broecke R. Sentinel node procedures in gynecologic cancers: an overview. Acta Obstet Gynecol Scand. 2012;91:174–181. doi: 10.1111/j.1600-0412.2011.01302.x. [DOI] [PubMed] [Google Scholar]
- 11.Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Lowik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104:323–332. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murawa D, Hirche C, Dresel S, Hunerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96:1289–1294. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 13.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J Surg Oncol. 2012 doi: 10.1002/jso.23045. [DOI] [PubMed] [Google Scholar]
- 15.Hutteman M, Mieog JS, van der Vorst JR, Liefers GJ, Putter H, Lowik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res Treat. 2011;127:163–170. doi: 10.1007/s10549-011-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010;19:210–3. doi: 10.1016/j.breast.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Hutteman M, van der Vorst JR, Gaarenstroom KN, Peters AA, Mieog JS, Schaafsma BE, Lowik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Optimization of near-infrared fluorescent sentinel lymph node mapping for vulvar cancer. Am J Obstet Gynecol. 2011 doi: 10.1016/j.ajog.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane LM, Themelis G, Arts HJ, Buddingh KT, Brouwers AH, Ntziachristos V, van Dam GM, Van Der Zee AG. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulvar cancer: First clinical results. Gynecol Oncol. 2010 doi: 10.1016/j.ygyno.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4:172–181. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 20.Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H, Gioux S, Kuppen PJ, Ashitate Y, Lowik CW, Smit VT, Oketokoun R, Ngo LH, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Toward Optimization of Imaging System and Lymphatic Tracer for Near-Infrared Fluorescent Sentinel Lymph Node Mapping in Breast Cancer. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H, Gioux S, Kuppen PJ, Ashitate Y, Lowik CW, Smit VT, Oketokoun R, Ngo LH, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol. 2011;18:2483–2491. doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Vorst JR, Schaafsma BE, Verbeek FP, Hutteman M, Mieog JS, Lowik CW, Liefers GJ, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Randomized Comparison of Near-infrared Fluorescence Imaging Using Indocyanine Green and 99(m) Technetium With or Without Patent Blue for the Sentinel Lymph Node Procedure in Breast Cancer Patients. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Poel HG, Buckle T, Brouwer OR, Valdes Olmos RA, van Leeuwen FW. Intraoperative Laparoscopic Fluorescence Guidance to the Sentinel Lymph Node in Prostate Cancer Patients: Clinical Proof of Concept of an Integrated Functional Imaging Approach Using a Multimodal Tracer. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Brouwer OR, Buckle T, Vermeeren L, Klop WM, Balm AJ, van der Poel HG, van Rhijn BW, Horenblas S, Nieweg OE, van Leeuwen FW, Valdes-Olmos RA. Validation of a multimodal radioactive/fluorescence imaging agent for lymphatic mapping and simultaneous radioguided and optical sentinel node detection. J Nucl Med; 2012 In press. [Google Scholar]
- 25.Schaafsma BE, van der Vorst JR, Gaarenstroom KN, Peters AA, Verbeek FP, de Kroon CD, Trimbos JB, van Poelgeest MI, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Randomized Comparison of Near-Infrared Fluorescence Lymphatic Tracers for Sentinel Lymph Node Mapping of Cervical Cancer. Gynecol Oncol. 2012 doi: 10.1016/j.ygyno.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]