Abstract
Drugs that cause liver injury often “stress” mitochondria and activate signal transduction pathways important in determining cell survival or death. In most cases, hepatocytes adapt to the drug-induced stress by activating adaptive signaling pathways, such as mitochondrial adaptive responses and erythroid 2-related factor 2 (Nrf-2), a transcription factor that upregulates antioxidant defenses. Due to adaptation, drugs alone rarely cause liver injury, with acetaminophen being the notable exception. Drug-induced liver injury (DILI) usually involves other extrinsic factors, such as the adaptive immune system, that cause “stressed” hepatocytes to become injured; leading to idiosyncratic DILI, the rare and unpredictable adverse drug reaction in the liver. Hepatocyte injury, due to drug and extrinsic insult, causes a second wave of signaling changes associated with adaptation, cell death, and repair. If the stress and injury reach a critical threshold, then death signaling pathways such as JNK become dominant and hepatocytes enter a failsafe mode to undergo self-destruction. DILI can be seen as an active process involving recruitment of death signaling pathways that mediate cell death rather than a passive process due to overwhelming biochemical injury. In this review, we highlight the role of signal transduction pathways, which frequently involve mitochondria, in the development of DILI.
DILI is an active process
Symptomatic idiosyncratic DILI is the rare, but serious liver injury caused by drugs in a small subset of patients (1 in ~ 1000-20,000) [1,2]. Drugs that cause idiosyncratic DILI usually pass clinical trials, which are generally too small in patient number (~ several thousand) for severe DILI to be observed. Idiosyncratic toxicants also usually pass toxicity testing in healthy animals. However, this does not mean drugs that cause idiosyncratic DILI exhibit no warning signs in preclinical development. On the contrary, with newer screening techniques a pattern has emerged showing that drugs that cause idiosyncratic DILI cause hepatocyte perturbations and stress, particularly to mitochondria. Although it is rare in patients, drug “toxicity” can often be seen in experimental models involving isolated organelles (mitochondria, microsomes), cultured hepatocytes, and “stressed” animal models. In these systems, it has been shown that idiosyncratic toxicants cause functional perturbations, often in mitochondria, that alter many signal transduction pathways. The stress responses caused by idiosyncratic toxicants often involve upregulation of signaling pathways involved in adaptation including antioxidant genes that help hepatocytes tolerate the drug.
Although drugs that cause idiosyncratic hepatotoxicity can stress hepatocytes and alter signaling pathways, these perturbations alone are rarely sufficient to induce liver injury. DILI is multi-factorial, potentially involving the adaptive immune system, infections, environment (age, diet) and genetics [3]. This is one reason that most drugs that cause DILI pass animal toxicity tests, because drug toxicity studies are generally performed in healthy animals. Extrinsic factors, such as the adaptive immune system, can cause “stressed” hepatocytes to become injured, which triggers a second wave of stress responses associated with adaptation, cell death, and repair. If the stress and injury reaches a critical threshold, then cell death signaling pathways become dominant and hepatocytes undergo self-destruction. DILI is, therefore, an active process involving recruitment of death signaling kinases such as c-Jun N-terminal kinase (JNK) that mediate cell death, even if the death is necrotic [4]. These recent findings overturn the traditional paradigm of cell death by toxic agents being a passive process directly due to overwhelming cell injury. In this review, we highlight recent evidence on the crucial role of signal transduction pathways, which frequently involve mitochondria, in the development of DILI.
Overview of DILI
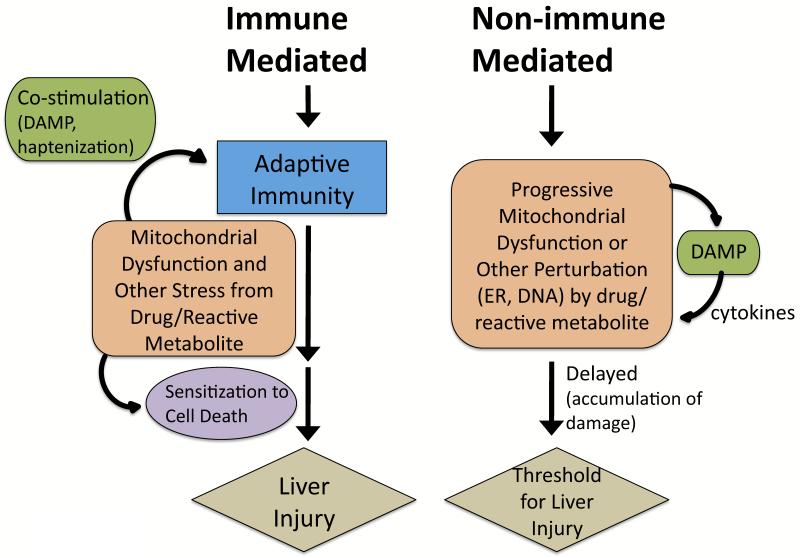
DILI is the leading cause of drug withdrawal from the market, black box warnings, or restricted use [1,2]. DILI can be subdivided into two categories: predictable and idiosyncratic. Acetaminophen (APAP) is one of the few marketed drugs that causes a predictable, dose-dependent DILI. Idiosyncratic DILI is not strictly dose-dependent, but a dose threshold exists because most drugs that cause liver injury require a minimum dose (50-100mg daily) for liver injury to occur. It is also possible that within each individual there is a dose dependency, and dose thresholds may vary among individuals. Idiosyncratic DILI has been traditionally divided into allergic (immune mediated) and non-allergic (non-immune mediated) sub-categories (Figure 1) [1,4]. Allergic DILI is associated with a short latency period and rapid recurrence of hepatotoxicity on re-exposure to drug (immune hypersensitivity), suggesting the involvement of the adaptive immune system in DILI. Non-allergic DILI was believed to be mediated by biochemical injury, and the adaptive immune system was not believed to be involved, due to the lack of features of immune hypersensitivity. However, recently many drugs thought to cause non-allergic hepatotoxicity (lumiracoxib, lapatanib, ticlopidine, amoxicillin-clavulanate, ximelagatran) have been found to be strongly associated with certain human leukocyte antigen (HLA; the human equivalent of MHC) haplotypes [5-8]. The strong relationship between idiosyncratic toxicants and HLA polymorphisms suggests that the adaptive immune system plays a role in a large number of cases of idiosyncratic DILI, and the categorization of allergic and non-allergic is difficult to distinguish. The lack of hypersensitivity to drugs in some DILI cases may be due to immune tolerance of the adaptive immune system leading to drug tolerance [9]. Having a certain HLA haplotype increases the risk for DILI, but the rates still remain low. For example, patients with the HLA B*5701 haplotype have an ~80-fold increased susceptibility to liver injury from flucloxacillin, but this translates to only ~1 in 500 individuals with this haplotype developing DILI [10]. Thus, although certain HLA haplotypes increase the risk for developing DILI, other factors, such as the direct stress induced by drug/metabolites on hepatocytes and the individual ability to adapt, as well as extrinsic factors (infections, environmental stresses), may also play important roles in the development of DILI [3].
Figure 1. Immune-mediated and non-immune-mediated idiosyncratic DILI.
Many drugs cause idiosyncratic DILI via an adaptive immune system mediated process and have strong associations with certain HLA haplotypes, even though they do not display classic characteristics of systemic immune hypersensitivity (i.e. fever, rash, eosinophilia, rapid positive rechallenge). Drugs/reactive metabolites may stress hepatocytes to stimulate the adaptive immune system. Excessive reactive metabolites bind to proteins (haptenization), and peptide-haptens exposed in the binding grove of HLA molecules then stimulate T-cells. Hepatocytes may become injured and release contents, such as mtDNA, that act as danger associated molecular pattern (DAMP) molecules that co-activate the immune system (sterile inflammation) [97]. Extensive drug-induced stress in hepatocytes may alter signaling pathways to sensitize hepatocytes to the adaptive immune system. Non-allergic DILI is believed to be mediated by biochemical injury, often involving mitochondria. The drug/reactive metabolite stress and injury to hepatocytes may need to accumulate (long latency) before manifestations of DILI are observed. DAMP molecules may also be released in this process and activate cytokines that may mediate injury. Mitochondria have a reserve respiratory capacity and significant inhibition of complexes is needed before bioenergetic capacity is impaired. Thus, drugs/reactive metabolites can damage mitochondria gradually, with no observable effects on mitochondrial respiration until a critical threshold is reached. How often non-immune idiosyncratic DILI occurs is unknown but remains plausible.
Hepatocellular injury following drug intake is the critical event underlying DILI. The key signaling pathways mediating DILI have been difficult to study in patients, due to the rarity of the disease and rapid disease progression at time of diagnosis. Consequently, most of our mechanistic knowledge of DILI has come from work with cell culture and animal studies using APAP, which is one of the few approved drugs that cause liver injury in animals [1]. Although APAP causes predictable DILI, some insights regarding idiosyncratic DILI have also been obtained using the APAP model. Susceptibility to APAP hepatotoxicity has many idiosyncratic features, and in animals APAP hepatotoxicity is affected by age, sex, diet, stress, and genetic factors. Because the APAP animal model has revealed much of our knowledge regarding signaling pathways involved in hepatotoxicity, we will begin with a consideration of the APAP model.
Signaling pathways involved in APAP-induced liver injury
APAP hepatotoxicity is caused by the formation of N-acetyl-p-benzo-quinoneimine (NAPQI), a reactive metabolite generated during metabolism of APAP by cytochrome P450, primarily by the CYP2EI isoform [11,12]. NAPQI is a highly reactive metabolite that forms covalent bonds with protein and non-protein thiols. At hepatotoxic doses of APAP, glutathione (GSH) becomes severely depleted in the cytoplasm and mitochondria, which contain separate GSH pools. The metabolism of APAP to NAPQI represents a minor pathway in APAP clearance and consequently high doses of APAP are required to deplete GSH in mitochondria.
Adaptation
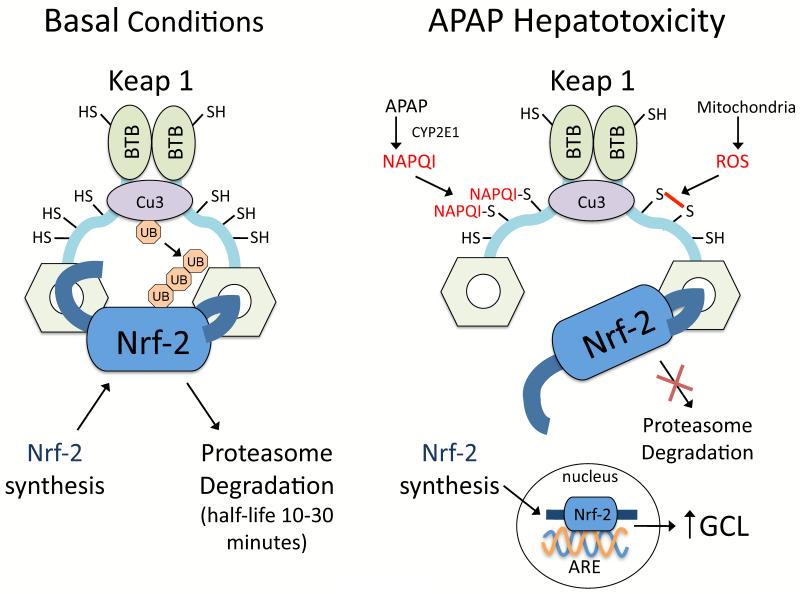
The consumption of even therapeutic doses of APAP (4 grams daily) is associated with some stress and injury to hepatocytes. Analysis of patients receiving therapeutic doses of APAP suggests that a high percentage (up to 81%) will develop elevated plasma ALT levels [13]. However, patients are believed to adapt to APAP, and in most cases continuous APAP intake does not lead to liver injury. In mice, it has been shown that activation of Nrf-2 plays a central role in the adaptation to APAP [14]. Nrf-2 is a transcriptional factor in the cytoplasm that is usually bound to Kelch-like ECH-associated protein 1 (Keap 1), a redox sensitive anchoring protein (Figure 2). Keap 1 has critical thiols (25 cysteine residues) that act as redox and electrophile sensors. NAPQI has been shown to directly bind to many thiols in Keap1 to promote translocation of newly synthesized Nrf-2 to the nucleus [15,16]. In mice, even low non-toxic doses of APAP cause Nrf-2 translocation to the nucleus to upregulate glutamate cysteine ligase (GCL, the rate limiting enzyme in GSH synthesis) and increase GSH synthesis in the liver [17].
Figure 2. Hinge and latch model of Nrf-2 regulation by Keap1.
Nrf-2 is bound to Keap1 (dimerized by binding of the BTB domains), which also has cullin-dependent E3 ubiquitin ligase complex (Cu3) attached. Cu3 ubiquitinates Nrf-2 attached to Keap1, causing Nrf-2 degradation by proteasomes. Nrf-2 bound to Keap1 consequently has a very short half-life (10-30 minutes) in hepatocytes due to continuous ubiquitination and degradation. Keap1 has critical thiols (25 cysteine residues) that act as redox and electrophile sensors. According to the “hinge and latch model”, when the thiols of Keap1 are oxidized or covalently bound by electrophillic agents such as NAPQI, a conformational change occurs in Keap1 that weakens its binding to Nrf-2. Consequently Nrf-2 becomes loosely bound to Keap1 and cannot be ubiquitinated by Cu3. Since Keap1 remains occupied by loosely attached Nrf-2 that is not degraded, newly synthesized Nrf-2 will translocate to the nucleus where it binds to the antioxidant response element (ARE) promoter on DNA important in transcribing GCL-c (catalytic component of glutamate-cysteine ligase) and other antioxidant proteins. The figure is modified from previous publications [14,16].
Death signaling pathway
Despite upregulation of GCL by Nrf-2, high doses of APAP will overwhelm the GSH synthesis machinery to cause extensive mitochondrial GSH depletion leading to hepatocyte death. Surprisingly, we found that APAP-induced liver injury is an active process involving the activation of death signaling pathways involving JNK, even though APAP primarily induces necrosis [18,19]. The silencing or inhibition of JNK protects against APAP-induced liver injury, even in the presence of excessive mitochondrial GSH depletion and covalent binding. JNK plays an important role in the stress response and can be activated by various stressors, including reactive oxygen species (ROS) and various cytokines [20,21]. Stress causes a transient activation of JNK that upregulates signaling pathways such as c-Jun to aid in cellular response to stress. However, when JNK activation is sustained, JNK promotes cell death by mediating mitochondrial dysfunction.
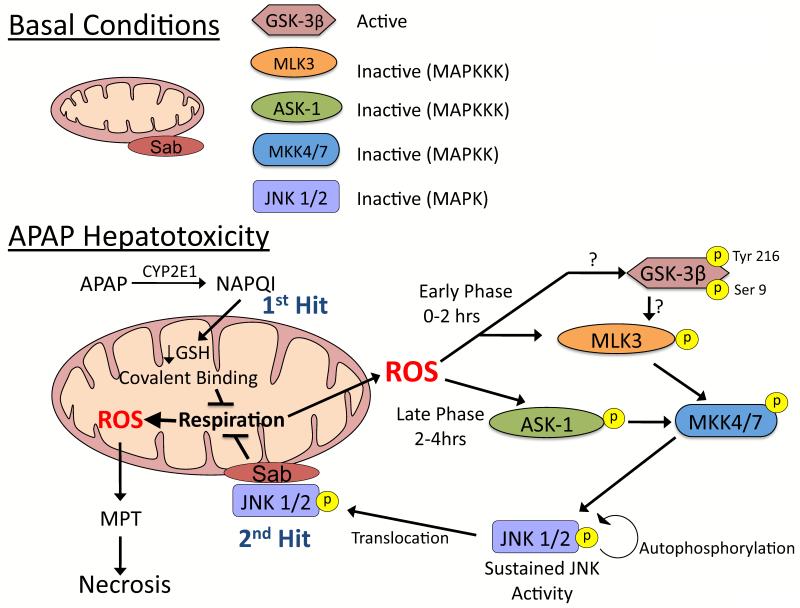
We hypothesize APAP hepatotoxicity involves a complex feed-forward loop that involves two hits to mitochondria (Figure 3) [4,22]. The first hit to mitochondria involves mitochondrial GSH depletion and covalent binding by NAPQI. NAPQI can bind to thiols in the respiratory complexes [23] and deplete mitochondrial GSH needed by GSH peroxidase that detoxifies H2O2 [19,24]. These factors are likely to be important in enhancing mitochondrial ROS generation following APAP treatment [19]. The increased mitochondrial ROS and/or redox alterations induced by NAPQI likely plays an important role in JNK activation during APAP hepatotoxicity. JNK activation only occurs after a critical threshold of mitochondrial GSH depletion occurs, probably > 90% depletion [19]. There are two phases of JNK activation during APAP hepatotoxicity, an early (1-2 hours) and a late (2-4 sustained hours) phase, which involve different signal transduction pathways. The early phase of JNK activation probably involves glycogen synthase kinase-3β (GSK-3β) and mixed-lineage kinase-3 (MLK3). We observed that silencing GSK-3β in mice prevented APAP-induced liver injury, which was associated with inhibition of JNK in the early phase of APAP-induced injury [25]. GSK-3β has also been shown to regulate JNK activation during fatty acid-induced apoptosis in primary hepatocytes [26] and activate JNK through mitogen-activated protein kinase/extracellular signal-regulated kinase-1 (MEKK1) or MLK dependent pathways [27,28]. Recently, it has been shown that MLK3 is involved in JNK activation during the early phase of APAP hepatotoxicity. MLK3 knockout mice are protected from APAP due to inhibition of JNK activation [29]. The late phase of JNK activation is believed to be mediated by apoptosis signal-regulating kinase-1 (ASK-1) in the liver. ASK-1 knockout mice treated with APAP experienced an early JNK activation, but the late phase of JNK activation was inhibited, which protected these mice from APAP hepatotoxicity [30]. ASK-1 and JNK may be activated and/or sustained by various redox modifications to a) thioredoxin, which binds and inhibits ASK-1 [31], b) GSH S-transferase Pi (GST-Pi), that binds and inhibits JNK [32], and c) phosphatases, important in dephosphorylating and inactivating JNK [33].
Figure 3. JNK activation during APAP-induced liver injury.
A. Under basal conditions most kinases, GSK-3β being a noted exception, are inactive and reside in the cytoplasm. B. APAP hepatotoxicity involves two hits to mitochondria. Mitochondrial GSH depletion and covalent binding by NAPQI (upstream hit to mitochondria) enhance mitochondrial ROS production. ROS cause two phases of MAPK activation during APAP hepatotoxicity, an early (0-2 hours) and a late (2-4 hours) phase, which involve different initiation events. The early phase likely involves GSK-3β activating MLK3, while the late phase is mediated by ASK-1 in the liver. Both then activate MKK 4/7, which in turn activates JNK. Once activated, JNK translocates to mitochondria and binds to Sab, a scaffold protein on the outer membrane of mitochondria. JNK binding to Sab leads to sustained enhancement of mitochondrial ROS generation which has two consequences: 1) self-amplifying activation of MAPK pathways and 2) MPT and collapse of mitochondrial function. MAPK = mitogen activated protein kinase; MAPKK = MAP kinase kinase; MAPKKK – MAPK kinase kinase kinase.
Once JNK translocates to mitochondria (2 second hit), it binds to Sab (SH3 domain-binding protein that preferentially associates with Btk), a scaffold protein on the outer membrane of mitochondria that contains a kinase interaction motif [34]. Sab is essential in APAP hepatotoxicity, and we observed that silencing Sab protected mice from liver injury caused by APAP, even in the presence of excessive mitochondrial GSH depletion and covalent binding [35]. JNK translocates to mitochondria, and binds to and phosphorylates Sab which leads to: i) further enhance mitochondrial ROS generation needed to sustain JNK activity and ii) induce MPT in damaged mitochondria. In Hela cells, the binding of JNK to Sab has been shown to enhance mitochondrial ROS generation by complex I up to 80% [36]. The increased mitochondrial ROS generation by JNK binding to Sab may be important in sustaining JNK activation through a feed-forward loop [35].
It appears that JNK modulation of mitochondrial ROS and/or MPT mainly occurs when mitochondria are first primed by stress. In Hela cells, JNK only enhanced ROS generation in mitochondria that had been primed by anisomycin treatment [36]. Anisomycin is a natural antibiotic and protein synthesis inhibitor that induces cell stress to activate JNK. Anisomycin treatment to Hela cells or mouse hepatocytes appears to induce some mitochondria stress that sensitizes to the actions of JNK [35,36]. Similarly, in isolated liver mitochondria, we observed JNK only induces MPT in mitochondria that have been stressed by NAPQI (GSH depleted with covalent binding), and not in control mitochondria [19]. Normal mitochondria appear less sensitive to the effects of JNK, suggesting a priming stressor to mitochondria is necessary. This may be important in DILI, where drugs and reactive metabolites can disrupt or injure mitochondria and set the stage for JNK to act as the executioner. Although we have identified Sab as a crucial binding partner in mitochondria important in mediating injury, the mechanism by which JNK-Sab modulates the electron transport chain (ETC), mitochondrial ROS generation, and MPT remains under investigation.
APAP toxicity, a model of “programmed necrosis”
Recently, clinical studies have shown that circulating levels of the cleaved K18 fragment (cK18), a marker of apoptosis, was much lower (15%) than levels of full length K18 (Fl-K18), a marker of necrosis (85%) in patients suffering from APAP hepatotoxicity [37]. This supports the notion that severe APAP hepatotoxicity in patients is primarily associated with necrosis in the liver with only a small amount of apoptosis occurring. The fact that APAP hepatotoxicity is primarily necrotic and yet regulated by signal transduction pathways involving JNK, suggests that APAP hepatotoxicity is a form of “programmed necrosis” [4]. The concept of programmed necrosis has been widely described in the literature, but mainly in an “artifactual” situation in which apoptosis is inhibited chemically by pan-caspase inhibitors (i.e. zVAD). For example, if Jurkat cells treated with tumor necrosis factor-α (TNFα) to induce apoptosis are also co-treated with zVAD, apoptosis is inhibited due to a lack of caspase activity. However, a failsafe mode occurs and necrosis mediated by receptor-interacting protein-1 (RIP1) ensues [38,39]. Thus cells appear to have many pathways to cell death if sufficient signaling stimulus like TNF or sufficient injury occurs. If one death pathway is blocked, as a failsafe another pathway can become activated. Normally during apoptosis, caspase 8 cleaves RIP1 along with other proteins to initiate apoptosis. The inhibition of caspases by zVAD, however, prevents RIP1 degradation, which subsequently binds to RIP3 to modulate various metabolic proteins including mitochondrial proteins, such as glutamate dehydrogenase 1 [40] and to promote mitochondrial fission. We have observed in preliminary studies that necrostatin, a selective RIP1 inhibitor [41], prevents APAP-induced hepatotoxicity in cultured primary hepatocytes, suggesting a possible involvement of RIP1 in APAP programmed necrosis [42]. APAP hepatotoxicity may be necrotic rather than apoptotic due the extensive GSH depletion and redox changes induced by NAPQI. Caspases have critical cysteine residues required for catalytic activity, and the extensive redox changes induced by NAPQI may prevent caspase activity. Consequently, a failsafe mode becomes activated leading hepatocytes to undergo a type of programmed necrosis mediated by JNK and possibly RIP1.
While we have focused on intrinsic hepatocellular signaling that mediates APAP signaling, it is clear that extrinsic factors (cytokines, diet, etc) can modulate APAP hepatotoxicity. These factors are beyond the scope of this review but have been reviewed elsewhere [43-45].
Mechanisms involved in idiosyncratic DILI
APAP is unique in that its toxicity is dependent on severe GSH depletion. Many drugs cause GSH depletion, either through oxidative stress or through formation of reactive metabolites, but never as extensively as APAP. For example, troglitazone forms a reactive metabolite that forms adducts with GSH [46], but does not cause GSH depletion in the liver [47]. It is clear that each drug undergoes drug-specific pathways (metabolism, reactive metabolite, etc) that result in a unique stress to hepatocytes. Although each drug is unique there are still many general features shared by drugs involved in liver injury. APAP and drugs that cause idiosyncratic DILI are generally associated with reactive metabolite formation, mitochondrial dysfunction, and oxidative stress. These stresses may result in many similar signaling changes including those involved in adaptation and in cell death.
Reactive metabolites
Reactive metabolites are formed during metabolism of most drugs that cause DILI [1,2]. Covalent binding of reactive metabolites to proteins can lead to functional changes that if extensive will stress cells and cause alterations in signal transduction pathways. In addition, reactive metabolite-protein adducts can form hapten-peptides which may be central in triggering the adaptive immune system when presented on specific HLA molecules (Figure 3) [9].
Mitochondrial dysfunction
Many idiosyncratic toxicants have been shown to directly disrupt mitochondria to stress hepatocytes [22,48]. The hepatotoxic effects of nucleoside reverse transcriptase inhibitors (NRT; didanosine, stavudine, zidovudine) amiodarone, and valproic acid have been strongly attributed to direct disruption of mitochondria [49,50]. However, many other idiosyncratic toxicants may also directly disrupt mitochondria. A screening of drugs that impede mitochondrial function (ROS, mitochondrial membrane potential, etc) in human hepatocytes revealed that of the 300 drugs tested, 50-60% of idiosyncratic toxicants caused some mitochondrial perturbation, whereas drugs that do not cause DILI had a 0-5% false positive rate [51]. Similarly, screening of drugs for disruption of isolated mouse liver mitochondria (membrane potential, respiration, cytochrome c release, etc) demonstrated a strong positive correlation between mitochondrial disruption and idiosyncratic toxicants (89% positive predictive value) [52]. Interestingly, antimicrobials represent 45.5% of idiosyncratic DILI cases. Because mitochondria are of bacterial ancestry and still share many genetic and structural similarities with bacteria, antibiotics may be harming mitochondria to promote DILI. The central role of mitochondria in DILI has also been supported in vivo using the heterozygous Mn-SOD, (SOD 2+/− mice), a model with a greater level of mitochondrial oxidative stress. Urs Boelsterli’s research team demonstrated that two idiosyncratic toxicants, troglitazone and flutamide, could cause liver injury in Mn-SOD+/− mice, but not in wild-type mice [47,53]. Troglitazone toxicity is, however, more controversial in this model [54].
Many pharmaceutical companies now incorporate some combination of the screening parameters (reactive metabolites, mitochondrial dysfunction, covalent binding, etc) in hepatocytes, usually cultured long-term (sandwich-cultured hepatocytes, micropatterned co-cultures) and isolated organelles to identify drugs that are potential risks for causing liver injury [51,52,55]. Overall, these screening approaches are good at identifying drugs with no idiosyncratic DILI liability (very high negative predictive value ~ 90-95%), but do not perform as well in positive predictions (~ 50-89%). Thus, we can reliably identify drugs that carry a low risk for causing DILI through mitochondrial stress, oxidative stress, reactive metabolites, extent of hepatic metabolism and dose, but cannot conclusively identify idiosyncratic toxicants. The drug screening in hepatocytes and other systems combined with the development of “omics” technology (genomics, metabolomics, etc) to screen patients will hopefully dramatically reduce DILI in the near future [56,57].
Signaling pathways involved in idiosyncratic hepatotoxicity
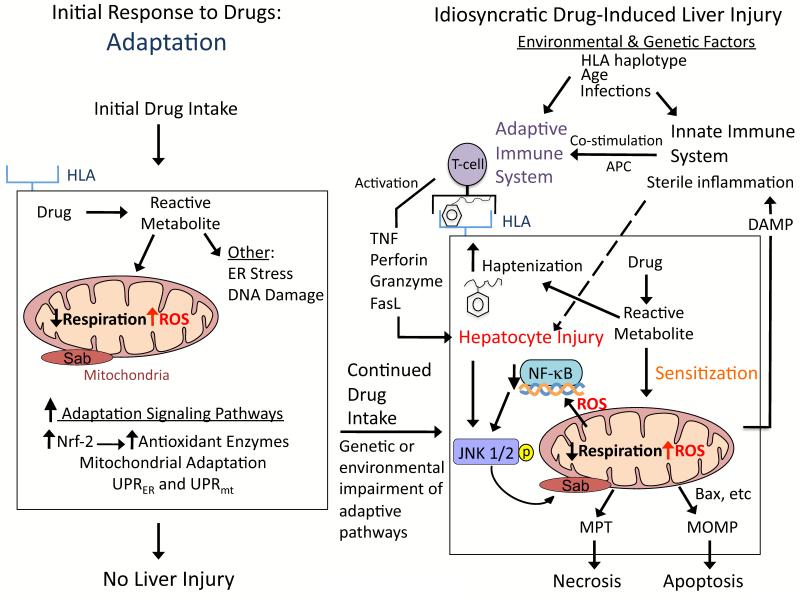
Signaling pathways mediating idiosyncratic DILI have been difficult to study, due to the rarity of the disease in patients and lack of good animal models. Based on various liver injury models and cultured hepatocyte studies, we hypothesize that signaling pathways that modulate hepatocellular injury during idiosyncratic DILI probably occur in multiple waves following drug intake (Figure 4): i) adaptive signaling pathways following drug intake; ii) signaling changes that sensitize hepatocytes to extrinsic factors after continual drug use; and iii) death signaling pathways when a critical threshold of hepatocyte injury is crossed. The extent of activation of specific signaling pathways and their temporal relationships likely play a major role in the outcome.
Figure 4. Hypothesis for idiosyncratic DILI.
Signaling pathways that modulate hepatocellular injury during idiosyncratic DILI probably occur in multiple waves following drug intake. The first signaling wave occurs as hepatocytes are exposed to the drug for the first time. The stress induced by the drug/reactive metabolite, in many cases due to disruption of mitochondria, causes activation of signal transduction pathways associated with adaptation to help hepatocytes cope with continuous drug intake. For most patients the activation of adaptation pathways such as Nrf-2 helps hepatocytes adjust to the drug, and no liver injury occurs. However in a minority of patients, due to genetic and environmental factors, the adaptation pathways may be overwhelmed and/or the stress imposed by continuous drug intake may cause signaling changes that sensitize hepatocytes to injury by extrinsic factors, such as the innate and/or adaptive immune system. According to the hapten model, hapten-peptides are processed and presented on HLA binding grooves of antigen-presenting cells (APC), which interact with T-cell receptors on CD4 T-cells. CD4 T-cells are activated and subsequently activate cytotoxic CD8 T-cells (CTL) that express surface FasL and release TNFα, perforin, granzyme and other cytotoxic factors. The activated CTL then target and kill the hepatocytes expressing the hapten peptide on HLA. Stressed hepatocytes may become injured because the drug/reactive metabolite inhibits pro-survival signaling pathways, such as the transcription factor, NF-κB, to sensitize hepatocytes to the immune system. If the stress and injury reach a critical threshold, then cell death signaling pathways including JNK become dominant and hepatocytes enter a failsafe mode of self-destruction. Activated JNK targets mitochondria to promote MPT and MOMP that induce apoptosis or necrosis, which manifests as DILI.
Adaptive signaling pathways
Large screening studies in hepatocytes examining changes in gene expression have shown that idiosyncratic toxicants (troglitazone, trovafloxacin) alter gene expression patterns much more than their non-hepatotoxic counterparts, possibly due to the stress and injury they inflict [58,59]. Many of these changes in hepatocytes are likely involved in adaptation to the drug. The liver is a very resilient organ, and clinical studies suggest that the liver readily adapts to chronic drug intake [10,60]. Idiosyncratic toxicants often cause higher rates of mild, asymptomatic, and usually transient liver injury, which is detected by transient elevation of serum ALT levels [10,61]. ALT levels return to normal despite continued drug use in nearly all instances. Idiosyncratic DILI has been hypothesized to be the consequence of failure to adapt to the injury caused by the drug in a small subset of patients. Thus, the majority of patients are “nonresponders” (no detectable injury) and “adaptors” that may upregulate signaling pathways to protect the liver, whereas a tiny minority (“susceptibles”) progress to severe injury which may be due to a failure of the liver to adapt. The adaptation to drugs in the liver may involve i) upregulation of protective cellular responses, such as Nrf-2 and antioxidant defense as observed with APAP; ii) upregulation and modification of routes of drug handling (i.e. detoxification, transport) reducing hepatic exposure to toxic parent drug or reactive metabolites; iii) development of immune tolerance by the adaptive immune system; or iv) adaptation of mitochondria to stress induced by drugs. Because a large number of drugs/reactive metabolites directly target mitochondria, the adaptation of mitochondria merits closer scrutiny.
Mitochondrial adaptation to drugs
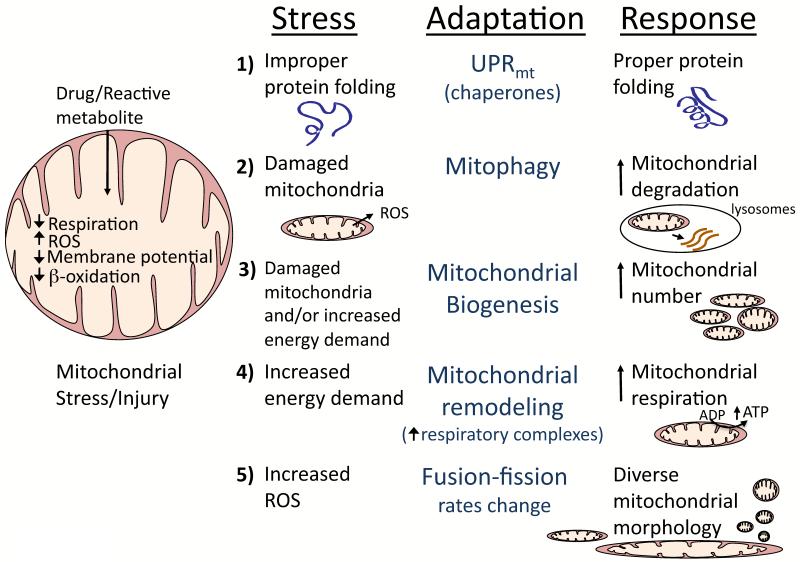
Following stress, various adaptations may occur in mitochondria (Figure 5). The rates of mitophagy (mitochondrial autophagy) may possibly increase to remove mitochondria damaged by drugs/reactive metabolites. Activation of autophagy has been shown to be protective during APAP-induced liver injury, and suppression of autophagy can worsen liver injury [62]. The mitochondrial respiratory chain may also undergo remodeling to increase respiratory capacity in response to stress. We recently observed that chronic alcohol feeding in mice causes remodeling of the mitochondrial respiratory chain involving increased incorporation of respiratory complexes (I, IV, V), which increases mitochondrial respiration to help the liver adapt to the metabolic stress of chronic alcohol feeding [63]. Similarly, the hepatotoxin CCl4, which induces extensive lipid peroxidation, has been shown to increase mitochondrial respiration and levels of respiratory complexes in the liver [64,65]. Mitochondrial stress and/or injury can stimulate mitochondrial biogenesis to replace injured or damaged mitochondria. We observed that the metabolic stress of alcohol or mitochondrial injury caused by rotenone increases mitochondrial biogenesis through upregulation of proliferator-activated receptor gamma coactivator-1α (PGC-1α), a co-activator and master regulator of mitochondria [63,66]. Studies with rotenone showed that PGC-1α activity was regulated by CREB-regulated transcription coactivator 3 (CRTC3), which is activated upon mitochondrial injury [66]. It is likely that idiosyncratic toxicants activate some of these mitochondrial adaptation pathways; however further studies are needed.
Figure 5. Mitochondrial adaptation to drugs.
Following stress, adaptations that may occur in mitochondria include: 1) Mitochondrial unfolded protein response (UPRmt). Similar to the UPR in ER, proteins in mitochondria may become misfolded with stress and chaperone proteins may be important in proper refolding. 2) Increase in mitophagy. The rates of mitophagy (mitochondrial autophagy) are likely to increase to remove damaged or dysfunctional mitochondria (i.e. mitochondrial generating increased ROS, etc) following stress. 3) Mitochondrial remodeling. The respiratory chain of mitochondria may undergo remodeling to increase respiratory capacity to deal with metabolic stresses. 4) Mitochondrial biogenesis. Mitochondrial stress and/or injury can stimulate mitochondrial biogenesis to replace injured or damaged mitochondria, which is regulated by various proteins including PGC-1〈 and CRTC3 in the liver. 5) Changes in mitochondrial fusion-fission. Toxic and metabolic stress can cause changes in mitochondrial fusion-fission rates, which will alter mitochondrial morphology and function. APAP and other drugs cause diverse mitochondrial morphology including fragmentation, enlargement and/or spheroid formation.
Mitochondria constantly undergo fusion-fission to exchange mtDNA, respiratory complexes and other mitochondrial constituents [67,68]. Toxic stress and metabolic stress can affect mitochondrial fusion-fission rates, which will alter mitochondrial morphology. In embryonic fibroblasts, starvation decreases mitochondrial fission to produce elongated mitochondria that are more resistant to mitophagy and have greater cristae surface area [69]. APAP hepatotoxicity is associated with dramatic morphological alterations in mitochondria including both fragmentation and elongation [70], as well as formation of spheroid-shaped mitochondria. Mitochondrial fusion and fission are primarily controlled by four highly conserved GTPases: mitofusins (MFN1, MFN2) in mitochondrial outer membranes promote fusion; optic atrophy (OPA1) in the inner membranes promotes fusion; and dynamin-related protein1 (DRP1) from the cytoplasm regulates fission [67]. DRP1-induced mitochondrial fission is a common occurrence in cell death and inhibition of DRP1 inhibits apoptosis in several models [67,71]. The role of DRP1 in cell death may be independent of actual fission; some evidence suggests that DRP1 mediated mitochondrial outer membrane remodeling or hemifission facilitates bax insertion, especially ER contact sites [72]. The signaling pathways regulating mitochondrial morphology with APAP appear to involve all the dynamin family members, but have not been fully characterized. Several other drugs have been shown to alter mitochondrial morphology in hepatocytes or hepatocyte cell lines. Chloramphenicol induced formation of large mitochondria (mega-mitochondria) associated with lower respiration in hepatocytes [73]. Troglitazone treatment causes formation of mega-mitochondria with enhanced H2O2 generation, but non-hepatotoxic pioglitazone and ciglitazone do not [74]. Trovafloxacin treatment in hepatocytes caused the down-regulation of many mitochondrial genes including Mfn1, suggesting possible morphological changes [59]. ROS appear to be important in triggering mega-mitochondria and spheroid-shaped mitochondria formation in various cell lines. Although mitochondrial morphological changes are often adaptive, as in the case of cell starvation, it remains uncertain whether mitochondrial morphology changes caused by drugs are protective or detrimental.
Sensitization of hepatocytes to the adaptive immune system and/or innate immune system
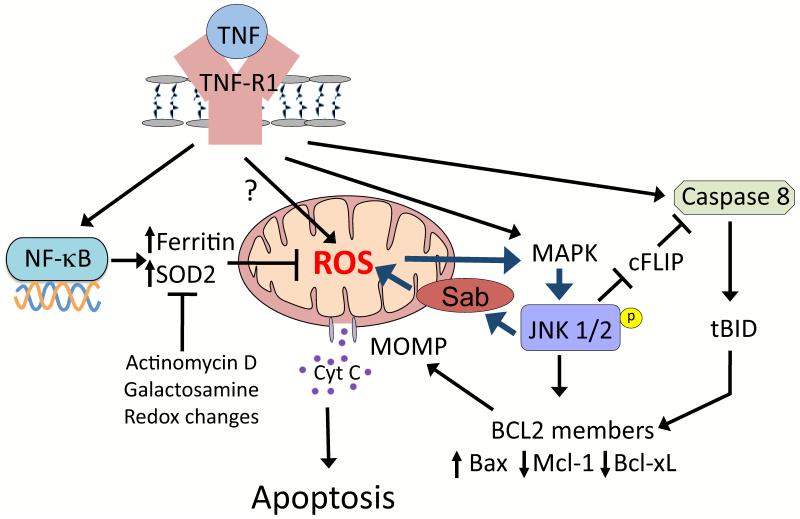
In animal and cell culture models, there is strong evidence that many drugs sensitize hepatocytes to cytokines, such as TNFα, through redox perturbation [21]. In the liver, TNFα binding to its receptor on hepatocytes will activate both a cell death pathway and a survival pathway involving NF-κB activation (Figure 6). Following activation, NF-κB initiates transcription of genes for antioxidant proteins (Mn-SOD, ferritin) and signaling proteins (GAPDD45b, XIAP, A20) responsible for turning off JNK activity [21,75,76]. TNFα-induced NF-κB activation makes JNK activation very transient, and thus, hepatocytes are normally resistant to the cytotoxic effects of TNFα and a pro-survival and proliferative response occurs. When NF-κB is inhibited chemically or through genetic means, JNK activity becomes sustained probably because the antioxidant effects of NF-κB (SOD2, ferritin transcription) are inhibited allowing TNFα to promote mitochondrial ROS, activate JNK and self amplify through the mitochondrial effects of JNK described above. We observed that redox perturbing agents (DEM, diamide), ROS, and mitochondrial inhibitors that increase ROS generation (antimycin, rotenone) sensitize hepatocytes to TNFα-induced apoptosis [77-80]. Redox perturbation and oxidative stress sensitize hepatocytes to TNFα by directly sustaining JNK activation and by inhibiting NF-κB, important in shutting down JNK (Figure 6). There are many sites involved in NF-κB signaling that are redox-regulated including IκB-α kinase (IKK), proteins involved in the ubiquitination of IκB-α, and NF-κB itself has critical thiols (cysteine-62 on p50) that are important in binding to DNA [21,80]. APAP, at sublethal doses, has been shown to sensitize primary mouse hepatocytes to the cytotoxic effects of TNFα by redox perturbations that inhibit NF-κB activity [78,80]. Many idiosyncratic toxicants similarly cause redox perturbation and oxidative stress that may activate JNK and/or inhibit NF-κB activity important in sensitization to TNFα. The idiosyncratic toxicant, chlorpromazine, was found to sensitize hepatocytes to TNF through a JNK dependent pathway [81].
Figure 6. Mitochondrial Sab plays a key role in TNFα-induced apoptosis.
In the liver, TNFα binding to hepatocytes will activate both a cell death pathway involving JNK and caspase 8, and a survival pathway involving NF-κB activation. Following activation, NF-κB initiates transcription of antioxidant proteins (Mn-SOD, ferritin) that lower ROS levels needed to sustain JNK. TNFα-induced NF-κB activation and transcription accounts for the transient nature of JNK activation so hepatocytes are normally resistant to the cytotoxic effects of TNFα. When NF-κB is inhibited chemically or by redox alterations, JNK activity becomes sustained through a self-amplifying pathway involving JNK binding to mitochondrial Sab that enhances ROS generation. JNK also inhibits the anti-apoptotic proteins, such as cFlip and mcl-1, and activate pro-apoptotic proteins such as bax. Sustained JNK activation will then lead to MOMP and apoptosis in hepatocytes. In contrast, in APAP hepatotoxicity sustained JNK activation does not induce apoptosis because ATP depletion and redox effects inhibit caspase activation and cell death proceeds through necrosis.
The sensitization of hepatocytes to TNFα by idiosyncratic toxicants has also been seen in vivo. Robert Roth’s research team has demonstrated that co-treatment of rats with a sublethal dose of lipopolysaccharide (LPS), which activates the innate immune system, can sensitize rats to drugs that cause idiosyncratic DILI in humans (chlorpromazine, trovafloxacin, ranitidine) [82-84]. Later studies revealed the sensitization effects of LPS were mediated by TNFα released by the innate immune system [85]. Whether the sensitization of rats to TNFα by idiosyncratic toxicants was due to NF-κB inhibition and/or sustained JNK activation has not been explored. While these in vivo experiments strongly support the notion that drug-induced stress may sensitize hepatocytes to TNFα, their relevance to idiosyncratic DILI in humans remains uncertain. Although idiosyncratic toxicants may sensitize the liver to TNFα released by the innate immune system, many idiosyncratic toxicants are associated with specific HLA haplotypes, suggesting the adaptive immune system is important in triggering DILI. The adaptive immune system also releases TNFα, as well as other cytotoxic agents (FasL, perforin, granulysin, granzyme) that can injure hepatocytes. Drug-related mitochondrial dysfunction might sensitize hepatocytes to adaptive immune (T-cell) killing. Drug related stress in hepatocytes (including mitochondria and ROS) might also present or promote danger signals, which co-stimulate the development of adaptive immune responses [1,2]. Furthermore, the innate immune system plays a role in the late stages of DILI, during which there is extensive inflammation, but whether it is important in the initiation and/or progression of DILI remains controversial. By contrast, infections and inflammatory conditions which activate the innate immune system are major risk factors for DILI. Therefore, there is a strong possibility that drug-induced stress may be sensitizing hepatocytes to TNFα in DILI associated with infections and inflammation [86].
Activation of death signaling pathways by idiosyncratic toxicants
During idiosyncratic DILI, the drug plus extrinsic factor(s) cause substantial stress and injury to hepatocytes leading to a second wave of signaling changes. If the stress and injury, probably involving mitochondria, reach a critical threshold, then death signaling pathways will become dominant and mitochondria will be targeted for self-destruction. JNK appears to play a central role in many forms of liver injury, and thus, is a strong candidate to mediate liver injury caused by a wide range of drugs. JNK is central to hepatocellular injury caused by bile acids, fatty acids, TRAIL-Fas, warm and cold ischemia/reperfusion, and all in vivo models involving TNFα-induced liver injury (Con A plus D-galactosamine, LPS plus D-galactosamine, and TNFα plus D-galactosamine) [20,26,35,87]. D-galactosamine inhibits RNA synthesis, thus inhibiting NF-κB transcription of genes that turn off JNK. It is likely that JNK binding to mitochondrial Sab is key in mediating liver injury in many of these models, because silencing Sab was found to protect against TNFα plus D-galactosamine-induced liver injury [35]. Most liver injury models in which mitochondrial dysfunction and cytochrome c release are central appear to be mediated by JNK involvement. For hepatotoxicity in which mitochondria does not play a major role (CCl4), JNK activation does not appear to be critical [18,30]. Interestingly, Fas-mediated death of hepatocytes does involve mitochondrial cytochrome c release but does not involve JNK; however, Fas-mediated liver injury amplified by TNFα-related apoptosis-inducing ligand (TRAIL) requires JNK activation [88]. Because mitochondrial dysfunction occurs with many idiosyncratic toxicants, we can surmise that JNK may be important in mediating liver injury caused by many drugs, but clearly further studies are needed.
Besides JNK, many other pro-death and pro-survival proteins may target or reside in mitochondria to mediate cell death. Although GSK-3β regulates JNK, GSK-3β has been shown to translocate to mitochondria following APAP treatment [25]. The implications of GSK-3β translocation to mitochondria in APAP hepatotoxcity remain uncertain. However, GSK-3β translocation to mitochondria is believed to be important in ischemia reperfusion injury in the heart. In this model, GSK-3β translocation to mitochondria promotes MPT through interactions with voltage-dependent anion channels (VDAC) and degradation of myeloid cell leukemia-1 (mcl-1), an antiapoptotic member of the bcl-2 family localized on the mitochondrial outer membrane [89-91]. In APAP hepatotoxicity, we observed mcl-1 degradation that corresponded with GSK-3β translocation to mitochondria [25]. The role of mcl-1 in APAP hepatotoxicity remains unclear, but silencing mcl-1 in liver causes spontaneous apoptosis [92]. JNK also activates the pro-apoptotic bax, which translocates to mitochondria during APAP hepatotoxicity [19]. However, bax knockout mice are not protected from APAP-induced liver injury, suggesting bax is not essential for APAP hepatotoxicity [93]. JNK has also been suggested to phosphorylate and inactivate bcl-2 and bcl-xl, two anti-apoptotic proteins on the outer membrane of mitochondria [94]. The bcl-2 family of proteins are important regulators of cell death in many models systems (mcl-1 in TNFα-induced apoptosis [95] and bid in cholestatic liver injury [96]), and thus are likely to play a role in liver injury caused by APAP and other drugs, although the extent remains uncertain. Mitochondria are key direct targets of JNK in a self-amplifying ROS cycle and subsequent necrosis by ROS-mediated MPT or bcl-2 family-mediated apoptosis, pointing to the role of mitochondria in a final common pathway of cell death. Drugs can act directly on mitochondria to initiate or sensitize to these pathways or act upstream with the participation of mitochondria due to extrinsic or intrinsic cell death pathways.
Concluding Remarks
With the advancement of genomic, proteomic, metabolomic, and other profiling technologies, we may soon be able to identify and screen patients for susceptibility to idiosyncratic DILI. These screening techniques have already shown that in a majority of cases the road to DILI goes through mitochondria [51,52]. Many drugs and/or reactive metabolites have been shown to directly stress mitochondria, causing increased ROS that activate or inhibit important signal transduction pathways. After a critical threshold of mitochondrial stress or injury occurs, JNK is activated and targets mitochondria to induce MPT or MOMP. During DILI, mitochondria are probably the central point where oxidative injury, pro-death proteins, and pro-survival proteins converge to determine the fate of cells. A greater understanding of signal transduction pathways regulating mitochondria will, therefore, provide a much greater understanding of the susceptibility and resistance to DILI. Furthermore, signal transduction pathways such as JNK are important mediators of cell death and therefore represent potential therapeutic targets for the treatment of DILI.
Acknowledgements
This work was supported by NIH grants AA016911 (DH), DK078586 (ZXL), DK067215 (NK), AA014428 (NK), and the USC Research Center for Liver Diseases (DK48522).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 2.Holt M, Ju C. Drug-induced liver injury. Handb Exp Pharmacol. 2010:3–27. doi: 10.1007/978-3-642-00663-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich RG. Idiosyncratic toxicity: a convergence of risk factors. Annu Rev Med. 2007;58:17–34. doi: 10.1146/annurev.med.58.072905.160823. [DOI] [PubMed] [Google Scholar]
- 4.Han D, et al. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol. 2010:267–310. doi: 10.1007/978-3-642-00663-0_10. [DOI] [PubMed] [Google Scholar]
- 5.Daly AK, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 6.Hirata K, et al. Ticlopidine-induced hepatotoxicity is associated with specific human leukocyte antigen genomic subtypes in Japanese patients: a preliminary case-control study. Pharmacogenomics J. 2008;8:29–33. doi: 10.1038/sj.tpj.6500442. [DOI] [PubMed] [Google Scholar]
- 7.Lucena MI, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfirevic A, et al. In silico analysis of HLA associations with drug-induced liver injury: use of a HLA-genotyped DNA archive from healthy volunteers. Genome Med. 2012;4:51. doi: 10.1186/gm350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uetrecht J. Immunoallergic drug-induced liver injury in humans. Semin Liver Dis. 2009;29:383–392. doi: 10.1055/s-0029-1240007. [DOI] [PubMed] [Google Scholar]
- 10.Watkins PB. Biomarkers for the diagnosis and management of drug-induced liver injury. Semin Liver Dis. 2009;29:393–399. doi: 10.1055/s-0029-1240008. [DOI] [PubMed] [Google Scholar]
- 11.Dahlin DC, et al. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, et al. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol. 1998;11:295–301. doi: 10.1021/tx9701687. [DOI] [PubMed] [Google Scholar]
- 13.Watkins PB, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 14.Copple IM, et al. The keap1-nrf2 cellular defense pathway: mechanisms of regulation and role in protection against drug-induced toxicity. Handb Exp Pharmacol. 2010:233–266. doi: 10.1007/978-3-642-00663-0_9. [DOI] [PubMed] [Google Scholar]
- 15.Copple IM, et al. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology. 2008;48:1292–1301. doi: 10.1002/hep.22472. [DOI] [PubMed] [Google Scholar]
- 16.Tong KI, et al. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldring CE, et al. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 2004;39:1267–1276. doi: 10.1002/hep.20183. [DOI] [PubMed] [Google Scholar]
- 18.Gunawan BK, et al. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 19.Hanawa N, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki E, et al. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han D, et al. Redox regulation of tumor necrosis factor signaling. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DP, et al. Mechanisms of pathogenesis in drug hepatotoxicity putting the stress on mitochondria. Mol Interv. 2010;10:98–111. doi: 10.1124/mi.10.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burcham PC, Harman AW. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem. 1991;266:5049–5054. [PubMed] [Google Scholar]
- 24.Han D, et al. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol. 2003;64:1136–1144. doi: 10.1124/mol.64.5.1136. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara M, et al. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–8255. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim SH, et al. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol. 2011;54:765–772. doi: 10.1016/j.jhep.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra R, et al. Glycogen synthase kinase-3beta induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem. 2007;282:30393–30405. doi: 10.1074/jbc.M705895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JW, et al. Glycogen synthase kinase 3 beta is a natural activator of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1) J Biol Chem. 2003;278:13995–14001. doi: 10.1074/jbc.M300253200. [DOI] [PubMed] [Google Scholar]
- 29.Sharma M, et al. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa H, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Saitoh M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. Embo J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler V, et al. Regulation of JNK signaling by GSTp. Embo J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 34.Wiltshire C, et al. A new c-Jun N-terminal kinase (JNK)-interacting protein, Sab (SH3BP5), associates with mitochondria. Biochem J. 2002;367:577–585. doi: 10.1042/BJ20020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Win S, et al. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chambers JW, Lograsso PV. Mitochondrial c-Jun N-terminal Kinase (JNK) Signaling Initiates Physiological Changes Resulting in Amplification of Reactive Oxygen Species Generation. J Biol Chem. 2011;286:16052–16062. doi: 10.1074/jbc.M111.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antoine DJ, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 39.Festjens N, et al. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 41.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han D, et al. Receptor mediating protein kinase 1 (RIP1) works in parallel with c-Jun N-terminal kinase to mediate acetaminophen-induced liver injury. Hepatology. 2011:54. [Google Scholar]
- 43.Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- 44.James LP, et al. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 45.Jaeschke H, et al. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez-Sanchez R, et al. Thiazolidinedione bioactivation: a comparison of the bioactivation potentials of troglitazone, rosiglitazone, and pioglitazone using stable isotope-labeled analogues and liquid chromatography tandem mass spectrometry. Chem Res Toxicol. 2006;19:1106–1116. doi: 10.1021/tx050353h. [DOI] [PubMed] [Google Scholar]
- 47.Ong MM, et al. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol Sci. 2007;97:205–213. doi: 10.1093/toxsci/kfl180. [DOI] [PubMed] [Google Scholar]
- 48.Boelsterli UA, Lim PL. Mitochondrial abnormalities--a link to idiosyncratic drug hepatotoxicity? Toxicol Appl Pharmacol. 2007;220:92–107. doi: 10.1016/j.taap.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Pessayre D, et al. Mitochondrial involvement in drug-induced liver injury. Handb Exp Pharmacol. 2010:311–365. doi: 10.1007/978-3-642-00663-0_11. [DOI] [PubMed] [Google Scholar]
- 50.Montessori V, et al. Hepatotoxicity of nucleoside reverse transcriptase inhibitors. Semin Liver Dis. 2003;23:167–172. doi: 10.1055/s-2003-39947. [DOI] [PubMed] [Google Scholar]
- 51.Xu JJ, et al. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol Sci. 2008;105:97–105. doi: 10.1093/toxsci/kfn109. [DOI] [PubMed] [Google Scholar]
- 52.Porceddu M, et al. Prediction of liver injury induced by chemicals in human with a multiparametric assay on isolated mouse liver mitochondria. Toxicol Sci. 2012;129:332–345. doi: 10.1093/toxsci/kfs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashimshetty R, et al. Underlying mitochondrial dysfunction triggers flutamide-induced oxidative liver injury in a mouse model of idiosyncratic drug toxicity. Toxicol Appl Pharmacol. 2009;238:150–159. doi: 10.1016/j.taap.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Fujimoto K, et al. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37:193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- 55.Khetani SR, et al. The Use of Micropatterned Co-cultures to Detect Compounds that Cause Drug induced Liver Injury in Humans. Toxicol Sci. 2012 doi: 10.1093/toxsci/kfs326. [DOI] [PubMed] [Google Scholar]
- 56.Winnike JH, et al. Use of pharmaco-metabonomics for early prediction of acetaminophen-induced hepatotoxicity in humans. Clin Pharmacol Ther. 2010;88:45–51. doi: 10.1038/clpt.2009.240. [DOI] [PubMed] [Google Scholar]
- 57.Daly AK. Using genome-wide association studies to identify genes important in serious adverse drug reactions. Annu Rev Pharmacol Toxicol. 2012;52:21–35. doi: 10.1146/annurev-pharmtox-010611-134743. [DOI] [PubMed] [Google Scholar]
- 58.Kier LD, et al. Applications of microarrays with toxicologically relevant genes (tox genes) for the evaluation of chemical toxicants in Sprague Dawley rats in vivo and human hepatocytes in vitro. Mutat Res. 2004;549:101–113. doi: 10.1016/j.mrfmmm.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Liguori MJ, et al. Microarray analysis in human hepatocytes suggests a mechanism for hepatotoxicity induced by trovafloxacin. Hepatology. 2005;41:177–186. doi: 10.1002/hep.20514. [DOI] [PubMed] [Google Scholar]
- 60.Watkins PB. Idiosyncratic liver injury: challenges and approaches. Toxicol Pathol. 2005;33:1–5. doi: 10.1080/01926230590888306. [DOI] [PubMed] [Google Scholar]
- 61.Watkins PB, et al. Using controlled clinical trials to learn more about acute drug-induced liver injury. Hepatology. 2008;48:1680–1689. doi: 10.1002/hep.22633. [DOI] [PubMed] [Google Scholar]
- 62.Ni HM, et al. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han D, et al. Dynamic adaptation of liver mitochondria to chronic alcohol feeding in mice: Biogenesis, remodeling, and functional alterations. J Biol Chem. 2012 doi: 10.1074/jbc.M112.377374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shiryaeva A, et al. Hepatocyte mitochondrion electron-transport chain alterations in CCl(4) and alcohol induced hepatitis in rats and their correction with simvastatin. J Bioenerg Biomembr. 2008;40:27–34. doi: 10.1007/s10863-008-9125-2. [DOI] [PubMed] [Google Scholar]
- 65.Morimoto T, et al. Changes in concentrations of respiratory components and cytochrome oxidase activity in mitochondria obtained from carbon tetrachloride-induced cirrhotic rat liver. Clin Sci (Lond) 1988;74:485–489. doi: 10.1042/cs0740485. [DOI] [PubMed] [Google Scholar]
- 66.Than TA, et al. Role of cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 3 (CRTC3) in the initiation of mitochondrial biogenesis and stress response in liver cells. J Biol Chem. 2011;286:22047–22054. doi: 10.1074/jbc.M111.240481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem. 2011;149:241–251. doi: 10.1093/jb/mvr002. [DOI] [PubMed] [Google Scholar]
- 68.Reddy PH, et al. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011;67:103–118. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomes LC, et al. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruepp SU, et al. Genomics and proteomics analysis of acetaminophen toxicity in mouse liver. Toxicol Sci. 2002;65:135–150. doi: 10.1093/toxsci/65.1.135. [DOI] [PubMed] [Google Scholar]
- 71.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 72.Hoppins S, Nunnari J. Cell Biology. Mitochondrial dynamics and apoptosis--the ER connection. Science. 2012;337:1052–1054. doi: 10.1126/science.1224709. [DOI] [PubMed] [Google Scholar]
- 73.Karbowski M, et al. Free radical-induced megamitochondria formation and apoptosis. Free Radic Biol Med. 1999;26:396–409. doi: 10.1016/s0891-5849(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 74.Shishido S, et al. Hydrogen peroxide overproduction in megamitochondria of troglitazone-treated human hepatocytes. Hepatology. 2003;37:136–147. doi: 10.1053/jhep.2003.50014. [DOI] [PubMed] [Google Scholar]
- 75.Wong GH, et al. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 76.Wullaert A, et al. Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090–1101. doi: 10.1016/j.bcp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Nagai H, et al. Reduced glutathione depletion causes necrosis and sensitization to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology. 2002;36:55–64. doi: 10.1053/jhep.2002.33995. [DOI] [PubMed] [Google Scholar]
- 78.Matsumaru K, et al. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425–1434. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- 79.Han D, et al. Hydrogen peroxide and redox modulation sensitize primary mouse hepatocytes to TNF-induced apoptosis. Free Radic Biol Med. 2006;41:627–639. doi: 10.1016/j.freeradbiomed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Lou H, Kaplowitz N. Glutathione depletion down-regulates tumor necrosis factor alpha-induced NF-kappaB activity via IkappaB kinase-dependent and -independent mechanisms. J Biol Chem. 2007;282:29470–29481. doi: 10.1074/jbc.M706145200. [DOI] [PubMed] [Google Scholar]
- 81.Gandhi A, et al. Role of c-Jun N-terminal kinase (JNK) in regulating tumor necrosis factor-alpha (TNF-alpha) mediated increase of acetaminophen (APAP) and chlorpromazine (CPZ) toxicity in murine hepatocytes. J Toxicol Sci. 2010;35:163–173. doi: 10.2131/jts.35.163. [DOI] [PubMed] [Google Scholar]
- 82.Shaw PJ, et al. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci. 2007;100:259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- 83.Tukov FF, et al. The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol Sci. 2007;100:267–280. doi: 10.1093/toxsci/kfm209. [DOI] [PubMed] [Google Scholar]
- 84.Luyendyk JP, et al. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther. 2003;307:9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- 85.Liguori MJ, et al. Comparison of TNFalpha to Lipopolysaccharide as an Inflammagen to Characterize the Idiosyncratic Hepatotoxicity Potential of Drugs: Trovafloxacin as an Example. Int J Mol Sci. 2010;11:4697–4714. doi: 10.3390/ijms11114697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roth RA, Ganey PE. Intrinsic versus idiosyncratic drug-induced hepatotoxicity--two villains or one? J Pharmacol Exp Ther. 2010;332:692–697. doi: 10.1124/jpet.109.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwabe RF. Cell death in the liver-all roads lead to JNK. Gastroenterology. 2006;131:314–316. doi: 10.1053/j.gastro.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 88.Corazza N, et al. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493–2499. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Das S, et al. Glycogen Synthase Kinase 3 Inhibition Slows Mitochondrial Adenine Nucleotide Transport and Regulates Voltage-Dependent Anion Channel Phosphorylation. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maurer U, et al. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 91.Juhaszova M, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vick B, et al. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajt ML, et al. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- 94.Latchoumycandane C, et al. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- 95.Kodama Y, et al. Antiapoptotic effect of c-Jun N-terminal Kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136:1423–1434. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 96.Higuchi H, et al. Bid antisense attenuates bile acid-induced apoptosis and cholestatic liver injury. J Pharmacol Exp Ther. 2001;299:866–873. [PubMed] [Google Scholar]
- 97.Krysko DV, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]