Abstract
A long-standing but poorly understood defect in autoimmune diseases is dysfunction of the hematopoietic cells. Leukopenia is often associated with systemic lupus erythematous (SLE) and other autoimmune diseases. In addition, homeostatic proliferation of T cells, which is a host response to T cell lymphopenia, has been implicated as potential cause of rheumatoid arthritis (RA) in human and experimental models of autoimmune diabetes in the NOD mice and the BB rats. Conversely, successful treatments of aplastic anemia by immune suppression suggest that the hematologic abnormality may have a root in autoimmune diseases. Traditionally, the link between autoimmune diseases and defects in hematopoietic cells has been viewed from the prism of antibody-mediated hemolytic cytopenia. While autoimmune destruction may well be part of pathogenesis of defects in hematopoietic system, it is worth considering the hypothesis that either leukopenia or pancytopenia may also result directly from defective hematopoietic stem cells (HSC). We have recently tested this hypothesis in the autoimmune Scurfy mice which has mutation Foxp3, the master regulator of regulatory T cells. Our data demonstrated that due to hyperactivation of mTOR, the HSC in the Scurfy mice are extremely poor in hematopoiesis. Moreover, rapamycin, an mTOR inhibitor rescued HSC defects and prolonged survival of the Scurfy mice. Our data raised the intriguing possibility that targeting mTOR dysregulation in the HSC may help to break the vicious cycle between cytopenia and autoimmune diseases.
Keywords: Cytopenia, homeostatic proliferation, CD24, mTOR, hematopoietic stem cells, autoimmune diseases, inflammatory cytokines, rapamycin
Introduction
Autoimmune diseases are caused by activation of self-reactive T and B cells that escape a multitude of mechanisms for immune tolerance [1–3]. Paradoxically, autoimmune diseases are also associated with immune deficiency and infections. For instance, at least two-thirds of patients with common variable immunodeficiency exhibit signs of autoimmune diseases [4–7]. In addition, patients with primary immune deficiency, such as DiGeorge syndrome, also exhibit signs of autoimmunity [8–12].
The cause and effect between autoimmune diseases and immunodeficiency remains unresolved. It is generally agreed that autoreactive antibodies may cause elimination of leukocytes and thus contribute to immune deficiency [4, 13]. Methotrexate and occasionally, prednisone, the popular drug for autoimmune diseases, are known to cause cytopenia [14–18]. On the other hand, lymphopenia has been shown to cause homeostatic proliferation (HP) of T cells [19–22]. HP has been suggested as a direct cause of autoimmune diseases in the NOD model of type I diabetes [23] and fatal autoimmune diseases in mice devoid of regulatory T cells [24]. One can thus envisage a vicious cycle between autoimmune diseases and immunodeficiency. How to untangle the web between autoimmunity and immunodeficiency is not only of interest for fundamental understanding of immunology, but also of practical significance in treatment of both autoimmune diseases and immunodeficiency. In this presentation, we will review literature in this less explored area and present our recent studies that address the impact of autoimmune diseases on hematopoiesis and the molecular pathway underlying such impact.
A vicious cycle between autoimmune diseases and cytopenia
The link between autoimmune diseases and defects of hematopoiesis system has its root in long-standing clinical observations. In the SLE patients, the cytopenia has emerged as a major hematologic criterion. The American College of Rheumatology (ACR) has the laboratory finding of hemolytic anemia, leukopenia, lymphopenia and thrombocytopenia as a diagnostic marker for SLE [25]. Likewise, cytopenia has been observed in rheumatoid arthritis patients and those with Sjogren’s syndrome [15, 26–28]. In addition to generalized cytopenia, more selective defects such as T cell lymphopenia have been reported in rheumatoid arthritis [29] and multiple sclerosis [30].
It is of interest to consider the cause-effect relationship between autoimmune diseases and cytopenia. A well-established autoimmune disease in both adults and children is autoimmune hemolytic anemia, in which autoreactive antibodies are abnormally produced and mediate elimination of both erythrocytes and/or leukocytes [4, 13]. In addition, it is increasingly clear that drugs frequently used for autoimmune patients, such as methotrexate and occasionally prednisone, may have cytopenia as a major adverse event [14–18]. A largely overlooked issue is whether autoimmune diseases may cause defective hematopoiesis. This issue will be revisited in the next section.
On the other hand, cytopenia may also be a fundamental cause of autoimmune diseases. An intriguing link between cytopenia and autoimmune diseases is T cell HP. HP refers to the ability of T cell to mount proliferation in response to paucity of T cells in the host. Physiologically, homeostatic proliferation occurs during the neonatal period. It has been suggested that such proliferation may complement T-cell lymphopoiesis in the thymus to fill the periphery lymphoid organs [20].
Importantly, homeostatic proliferation not only increases the number of T cells in the host, but also fundamentally changes the T cells in at least two ways. First, since HP is primarily driven by self-antigens [19, 21, 22], it is to be expected that homeostatic proliferation would increase the overall autoreactivity of T cells. This has been confirmed in mice with neonatal thymectomy [31]. Second, T cells that have undergone HP acquire features of memory T cells and thus have lower activation threshold [32, 33]. Both features suggest that homeostatic proliferation may increase the risk of autoimmune diseases. To test this notion, we used mice with a fetal autoimmune disease, called Scurfy [34–36]. The Scurfy mice were chosen as they are known to exhibit both cytopenia and severe autoimmune diseases [34–36]. Moreover, subsequent studies have identified a similar X-linked autoimmune disease, known as IPEX for immune dysregulation, polyendocrinopathy, enteropathy, and x-linked syndrome [37]. The genetic bases for both diseases were identified about 10 years ago, as inactivating mutations of the FOXP3 gene [38–41]. As the first step to determine if T cell production was defective in the thymus, we analyzed T cell development during the perinatal period. We showed that, in the Scurfy mice, the production of T cells in the thymus was reduced as proliferation of T cell progenitors was hampered by an increased Erbb2 expression in the thymus [42]. Corresponding to defective T cell production, the Scurfy mice had exacerbated homeostatic proliferation [24]. Since increased survival of the Scurfy mice can be achieved only by adoptive transfer of a combination of regulatory T cells and non-regulatory T cells [24], homeostatic proliferation of T cells must be suppressed to prevent the fatal autoimmunity in the Scurfy mice.
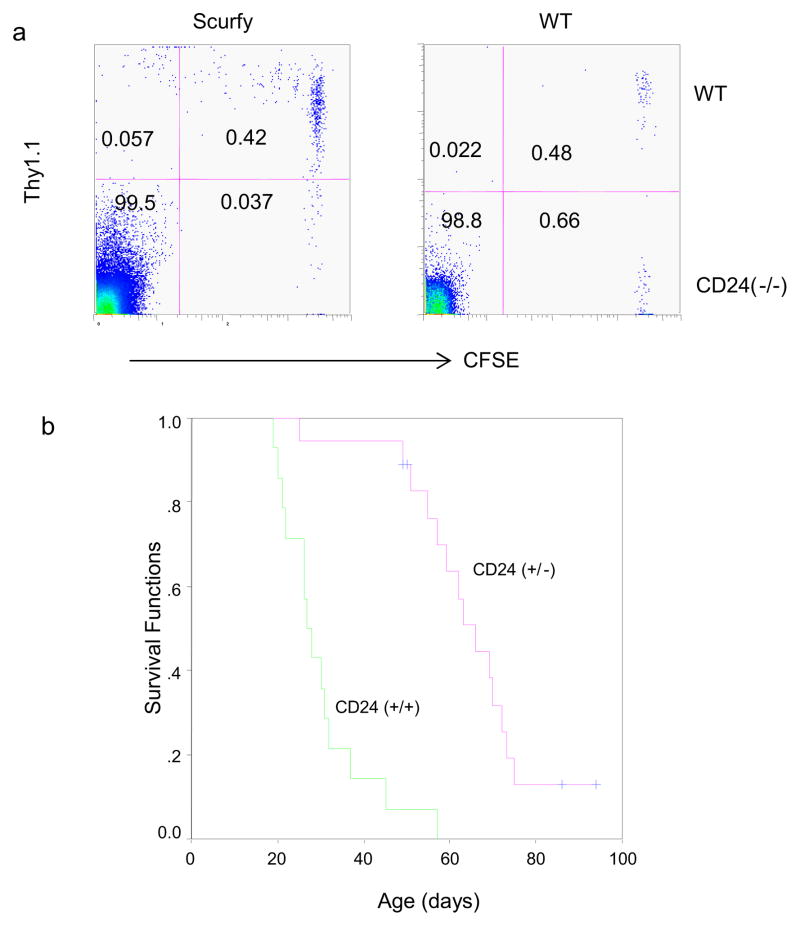
In order to test this hypothesis by genetic manipulation, one needs to identify a T-cell intrinsic regulator for homeostatic proliferation. In this context, we have reported that a functional CD24 gene on T cells is critical for homeostatic proliferation in a lymphopenic host [43]. To test whether a similar requirement also holds true in the Scurfy mice, we adoptive transferred a mixture of WT and CD24-deficient T cells to the Scurfy mice. As shown in Fig. 1a, while wild-type T cells mounted a vigorous proliferation, CD24−/− T cells were largely undivided. Thus, much like the lymphopenic host, the homeostatic proliferation in the Scurfy mice also requires CD24 expression in T cells. The requirement for CD24 in homeostatic proliferation in the Scurfy mice provides us with a model to evaluate its contribution to the pathogenesis of autoimmune diseases in the Scurfy mice. We crossed the CD24-null alleles into the Scurfy mice and monitored survival of Scurfy mice with different CD24 genotypes. As shown in Fig. 1b, CD24-deficiency significantly extended the survival of the Scurfy mice. These data make a compelling case that homeostatic proliferation is a missing link between lymphopenia and autoimmune diseases
Fig. 1.
Genetic evidence for a critical role for CD24-mediated homeostatic proliferation in the pathogensis of autoimmune diseases in the Scurfy mice. a.. CD24-dependent homeostatic proliferation of Foxp3WT T cells in the Scurfy mice. 4×106 total T cells from either WT Thy1.1+ B6 or Thy1.1− CD24-deficient B6 mice were mixed at a 1:1 ratio and injected into four day old Scurfy B6 mice or wild type littermates. Four days later, the recipient mice were sacrificed and the spleen and lymph node cells were stained with anti-CD4, Thy1.1 antibodies. Data shown are profiles of gated CD4 T cells in the lymph nodes and have been repeated twice. Note that in the WT mice, donor T cells did not dilute CFSE regardless of CD24 genotype. In contrast, WT but not CD24-deficient T cells divided in the Scurfy host. CD24-deficiency abrogated homeostatic proliferation of T cells in the Scurfy mice. b. Heterozygous deletion of CD24 is sufficient to prolong survival of the Scurfy mice. Life span of CD24+/+ and CD24+/− Foxp3sf mice. The mice that have not reached the endpoint of analysis are shown as censored samples, marked by a cross. An extremely significant difference was observed between the life spans of the two strains of mice (P<0.00001).
Apart from the Scurfy model, studies by others have demonstrated that T lymphopenia is associated with exacerbation of autoimmune diseases in type I diabetes in the NOD mice [23]. More importantly, the development of diabetes can be prevented by adoptive transfer of naïve T cells [23]. Corresponding to mouse data, defective T cell production and homeostatic proliferation was observed in RA patients [29, 44].
The link between lymphopenia and autoimmune diseases is strengthened by genetic studies in mice, rats and humans. Lymphopenia was observed in the Y chromosome-associated lupus in mice [45]. In the BB rat, the immune-associated nucleotide (Ian)-related genes are associated with lymphopenia and risk of type I diabetes [46, 47]. More importantly, DiGeorge syndrome, which is a prototype of primary immune deficiency due to defective T cell production, is associated with autoimmune diseases, including juvenile arthritis and Grave’s disease [8–12, 48].
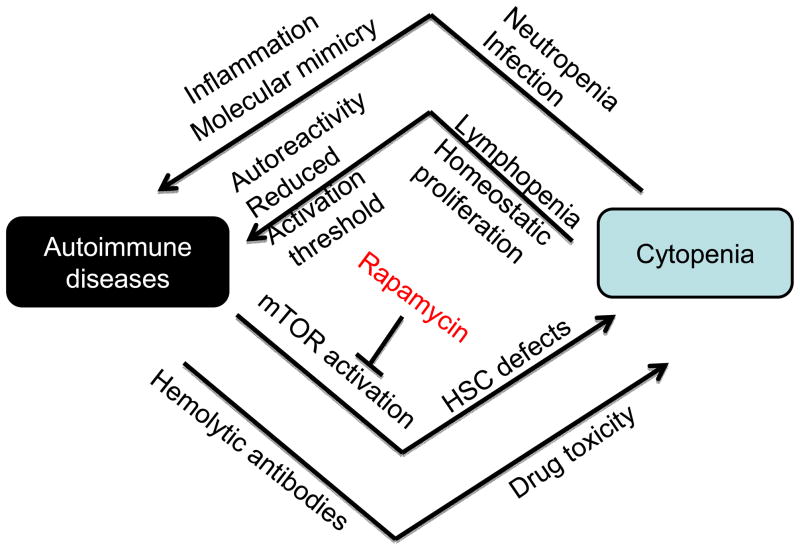
Taken together, a compelling case can be made that cytopenia may be an important cause of autoimmune diseases (Fig. 2). While lymphopenia provides the most compelling link between autoimmune diseases and cytopenia, it is also likely that additional associations with cytopenia can exacerbate autoimmune diseases. For instance, neutropenia is often associated with infections [49]. Infections may initiate or exacerbate autoimmune diseases through both activation of Toll-like receptors [50] and/or through molecular mimicry [51, 52].
Fig. 2.
A vicious cycle between cytopenia and autoimmune diseases. Cytopenia, as observed in autoimmune patients, has been shown to be caused by autoreactive hemolytic antibodies, drug toxicity or HSC defects associated with mTOR-hyperactivation. Cytopenia may exacerbate autoimmune diseases by increased infection and lymphopenia. Infection has been shown to exacerbate autoimmune diseases, both through molecular mimicry and activation of TLR. On the other hand, since lymphopenia-induced HP requires self MHC-peptide complex, homeostatic proliferation may increase the frequency of autoreactive T cells. Furthermore, since HP converts naïve T cells into memory-like T cells with a lower activation threshold, HP will likely facilitate activation of autoreactive T cells. Given the central role of mTOR activation in HSC defects, we propose that rapamycin may be used to break the vicious cycle between cytopenia and autoimmune diseases.
Hematopoietic stem cells and autoimmune diseases
As outlined above, autoantibodies and drug side effects are two accepted causes of cytopenia in autoimmune patients. It is largely unresolved whether autoimmune diseases may affect the function of HSC. The most important clue that autoimmune diseases may affect the HSC functions comes from clinical experience with aplastic anemia, a pancytopenia attributed to stem cell defects. Aplastic anemia is caused by defective stem cell function and manifests as defective production of both erythroid, myeloid and lymphoid cells. Most cases of aplastic anemia are considered idiopathic. Transplantation is recommended when histocompatible donors are available. Since this is not an option for most patients with aplastic anemia, immune suppression therapy, typically a combination of anti-thymocyte globulin (ATG), which eliminates T lymphocytes, and cyclosporine A, a commonly used immune suppressant, are adopted. Since immune suppression results in complete response in 50–70% of patients, it is generally accepted that most acquired aplastic anemia is a result of concurrent autoimmunity [53].
To directly demonstrate a link between autoimmune diseases and HSC function, we first analyzed the hematopoiesis in the Scurfy mice, which is devoid of regulatory T cells [54], and developed fatal autoimmune diseases and pancytopenia [34–36]. We observed a progressive loss of bone marrow cellularity that closely correlated with the progression of autoimmune diseases. Interestingly, the number of HSC (Flt2−Lin-Sca-1+c-Kit+CD150+CD48−CD34−) temporally expanded in the Scurfy mice at three weeks of age when the autoimmune diseases initiated but dropped precipitously by 4 weeks of age when autoimmune symptoms reached their peak, to a level that is 5–10 fold lower than wild-type littermates. In order to compare HSC activity in the bone marrow of WT and Scurfy mice, we harvested bone marrow from 1, 3, and 4 week old mice and carried out competitive transplantation. Our data demonstrated that while bone marrow cells from day 7 old Scurfy mice were as competent as WT cells in long-term hematopoiesis, bone marrow cells from 3 and 4 week old Scurfy mice had greatly diminished hematopoiesis. The defects were observed in all lineages of lymphocytes and myeloid cells. These data provide direct evidence that autoimmune diseases have severe effects on both the number and function of HSC [55]. This model is also valuable for therapeutic intervention of cytopenia associated with autoimmune diseases.
mTOR hyperactivation as the underlying cause for defective hematopoiesis in autoimmune diseases
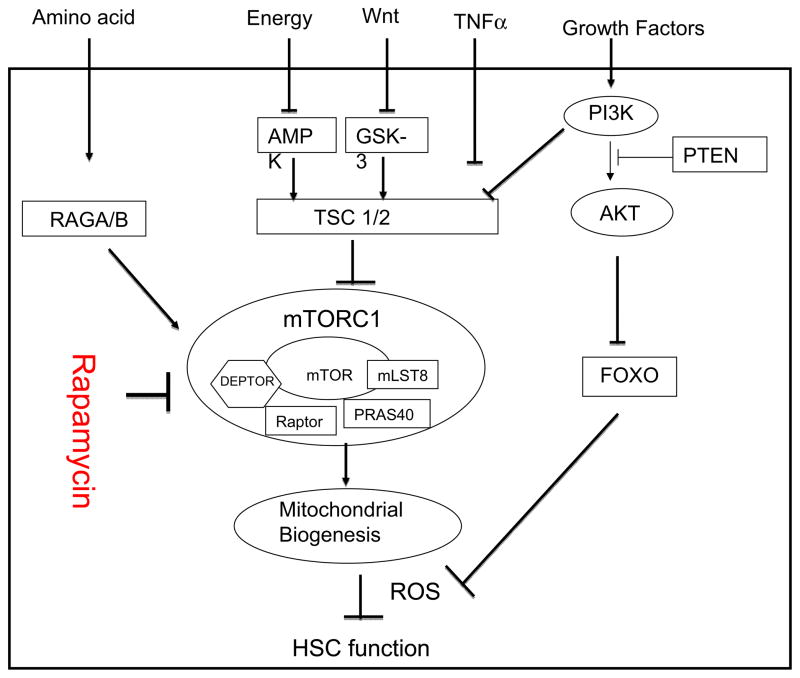
Mammalian targets of rapamycin (mTOR) have emerged as a key cellular sense for environmental changes, including nutrition, energy, inflammatory stimuli and growth signals such as hormones [56–59]. mTOR is stimulated by signals that activate AKT, which inactivates TSC1/TSC2 complex [57, 60–62]. Conversely, PTEN, a multi-functional negative regulator of cellular signaling and genomic stability, is known to inhibit mTOR activation, perhaps through inactivation of AKT [63]. TSC function is maintained by GSK but abrogated by Wnt signaling pathway [58]. In addition, mTOR sense cellular energy level through AMPK, which is in turn activated by energy-deprivation [59]. Inactivation of TSC complex by IKKβ links mTOR to inflammation [64]. The levels of free amino acids are sensed by RAGA/B complex, which in turn activates mTOR [65]. mTOR forms two distinct signaling complexes, known as TORC1 and TORC2, by interacting with either Raptor or Rictor, respectively [66, 67]. While the TORC1 is activated by AKT, TORC2 regulates activation of AKT1. Moreover, TORC1 and TORC2 are differentially affected by TSC complex. While inactivation of TSC increases TORC1 activity, deletion of TSC appears to inactivate TORC2 [68, 69], perhaps through a negative feedback mechanism. As depicted in Fig. 3, mTOR pathway has now emerged as one of the best characterized central pathways for cell-environment interaction, including HSC function. Since hematopoiesis is dynamically regulated by the environment, the role for mTOR in HSC function is of great interest.
Fig 3.
A putative molecular mechanism underlying the hematopoietic defects in autoimmune patients. mTOR is negatively regulated by the TSC1/2 complex, which senses energy levels, inflammatory environments, growth signals from Wnt and other growth factors. Although apparently down-stream of the TSC1/2 complex, amino acid levels also regulate mTOR activity through the GTPase RAGA/B complex. Hyperactivation of mTOR disrupts the quiescence and function of HSC through increased mitochondrial biogenesis and ROS production. By suppressing TORC1 activation, short-term treatment of Scurfy mice with rapamycin resulted in long-term restoration of HSC function. Therefore, it is worth exploring whether the drug may be used to restore hematopoiesis in autoimmune patients.
Two groups have reported that targeted mutation of the Pten gene in the HSC results in transient expansion and their loss of stem cell activity as demonstrated by dysregulation in hematopoiesis in the host and lack of hematopoiesis in bone marrow transplantation studies [70, 71]. Since Pten is a negative regulator of mTOR, it has been suggested that functional loss caused by Pten may be due to hyperactivation of mTOR [70, 71]. However, other studies have raised the possibility that this is achieved by dysregulation of FOXO and genomic instability [72]. To address this issue, we tested the HSC function after inactivation of TSC, which is a more specific regulator of mTOR activity. Our data showed that deletion of the Tsc1 gene results in loss of quiescence and stem cell activation in the HSC, even though loss of HSC function does not correspond to reduction of cells with HSC markers [73]. The defects can be attributed to mTOR hyperactivation as treatment with rapamycin restores the stemness of the HSC. More importantly, mTOR activation causes increased production of radical oxygen species and mitochondrion biogenesis, which is responsible for the defective stem cell function.
Given the broad similarity in stem cell behavior in autoimmune mice and those with Tsc1 deletion, we tested if mTOR activation is responsible for the stem cell defects in the autoimmune mice. We have provided several lines of evidence for the hypothesis [55]. First, we observed that inflammatory cytokines, such as IL-6 and TNFα, which were highly elevated in the Scurfy mice, induced activation of mTOR within 30 minutes. Second, we showed that HSC in the Scurfy mice had highly elevated levels of mTOR activation, as revealed by the phosphorylation of mTOR and its downstream substrate S6. Third, we showed that short-term treatment of rapamycin significantly restored bone marrow cellularity and increased production of lymphoid, myeloid and erythroid lineages in the bone marrow. Fourth, we reported that rapamycin treated bone marrow cells showed vastly improved activity in long-term reconstitution in competitive bone marrow transplantation. Last but not least, we observed a very significant improvement of survival of Scurfy mice by short-term treatment with rapamycin.
The genetic basis of Scurfy mice is the mutation in Foxp3 gene. In human, the FOXP3 mutations result in the syndrome of immune dysregulation, polyendocrinopathy, autoimmune-enteropathy (IPEX; OMIM304930) which is a fatal X-linked recessive disorder of early childhood. Protean symptoms of IPEX are severe secretory enteropathy causing failure to thrive, early onset insulin-dependent diabetes mellitus, and eczema [74–77]. Impressively, Bindl, et al reported that sirolimus successfully controlled the gastrointestinal and dermatologic symptoms of IPEX and reduced the systemic inflammatory reaction in three patients for up to 5 years without significant side effects [78]. Given the lack of effective treatment for IPEX patients, it is surprising that only few follow up clinical reports on using sirolimus in IPEX and IPEX-like children have been reported with variable results since the initial report [79, 80].
Implications for the treatment of hematopoiesis defects in autoimmune diseases in humans
Taken together, our studies have demonstrated that mTOR activation is an underlying cause of hematopoiesis defects in autoimmune patients. The above discussion highlights the fact that cytopenia is both a cause and effect of autoimmune diseases (Fig. 2). Therefore, how to break the vicious cycle of cytopenia and autoimmune diseases has significant implication in the treatment of autoimmune diseases. Since mTOR hyperactivation is a root cause of HSC defects in autoimmune diseases, an obvious question is whether it is feasible to use rapamycin to restore hematopoiesis in autoimmune patients.
Perhaps the first issue is that of safety. A number of transplantation studies have led to a labeling of cytopenia and lipidemia as rapamycin side effects [81, 82]. However, close examination of the trials suggests that the side effects were observed in patients with multiple drug combinations [82, 83], but rarely in rapamycin monotherapy [84, 85]. Even in multi-drug combinations, the side effect was observed in patients with trough concentrations equal or greater than 16 ng/ml [86]. These studies raise the possibility that when doses and drug combinations are carefully managed, it is possible to implement a regimen to use rapamycin for autoimmune diseases. Importantly, in a number of pilot studies, rapamycin appears to have conferred clinical benefits for patients with SLE [87] and type I diabetes [84]. However, to our knowledge, no clinical trial has been conducted to test the concept that hematological defects in autoimmune diseases can be corrected with administration of rapamycin.
It is important to bear in mind that our proposed use of rapamycin to correct hematological defects in autoimmune diseases is based on reprogramming of HSC rather than immune suppression. As such, one may expect a long-lasting therapeutic effect after a short treatment window. In our experience with animal models, short-term treatment of rapamycin during the perinatal period has a long-term effect in adult mice [55]. Thus, for survival studies, the treatment lasted for only one week but the effect was observed for several months after the treatment. For the test of long-term HSC function, the treatment lasted for only two weeks and the impact on long-term HSC could be observed in a new host that received no rapamycin. This is consistent with the notion that rapamycin reprogrammed HSC to increase its stemness. The long-term impact of transient treatment suggests that it may be feasible to identify a therapeutic window to avoid the adverse effects of rapamycin.
Conclusions and future directions
Autoimmune diseases and leukopenia form a vicious cycle. The leukopenia is caused by both direct autoimmune destruction and drug toxicity of leukocyte and inflammation-induced aging of HSC. Leukopenia exacerbates autoimmune diseases by increasing the risk of infection and by inducing homeostatic proliferation. Recent studies suggested the possibilities that environmental features and epigenetics may also be important in pathogenesis of leukopenia in autoimmune diseases [88–90]. Since the HSC defect was caused by mTOR hyperactivation, it may be possible to break this vicious cycle through mTOR targeting. Further studies are needed to evaluate the feasibility of restoring hematopoiesis in cytopenic autoimmune patients through judicious use of mTOR inhibitors and other drugs available for the specific indications. A successful restoration of hematopoiesis in autoimmune patients will not only fulfill an unmet medical need, but also provide us an opportunity to evaluate the contribution of cytopenia to the progression of autoimmune diseases.
Highlights.
Cytopenia and autoimmunity paradoxically co-exist in patients with autoimmune diseases;
Cytopenia promotes autoimmune diseases through increased risk of infection and lymphopenia-driven homeostatic proliferation;
Autoimmune diseases cause mTOR dysregulation and loss of stemness of HSC;
mTOR inhibitors may break the vicious cycle between cytopenia and autoimmune diseases.
Acknowledgments
We thank Dawn Griffiths for editorial assistance. This work is supported by grants from the National Institutes of Health and the U.S. Department of Defense.
Footnotes
The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Cunningham-Rundles C. Treatment and outcome of autoimmune hematologic disease in common variable immunodeficiency (CVID) J Autoimmun. 2005;25:57–62. doi: 10.1016/j.jaut.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Boileau J, Mouillot G, Gerard L, Carmagnat M, Rabian C, Oksenhendler E, et al. Autoimmunity in common variable immunodeficiency: correlation with lymphocyte phenotype in the French DEFI study. J Autoimmun. 2011;36:25–32. doi: 10.1016/j.jaut.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Haymore BR, Mikita CP, Tsokos GC. Common variable immune deficiency (CVID) presenting as an autoimmune disease: role of memory B cells. Autoimmun Rev. 2008;7:309–12. doi: 10.1016/j.autrev.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Rensing-Ehl A, Warnatz K, Fuchs S, Schlesier M, Salzer U, Draeger R, et al. Clinical and immunological overlap between autoimmune lymphoproliferative syndrome and common variable immunodeficiency. Clin Immunol. 2010;137:357–65. doi: 10.1016/j.clim.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Kratz CP, Niehues T, Lyding S, Heusch A, Janssen G, Gobel U. Evans syndrome in a patient with chromosome 22q11. 2 deletion syndrome: a case report. Pediatr Hematol Oncol. 2003;20:167–72. doi: 10.1080/0880010390158685. [DOI] [PubMed] [Google Scholar]
- 9.Pelkonen P, Lahdenne P, Lantto R, Honkanen V. Chronic arthritis associated with chromosome deletion 22q11. 2 syndrome. J Rheumatol. 2002;29:2648–50. [PubMed] [Google Scholar]
- 10.Davies K, Stiehm ER, Woo P, Murray KJ. Juvenile idiopathic polyarticular arthritis and IgA deficiency in the 22q11 deletion syndrome. J Rheumatol. 2001;28:2326–34. [PubMed] [Google Scholar]
- 11.Kawamura T, Nimura I, Hanafusa M, Fujikawa R, Okubo M, Egusa G, et al. DiGeorge syndrome with Graves’ disease: A case report. Endocr J. 2000;47:91–5. doi: 10.1507/endocrj.47.91. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan KE, McDonald-McGinn DM, Driscoll DA, Zmijewski CM, Ellabban AS, Reed L, et al. Juvenile rheumatoid arthritis-like polyarthritis in chromosome 22q11. 2 deletion syndrome (DiGeorge anomalad/velocardiofacial syndrome/conotruncal anomaly face syndrome) Arthritis Rheum. 1997;40:430–6. doi: 10.1002/art.1780400307. [DOI] [PubMed] [Google Scholar]
- 13.Ortega-Hernandez OD, Agmon-Levin N, Blank M, Asherson RA, Shoenfeld Y. The physiopathology of the catastrophic antiphospholipid (Asherson’s) syndrome: compelling evidence. J Autoimmun. 2009;32:1–6. doi: 10.1016/j.jaut.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Gispen JG, Alarcon GS, Johnson JJ, Acton RT, Barger BO, Koopman WJ. Toxicity of methotrexate in rheumatoid arthritis. J Rheumatol. 1987;14:74–9. [PubMed] [Google Scholar]
- 15.Berthelot JM, Maugars Y, Hamidou M, Chiffoleau A, Barrier J, Grolleau JY, et al. Pancytopenia and severe cytopenia induced by low-dose methotrexate. Eight case-reports and a review of one hundred cases from the literature (with twenty-four deaths) Rev Rhum Engl Ed. 1995;62:477–86. [PubMed] [Google Scholar]
- 16.Czaja AJ. Safety issues in the management of autoimmune hepatitis. Expert Opin Drug Saf. 2008;7:319–33. doi: 10.1517/14740338.7.3.319. [DOI] [PubMed] [Google Scholar]
- 17.Rahman P, Humphrey-Murto S, Gladman DD, Urowitz MB. Cytotoxic therapy in systemic lupus erythematosus. Experience from a single center. Medicine (Baltimore) 1997;76:432–7. doi: 10.1097/00005792-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Shoda H, Inokuma S, Yajima N, Tanaka Y, Oobayashi T, Setoguchi K. Higher maximal serum concentration of methotrexate predicts the incidence of adverse reactions in Japanese rheumatoid arthritis patients. Mod Rheumatol. 2007;17:311–6. doi: 10.1007/s10165-007-0582-y. [DOI] [PubMed] [Google Scholar]
- 19.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–90. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–40. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 21.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–81. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 22.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 24.Chang X, Zheng P, Liu Y. Homeostatic proliferation in mice with germline FoxP3 mutation and its contribution to fatal autoimmunity. J Immunol. 2008;181:2399–406. doi: 10.4049/jimmunol.181.4.2399. [DOI] [PubMed] [Google Scholar]
- 25.Kao AH, Manzi S, Ramsey-Goldman R. Review of ACR hematologic criteria in systemic lupus erythematosus. Lupus. 2004;13:865–8. doi: 10.1191/0961203304lu2025oa. [DOI] [PubMed] [Google Scholar]
- 26.Quintero OL, Rojas-Villarraga A, Mantilla RD, Anaya JM. Autoimmune diseases in the intensive care unit. An update. Autoimmun Rev. 2012 doi: 10.1016/j.autrev.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-Casals M, Cervera R, Garcia-Carrasco M, Vidal J, Trejo O, Jimenez S, et al. Cytopenia and past human parvovirus B19 infection in patients with primary Sjogren’s syndrome. Semin Arthritis Rheum. 2000;29:373–8. doi: 10.1053/sarh.2000.7024. [DOI] [PubMed] [Google Scholar]
- 28.Ferraccioli GF, Tonutti E, Casatta L, Pegoraro I, De Vita S, Sala P, et al. CD4 cytopenia and occasional expansion of CD4+CD8+lymphocytes in Sjogren’s syndrome. Clin Exp Rheumatol. 1996;14:125–30. [PubMed] [Google Scholar]
- 29.Goronzy JJ, Weyand CM. Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol. 2001;22:251–5. doi: 10.1016/s1471-4906(00)01841-x. [DOI] [PubMed] [Google Scholar]
- 30.Hug A, Korporal M, Schroder I, Haas J, Glatz K, Storch-Hagenlocher B, et al. Thymic export function and T cell homeostasis in patients with relapsing remitting multiple sclerosis. J Immunol. 2003;171:432–7. doi: 10.4049/jimmunol.171.1.432. [DOI] [PubMed] [Google Scholar]
- 31.Smith H, Chen IM, Kubo R, Tung KS. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989;245:749–52. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- 32.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–56. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T Cells Transiently Acquire a Memory-like Phenotype during Homeostasis-driven Proliferation. J Exp Med. 2000;192:557–64. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godfrey VL, Rouse BT, Wilkinson JE. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am J Pathol. 1994;145:281–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A. 1991;88:5528–32. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1990;87:2433–7. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baud O, Goulet O, Canioni D, Le Deist F, Radford I, Rieu D, et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med. 2001;344:1758–62. doi: 10.1056/NEJM200106073442304. [DOI] [PubMed] [Google Scholar]
- 38.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 39.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 40.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 42.Chang X, Gao JX, Jiang Q, Wen J, Seifers N, Su L, et al. The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J Exp Med. 2005;202:1141–51. doi: 10.1084/jem.20050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med. 2004;200:1083–9. doi: 10.1084/jem.20040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner U, Pierer M, Wahle M, Moritz F, Kaltenhauser S, Hantzschel H. Ex vivo homeostatic proliferation of CD4+ T cells in rheumatoid arthritis is dysregulated and driven by membrane-anchored TNFalpha. J Immunol. 2004;173:2825–33. doi: 10.4049/jimmunol.173.4.2825. [DOI] [PubMed] [Google Scholar]
- 45.Lawson BR, Koundouris SI, Barnhouse M, Dummer W, Baccala R, Kono DH, et al. The role of alpha beta+ T cells and homeostatic T cell proliferation in Y-chromosome-associated murine lupus. J Immunol. 2001;167:2354–60. doi: 10.4049/jimmunol.167.4.2354. [DOI] [PubMed] [Google Scholar]
- 46.MacMurray AJ, Kwitek AE, Rutledge EA, Van Yserloo B, Gohlke P, Speros SJ, et al. Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res. 2002;12:1029–39. doi: 10.1101/gr.412702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hornum L, Romer J, Markholst H. The diabetes-prone BB rat carries a frameshift mutation in Ian4, a positional candidate of Iddm1. Diabetes. 2002;51:1972–9. doi: 10.2337/diabetes.51.6.1972. [DOI] [PubMed] [Google Scholar]
- 48.Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and Its D. melanogaster homolog are required for miRNA biogenesis. Curr Biol. 2004;14:2162–7. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Rolston KV. Management of infections in the neutropenic patient. Annu Rev Med. 2004;55:519–26. doi: 10.1146/annurev.med.55.091902.103826. [DOI] [PubMed] [Google Scholar]
- 50.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 51.Elde NC, Malik HS. The evolutionary conundrum of pathogen mimicry. Nat Rev Microbiol. 2009;7:787–97. doi: 10.1038/nrmicro2222. [DOI] [PubMed] [Google Scholar]
- 52.Kountouras J, Deretzi G, Grigoriadis N, Zavos C, Boziki M, Gavalas E, et al. Guillain-Barre syndrome. Lancet Neurol. 2008;7:1080–1. doi: 10.1016/S1474-4422(08)70247-3. author reply 3–5. [DOI] [PubMed] [Google Scholar]
- 53.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185–96. doi: 10.1182/blood-2011-12-274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 55.Chen C, Liu Y, Zheng P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J Clin Invest. 2010;120:4091–101. doi: 10.1172/JCI43873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 57.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 58.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 59.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 60.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–92. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potter CJ, Huang H, Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–68. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 62.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–65. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 63.Potter CJ, Pedraza LG, Huang H, Xu T. The tuberous sclerosis complex (TSC) pathway and mechanism of size control. Biochem Soc Trans. 2003;31:584–6. doi: 10.1042/bst0310584. [DOI] [PubMed] [Google Scholar]
- 64.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 65.Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138:3343–56. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–81. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 67.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–32. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–15. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Q, Inoki K, Kim E, Guan KL. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci U S A. 2006;103:6811–6. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–82. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 72.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Ruemmele FM, Brousse N, Goulet O. Autoimmune enteropathy: molecular concepts. Curr Opin Gastroenterol. 2004;20:587–91. doi: 10.1097/00001574-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 76.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–45. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. doi: 10.3389/fimmu.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bindl L, Torgerson T, Perroni L, Youssef N, Ochs HD, Goulet O, et al. Successful use of the new immune-suppressor sirolimus in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) J Pediatr. 2005;147:256–9. doi: 10.1016/j.jpeds.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 79.Gambineri E, Perroni L, Passerini L, Bianchi L, Doglioni C, Meschi F, et al. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol. 2008;122:1105–12. e1. doi: 10.1016/j.jaci.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 80.Yong PL, Russo P, Sullivan KE. Use of sirolimus in IPEX and IPEX-like children. J Clin Immunol. 2008;28:581–7. doi: 10.1007/s10875-008-9196-1. [DOI] [PubMed] [Google Scholar]
- 81.Sofroniadou S, Goldsmith D. Mammalian target of rapamycin (mTOR) inhibitors: potential uses and a review of haematological adverse effects. Drug Saf. 2011;34:97–115. doi: 10.2165/11585040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 82.Fishbane S, Cohen DJ, Coyne DW, Djamali A, Singh AK, Wish JB. Posttransplant anemia: the role of sirolimus. Kidney Int. 2009;76:376–82. doi: 10.1038/ki.2009.231. [DOI] [PubMed] [Google Scholar]
- 83.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61:2340–8. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piemonti L, Maffi P, Monti L, Lampasona V, Perseghin G, Magistretti P, et al. Beta cell function during rapamycin monotherapy in long-term type 1 diabetes. Diabetologia. 2011;54:433–9. doi: 10.1007/s00125-010-1959-6. [DOI] [PubMed] [Google Scholar]
- 85.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 86.Hong JC, Kahan BD. Sirolimus-induced thrombocytopenia and leukopenia in renal transplant recipients: risk factors, incidence, progression, and management. Transplantation. 2000;69:2085–90. doi: 10.1097/00007890-200005270-00019. [DOI] [PubMed] [Google Scholar]
- 87.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983–8. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ngalamika O, Zhang Y, Yin H, Zhao M, Gershwin ME, Lu Q. Epigenetics, autoimmunity and hematologic malignancies: A comprehensive review. J Autoimmun. 2012;39:451–65. doi: 10.1016/j.jaut.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Selmi C, Leung PS, Sherr DH, Diaz M, Nyland JF, Monestier M, et al. Mechanisms of environmental influence on human autoimmunity: A national institute of environmental health sciences expert panel workshop. J Autoimmun. 2012;39:272–84. doi: 10.1016/j.jaut.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 90.Selmi C, Lu Q, Humble MC. Heritability versus the role of the environment in autoimmunity. J Autoimmun. 2012;39:249–52. doi: 10.1016/j.jaut.2012.07.011. [DOI] [PubMed] [Google Scholar]