Abstract
Schizophrenia has been associated with low glutathione (GSH), one of the most important substrates for natural defense against oxidative stress. This abnormality is often attributed to genetic or other pathological causes. However, low GSH in schizophrenia could also be due to insufficient antioxidant consumption or other exogenous factors. We evaluated GSH in relation to diet, smoking, and medication status in schizophrenia patients. We recruited 54 participants (29 schizophrenia patients and 25 normal controls). The Antioxidant Dietary Source Questions was used to estimate the total antioxidant capacity (TAC) from participants’ diet. GSH and the oxidized form of glutathione (GSSG) were assayed. We found that GSH was significantly lower (p < 0.001) while %GSSG was 2 to 5 fold higher (p = 0.023) in patients compared with controls. No evidence for lower TAC dietary intake was found in schizophrenia patients compared with controls; rather nominally higher TAC level was found in the patients diet (p=0.02). Analysis of consumption of individual food categories also failed to find evidence of reduced dietary antioxidant intake in schizophrenia patients. Smoking and medications did not significantly predict the GSH deficit either. However, there was a significant smoking by diagnosis interaction on GSH (p=0.026) such that smoking was associated with higher GSH level in controls while smoking in patients was not associated with this effect. Schizophrenia patients may have an impaired upregulation of GSH synthesis that normally occurs due to smoking-induced antioxidative response. Lower GSH was independently present in patients on clozapine (p = 0.005) and patients on other antipsychotics (p < 0.001) compared with controls. In conclusion, none of the exogenous sources played a major role in explaining abnormalities in the glutathione pathway in patients. The state of abnormal glutathione redox may therefore be a part of schizophrenia pathophysiology.
Keywords: Antioxidant, food, smoking, antipsychotic, glutathione, GSH, GSSG
1. INTRODUCTION
Schizophrenia has been associated with increased oxidative stress (Do et al., 2009; Kano et al., 2012). In aerobic cells, reactive oxygen species (ROS) and free radicals are byproducts of oxidative metabolism. The defense against oxidative stress is complex, involving multiple enzymes and antioxidant compounds. One of the major mechanisms for sequestration of ROS and free radicals is through the glutathione pathway where the reduced form of glutathione (monomeric glutathione or GSH) is converted to its oxidized form (glutathione disulfide or GSSG). Glutathione is present mainly (~99%) in GSH form in the body (Lenton et al., 1999). Although there are multiple defense systems and several of them have shown to be abnormal in schizophrenia, low GSH concentration is perhaps among the most consistently reported in schizophrenia. Low GSH has been found in post-mortem brain samples (Yao et al., 2006; Gawryluk et al., 2011) and in-vivo magnetic resonance spectroscopy studies (Do et al., 2000; Wood et al., 2009). GSH levels were decreased in the blood of antipsychotics-free and antipsychotics-treated patients (Raffaet al., 2009) and in cerebrospinal fluid of drug-naive patients (Do et al., 2000) suggesting that it is not secondary to antipsychotics. Taken together, low GSH level in schizophrenia provides a good index of an abnormal redox state associated with this illness, which is relatively consistently observed and not attributed solely to treatment with antipsychotics. Administration of the glutathione precursor n-acetylcysteine (NAC) improved clinical symptoms in patients (Berk et al., 2008), further supporting the association of oxidative stress with schizophrenia. However, it is unclear whether the mechanism that produces such effects is a direct result of pathophysiology associated with schizophrenia, an indirect result of other related pathology, or arising from sources outside the primary disease process that may differ between patients and controls. Hence, we examined the potential role of three exogenous factors in mediating oxidative stress, as indexed by abnormal GSH and GSSG levels, in schizophrenia: diet, smoking and antipsychotics.
First, diet is an important source of variance in oxidative stress in the general population (Watsonet al., 2005; Jenkinson et al., 1999). We assessed whether there is a deficit in intake of antioxidants in food in schizophrenia, as dietary antioxidant contents provide the substrate and also regulate enzymes in the redox pathways (Bagchi et al., 1997; Huber et al.,2002). One prior study found no evidence of reduced antioxidant intake in schizophrenia (Strassnig et al., 2005). Another study showed that lower plasma antioxidant levels were not correlated with body mass (Reddy et al., 2003). However, to date no study has assessed whether dietary antioxidant intake affects the reported glutathione deficit in schizophrenia.
Second, smoking can contribute to oxidative stress by increasing lipid peroxidation (Solak et al., 2005). However, smoking also induces a potent antioxidant response and increases systemic production of glutathione (Gould et al 2011). One study found increases in antioxidant enzymes in schizophrenic patients who smoke (Zhang et al., 2007). More data are needed to clarify if smoking contributes to the decreased glutathione in schizophrenia.
Third, the oxidative profile of antipsychotics remains unclear; some studies show antioxidant properties (Parikh et al., 2003; Miljevic et al., 2010; Zhang et al., 2012; Stojković et al., 2012) or improvement of peripheral glutathione and antioxidant enzymes by antipsychotics (Raffa et al., 2009; Zhang et al., 2012). On the other hand, patients on clozapine have shown higher superoxide dismutase and lower glutathione peroxidase, which was interpreted as secondary to increased oxidative stress by clozapine (Miljevic et al., 2010). Hence, we tested if clozapine versus non-clozapine antipsychotics were correlated with glutathione abnormalities in schizophrenia.
2. METHODS
2.1. Participants
We recruited 54 participants (age range 18 – 62 years): 29 medicated patients and 25 normal controls, frequency-matched on age, gender and smoking status (Table 1). Patients were recruited from outpatient clinics of Maryland Psychiatric Research Center and neighboring community clinics. Controls were recruited using local media advertisements. Exclusion criteria included major medical and neurological illnesses, history of head injury with cognitive sequelae, mental retardation, substance dependence within the past 6 months or current alcohol or illicit drug abuse. Participants taking dietary supplements with antioxidant contents or drugs that inhibit beta-oxidation including acetaminophen and ibuprofen (Pessayre et al., 1999) on regular basis were excluded. The Structured Clinical Interview for DSM-IV (SCID) was administered to all subjects to obtain DSM-IV diagnoses. Controls were interviewed with SCID and had no DSM IV Axis I diagnosis and no family history of psychosis in 3 generations. Patients were clinically stable individuals with DSM-IV schizophrenia on antipsychotics. The dose of the antipsychotics was converted to chlorpromazine equivalent (CPZ). Among these patients, 3 were on first generation antipsychotic medications (CPZ of daily dose = 117.1 ± 23.4 mg) and the rest was on second generation antipsychotics: including 12 on clozapine (368.8 ±137.5 mg), 3 on aripiprazole (18.3 ± 10.4 mg), 3 on risperidone (6.7 ± 4.6 mg),2 on olanzapine (10.0 ± 7.1 mg), 2 on quetiapine (800.0 ± 282.8 mg) and the remaining on 2 or more antipsychotic medications. All subjects gave written informed consent in accordance with local Institutional Review Board guidelines.
Table 1.
Demographic, clinical characteristics, and dietary total antioxidant capacity (TAC) intake.
| Normal Controls (N = 25) | Schizophrenia Patients (N = 29) | Statistics | P values | |
|---|---|---|---|---|
| Age (years) | 38.76±13.721 | 41.10±13.81 | 0.009 | 0.55 |
| Sex (male:female) | 11:14 | 20:9 | 2.48 | 0.12 |
| Smoker:Nonsmoker | 7:18 | 8:21 | 0.00 | 1.00 |
| Education (year) | 13.4±1.6 | 12.1±2.0 | 5.77 | 0.020 |
| Age of illness onset (year) | n/a | 21.4±8.4 | n/a | n/a |
| Duration of illness (year) | n/a | 20.0±13.4 | n/a | n/a |
| BPRS score | n/a | 43.28±9.93 | n/a | n/a |
| UPSA-2 | 102.9±10.3 | 90.7±10.5 | 17.62 | <0.001 |
| Fasting duration (hours) | 12.18±2.81 | 12.48±3.64 | 0.11 | 0.74 |
| GSH (μmol/L) | 800.50±201.73 | 516.50±238.40 | 21.06 | <0.001 |
| GSSG (μmol/L) | 19.96±24.82 | 27.94±23.19 | 8.56 | 0.005 |
| %GSSG | 2.40±3.02 | 12.43±24.18 | 2.28 | 0.023 |
| TAC intake/week (μmol FRAP/week) | 12620.6 + 1046.2 | 15257.7 + 1800.0 | 1.48 | 0.23 |
Values are mean ± sd. BPRS: Brief Psychiatric Rating Scale total score. UPSA-2: the University of California San Diego Performance-based Skills Assessment, 2nd edition total score (the data for controls was from 23 subjects because 2 controls did not complete UPSA-2).
2.2. Measurement of GSH and GSSG
Participants were instructed to avoid strenuous physical exercise 24 hours before, to fast overnight and not to smoke in the morning on the day of the blood draw. Venous whole blood sample was drawn between 8:30AM to 10:30AM. The key step of sample preparation was to prevent GSH, the predominant form of glutathione, to be oxidized into GSSG (so that GSSG can be accurately measured). GSSG samples were mixed with a pyridine derivative thiol-scavenging reagent before freezing. This redox scavenger overcomes shortfalls of other methods associated with undesirable enzyme reactivity or slow rate reaction (Griffith, 1980; Güntherberg and Rost, 1966). The scavenging reagent is a proprietary product by Oxford Biomedical Research Inc., Rochester Hills. MI, USA. It is related to the M2VP (1-methyl-2-vinylpyridinium trifluoromethane-sulfonate) described in the literature (Tietze 1969), an approach with the advantage of complete scavenging of GSH in less than one minute, compared to other methods that could take 60 minutes to remove GSH in the sample during which time oxidation of GSH may occur, resulting in overestimation of the GSSG concentration. Scavenger-processed (for GSSG) and unprocessed (for GSH) whole blood samples were immediately stored at −80°C to avoid in vitro oxidation by room temperature and cellular lysation (blood draw occurred in the freezer room). At the end of the study, all samples were shipped using a secured dry ice container to Oxford Biomedicals, who performed GSH and GSSG assays in one batch blind to subject information. The reaction of GSH with Ellman’s reagent (5,5′-dithiobis-2-nitrobenzoic acid (DTNB)) gave rise to a product quantified spectrophotometrically at 412 nm. This reaction was used to measure the reduction of GSSG to GSH and its rate is proportional to GSH and GSSG concentrations (Iwasaki et al., 2009). The assay uses an eight-point standard curve for both total GSH and GSSG determinations. Given the predominant form is GSH (Lenton et al., 1999), GSSG is more meaningful when expressed as %GSSG [(GSSG/total glutathione) × 100], which was the primary measure for GSSG. Note that whole blood, not serum or plasma, GSH and GSSG were measured here.
2.3. Antioxidant Dietary Source Questions (ADS)
We developed the ADS Questions based on similar studies (Ellinger et al., 2011; Rietveld and Wiseman, 2003; Santarelli et al., 2010) by including types of food with antioxidant contents common in the dietary habits of people in the Northeast and Mid-Atlantic regions of the U.S., such as fruits, vegetables, nuts, and cereals, among others. Each nutrient was recorded as weekly average of portion sizes over the previous 6 months using a self-reporting format. Items and portion sizes were derived from USDA National Nutrient Database for Standard Reference and similar guides (e.g., http://usda.mannlib.cornell.edu/MannUsda/; http://www.ars.usda.gov/SP2UserFiles/; http://www.nutfruit.org/; Wu et al. 2004). Intake information was converted to total antioxidant capacity (TAC), calculated on the ferric reducing antioxidant power (FRAP) based on reference values from the standard food composition table (Carlsen et al., 2010). A summary for included food categories is in Table 2. The actual ADS Questionnaire and the ADS Conversion table are freely available to readers upon request.
Table 2.
Weekly total antioxidant capacity (TAC) of each dietary item or category, based on the Antioxidant Dietary Source Questions.
| Dietary Food Items | Total Antioxidant Capacity/week(μmol FRAP/week)
|
F | P | |
|---|---|---|---|---|
| Normal Controls (N=25) | Schizophrenia Patients (N=29) | |||
| Fruit | 2582.73 ± 469.881 | 3223.63±478.62 | 0.9 | 0.35 |
| Vegetable | 2711.62 ± 352.15 | 2515.71±304.14 | 0.18 | 0.67 |
| Wine | 212.5351± 58.30 | 215.94 ± 103.05 | 0.01 | 0.98 |
| Juice | 4606.69 ± 915.68 | 8362.29 ± 858.01 | 8.945 | 0.04* |
| Nut products | 2120.48 ± 513.29 | 2307.85 ± 597.07 | 0.06 | 0.82 |
| Dry fruit | 635.58 ± 170.39 | 934.67 ± 333.70 | 0.58 | 0.45 |
| Bread | 2334.96 ± 363.07 | 2265.72 ± 355.43 | 0.02 | 0.89 |
| Cereal | 900.57 ± 186.57 | 1368.37 ±221.83 | 2.51 | 0.12 |
| Baking chocolate | 704.49 ± 272.54 | 1735.19 ± 661.96 | 1.86 | 0.18 |
| Milk chocolate | 197.31 ± 46.18 | 283.50 ± 68.66 | 1.02 | 0.32 |
| Total | 22580.72 ± 2124.20 | 24307.89 ± 2204.54 | 5.78 | 0.02* |
Values are mean ± mean error (sd).
2.4. Clinical and functional assessments
To determine whether GSH and GSSG are related to clinical symptoms, we used the 20-item Brief Psychiatric Rating Scale (BPRS) total score to estimate overall symptom severity. To assess psychosocial functioning in all subjects, we used the University of California San Diego Performance-based Skills Assessment, 2nd edition (UPSA-2). UPSA-2 is a proxy measure of function that assesses simulated life and community skills in 5 areas (Patterson et al., 2001; Twamley et al., 2002; Harvey et al., 2007). The total score was used to estimate overall everyday functioning.
2.5. Statistical Analysis
ANOVA was used to assess patient-control differences on GSH, GSSG, and %GSSG. Univariate ANOVA was used to examine smoking status by group interaction on GSH and GSSG. Data normality was verified using the Kolmogorov-Smirnov goodness-of-fit test. Mann-Whitney U tests were used when data did not follow normal distribution. Correlations were performed using Spearman’s rank order tests. GSH and GSSG contributions to clinical symptom and function were assessed by linear regressions where a clinical assessment was the dependent variable and GSH and GSSG were predictors. GSSG instead of %GSSG was used in regression analysis for maximizing the independence of the two predictors. All tests were two-tailed.
3. RESULTS
3.1. GSH and %GSSG levels
Fasting whole blood GSH levels were significantly lower in patients compared with controls (p<0.001) (Figure 1A). Fasting GSSG levels were significantly higher in patients vs. controls: patients showed over 5-fold higher in %GSSG compared with controls (p=0.023) (Figure 1B). %GSSG in patients also showed large variance: three patients had %GSSG > 50%, largely because they had GSH levels close to zero. The difference remained significant (p=0.027) even upon removal of these extreme cases: patients still had over 2-fold higher %GSSG compared with controls (Figure 1C). GSH and GSSG were not significantly correlated in either controls (r=0.03, p=0.90) or patients (r=−0.09, p=0.66). Age was not significantly associated with blood GSH level in patients or controls (r=−0.21, p= 0.27 and r=−0.26, p= 0.21, respectively). Using age as covariate in comparisons of GSH between patients and controls, we found that age had a trend effect (F=3.17, p=0.08) but little effect on the difference in GSH between the two groups (F=10.68, p<0.001). We further divided the subjects into two groups: younger adults age 45 or below and older adults age above 45. We found that GSH remained significantly different between controls and patients in the younger age range (832.3±239.5 vs. 567.4±174.5, respectively, F=12.20, p=0.002) and in the older age range (760.0±140.9 vs. 453.9±294.5, respectively, F=9.91, p=0.005).
Figure 1.
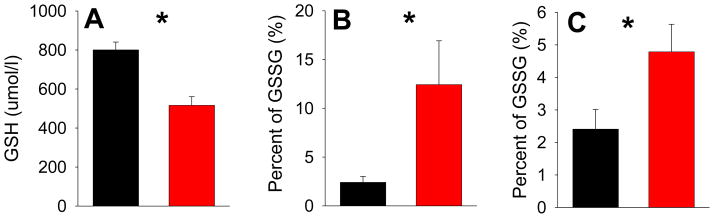
(A) The reduced form of glutathione (GSH) in schizophrenia patients (red bar) was significantly lower compared with controls (black bar) (* p<0.001). (B) Percent of the oxidized form of glutathione (GSSG) was significantly increased in schizophrenia patients compared with controls (* p=0.023). Note the large variance in the patient group, due to %GSSG over 50% in 3 patients. (C) However, even after removing the 3 patients with extremely high %GSSG, patients were still significant different from controls in %GSSG (* p=0.027).
3.2. Dietary total antioxidant capacity (TAC)
Based on ADS, average recent dietary TAC was not reduced in patients compared with controls. Rather, a nominally significantly higher TAC was observed in patients compared with controls (p=0.02) (Table 2). Of individual dietary items or categories, only fruit juice showed a nominally significant group difference such that patients obtained more TAC from fruit juice than controls (p=0.04). None of the comparisons were statistically significant after Bonferroni corrections for multiple comparisons. In controls, total dietary TAC demonstrated a positive but non-significant correlation with GSH (r=0.34, p=0.10) and a negative, but non-significant correlation with %GSSG (r=− 0.29, p=0.16). In patients, correlations between TAC and GSH (r=−0.10, p=0.61) and %GSSG (r=0.21, p=0.28) were also non-significant. These findings suggested that dietary TAC may contribute to more GSH and less GSSG in normal controls, albeit only at trend levels, and this relationship appeared more obscure in patients.
3.3. Smoking
There was a significant smoking by diagnosis interaction on GSH (p=0.026). There was no difference in GSH concentrations between smokers and non-smokers in the whole sample (651.5±329.8 vs. 646.6 ±236.6, respectively, p=0.93) or in patients (417.3±121.5 vs. 554.3±262.6, respectively, p=0.17). The difference approached significance in controls (smokers: 919.0 ±282.8 vs. nonsmokers: 754.4±145.6, p=0.06). The significant interaction was due to an opposite trend of high GSH in control smokers but lower GSH in patient smokers compared with the respective nonsmokers. There was no significant smoking x diagnosis interaction on %GSSG (p=0.38). %GSSG was not significantly different between smokers and nonsmokers in the combined sample (p=0.42), normal controls (p=0.62), or patients (p=0.38). The three patients with high %GSSG were all nonsmokers. Finally, there were no significant correlations between number of cigarettes smoked per day and GSH (r=0.11, p=0.74) or %GSSG (r=0.16, p=0.64).
3.4. Antipsychotic medications
Correlations between antipsychotic CPZ and GSH and GSSG were not significant (r=−0.26 and −0.25, respectively, n=29, all p > 0.40). Separating patients into clozapine vs. other antipsychotics subgroup, lower GSH was present independently in clozapine (559.2±238.3) (p = 0.005) and other antipsychotics subgroup (486.4±241.0) (p<0.001) compared with normal controls (800.5±201.7). Increased %GSSG was also present in both clozapine (10.6±23.6%) and other antipsychotics subgroups (13.7±25.3%) compared with controls (2.4±3.0%) although they were not statistically significant (p=0.051–0.20). Clozapine dose was not significantly correlated with GSH level (r=0.01, p=0.97), but significantly and inversely correlated with %GSSG level (r=−0.65, n=12, p=0.02) such that higher clozapine doses were associated with lower GSSG.
3.5. Clinical and functional implications
GSH and GSSG were not significantly correlated with age of psychosis illness onset (r=0.04, p=0.83 and r=0.20, p=0.32, respectively) or duration of illness (r=−0.18, p=0.36 and r=−0.10, p=0.62, respectively) in schizophrenia patients. In the regression analysis of GSH and GSSG contributions to BPRS total score in patients, the model was not significant (F(2, 26)=0.30, p=0.74). In the regression analysis of GSH and GSSG contributions to UPSA-2, the model was significant (F(2, 49)=4.36, ΔR2=15.1%, p=0.018) with tolerable colinearity statistics (VIF=1.02). GSH level significantly contributed to higher function (t=2.08, ΔR2=8.0%, p=0.045) while GSSG level significantly contributed to lower function (t=−2.22, ΔR2=7.1%, p=0.031).
4. DISCUSSION
This study tested the hypothesis that dietary antioxidant intake, smoking, and antipsychotic medication contribute to abnormal GSH and GSSG levels in schizophrenia. We found no evidence to support the hypothesis that these factors are the primary sources for the abnormal glutathione redox state in patients (Table 2).
Smoking is highly prevalent in schizophrenia (Cooper et al., 2012) although its potential role in the oxidative stress abnormalities in schizophrenia remains unclear. Interestingly, control smokers showed a trend of higher GSH compared with control nonsmokers, whereas the opposite trend was observed in patients. This may seem paradoxical. However, increased redox response to smoking has been observed in chronic obstructive pulmonary disorder patients (Gould et al., 2011). It is possible that in control subjects smoking activated the available redox response, leading to increase in GSH levels; while in patients smoking did not change or even led to a decrease in GSH levels, which would be consistent with their impaired upregulation of GSH synthesis under oxidative stress conditions as reported by Gysin et al (Gysin et al., 2007). Beyond that, we found no evidence that smoking accounted for the low GSH concentration in patients. Low GSH/high GSSG state in patients remained significantly different from controls even when smoking status was matched between patients and controls. Smoking severity was also not significantly correlated with GSH or GSSG levels.
We also found no evidence that GSH or GSSG were influenced by medication levels as measured by daily dose, but this does not rule out potential effect from chronic medication exposure. Lower GSH has been seen in treatment-naive or unmedicated patients (Raffa et al., 2011, 2009; Do et al., 2000), supporting that decreased GSH is present even without antipsychotics. We also showed that GSH was significantly decreased in patients regardless of clozapine use, ruling out a substantial impact of clozapine on the observed deficit. We found an inverse relationship between clozapine dose and concentration of oxidized glutathione (GSSG), which may complement previous findings of reduced glutathione peroxidase activity in clozapine-treated patients (Miljevic et al. 2010). Dose-dependent inhibition of glutathione peroxidase activity by clozapine may contribute to conflicting results regarding activity of this enzyme in schizophrenia vs. healthy controls (Dakhale et al., 2004; Kuloglu et al., 2002; Raffa et al., 2011; Ben Othmen et al., 2008; Ranjekar et al., 2003; Yao et al., 2006).
GSH and GSSG levels were significantly associated with everyday functioning as measured by UPSA-2, suggesting that 1) GSH is related to better functioning; 2) GSSG may have the opposite effect, and 3) UPSA-2 appears sensitive to glutathione redox state. UPSA-2 is a well-validated measure of psychosocial functioning (Patterson et al. 2001; Twamley et al. 2002; Harvey et al. 2007). If replicated by other studies, this would underscore the clinical relevance of oxidative stress abnormalities in schizophrenia by connecting a pathophysiological mechanism to real-world functioning. However, we did not find a significant correlation with BPRS. We speculate that perhaps BPRS is a measure that is more biased by various medications while UPSA is a trait-like measure of functional capacity and thus more likely to demonstrate significant correlations with GSH/GSSG. Further studies are necessary to more fully understand how oxidative stress can contribute to poor psychosocial functioning.
A growing body of evidence implicates oxidative stress as an important component of schizophrenia pathophysiology, with deficits in glutathione synthesis (Gysin et al., 2007), regeneration (Yao et al., 2006) and impaired antioxidant enzyme activity contributing to abnormal redox balance. Oxidative stress appears to be an important mechanism in schizophrenia neurodevelopmental models. Genetic factors and early environmental insults may create a state of redox dysregulation, with downstream effects on NMDA regulation, myelination, and functional connectivity between brain regions (Do et al., 2009). Indeed, developmental animal models converge in identifying cortical inhibitory interneurons as a cell type vulnerable to the effects of oxidative stress (Sullivan and O’Donnell, 2012).
The study is limited by its retrospective, recall-based design of the ADS; the precision of data on dietary antioxidant intake is likely biased by recall related errors, which could mask small but still significant relationships between diet and GSH/GSSG levels. The sample size here was small, which may in part also explain an insignificant correlation between GSH and dietary TAC (r=0.34, p=0.10). It is also possible that dietary TAC may have a more direct relationship with other antioxidant defense mechanisms that were not captured by the GSH/GSSG indices. Another limitation is that GSH was peripherally measured; the relationship between levels of GSH in the periphery and in brain tissue remains unclear. However, oral administration of glutathione precursor has shown to robustly enhance GSH level in brain tissue (Nehru et al., 2007), which indirectly supports a central-peripheral correlation because by definition, dietary supplements are peripheral. Lower total glutathione blood level also significantly predicts greater brain volume decrease in a two-year longitudinal study of first episode psychosis patients (Fraguas et al., 2012). Such evidence provides reasonable support that some associations are likely present between peripheral GSH and brain functions.
We replicated the finding of decreased GSH and elevated GSSG in schizophrenia. Our study supports that this state of redox dysregulation is likely to be intrinsic to the disorder. Evidence found in this study demonstrates the importance of this physiological index in terms of psychosocial functioning. Further study of impairments in antioxidant defenses should contribute to our understanding of the etiology of schizophrenia and identify targets for intervention.
Acknowledgments
Role of funding source
Support was received from National Institutes of Health grants R01MH085646, R01DA027680, and R21DA033817.
There are no further acknowledgements.
Footnotes
Contributors
Elliot Hong and Alejandro Ballesteros designed the study. Ann Summerfelt, Pan Jiang and Alejandro Ballesteros collected the data. Elliot Hong and Alejandro Ballesteros managed literature searches and data analyses, and wrote the first draft of the manuscript. All authorscontributed to interpretation of results, participated in critical revision of manuscript drafts and approved the final version.
Conflict of interest
All authors report no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95(2):179–89. [PubMed] [Google Scholar]
- Ben Othmen L, Mechri A, Fendri C, Bost M, Chazot G, Gaha L, Kerkeni A. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog Neuro-psychopharmacol Biol Psychiatry. 2008;32:155–159. doi: 10.1016/j.pnpbp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64(5):361–8. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I, Berhe N, Willett WC, Phillips KM, Jacobs DR, Jr, Blomhoff R. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3. doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J, Mancuso SG, Borland R, Slade T, Galletly C, Castle D. Tobacco smoking among people living with a psychotic illness: The second Australian survey of psychosis. Aust N Z J Psychiatry. 2012;46(9):851–63. doi: 10.1177/0004867412449876. [DOI] [PubMed] [Google Scholar]
- Dakhale G, Khanzode S, Khanzode S, Saoji A, Khobragade L, Turankar A. Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–209. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19(2):220–30. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuénod M. Schizophrenia glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12(10):3721–8. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Ellinger S, Müller N, Stehle P, Ulrich-Merzenich G. Consumption of green tea or green tea products: is there an evidence for antioxidant effects from controlled interventional studies? Phytomedicine. 2011;18(11):903–15. doi: 10.1016/j.phymed.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Fraguas D, Gonzalez-Pinto A, Micó JA, Reig S, Parellada M, Martínez-Cengotitabengoa M, Castro-Fornieles J, Rapado-Castro M, Baeza I, Janssen J, Desco M, Leza JC, Arango C. Decreased glutathione levels predict loss of brain volume in children and adolescents with first-episode psychosis in a two-year longitudinal study. Schizophr Res. 2012;137:58–65. doi: 10.1016/j.schres.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Fruit and Tree Nut Yearbook Spreadsheet Files 89022) of United States Department of Agriculture. [Accesed October 14 2012]; http://usda.mannlib.cornell.edu/MannUsda/viewStaticPage.do?url=http://usda01.library.cornell.edu/usda/ers/./89022/2010/index.html.
- Gawryluk JW, Wang JF, Andreazza AC, Shao L, Yatham LN, Young LT. Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. Int J Neuropsychopharmacol. 2011;14(8):1069–74. doi: 10.1017/S1461145711000617. [DOI] [PubMed] [Google Scholar]
- Gould NS, Min E, Gauthier S, Martin RJ, Day BJ. Lung glutathione adaptive responses to cigarette smoke exposure. Respir Res. 2011;12:133. doi: 10.1186/1465-9921-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–12. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Güntherberg H, Rost J. The true oxidized glutathione content of red blood cells obtained by new enzymic and paper chromatographic methods. Anal Biochem. 1966;15(2):205–10. doi: 10.1016/0003-2697(66)90025-x. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P, Tosic M, Werge T, Cuénod M, Do KQ. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104(42):16621–6. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Velligan DI, Bellack AS. Performance-based measures of functional skills: usefulness in clinical treatment studies. Schizophr Bull. 2007;33:1138–1148. doi: 10.1093/schbul/sbm040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber WW, Prustomersky S, Delbanco E, Uhl M, Scharf G, Turesky RJ, Thier R, Schulte-Hermann R. Enhancement of the chemoprotective enzymes glucuronosyl transferase and glutathione transferase in specific organs of the rat by the coffee components kahweol and cafestol. Arch Toxicol. 2002;76(4):209–17. doi: 10.1007/s00204-002-0322-1. [DOI] [PubMed] [Google Scholar]
- International Nut and Dried Fruit Foundation. [Accessed June 15, 2011]; http://www.nutfruit.org/es/consumidores.htm.
- Iwasaki Y, Saito Y, Nakano Y, Mochizuki K, Sakata O, Ito R, Saito K, Nakazawa H. Chromatographic and mass spectrometric analysis of glutathione in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(28):3309–17. doi: 10.1016/j.jchromb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Jenkinson A, Franklin MF, Wahle K, Duthie GG. Dietary intakes of polyunsaturated fatty acids and indices of oxidative stress in human volunteers. Eur J Clin Nutr. 1999;53(7):523–8. doi: 10.1038/sj.ejcn.1600783. [DOI] [PubMed] [Google Scholar]
- Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, Takayanagi Y, Lee Y, Rapoport J, Eaton W, Cascella N, Ji H, Goldman D, Sawa A. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.120. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–175. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- Lenton KJ, Therriault H, Wagner JR. Analysis of glutathione and glutathione disulfide in whole cells and mitochondria by postcolumn derivatization high-performance liquid chromatography with ortho-phthalaldehyde. Anal Biochem. 1999;274(1):125–30. doi: 10.1006/abio.1999.4258. [DOI] [PubMed] [Google Scholar]
- Miljevic C, Nikolic M, Nikolic-Kokic A, Jones DR, Niketic V, Lecic-Tosevski D, Spasic MB. Lipid status, antioxidant enzyme defence and haemoglobin content in the blood of long-term clozapine-treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:303–7. doi: 10.1016/j.pnpbp.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Nehru B, Kanwar SS. Modulation by N-acetylcysteine of lead-induced alterations in rat brain: reduced glutathione levels and morphology. Toxicol Mech Methods. 2007;17:289–93. doi: 10.1080/15376510601017769. [DOI] [PubMed] [Google Scholar]
- Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2003;37(1):43–51. doi: 10.1016/s0022-3956(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27(2):235–45. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Mansouri A, Haouzi D, Fromenty B. Hepatotoxicity due to mitochondrial dysfunction. Cell Biol Toxicol. 1999;15(6):367–73. doi: 10.1023/a:1007649815992. [DOI] [PubMed] [Google Scholar]
- Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naïve first-episode schizophrenic patients. BMC Psychiatry. 2011;11:124. doi: 10.1186/1471-244X-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(7):1178–83. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121(2):109–22. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- Reddy R, Keshavan M, Yao JK. Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr Res. 2003;62(3):205–12. doi: 10.1016/s0920-9964(02)00407-3. [DOI] [PubMed] [Google Scholar]
- Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J Nutr. 2003;133(10):3285S–3292S. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- Santarelli RL, Vendeuvre JL, Naud N, Taché S, Guéraud F, Viau M, Genot C, Corpet DE, Pierre FH. Meat processing and colon carcinogenesis: cooked, nitrite-treated, and oxidized high-heme cured meat promotes mucin-depleted foci in rats. Cancer Prev Res (Phila) 2010;3(7):852–64. doi: 10.1158/1940-6207.CAPR-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solak ZA, Kabaroglu C, Cok G, Parildar Z, Bayindir U, Ozmen D, Bayindir O. Effect of different levels of cigarette smoking on lipid peroxidation, glutathione enzymes and paraoxanase activity in healthy people. ClinExp Med. 2005;5 (3):99–105. doi: 10.1007/s10238-005-0072-5. [DOI] [PubMed] [Google Scholar]
- Stojković T, Radonjić NV, Velimirović M, Jevtić G, Popović V, Doknić M, Petronijević ND. Risperidone reverses phencyclidine induced decrease in glutathione levels and alterations of antioxidant defense in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):192–9. doi: 10.1016/j.pnpbp.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Singh Brar J, Ganguli R. Dietary fatty acid and antioxidant intake in community-dwelling patients suffering from schizophrenia. Schizophr Res. 2005;76(2–3):343–51. doi: 10.1016/j.schres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EM, O’Donnell P. Inhibitory interneurons, oxidative stress, and schizophrenia. Schizophrenia Bulletin. 2012;38(3):373–6. doi: 10.1093/schbul/sbs052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, Patterson TL, Jeste DV. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry. 2002;159(12):2013–20. doi: 10.1176/appi.ajp.159.12.2013. [DOI] [PubMed] [Google Scholar]
- [Accesed June 15, 2011];USDA National Nutrient Database for Standard Reference, Release 23. http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/SR23/nutrlist/sr23a255.pdf.
- [Accesed in 14th October 2012];USDA National Nutrient Database for Standard Reference. http://usda.mannlib.cornell.edu/MannUsda/viewStaticPage.do?url=http://usda01.library.cornell.edu/usda/ers/./89022/2010/index.html.
- Watson TA, Callister R, Taylor RD, Sibbritt DW, MacDonald-Wicks LK, Garg ML. Antioxidant restriction and oxidative stress in short-duration exhaustive exercise. Med Sci Sports Exerc. 2005;37(1):63–71. doi: 10.1249/01.mss.0000150016.46508.a1. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Wellard RM, Proffitt TM, McConchie M, Berk M, McGorry PD, Pantelis C. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis. 2009;33(3):354–7. doi: 10.1016/j.nbd.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52(12):4026–37. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22(1–2):83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Zhou DF, Haile CN, Wu GY, Cao LY, Kosten TA, Kosten TR. Nicotine dependence, symptoms and oxidative stress in male patients with schizophrenia. Neuropsychopharmacology. 2007;32(9):2020–4. doi: 10.1038/sj.npp.1301317. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Shen YC, Zhang PY, Liang J, Chenda C, Xiu MH, Kosten TA, Kosten TR. Effects of risperidone and haloperidol on superoxide dismutase and nitric oxide in schizophrenia. Neuropharmacology. 2012;62(5–6):1928–34. doi: 10.1016/j.neuropharm.2011.12.014. [DOI] [PubMed] [Google Scholar]


