Abstract
Cognitive dysfunction, including significant impairments in learning, behavior, and attention, is found in over 10% of children in the general population. However, in the common inherited cancer predisposition syndrome, Neurofibromatosis type 1 (NF1), the prevalence of these cognitive deficits approaches 70%. As a monogenic disorder, NF1 provides a unique genetic tool to identify and mechanistically dissect the molecular and cellular bases underlying cognitive dysfunction. In this review, we discuss Nf1 fly and mouse systems that mimic many of the cognitive abnormalities seen in children with NF1. Further, we describe discoveries from these models that have uncovered defects in the regulation of Ras activity, cAMP generation, and dopamine homeostasis as key mechanisms important for cognitive dysfunction in children with NF1.
Keywords: neurofibromin, cyclic AMP, dopamine, NF1, learning, attention deficits
Introduction
Neurofibromatosis type 1 (NF1) is one of the most common neurogenetic disorders affecting the nervous system. The hallmark of NF1 is the development of tumors involving the central and peripheral nervous systems. In this condition, affected individuals are prone to the formation of peripheral nerve sheath tumors (ie. neurofibromas, plexiform neurofibromas, and malignant peripheral nerve sheath tumors) and brain tumors (optic pathway gliomas and malignant gliomas). Moreover, 50–70% of children with NF1 manifest specific cognitive impairments, including difficulties with attention, executive function, language, visual perception and learning [1–4]. While most children exhibit some form of cognitive deficit that negatively impacts their scholastic performance, the specific cognitive abnormality present (ie. attention deficit, spatial memory impairment, fine motor delay) and the severity of the deficit varies greatly from child to child.
In concert with clinical studies characterizing the spectrum of learning, behavioral and motor delays in children with NF1, laboratory investigations have begun to define the molecular and cellular etiologies for these common problems. Using Nf1 genetically-engineered strains of mice and flies, investigators have successfully modeled many of the cognitive and behavioral deficits seen in children with NF1, and employed these model systems to better define the role of the NF1 protein (neurofibromin) in normal central nervous system (CNS) neuronal function. This review will highlight the basic neurobiological insights that have derived from the use of these robust preclinical strains as well as their importance for the identification and validation of new therapeutic drug targets relevant to the treatment of children with NF1.
Clinical features of NF1
Neurofibromatosis-1 (NF1) is a common nervous system disorder, affecting 1 in 3500 people globally [5]. NF1 is inherited in an autosomal dominant manner, although ~50% of patients present with de novo mutations, and represent the first member of their family with NF1 [6]. While NF1 genetic testing is available for select individuals, the diagnosis of NF1 is most often established on clinical grounds (Table 1). To be given the diagnosis of NF1, individuals must have at least two features of the condition, including greater than 6 café-au-lait macules (birthmarks), skinfold (underarm or groin) freckling, Lisch nodules (iris hamartomas), neurofibromas, an optic pathway glioma, a distinctive bone abnormality (tibial dysplasia), or a first degree relative with NF1 [7]. In addition to these features, individuals with NF1 may also manifest learning/behavioral problems, malignant gliomas, T2-hyperintensities on neuroimaging (ie. magnetic resonance imaging) studies, enlarged heads (macrocephaly), gross and fine motor delays, short stature, and other less common cancers [8–13].
Table 1.
NF1 Clinical Manifestations
| Clinical Feature | Diagnostic criteria (Fulfillment of ≥2) | Prevalence [114, 115] (% of NF1 population) |
|---|---|---|
| Family history | 50% | |
| Neurocutaneous | ||
| Café-au-lait spots | 6 or more | 99% in adults |
| Axillary/inguinal freckling | 2 or more | 85% |
| Lisch nodules | 2 or more | 95% in adults |
| Neurofibromas | 2 or more | 99% in adults |
| Plexiform neurofibromas | 1 or more | 20–45% |
| Skeletal | ||
| Scoliosis | ND | 5–10% |
| Bone dysplasia | 1–5% | |
| Pseudarthrosis | 2% | |
| Short stature | ND | 30% |
| Macrocephaly | ND | 45% |
| Tumors | ||
| Optic pathway glioma | 15% | |
| Malignant peripheral nerve sheath tumor | ND | 2–5% |
| Low-grade glioma | ND | 2–3% |
| Malignant glioma | ND | 1–2% |
Note: ND=non-diagnostic; denotes NF1 clinical features associated with disease, but are not assessed for diagnosis
Cognitive and behavioral deficits in children with NF1
Cognitive problems are the most frequently observed neurological impairments in children with NF1. The majority of children display some degree of cognitive deficits [1], which limit their full academic achievement and overall quality of life. Clinical studies examining cognitive problems in NF1 have revealed a left shift in average IQ, ranging from low-to-normal IQs, with specific learning deficits observed in 30–70% of children [1, 14, 15]. Additionally, children with NF1 exhibit poor performance on tasks of reading, spelling and mathematics, impaired expressive and receptive language skills, deficits in visuospatial and visuoperceptual skills, and defects in executive function (planning and concept formation) [1, 16, 17]. While less common, there is also an increased incidence of autistic spectrum disorder in children with NF1 [18]. Problems with attention and behavior in children with NF1 can also negatively impact school performance and social interactions [19–21]. Nearly 70% of children with NF1 report deficits in one or more of the attention system domains (sustained, selective, divided and shifting attention) [1, 22, 23], and one-third to one-half of children are diagnosed with attention-deficit hyperactivity disorder (ADHD) [1, 2]. Moreover, children with NF1 tend to be impulsive, and often have difficulty detecting and responding to social cues.
Neurofibromin structure and function
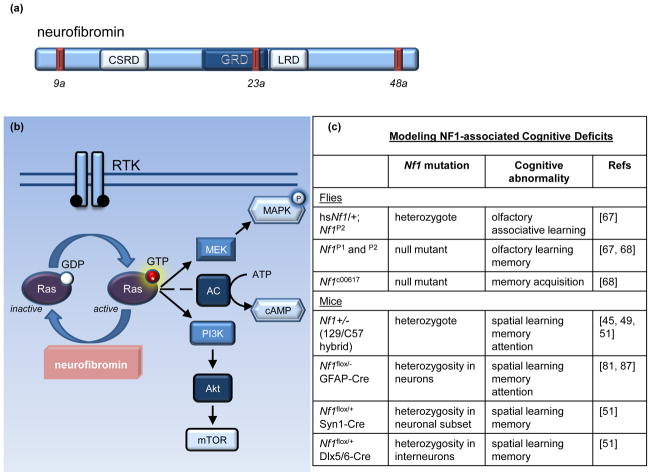
The NF1 gene resides on chromosome 17 [5, 24] and encodes a large cytoplasmic protein (neurofibromin), encompassing 2818 amino acids and over 60 exons. Neurofibromin contains several domains, including a cysteine-rich domain (CSRD), a leucine repeat domain (LRD), and a Ras-GAP domain (GRD) (Figure 1a). The NF1 gene also contains at least 3 alternatively spliced exons, 9a, 23a, and 48a, each with unique properties. Exon 9a-containing neurofibromin is a neuron-specific isoform [25, 26], whereas exon 48a-containing neurofibromin is expressed in muscle [27]. Exon 23a neurofibromin interrupts the normal function of the GAP domain, but has a more widespread tissue distribution [28–30]. Interestingly, mice engineered to lack exon 23a do not have an increased tumor predisposition, but manifest specific learning impairments [28]. Current studies are focused on defining the functional consequences of NF1 alternative splicing, especially exon 9a, on neurofibromin signaling and NF1-associated tumor and cognitive deficits.
Figure 1. Neurofibromin structure and function.
(a) Neurofibromin is a 2818 amino acid protein that contains multiple alternatively spliced exons (9a, 23a, and 48a shown as red bars) and encodes several distinct functional domains, including a cysteine-rich domain (CSRD), a Ras-GAP domain (GRD), and a leucine repeat domain (LRD) [5, 24–30]. (b) Neurofibromin serves as a negative regulator of Ras by accelerating the hydrolysis of the GTP-bound active Ras, producing inactive GDP-bound Ras [31–34]. Upon receptor tyrosine kinase (RTK) activation, Ras guanosine exchange (GDP to GTP) promotes Ras activity. Activated Ras, in turn, stimulates its downstream effectors, including MEK/MAPK and PI3K/Akt/mTOR. Ras can also positively regulate adenylate cyclase (AC) activity in some cell types. (c) Current fly and mouse models of NF1-associated learning and attention abnormalities. Abbreviations: GFAP, glial fibrillary acidic protein; Syn1, synapsin 1; Dlx5/6, distal-less homeobox 5 and 6.
The neurofibromin GRD functions in a similar fashion to other GTPase-activating proteins (GAPs), which negatively regulate the activity of the p21-Ras proto-oncogene (Figure 1b). Ras is recruited to the plasma membrane by adaptor proteins and activated by receptor tyrosine kinases (RTKs) following growth factor binding. At the membrane, guanine nucleotide exchange factors (GEFs) enable Ras to bind guanosine triphosphate (GTP) to become active and transmit growth-promoting signals. In its active state, Ras signals to several downstream effectors, including the mitogen-activated protein kinase (MAPK) and phosphoinositide 3 kinase (PI3K)/mammalian target of rapamycin (mTOR) signaling pathways. Neurofibromin acts as an inhibitor of Ras activity by catalyzing the hydrolysis of active GTP-bound Ras to inactive GDP-bound Ras, such that in cells lacking neurofibromin, there are high levels of Ras pathway (ie. MEK/MAPK and PI3K/mTOR) activity, and increased cell growth [31–34].
In addition to Ras regulation, neurofibromin is also a positive regulator of intracellular cyclic AMP (cAMP) levels. Studies initially performed in Nf1 mutant flies revealed that Drosophila larval size did not result from de-regulated Ras signaling, but rather was dependent on cAMP-mediated Protein Kinase A (PKA) activity [35, 36]. Subsequent studies in mice likewise demonstrated that both Nf1-deficient embryonic brains and postnatal astrocyte cultures had reduced cAMP levels [37, 38]. While the precise mechanism(s) underlying neurofibromin control of cAMP homeostasis has yet to be fully elucidated, there appear to be both Ras-dependent and Ras-independent modes of regulation [36, 37, 39].
Nf1 Models of Learning, Memory, and Attention Deficits
Inspection of the predicted protein sequence of the Drosophila and murine Nf1 homologs reveals 60% and greater than 98% identity to the human NF1 gene product, respectively, supporting the use of Nf1 mutant mouse and fly models to study many of the cognitive and behavioral features seen in the human condition [35, 40, 41]. In this regard, robust mouse and fly models have been developed and employed for preclinical discovery and validation initiatives aimed at improving clinical outcomes for children and adults with NF1 (Figure 1c).
The Role of Ras Activation in Learning and Memory
Studies pioneered in the late 1990s by Alcino Silva and colleagues employed mice harboring a germline inactivating mutation in one Nf1 allele. These Nf1 heterozygous (Nf1+/−) mice are genetically similar to children with NF1 who start life with one functional and one non-functional copy of the NF1 gene in every cell. Nf1+/− mice exhibit impaired spatial learning in the Morris water maze, a behavioral task used to assess hippocampal-based learning [42, 43]. These learning defects improve with training time, supporting clinical observations that children with NF1 can learn with additional focused training and resources [3]. Concomitant with defects in spatial learning, Nf1+/− mice also have decreased CA1 hippocampal long-term potentiation (LTP) following theta-burst stimulation [43]. Further characterization of this LTP deficit revealed abnormalities in both early-phase and long-term LTP maintenance [43, 44]. Whereas early-phase LTP is thought to underlie immediate learning, late-phase LTP regulates long-term memory formation [45]. In this manner, abnormalities in both early-phase and late-phase LTP could contribute to the learning and memory deficits in Nf1+/− mice.
To better define the connection between reduced neurofibromin and impaired hippocampal function, Silva and colleagues explored the possibility that increased Ras activity was responsible for the observed cognitive deficits [43]. Their demonstration of increased Ras and MEK activity in the brains of Nf1+/− mice prompted genetic rescue experiments, where Nf1+/− mice were crossed with mice to reduce Ras (K-Ras and N-Ras) expression (Nf1+/−;KRas+/− or Nf1+/−;NRas+/− mice). In these studies, performance of Nf1+/− mice with either reduced K-Ras or N-Ras expression was comparable to that found in wild-type control mice [43]. Similarly, pharmacological inhibition of Ras using a drug that blocks the post-translational farnesylation of Ras (ie. Lovastatin), improved performance of Nf1+/− mice on hippocampal-based spatial learning tests [43, 46]. Collectively, these findings provide strong evidence for a functional link between reduced brain neurofibromin expression, elevated Ras activity and cognitive deficits, and prompted clinical trials using Lovastatin-like treatments for children with NF1 [47].
The cellular and neurochemical bases for impaired LTP and learning in Nf1+/− mice have also been investigated. Using Nf1 conditional knockout (CKO) mice in which one copy of the Nf1 gene was selectively inactivated in neurons or astrocytes, the consequence of reduced neurofibromin expression in distinct cell populations on mouse learning and memory was evaluated [48]. While reduced Nf1 expression in neurons recapitulated the learning and memory deficits observed in Nf1+/− mice, it had no effect in Glial fibrillary acidic protein (GFAP) positive astrocytes. Neuron subpopulation-specific Cre driver lines have also been employed to address learning deficits after selective inactivation of Nf1. Specifically, reduced Nf1 expression in excitatory and inhibitory neurons (Synapsin-I-Cre mice) or GABA-containing neurons [Distal-less Homeobox 5/6 (Dlx5/6)-Cre mice], but not forebrain pyramidal neurons [ie. Ca 2+/Calmodulin-Dependent Protein Kinase II (CAMKII)-Cre mice], resulted in defects in learning and memory similar to that observed in Nf1+/− mice. These observations demonstrate a primary neuronal defect as a responsible cellular etiology underlying murine Nf1 learning and memory dysfunction.
Based on the finding that inhibitory neurons were sensitive to the effects of reduced Nf1 gene expression, it was subsequently shown that Nf1+/− mice had increased hippocampal GABA inhibitory tone. In these studies, elevated MAPK-mediated synapsin-I phosphorylation and increased GABA release in hippocampal inhibitory interneurons were shown to cause impaired Nf1+/− mouse LTP and learning [43, 48]. Consistent with this model, treating Nf1 mutant mice with a GABAA receptor antagonist (ie. picrotoxin) restored normal LTP in the hippocampus [43]. These findings established a direct relationship between Ras regulation and GABAergic signaling relevant to the Nf1 learning deficits.
The notion that de-regulated Ras activation can cause cognition dysfunction is further underscored by several human disorders caused by genetic mutations that lead to increased Ras signaling. For example, patients with Legius syndrome, a NF1-like disorder that results from inactivating mutations in the SPRED1 Ras-MEK/MAPK regulator, exhibit mild neurocognitive deficits [49–51]. Similar to Nf1+/− mice, defective spred1 function in mice resulted in increased MAPK signaling [49, 52, 53], in addition to deficits in learning and memory [54]. Costello syndrome is a Ras disorder (“Ras-opathy”) caused by an activating mutation in the HRAS gene, leading to constitutive Ras signaling [55]. Children with Costello syndrome often have cognitive impairments, low IQs, and increased anxiety [56]. Similar to their human counterparts, HRasG12V knock-in mice exhibit increased anxiety behaviors and cognitive impairments [57]. Lastly, in additional models of aberrant Ras activity, pharmacological inhibition of PI3K or the MAPK kinase MEK was effective at restoring normal synaptic plasticity and learning in rodents [58–61]. Taken together, these studies demonstrate that inappropriate regulation of Ras activity can disrupt cognitive function, providing a preclinical rationale for Ras pharmacological treatments for NF1-associated cognitive deficits.
The Role of cAMP in Learning and Memory
In Drosophila, neurofibromin modulates cAMP by activating the adenylyl cyclase 1 (AC1) homolog, rutabaga (rut) [35, 36]. While the exact mechanism remains to be elucidated, activation of rut-AC is initiated by an interaction with the C-terminus of neurofibromin [39]. Further, this interaction is essential for learning and memory in flies [62, 63]. Using a classic Pavlovian olfactory behavioral paradigm, flies lacking Nf1 or rut-AC show reduced performance, indicative of learning and memory impairments [62–66]. Restoration of neurofibromin expression using dNf1 heat-shock transgenes or correction of the cAMP defect by ectopic protein kinase A (PKA) expression restored normal learning and memory in Nf1 mutant flies [62]. Recent studies have revealed that distinct domains of Drosophila neurofibromin can modulate different components of learning and memory. Whereas mutations in the GAP domain cause defects in long-term memory, the cAMP-activating C-terminal region of neurofibromin mediates immediate memory or learning [45]. A direct link between cAMP homeostasis and cognitive defects has not been firmly established in Nf1 mouse models, yet support for a role of cAMP in learning and memory derives from studies in other rodent models. In these reports, pharmacological and genetic manipulation of cAMP levels enhanced LTP, learning, memory, and hippocampal neurogenesis [67–71].
In Nf1 mutant mice, some of the defects observed in CNS neurons were found to be dependent on cyclic AMP signaling. Following complete Nf1 loss in neural stem cells (NSCs), reduced secondary somatosensory cortex thickness was observed [72]. Further examination of these Nf1 CKO (ie. Nf1BLBPCKO) mice revealed that this reduction in cortical thickness resulted from decreased neuronal arborization, rather than from reductions in the total number of neurons. Using in vitro methods, it was demonstrated that neurons differentiating from Nf1-deficient NSCs also had shortened neuronal processes [72]. The biochemical basis for this defect in neuronal morphology was shown to result from impaired cAMP generation, as both Nf1BLBPCKO mouse brains and Nf1+/− primary neurons had lower cAMP levels in vitro and in vivo [72–74]. Re-expression of the Ras-regulatory NF1-GAP domain in Nf1BLBPCKO mice did not correct the cortical thickness deficit. Similarly, expression of an activated K-Ras allele in NSCs both in vitro and in vivo did not alter neurite length. Instead, treatment of Nf1BLBPCKO mice with pharmacological agents that increase cAMP levels restored normal cortical thickness in vivo and neurite lengths in primary Nf1+/− neurons in vitro [72–74].
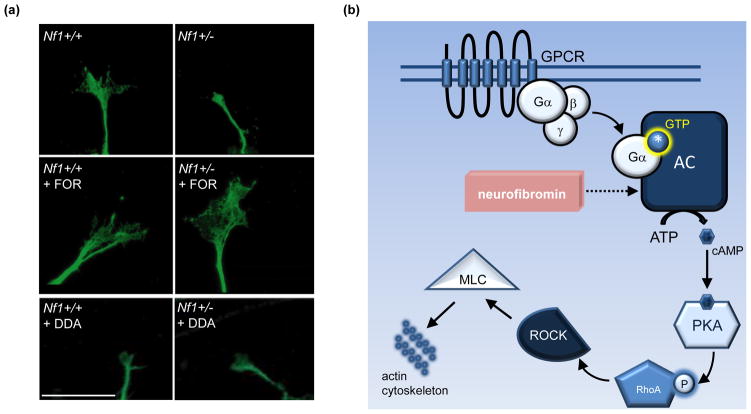
Since the brains of children with NF1 are composed of neurons with reduced, not absent, NF1 gene expression, subsequent studies have employed Nf1+/− CNS neurons as a relevant model (Figure 2a). Similar to Nf1-deficient neurons, Nf1+/− CNS neurons exhibited 25% and 40% reductions in neurite (axonal) length and growth cone area, respectively [72, 73]. These abnormalities resulted from impaired cAMP generation, and were corrected by treatments that elevated intracellular cAMP levels, including Rolipram (a phosphodiesterase-4 inhibitor), non-hydrolyzable cAMP analogs, and adenylyl cyclase (AC) activators (eg. Forskolin). Conversely, blocking cAMP production, using 2,3 dideoxyadenosine (DDA) to inhibit adenylyl cyclase function, was sufficient to abrogate neurite extension in wild-type neurons [73, 74]. Lastly, neurofibromin regulation of actin cytoskeletal dynamics was found to be dependent on cAMP-mediated PKA activation of Rho/ROCK/MLC signaling [74], such that correcting the defective signaling conferred by any component along this pathway normalized Nf1+/− axon lengths and growth cone areas in vitro (Figure 2b).
Figure 2. Neurofibromin regulation of neuronal morphology is cAMP-mediated.
(a) Neuronal morphology (growth cone areas and neurite length) is attenuated by reduced neurofibromin expression and cAMP levels. Top panel: Primary cultured Nf1+/− hippocampal neurons have decreased neurite lengths (not shown) and growth cone areas as a result of reduced cAMP generation [73]. Middle panel: Stimulating adenylyl cyclase (AC) with Forskolin (FOR) elevates cAMP levels and rescues growth cone areas in Nf1+/− hippocampal neurons. Bottom panel: Decreasing cAMP levels in wild-type neurons by blocking adenylyl cyclase activity with the AC inhibitor 2,3-dideoxyadenosine (DDA) blunts growth cone areas, comparably to Nf1+/− neurons. Scale bar = 20μm. Adapted, with permission, from [73]. (b) Schematic diagram illustrating mechanism of cAMP-mediated morphological changes. In neurons, low cAMP generation due to reduced Nf1 expression, in turn, leads to decreased PKA activation, RhoA/ROCK activity, and MLC phosphorylation. These changes contribute to impaired actin cytoskeletal dynamics, resulting in smaller growth cones and shortened axons [74]. Abbreviations: GPCR, G protein-coupled receptor; MLC, myosin light chain; PKA, protein kinase A; ROCK, Rho-associated protein kinase.
Further examination of these abnormal Nf1+/− CNS neurons also revealed small increases in programmed cell death. In studies examining the impact of reduced neurofibromin expression on retinal ganglion cells (RGCs), optic glioma formation in Nf1 mutant strains was associated with increased RGC apoptosis [73, 76]. As observed in vitro, treatment of Nf1 optic glioma-bearing mice with Rolipram (to raise intracellular cAMP levels) almost completely ameliorated the increased RGC programmed cell death in vivo [73], underscoring the importance of neurofibromin-mediated cAMP homeostasis in the maintenance of normal CNS neuronal function. Interestingly, CNS neurons with reduced Nf1 expression had similar levels of cAMP as neurons completely lacking neurofibromin expression, emphasizing the profound effect of Nf1 heterozygosity on CNS neuronal morphology and function [74]. Further studies using hippocampal neurons demonstrated a requirement of neurofibromin for dendritic spine formation, such that Nf1 knockdown reduced spine formation in mature neurons in vitro [77]. Correspondingly, dendritic spine densities were also reduced in the brains of Nf1+/− mice [78]. Together, altered dendritic spine density, axonal morphological defects, and reduced neuronal survival may lead to impaired synaptic efficacy and reduced cognitive function in children with NF1.
The Role of Dopamine Homeostasis in Learning, Memory, and Attention
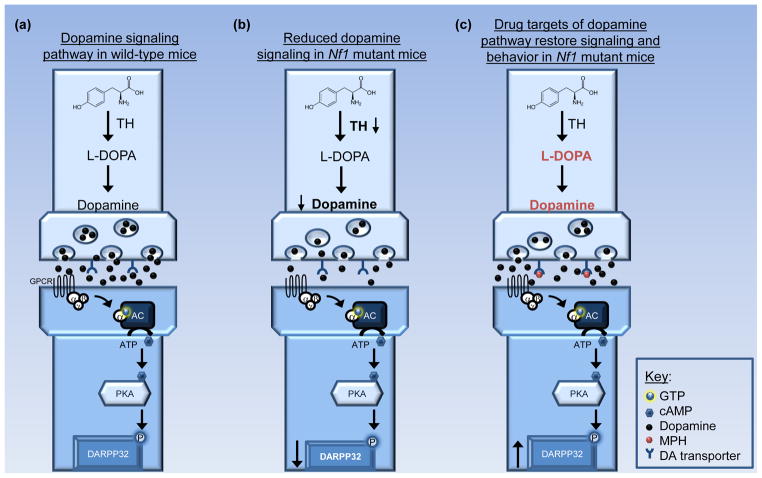
Due to the high prevalence of attention problems in children with NF1, stimulant medications, such as methylphenidate (MPH), have proven to be effective treatments in this affected population [2]. MPH restores dopamine homeostasis by blocking dopamine reuptake through the DA transporter (DAT), allowing for increased extracellular dopamine availability in dopaminergic neurons [79, 80]. While brain dopaminergic defects have yet to be reported in children with NF1, impaired dopamine homeostasis has been shown in an Nf1 genetically-engineered mouse strain [81]. In these studies, Nf1+/− mice with total Nf1 loss in GFAP+ astroglial cells (ie. Nf1+/−GFAPCKO mice) were used. Similar to Nf1+/− mice, Nf1+/−GFAPCKO mice had reduced exploratory behaviors and performed poorly on tests of selective and non-selective attention. Moreover, there were reduced dopamine levels and reduced post-synaptic dopamine signaling in the striatum, as measured by decreased dopamine- and cAMP-regulated phosphoprotein- 32 (DARPP32) phosphorylation. In addition, cell-autonomous decreases in the growth cone areas and neurite lengths of dopaminergic neurons in vitro were observed, resulting in attenuated cell projections to the striatum in vivo. While post-synaptic dopamine receptor expression was normal, presynaptic dopamine transporters [ie. DAT and vesicular monoamine transporter (VMAT)] and tyrosine hydroxylase (TH) expression were significantly reduced in Nf1+/−GFAPCKO mice [81] (Figure 3). To provide a preclinical measure of reduced striatal dopamine levels, positron emission tomography (PET) imaging with 11C-raclopride demonstrated increased binding in the striata of Nf1+/−GFAPCKO mice, indicative of low endogenous dopamine [75]. Consistent with a dopamine availability deficit, and similar to children with NF1, treatment with dopamine-elevating drugs, such as MPH and L-DOPA, increased striatal dopamine levels and ameliorated attention defects in these mice [75, 81].
Figure 3. Role of dopamine in NF1-associated behavior.
(a) Tyrosine hydroxylase (TH)-positive neurons in the substantia nigra project to the striatum and modulate dopamine signaling. Presynaptically, TH converts tyrosine to L-DOPA. Upon release, dopamine returns to the presynaptic dopamine pool by binding dopamine transporters or it activates adenylyl cyclase (AC) by binding post-synaptic dopamine receptors. AC activation stimulates cAMP generation and dopamine- and cAMP-regulated phosphoprotein-32 (DARPP32) phosphorylation in the striatum. (b) In Nf1 mutant mice, TH expression is reduced, leading to lower striatal dopamine levels and lower effector signaling, as indicated by reduced DARPP32 phosphorylation [81]. Abnormal dopamine homeostasis also results in attention system dysfunction in the Nf1 mutant model [75, 81]. (c) Treatment with drugs that increase dopamine synthesis (L-DOPA), exogenous dopamine levels (dopamine), or block dopamine reuptake [eg. methylphenidate (MPH)] restores striatal dopamine levels and reverses the attention phenotype in Nf1 mutant mice [75, 81]. Abbreviations: GPCR, G-protein coupled receptors.
Dopamine system function is also critical for learning and memory in rodents, such that disruption of hippocampal dopaminergic innervations and loss of hippocampal D1/D5 dopamine receptor function result in spatial learning defects [82–84]. A recent study has explored the relationship between dopamine homeostasis and learning in Nf1+/−GFAPCKO mice further [85]. This study found Nf1+/−GFAPCKO mice to have reduced dopamine levels in the hippocampus, similar to the striatum. Moreover, L-DOPA administration rescued the post-acquisition Morris Water Maze probe trial deficits in vivo and dopamine D1 receptor agonist (ie. SKF-38393) treatment corrected the LTP abnormalities in hippocampal slice preparations in vitro. These findings, coupled with previous observations, establish defective dopaminergic function as a contributing factor that is important for both spatial learning/memory and attention system dysfunction in Nf1 mutant mice.
While the precise mechanism by which neurofibromin modulates dopamine signaling has yet to be identified, these findings suggest that dopamine-targeted therapies may be useful treatments for some children with NF1-associated cognitive abnormalities. Furthermore, these studies highlight the potential utility of raclopride PET imaging to stratify children with NF1 into subgroups most likely to respond to dopamine-elevating treatments.
Clinical heterogeneity and implications
While it is convenient to regard monogenetic disorders as homogenous medical conditions, the marked clinical variability between members of the same family with the same germline NF1 genetic mutation argues to the contrary. Instead, it is more likely that NF1 is composed of numerous distinct diseases, each defined by factors including patient age, patient sex, the timing of NF1 inactivation, the specific cell type, genomic modifiers, and microenvironmental influences. These factors have begun to be elucidated in cancers arising in Nf1 genetically-engineered mice where the timing of bi-allelic Nf1 gene inactivation [86–88], the cell type (cell of origin) [89–96], the genetic (strain) background [97–99], and the non-neoplastic cell microenvironment [93, 100–102] play critical deterministic roles.
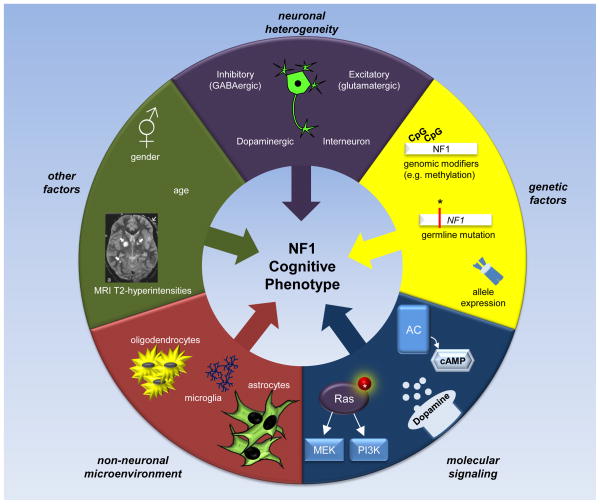
Based on these observations, we propose a model of NF1-associated cognitive abnormalities in which the wide range of clinical features represent an admixture of distinct cellular and molecular etiologies. As such, there is no single molecular abnormality that underlies all of the cognitive dysfunction observed in children with NF1. Rather, the specific collection of cognitive and behavioral deficits observed in any given child with NF1 reflects the relative contributions of multiple cellular and molecular defects (Figure 4). Emerging evidence implicates specific neuronal populations, several molecular defects, and other contributing factors; as such, we envision a model of cognitive dysfunction in which the resulting clinical phenotype reflects the interplay of all of these potential abnormalities.
Figure 4. Factors Influencing the NF1 Cognitive Phenotype.
The specific cognitive phenotype observed in any given individual with NF1 reflects the confluence of genomic, molecular, cellular, and environmental factors. The specific germline NF1 gene mutation, allele expression, and genomic modifying events (including methylation), may influence clinical heterogeneity and create variations in neurofibromin expression in different cell types. Heterogeneity in neuronal subpopulations may also factor into the overall cognitive phenotype. In this manner, the relative contributions of defects in different populations of CNS neurons (e.g., GABA-ergic, dopaminergic) may lead to a distinct spectrum of cognitive and behavioral abnormalities. Similarly, less well-studied characteristics, such as patient sex, age, and NF1-associated brain abnormalities (e.g., T2-hyperintensities), may also contribute to the observed patient cognitive profile. In addition, the effects of abnormal signaling through specific molecular pathways (ie. signaling heterogeneity) could likewise differentially impact distinct neuronal populations and lead to unique cognitive phenotypes. While understudied to date, it is also possible that NF1+/− oligodendrocytes, microglia, and/or astrocytes contribute to abnormal neuronal function as a result of disrupted axonal signal propagation, impaired synaptic pruning, and/or changes in glutamate or other neurotransmitter availability.
First, genomic factors likely influence the specific constellation of cognitive and behavioral deficits observed in children with NF1. In this regard, attention deficits are more common in boys in the general population, but are equally represented in both sexes in children with NF1 [1]. This finding suggests that sexual dimorphic genomic factors may contribute to the phenotypes observed in pre-pubescent children, as have been reported for other CNS abnormalities in mice [103–105]. Second, not all CNS neuronal populations appear to be equally affected by reduced Nf1 gene expression. The use of conditional knockout mouse strains has already revealed that Nf1+/− forebrain pyramidal neurons do not contribute to the abnormal learning and memory [48]. It is possible that distinct neuronal populations differentially respond to reduced neurofibromin expression, and contribute in unique fashions to the learning, memory, and attention deficits that characterize mice and people with a germline inactivating Nf1 gene mutation. Third, the specific germline NF1 gene mutation may create different abnormalities in CNS neurons that reflect the degree of neurofibromin reduction or a specific impairment in cAMP or Ras signaling. While there are few studies examining the effect of specific NF1 gene mutations on neurofibromin expression and function, Nf1+/− mice maintained on different genetic backgrounds exhibit a wide spectrum of reduced Nf1 mRNA expression [99]. Fourth, it is possible that some of the more severe neurocognitive abnormalities occasionally seen in children with NF1 may reflect total loss of neurofibromin expression in select populations of CNS neurons. Recent evidence demonstrates gross brain abnormalities following Nf1 biallelic inactivation in developing neural stem cells [106]. Further study will be required to formally evaluate this intriguing possibility. Fifth, Nf1 mutant mouse strains have been instructive in elucidating the major neurochemical defects responsible for learning, memory and attention abnormalities associated with NF1. Intelligent use of these preclinical mouse strains may provide insights into potential treatments that reflect the predominant signaling defect (e.g., cAMP, Ras, dopamine) in individuals with NF1.
Finally, it is possible that non-neuronal cell types, including NF1+/− oligodendrocytes, astrocytes, and microglia, may contribute to neuronal dysfunction in the brains of children and adults with NF1. In this regard, astrocytes are important for glutamate homeostasis in one tuberous sclerosis complex (TSC) mouse model of CNS disease, such that impaired astrocyte glutamate transport is partly responsible for the seizures and synaptic plasticity defects in Tsc1 conditional knockout mice [107, 108]. Similarly, oligodendrocytes can maintain neuronal integrity and promote axonal signal propagation. Previous studies revealed that spinal cord oligodendrocyte precursor differentiation is regulated by neurofibromin [109], such that two-fold more NG2+ progenitor cells reside in the spinal cords of Nf1+/− mice. Lastly, microglia can regulate synaptic plasticity and pruning [110, 111]. Increased numbers of microglia have been observed in Nf1+/− mouse brains [100], where they represent critical modulators of optic glioma growth [101, 102]. Their roles in NF1-associated learning, memory, and attention deficits have not been explored.
Concluding Remarks
In this review, we have highlighted the roles of Ras, cyclic AMP, and dopamine as key molecular targets that modulate NF1-associated cognitive dysfunction. Each of these factors represents a potential therapeutic target; however, the efficacy of any particular treatment regimen will rely heavily on the specific constellation of molecular and cellular abnormalities present in a given child with NF1 (Figure 5). This idea is underscored by the outcomes of several recent NF1 clinical trials. While treatment with the Lovastatin analog, Simvastatin, did not improve cognitive function in the group as a whole, it did enhance performance on some features of learning and memory in a subgroup of patients [47]. A more recent phase I Lovastatin trial demonstrated some improvement in verbal and nonverbal memory, without any effect on attention system dysfunction [112]. Similarly, some reports have shown that MPH can improve working memory in children with ADHD [113] as well as learning in NF1 patients [2]. By considering the interplay between each of these cellular and molecular abnormalities, future approaches to managing the learning, memory, and attention system deficits in children with NF1 may consider integrating targeted therapies specific to the biochemical and neurochemical abnormalities unique to each child (Box 1).
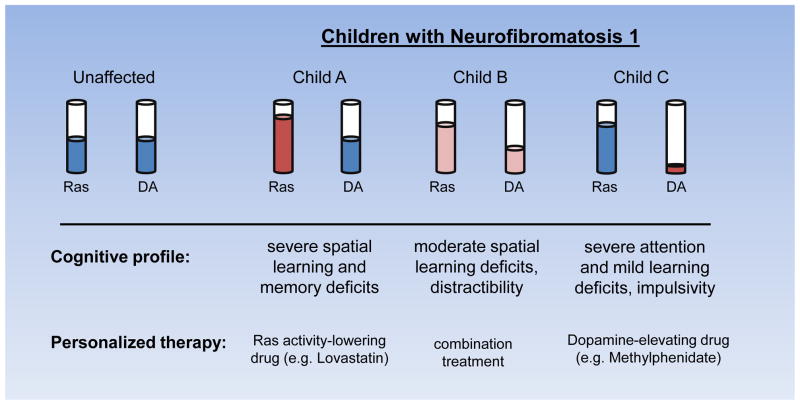
Figure 5. Model of clinical heterogeneity and implications for treatment.
The cognitive profile of children with NF1 likely includes a diverse set of learning, memory, attention, and motor deficits, each linked to a distinct molecular abnormality (i.e. increased Ras, reduced cAMP, or low dopamine). In this regard, each child with NF1 has a different level of Ras, dopamine, and cAMP (not shown) signaling that collectively contribute to the overall cognitive phenotype. Identifying the primary set of cellular and molecular abnormalities may lead to individualized treatments with a higher likelihood of improving the neurocognitive deficits specific to that child or adult with NF1.
Box 1. Outstanding questions.
How does neurofibromin regulate cAMP homeostasis in CNS neurons?
How does neurofibromin regulate dopamine homeostasis in the brain?
What is the relationship between neurofibromin cAMP, Ras, and dopamine regulation in CNS neurons?
Does reduced neurofibromin function in distinct CNS neuronal populations differentially contribute to the cognitive and behavioral abnormalities observed in children with NF1?
How do non-neuronal cells (such as microglia, oligodendrocytes, and astrocytes) influence the cognitive and behavioral abnormalities in children with NF1?
How do combinations of molecular and cellular abnormalities result in specific cognitive profiles in children with NF1?
What underlies the clinical heterogeneity in children with NF1-associated cognitive and behavioral abnormalities (genomic modifiers, environmental factors, patient sex, etc.)? What are the implications for treatment?
How can we stratify children with NF1-associated cognitive and behavioral abnormalities based on their unique molecular and cellular defects? What are the implications for personalized therapy?
Acknowledgments
This work was supported by grants from the Department of Defense (W81XWH-10-1-0884) and National Cancer Institute (U01-CA141549) to D.H.G.. K.D.A. is supported by a Diversity Supplement from the National Cancer Institute (U01-CA141549).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hyman SL, et al. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- 2.Mautner VF, et al. Treatment of ADHD in neurofibromatosis type 1. Developmental medicine and child neurology. 2002;44:164–170. doi: 10.1017/s0012162201001876. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SL, et al. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Developmental medicine and child neurology. 2006;48:973–977. doi: 10.1017/S0012162206002131. [DOI] [PubMed] [Google Scholar]
- 4.Ferner RE. The neurofibromatoses. Practical neurology. 2010;10:82–93. doi: 10.1136/jnnp.2010.206532. [DOI] [PubMed] [Google Scholar]
- 5.Wallace MR, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 6.Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Seminars in pediatric neurology. 2006;13:2–7. doi: 10.1016/j.spen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Archives of neurology. 1988;45:575–578. [PubMed] [Google Scholar]
- 8.Sabol Z, et al. Clinical sensitivity and specificity of multiple T2-hyperintensities on brain magnetic resonance imaging in diagnosis of neurofibromatosis type 1 in children: diagnostic accuracy study. Croatian medical journal. 2011;52:488–496. doi: 10.3325/cmj.2011.52.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman SL, et al. Natural history of cognitive deficits and their relationship to MRI T2-hyperintensities in NF1. Neurology. 2003;60:1139–1145. doi: 10.1212/01.wnl.0000055090.78351.c1. [DOI] [PubMed] [Google Scholar]
- 10.Johnson BA, et al. Motor proficiency in children with neurofibromatosis type 1. Pediatric physical therapy: the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2010;22:344–348. doi: 10.1097/PEP.0b013e3181f9dbc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soucy EA, et al. Developmental delays in children with neurofibromatosis type 1. Journal of child neurology. 2012;27:641–644. doi: 10.1177/0883073811423974. [DOI] [PubMed] [Google Scholar]
- 12.Soucy EA, et al. Height Assessments in Children With Neurofibromatosis Type 1. Journal of child neurology. 2012 doi: 10.1177/0883073812446310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutmann DH, et al. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1) Neurology. 2002;59:759–761. doi: 10.1212/wnl.59.5.759. [DOI] [PubMed] [Google Scholar]
- 14.North KN, et al. Cognitive function and academic performance in neurofibromatosis. 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121–1127. doi: 10.1212/wnl.48.4.1121. [DOI] [PubMed] [Google Scholar]
- 15.Acosta MT, et al. Neurofibromatosis type 1: new insights into neurocognitive issues. Current neurology and neuroscience reports. 2006;6:136–143. doi: 10.1007/s11910-996-0036-5. [DOI] [PubMed] [Google Scholar]
- 16.Payne JM, et al. Paired associate learning in children with neurofibromatosis type 1: implications for clinical trials. Journal of neurology. 2012 doi: 10.1007/s00415–012–6620–5. [DOI] [PubMed] [Google Scholar]
- 17.Acosta MT, et al. The Learning Disabilities Network (LeaDNet): using neurofibromatosis type 1 (NF1) as a paradigm for translational research. American journal of medical genetics. Part A. 2012;158A:2225–2232. doi: 10.1002/ajmg.a.35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson H, et al. Psychological disturbance and sleep disorders in children with neurofibromatosis type 1. Developmental medicine and child neurology. 2005;47:237–242. doi: 10.1017/s0012162205000460. [DOI] [PubMed] [Google Scholar]
- 19.Coude FX, et al. Academic impairment is the most frequent complication of neurofibromatosis type-1 (NF1) in children. Behavior genetics. 2006;36:660–664. doi: 10.1007/s10519-005-9040-9. [DOI] [PubMed] [Google Scholar]
- 20.Descheemaeker MJ, et al. Behavioural, academic and neuropsychological profile of normally gifted Neurofibromatosis type 1 children. Journal of intellectual disability research: JIDR. 2005;49:33–46. doi: 10.1111/j.1365-2788.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 21.Barton B, North K. Social skills of children with neurofibromatosis type 1. Developmental medicine and child neurology. 2004;46:553–563. doi: 10.1017/s0012162204000921. [DOI] [PubMed] [Google Scholar]
- 22.Maddrey AM, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. Journal of neuro-oncology. 2005;72:245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- 23.Isenberg JC, et al. Attention Skills in Children With Neurofibromatosis Type 1. Journal of child neurology. 2012 doi: 10.1177/0883073812439435. [DOI] [PubMed] [Google Scholar]
- 24.Viskochil D, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- 25.Gutmann DH, et al. Developmental regulation of a neuron-specific neurofibromatosis 1 isoform. Annals of neurology. 1999;46:777–782. doi: 10.1002/1531-8249(199911)46:5<777::aid-ana15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 26.Geist RT, Gutmann DH. Expression of a developmentally-regulated neuron-specific isoform of the neurofibromatosis 1 (NF1) gene. Neuroscience letters. 1996;211:85–88. doi: 10.1016/0304-3940(96)12730-0. [DOI] [PubMed] [Google Scholar]
- 27.Gutmann DH, et al. Expression of two new protein isoforms of the neurofibromatosis type 1 gene product, neurofibromin, in muscle tissues. Developmental dynamics: an official publication of the American Association of Anatomists. 1995;202:302–311. doi: 10.1002/aja.1002020309. [DOI] [PubMed] [Google Scholar]
- 28.Costa RM, et al. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nature genetics. 2001;27:399–405. doi: 10.1038/86898. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Gutmann DH. Mutations in the GAP-related domain impair the ability of neurofibromin to associate with microtubules. Brain research. 1997;759:149–152. doi: 10.1016/s0006-8993(97)00328-4. [DOI] [PubMed] [Google Scholar]
- 30.Gutmann DH, et al. Expression of the neurofibromatosis 1 (NF1) isoforms in developing and adult rat tissues. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1995;6:315–323. [PubMed] [Google Scholar]
- 31.Basu TN, et al. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- 32.Bollag G, et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nature genetics. 1996;12:144–148. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]
- 33.DeClue JE, et al. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992;69:265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- 34.Lau N, et al. Loss of neurofibromin is associated with activation of RAS/MAPK and PI3-K/AKT signaling in a neurofibromatosis 1 astrocytoma. Journal of neuropathology and experimental neurology. 2000;59:759–767. doi: 10.1093/jnen/59.9.759. [DOI] [PubMed] [Google Scholar]
- 35.The I, et al. Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science. 1997;276:791–794. doi: 10.1126/science.276.5313.791. [DOI] [PubMed] [Google Scholar]
- 36.Guo HF, et al. Requirement of Drosophila NF1 for activation of adenylyl cyclase by PACAP38-like neuropeptides. Science. 1997;276:795–798. doi: 10.1126/science.276.5313.795. [DOI] [PubMed] [Google Scholar]
- 37.Tong J, et al. Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nature neuroscience. 2002;5:95–96. doi: 10.1038/nn792. [DOI] [PubMed] [Google Scholar]
- 38.Dasgupta B, et al. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:8949–8954. doi: 10.1523/JNEUROSCI.23-26-08949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannan F, et al. Effect of neurofibromatosis type I mutations on a novel pathway for adenylyl cyclase activation requiring neurofibromin and Ras. Human molecular genetics. 2006;15:1087–1098. doi: 10.1093/hmg/ddl023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacks T, et al. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature genetics. 1994;7:353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- 41.Cichowski K, et al. Nf1 gene targeting: toward models and mechanisms. Seminars in cancer biology. 1996;7:291–298. doi: 10.1006/scbi.1996.0037. [DOI] [PubMed] [Google Scholar]
- 42.Silva AJ, et al. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nature genetics. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- 43.Costa RM, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 44.Guilding C, et al. Restored plasticity in a mouse model of neurofibromatosis type 1 via inhibition of hyperactive ERK and CREB. The European journal of neuroscience. 2007;25:99–105. doi: 10.1111/j.1460-9568.2006.05238.x. [DOI] [PubMed] [Google Scholar]
- 45.Ho IS, et al. Distinct functional domains of neurofibromatosis type 1 regulate immediate versus long-term memory formation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6852–6857. doi: 10.1523/JNEUROSCI.0933-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Current biology: CB. 2005;15:1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 47.Krab LC, et al. Effect of simvastatin on cognitive functioning in children with neurofibromatosis type 1: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2008;300:287–294. doi: 10.1001/jama.300.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui Y, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brems H, et al. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nature genetics. 2007;39:1120–1126. doi: 10.1038/ng2113. [DOI] [PubMed] [Google Scholar]
- 50.Denayer E, et al. Observations on intelligence and behavior in 15 patients with Legius syndrome. American journal of medical genetics. Part C, Seminars in medical genetics. 2011;157:123–128. doi: 10.1002/ajmg.c.30297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasmant E, et al. SPRED1 germline mutations caused a neurofibromatosis type 1 overlapping phenotype. Journal of medical genetics. 2009;46:425–430. doi: 10.1136/jmg.2008.065243. [DOI] [PubMed] [Google Scholar]
- 52.Wakioka T, et al. Spred is a Sprouty-related suppressor of Ras signalling. Nature. 2001;412:647–651. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- 53.Nonami A, et al. Spred-1 negatively regulates interleukin-3-mediated ERK/mitogen-activated protein (MAP) kinase activation in hematopoietic cells. The Journal of biological chemistry. 2004;279:52543–52551. doi: 10.1074/jbc.M405189200. [DOI] [PubMed] [Google Scholar]
- 54.Denayer E, et al. Spred1 is required for synaptic plasticity and hippocampus-dependent learning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:14443–14449. doi: 10.1523/JNEUROSCI.4698-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoki Y, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nature genetics. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 56.Axelrad ME, et al. Neurocognitive, adaptive, and behavioral functioning of individuals with Costello syndrome: a review. American journal of medical genetics. Part C, Seminars in medical genetics. 2011;157:115–122. doi: 10.1002/ajmg.c.30299. [DOI] [PubMed] [Google Scholar]
- 57.Viosca J, et al. Germline expression of H-Ras(G12V) causes neurological deficits associated to Costello syndrome. Genes, brain, and behavior. 2009;8:60–71. doi: 10.1111/j.1601-183X.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- 58.Atkins CM, et al. The MAPK cascade is required for mammalian associative learning. Nature neuroscience. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 59.Giese KP, et al. Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1) Neuropharmacology. 2001;41:791–800. doi: 10.1016/s0028-3908(01)00096-x. [DOI] [PubMed] [Google Scholar]
- 60.Chen AP, et al. Forebrain-specific knockout of B-raf kinase leads to deficits in hippocampal long-term potentiation, learning, and memory. Journal of neuroscience research. 2006;83:28–38. doi: 10.1002/jnr.20703. [DOI] [PubMed] [Google Scholar]
- 61.Lin CH, et al. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- 62.Guo HF, et al. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature. 2000;403:895–898. doi: 10.1038/35002593. [DOI] [PubMed] [Google Scholar]
- 63.Buchanan ME, Davis RL. A distinct set of Drosophila brain neurons required for neurofibromatosis type 1-dependent learning and memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:10135–10143. doi: 10.1523/JNEUROSCI.0283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of comparative physiology. A, Sensory, neural, and behavioral physiology. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 65.Margulies C, et al. Deconstructing memory in Drosophila. Current biology: CB. 2005;15:R700–713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams JA, et al. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293:2251–2256. doi: 10.1126/science.1063097. [DOI] [PubMed] [Google Scholar]
- 67.Rutten K, et al. Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. The European journal of neuroscience. 2008;28:625–632. doi: 10.1111/j.1460-9568.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- 68.Rutten K, et al. Sub-chronic rolipram treatment leads to a persistent improvement in long-term object memory in rats. Neurobiology of learning and memory. 2008;90:569–575. doi: 10.1016/j.nlm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 69.Li YF, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodefer JS, et al. Selective phosphodiesterase inhibitors improve performance on the ED/ID cognitive task in rats. Neuropharmacology. 2012;62:1182–1190. doi: 10.1016/j.neuropharm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Wiescholleck V, Manahan-Vaughan D. PDE4 inhibition enhances hippocampal synaptic plasticity in vivo and rescues MK801-induced impairment of long-term potentiation and object recognition memory in an animal model of psychosis. Translational psychiatry. 2012;2:e89. doi: 10.1038/tp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hegedus B, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell stem cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 73.Brown JA, et al. Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:5579–5589. doi: 10.1523/JNEUROSCI.3994-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown JA, et al. Neurofibromatosis-1 heterozygosity impairs CNS neuronal morphology in a cAMP/PKA/ROCK-dependent manner. Molecular and cellular neurosciences. 2012;49:13–22. doi: 10.1016/j.mcn.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown JA, et al. PET imaging for attention deficit preclinical drug testing in neurofibromatosis-1 mice. Experimental neurology. 2011;232:333–338. doi: 10.1016/j.expneurol.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hegedus B, et al. Optic nerve dysfunction in a mouse model of neurofibromatosis-1 optic glioma. Journal of neuropathology and experimental neurology. 2009;68:542–551. doi: 10.1097/NEN.0b013e3181a3240b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin YL, et al. Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. The Journal of cell biology. 2007;177:829–841. doi: 10.1083/jcb.200608121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang HF, et al. Valosin-containing protein and neurofibromin interact to regulate dendritic spine density. The Journal of clinical investigation. 2011;121:4820–4837. doi: 10.1172/JCI45677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Volkow ND, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spencer TJ, et al. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. The American journal of psychiatry. 2006;163:387–395. doi: 10.1176/appi.ajp.163.3.387. [DOI] [PubMed] [Google Scholar]
- 81.Brown JA, et al. Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Human molecular genetics. 2010;19:4515–4528. doi: 10.1093/hmg/ddq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gasbarri A, et al. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–1044. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- 83.El-Ghundi M, et al. Spatial learning deficit in dopamine D(1) receptor knockout mice. European journal of pharmacology. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 84.Xing B, et al. Dopamine D1 but not D3 receptor is critical for spatial learning and related signaling in the hippocampus. Neuroscience. 2010;169:1511–1519. doi: 10.1016/j.neuroscience.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 85.Diggs-Andrews KA, et al. Dopamine deficiency underlies learning deficits in Neurofibromatosis-1 mice. Annals of neurology. 2012 doi: 10.1002/ana.23793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Le LQ, et al. Susceptible stages in Schwann cells for NF1-associated plexiform neurofibroma development. Cancer research. 2011;71:4686–4695. doi: 10.1158/0008-5472.CAN-10-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bajenaru ML, et al. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Molecular and cellular biology. 2002;22:5100–5113. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin L, et al. Mice lacking neurofibromin develop gastric hyperplasia. American journal of physiology. Gastrointestinal and liver physiology. 2009;297:G751–761. doi: 10.1152/ajpgi.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le LQ, et al. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell stem cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alcantara Llaguno S, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu Y, et al. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132:5577–5588. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajenaru ML, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer research. 2003;63:8573–8577. [PubMed] [Google Scholar]
- 94.Zhu Y, et al. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Y, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes & development. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gitler AD, et al. Tie2-Cre-induced inactivation of a conditional mutant Nf1 allele in mouse results in a myeloproliferative disorder that models juvenile myelomonocytic leukemia. Pediatric research. 2004;55:581–584. doi: 10.1203/01.PDR.0000113462.98851.2E. [DOI] [PubMed] [Google Scholar]
- 97.Reilly KM, et al. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nature genetics. 2000;26:109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- 98.Reilly KM, et al. Susceptibility to astrocytoma in mice mutant for Nf1 and Trp53 is linked to chromosome 11 and subject to epigenetic effects. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13008–13013. doi: 10.1073/pnas.0401236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hawes JJ, et al. Nf1 expression is dependent on strain background: implications for tumor suppressor haploinsufficiency studies. Neurogenetics. 2007;8:121–130. doi: 10.1007/s10048-006-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Human molecular genetics. 2007;16:1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 101.Daginakatte GC, et al. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer research. 2008;68:10358–10366. doi: 10.1158/0008-5472.CAN-08-2506. [DOI] [PubMed] [Google Scholar]
- 102.Simmons GW, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. Journal of neuropathology and experimental neurology. 2011;70:51–62. doi: 10.1097/NEN.0b013e3182032d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang X, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome research. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nature neuroscience. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brennan KC, et al. Reduced threshold for cortical spreading depression in female mice. Annals of neurology. 2007;61:603–606. doi: 10.1002/ana.21138. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, et al. ERK inhibition rescues defects in fate specification of Nf1- deficient neural progenitors and brain abnormalities. Cell. 2012;150:816–830. doi: 10.1016/j.cell.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong M, et al. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Annals of neurology. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- 108.Zeng LH, et al. Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiology of disease. 2007;28:184–196. doi: 10.1016/j.nbd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bennett MR, et al. Aberrant growth and differentiation of oligodendrocyte progenitors in neurofibromatosis type 1 mutants. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7207–7217. doi: 10.1523/JNEUROSCI.23-18-07207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 112.Acosta MT, et al. Lovastatin as treatment for neurocognitive deficits in neurofibromatosis type 1: phase I study. Pediatric neurology. 2011;45:241–245. doi: 10.1016/j.pediatrneurol.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 113.Strand MT, et al. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. Journal of abnormal child psychology. 2012;40:1193–1207. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferner RE, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. Journal of medical genetics. 2007;44:81–88. doi: 10.1136/jmg.2006.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Williams VC, et al. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124–133. doi: 10.1542/peds.2007-3204. [DOI] [PubMed] [Google Scholar]