Abstract
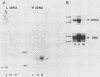
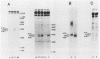
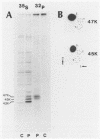
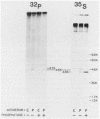
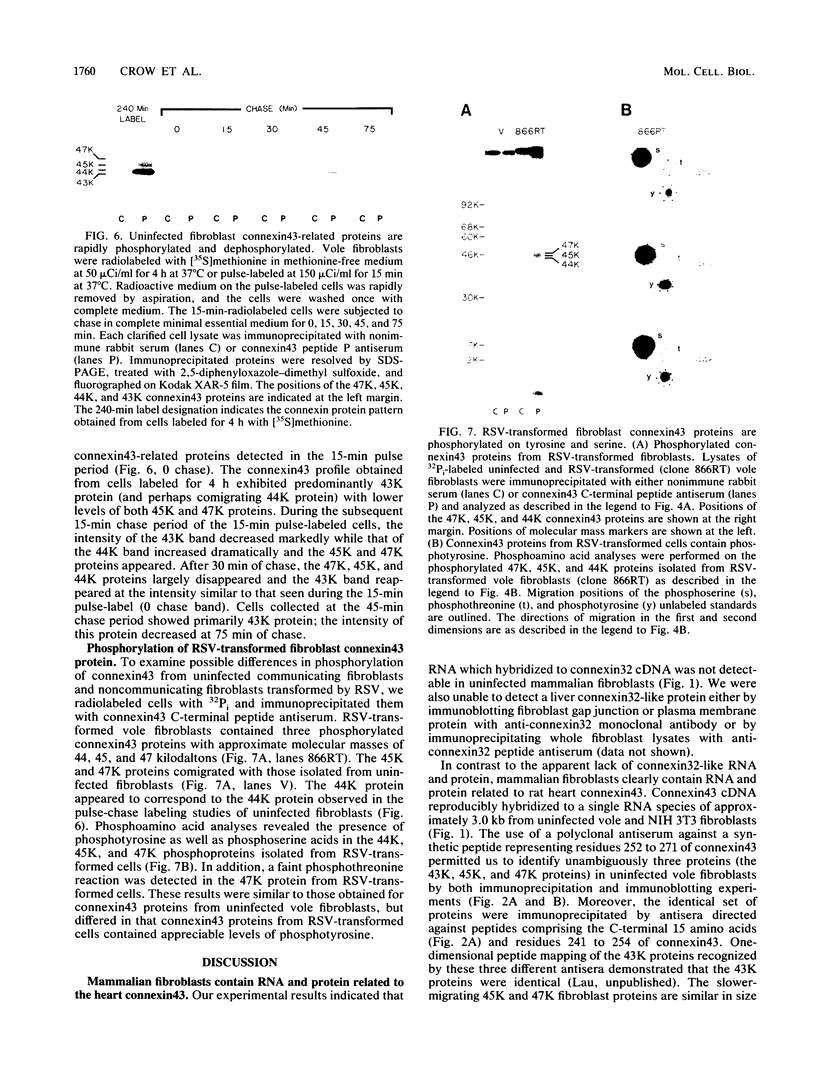
Gap junctions are membrane channels that permit the interchange of ions and other low-molecular-weight molecules between adjacent cells. Rous sarcoma virus (RSV)-induced transformation is marked by an early and profound disruption of gap-junctional communication, suggesting that these membrane structures may serve as sites of pp60v-src action. We have begun an investigation of this possibility by identifying and characterizing putative proteins involved in junctional communication in fibroblasts, the major cell type currently used to study RSV-induced transformation. We found that uninfected mammalian fibroblasts do not appear to contain RNA or protein related to connexin32, the major rat liver gap junction protein. In contrast, vole and mouse fibroblasts contained a homologous 3.0-kilobase RNA similar in size to the heart tissue RNA encoding the gap junction protein, connexin43. Anti-connexin43 peptide antisera specifically reacted with three proteins of approximately 43, 45 and 47 kilodaltons (kDa) from communicating fibroblasts. Gap junctions of heart cells contained predominantly 45- and 47-kDa species similar to those found in fibroblasts. Uninfected fibroblast 45- and 47-kDa proteins were phosphorylated on serine residues. Phosphatase digestions of 45- and 47-kDa proteins and pulse-chase labeling studies indicated that these proteins represented phosphorylated forms of the 43-kDa protein. Phosphorylation of connexin protein appeared to occur shortly after synthesis, followed by an equally rapid dephosphorylation. In comparison with these results, connexin43 protein in RSV-transformed fibroblasts contained both phosphotyrosine and phosphoserine. Thus, the presence of phosphotyrosine in connexin43 correlates with the loss of gap-junctional communication observed in RSV-transformed fibroblasts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. M., Menko A. S., Johnson R. G., Sheppard J. R., Sheridan J. D. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J Cell Biol. 1981 Nov;91(2 Pt 1):573–578. doi: 10.1083/jcb.91.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Intercellular communication and the control of growth: X. Alteration of junctional permeability by the src gene. A study with temperature-sensitive mutant Rous sarcoma virus. J Membr Biol. 1984;82(3):191–205. doi: 10.1007/BF01871629. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Intercellular communication and the control of growth: XI. Alteration of junctional permeability by the src gene in a revertant cell with normal cytoskeleton. J Membr Biol. 1984;82(3):207–212. doi: 10.1007/BF01871630. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Polyomavirus middle T antigen downregulates junctional cell-to-cell communication. Mol Cell Biol. 1987 Feb;7(2):946–950. doi: 10.1128/mcb.7.2.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarnia R., Reddy S., Kmiecik T. E., Shalloway D., Loewenstein W. R. The cellular src gene product regulates junctional cell-to-cell communication. Science. 1988 Jan 22;239(4838):398–401. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Kistler J., Paul D. L., Goodenough D. A. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989 Feb;108(2):595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Trosko J. E., Kung H. J., Bombick D., Matsumura F. Potential role of the src gene product in inhibition of gap-junctional communication in NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5360–5364. doi: 10.1073/pnas.82.16.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Belzer S. K., Purchio A. F. Structurally and functionally modified forms of pp60v-src in Rous sarcoma virus-transformed cell lysates. Mol Cell Biol. 1984 Jul;4(7):1213–1220. doi: 10.1128/mcb.4.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G., Miller T., Paul D., Voellmy R., Werner R. Expression of functional cell-cell channels from cloned rat liver gap junction complementary DNA. Science. 1987 Jun 5;236(4806):1290–1293. doi: 10.1126/science.3035715. [DOI] [PubMed] [Google Scholar]
- Erikson E., Brugge J. S., Erikson R. L. Phosphorylated and nonphosphorylated forms of avian sarcoma virus polypeptide p19. Virology. 1977 Jul 1;80(1):177–185. doi: 10.1016/0042-6822(77)90390-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L. A detergent-independent procedure for the isolation of gap junctions from rat liver. J Biol Chem. 1984 Aug 10;259(15):9936–9943. [PubMed] [Google Scholar]
- Hertzberg E. L. Antibody probes in the study of gap junctional communication. Annu Rev Physiol. 1985;47:305–318. doi: 10.1146/annurev.ph.47.030185.001513. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Vogt P. K., Faras A. J. Quantitation and localization of Rous sarcoma virus-specific RNA in transformed and revertant field vole cells. J Virol. 1978 Feb;25(2):518–526. doi: 10.1128/jvi.25.2.518-526.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986 Sep;103(3):767–776. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. F., Krzyzek R. A., Faras A. J. Loss of tumorigenicity correlates with a reduction in pp60src kinase activity in a revertant subclone of avian sarcoma virus-infected field vole cells. Cell. 1981 Mar;23(3):815–823. doi: 10.1016/0092-8674(81)90446-3. [DOI] [PubMed] [Google Scholar]
- Lau A. F. Phosphotyrosine-containing 120,000-dalton protein coimmunoprecipitated with pp60v-src from Rous sarcoma virus-transformed mammalian cells. Virology. 1986 May;151(1):86–99. doi: 10.1016/0042-6822(86)90106-6. [DOI] [PubMed] [Google Scholar]
- Manjunath C. K., Goings G. E., Page E. Proteolysis of cardiac gap junctions during their isolation from rat hearts. J Membr Biol. 1985;85(2):159–168. doi: 10.1007/BF01871268. [DOI] [PubMed] [Google Scholar]
- Manjunath C. K., Nicholson B. J., Teplow D., Hood L., Page E., Revel J. P. The cardiac gap junction protein (Mr 47,000) has a tissue-specific cytoplasmic domain of Mr 17,000 at its carboxy-terminus. Biochem Biophys Res Commun. 1987 Jan 15;142(1):228–234. doi: 10.1016/0006-291x(87)90475-x. [DOI] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src-gene expression on the synthesis and phosphorylation of cellular polypeptides. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):975–982. doi: 10.1101/sqb.1980.044.01.105. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Zwiller J., Ogasawara E. M., Nakamoto S. S., Boynton A. L. Stimulation by inositol trisphosphate and tetrakisphosphate of a protein phosphatase. Biochem Biophys Res Commun. 1988 Sep 15;155(2):767–772. doi: 10.1016/s0006-291x(88)80561-8. [DOI] [PubMed] [Google Scholar]