Abstract
Most microbes live in complex communities, where they interact both synergistically and competitively. In order to explore the relationship between environmental heterogeneity and the spatial structure of well-defined biofilms, single- and mixed-species biofilms of Pseudomonas aeruginosa PAO1 and Flavobacterium sp. CDC-65 were grown in a planar flow cell under highly controlled flow gradients. Both organisms behaved differently in mixed cultures than in single-species cultures due to inter-species interactions, and these interactions were significantly affected by external flow conditions. Pseudomonas and Flavobacterium showed a competitive relationship under slow inflow conditions, where the supply of growth medium was limited. Under such competitive conditions, the faster specific growth rate of Flavobacterium allowed it to secure access to favorable regions of the biofilm by over-growing Pseudomonas. In contrast, Pseudomonas was restricted to nutritionally depleted habitat near the base of the biofilm, and its growth was significantly inhibited. Conversely, under higher inflow conditions providing greater influx of growth medium, both organisms accumulated greater biomass in mixed biofilms than in single-species biofilms. Spatial segregation of the two organisms within the biofilms contributed to enhanced overall exploitation of available nutrients and substrates, while morphological changes favored better adherence to the surface under high hydrodynamic shear. These results indicate that synergy and competition in biofilms varies with flow conditions. Limited resource replenishment favors competition under low-flow conditions, while high flow reduces competition and favors synergy by providing greater resources and simultaneously imposing increased hydrodynamic shear that makes it more difficult to accumulate biomass on the surface. Ecological interactions that produce mechanically stronger and more robust biofilms will support more extensive growth on surfaces subject to high hydrodynamic shear, but these interactions are difficult to predict from observations of the behavior of individual organisms.
Keywords: dual-species biofilm, multi-species interaction, flow gradients, Pseudomonas aeruginosa, Flavobacterium
1 Introduction
Culture-based and non-culture approaches have revealed that microorganisms rarely live alone, but rather interact with each other to form complex communities. Although some microbial communities consist of free-swimming cells in aquatic environments, most microbial communities grow on interfaces as biofilms (Ward, et al., 1998, Davey & O’toole, 2000, Kolenbrander, 2000, Zengler, 2009). Organisms in biofilms are involved in both symbiosis and competition for resources, and these interactions are affected by organism distributions, biofilm structures, and spatial heterogeneity. Well-defined multi-species biofilms have been used as simplified model systems in microbial ecology to follow community development, identify the role of individual populations in the community, and observe responses of microbial communities to environmental gradients. Such interactions have been observed in layered sludge granules, organized phototrophic consortia, and colonization of biofilms by planktonic organisms (Sekiguchi, et al., 1999, Overmann & van Gemerden, 2000, An, et al., 2006, Hansen, et al., 2007, Beaufort, et al., 2012).
Pseudomonas aeruginosa and Flavobacterium spp. are two of the most ubiquitous opportunistic pathogens found in rivers, lakes, and engineered water systems (Ford, 1999, Manz, et al., 1999, Brinkmeyer, et al., 2003). They are major sources of nosocomial pneumonias and bacteremia from hospital devices and water supply systems, and they have been found to be major contributors of biofilms associated with outbreaks of Legionellosis (Stamm, et al., 1975, Orrison, et al., 1983, Pearson, et al., 1996, Anaissie, et al., 2002). These two organisms have previously been used to form a well-defined consortium with Klebsiella pneumoniae to examine the survival and growth of Legionella pneumophila in biofilms (Murga, et al., 2001), and to study biofilm colonization and disinfection in pilot-scale cooling tower systems (Liu, et al., 2009, Liu, et al., 2011). P. aeruginosa has been observed to dominate the community in closed batch systems while Flavobacterium has been found to dominate in open (flow-through) systems (Liu, et al., 2009). However, the specific environmental conditions responsible for this behavior are still unclear. Many studies have indicated that the development and morphology of biofilms is significantly affected by external hydrodynamic forces, inputs and outputs of nutrients and substrates, and chemical transport. Replenishment of nutrients and substrates (such as carbon sources, nitrogen sources and oxygen) is essential for biofilm growth, and this replenishment increases with flow rate, but the accompanying hydrodynamic forces also affect biofilm structure and can lead to detachment from the substratum (Chopp, et al., 2002, Liu & Tay, 2002, Purevdorj, et al., 2002, Battin, et al., 2003).
Here the behavior of a model ecosystem composed of Pseudomonas aeruginosa PAO1 and Flavobacterium sp. CDC-65 was examined under well-defined environmental gradients in a planar flow cell. The flow cell system allowed biofilm growth to be monitored non-invasively for seven days under imposed gradients of local velocity, hydrodynamic shear stress, and influx of growth medium. We hypothesized that patterns of biofilm growth would be directly related to the imposed environmental heterogeneity, and that each organism would colonize regions of the biofilm where local conditions particularly favored its growth.
2 Methods and Materials
2.1 Bacteria strains and propagation
Pseudomonas aeruginosa strain PAO1 with chromosomally encoded green fluorescent protein (GFP) and Flavobacterium sp. CDC-65 were used in flow-cells experiments (Orrison, et al., 1983, Stover, et al., 2000, Murga, et al., 2001). R2A medium was used to simulate typical depleted environmental water conditions. R2A medium contained (per liter): 0.05 g of yeast extract, 0.05 g of proteose peptone No. 3, 0.05 g of casamino acids, 0.05 g of dextrose, 0.03 g of sodium pyruvate, 0.03 g of dipotassium phosphate, and 0.005 g of magnesium sulfate (Murga, et al., 2001). Stock cultures of Pseudomonas and Flavobacterium were streaked onto R2A agar plates and incubated at 30°C for 24 hours. R2Aagar was R2A medium with the addition of 15 g per liter agar. A single colony of each strain was transferred into 5 ml of R2A medium, and grown at 30°C with shaking to stationary phase. Each culture was then transferred to fresh medium and diluted to OD600 = 0.1 for inoculation into the flow cell.
Flow cell experiments were conducted at room temperature, 25°C. In order to help interpret the relative growth of the two organisms in flow cells, the growth curve of each strain in R2A liquid medium was measured at 25°C in triplicate. Specific growth rates at exponential phase of each organism were then calculated from these results (Elvers, et al., 1998). To confirm that metabolic products of each organism didn’t inhibit the other organism’s growth, disk sensitivity tests were performed using suspensions of each species following the methods of Nikoskelainen et al. (Nikoskelainen, et al., 2001).
2.2 Flow cell experiments
We cultivated biofilms in the planar flow cell system described in Zhang et al. (Zhang, et al., 2011) to support biofilm growth under essentially two-dimensional flow conditions. The flow cell chamber has dimensions of 3.5 cm × 3.5 cm × 0.06 cm. The flow configuration is shown in Figure 1A, and detailed descriptions of the geometry and performance of this flow cell is available in Zhang et al. (2011). Biofilms were grown under the right-angle flow pattern shown in Figure 1B. This flow configuration produces distinct gradients of hydrodynamic shear and influx of growth medium from the bottom-right to the upper-left of the flow chamber. Biofilm growth was observed in the center region of the flow cell where there is a regular flow gradient, as shown in Figure 1C. Results are presented for each of the nine observation locations denoted R1 to R9 in Figure 1C. The lattice Boltzmann method was used to simulate the flow field throughout the flow cell and solute transport from inlets to observation regions (Zhang, et al., 2011).
Figure 1.
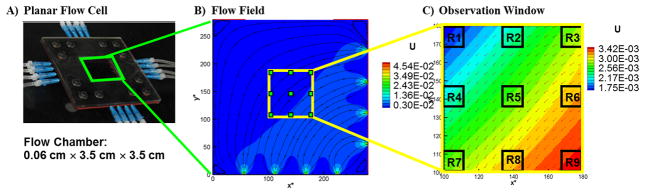
A) Photograph of the planar flow cell. B) The flow field in the flow chamber with streamlines under inflow rate of 0.16 mL min−1. C) The observation window in the center of the flow chamber, including locations of nine regions observed by confocal microscopy. Coordinates are lattice locations normalized by lattice spacing (Zhang, et al., 2011), and color bars of U represent lattice dimensionless fluid velocity increased from blue to red.
Flow cell experiments were performed with both single-species cultures of Pseudomonas and Flavobacterium and mixed-species cultures of the two organisms. In the multi-species experiments, separate batch cultures of Pseudomonas and Flavobacterium were grown to the stationary phase, and then inoculated at a ratio of 1:1 into flow cells. After inoculation, the flow cell was inverted for one hour to facilitate adherence of cells to the glass cover slip, and then returned back to the original position. Microscopic observations confirmed that this procedure evenly distributed Pseudomonas and Flavobacterium on the substratum (results not shown). Then R2A media was introduced by means of a peristaltic pump (Gilson Mini Plus3) particularly suitable for biofilm experiments because it has minimal flow pulsation. Flow cell experiments were performed under two flow rates, a slower inflow rate of 0.16 mL min−1 and a faster inflow rate of 0.80 mL min−1, in duplicate. We chose these conditions because we previously observed that they produced distinct patterns of growth and detachment in P. aeruginosa biofilms (Zhang, et al., 2011). The slow inflow rate provided a velocity gradient from 2.52 cm min−1 in R1 to 3.72 cm min−1 in R9 in the observation window spanning the nine regions R1 to R9 shown in Figure 2A. The fast inflow rate provided velocities ranging from 5.76 cm min−1 in R1 to 10.4 cm min−1 in R9, as shown in Figure 2B.
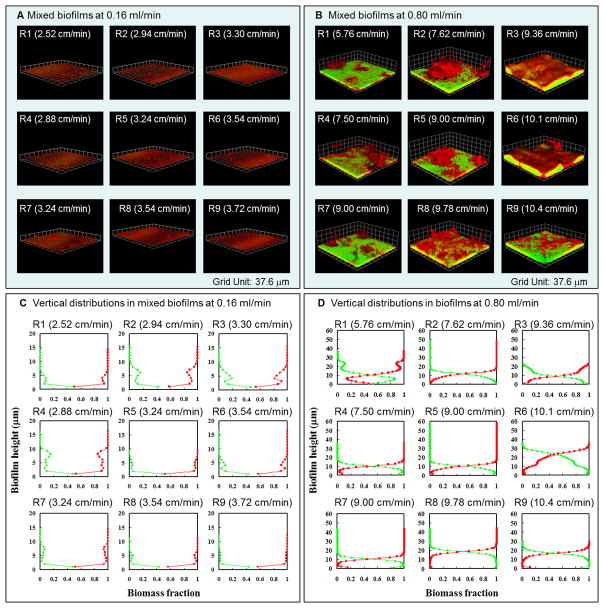
Figure 2.
Dual-species seven-day-old biofilms of Pseudomonas aeruginosa PAO1 (green) and Flavobacterium sp. CDC-65 (red) under inflow rates of 0.16 mL min−1 and 0.8 mL min−1. A) and B) show the representative biofilm morphology on the 7th day at each of the nine regions indicated in Figure 1C. Local velocities in each region are given in parentheses, and the grid unit is 37.6 μm. C) and D) show the representative vertical distributions of Pseudomonas and Flavobacterium at each region of the biofilm. Red lines represent the fraction of Flavobacterium and green lines represent the fraction of Pseudomonas.
2.3 Characterizing biofilm morphologies
A LSM 510 META confocal laser scanning microscope (Carl Zeiss) with a Plan-Apochromat 63×/1.4 NA oil immersion objective lens was used to image the biofilms. Three image stacks were collected in each region shown in Figure 1C. Pseudomonas was detected by its constitutively expressed green fluorescent protein (GFP). Flavobacterium was detected by counterstaining with nucleic acid dye, SYTO-62 (Molecular Probes). 50 μM of SYTO-62 was added into the flow cell and allowed to bind under stagnant conditions for 20 min in the dark, and then flow was resumed to rinse unbound dye. GFP and SYTO-62 was imaged using CLSM at excitation wavelengths of 488 nm and 633 nm, respectively. The VOLOCITY software package (Improvision Inc.) was used to generate three-dimensional images from stacks of CLSM images, and the COMSTAT software was used for quantitative analysis of these three-dimensional images (Heydorn, et al., 2000, Heydorn, et al., 2000).
2.4 Statistical analysis
The one-factor t-test was used to assess differences in the biofilm structural characteristics associated with local velocity and solute travel time. Two-way analysis of variance was used to evaluate the changes in morphology of single- and multi-species biofilms under different flow conditions.
3 Results
3.1 Mixed-species biofilms under flow gradients
After 5 days of growth, the system reached a steady state in which the overall pattern of biofilm morphology and the distribution of the two species within the biofilm no longer changed with time. Figure 2 shows the biofilm morphology and spatial distributions of each organism within seven-day-old mixed-species biofilms.
Under slow inflow conditions, thin biofilms composed of both Pseudomonas and Flavobacterium were detected in all regions. Pseudomonas was present as sporadic small colonies and single cells, while Flavobacterium formed relatively continuous biofilms with larger clusters (Figure 2A). Regions R1, R2 and R4 had thinner Flavobacterium biofilms and more Pseudomonas colonies. Vertical distributions, presented in Figure 2C, indicate that Pseudomonas mainly occurred at the base of mixed biofilms and was overgrown by Flavobacterium. In all regions, at all elevations, Flavobacterium was the dominant component of biomass in the seven-day biofilm.
Under fast inflow conditions, more complex intergrowth of the two organisms was observed. The biofilm morphologies and the vertical distributions of each organism are shown in Figure 2B and 2D, respectively. Pseudomonas always formed the base of mixed biofilms while Flavobacterium dominated the upper part of the biofilm canopy. In regions R1, R4 and R7, Pseudomonas occurred in a smooth, continuous, and thin base layer, while Flavobacterium formed discontinuous large tufts and clusters on top of Pseudomonas biofilms. In these regions, Pseudomonas was the dominant organism at the base of the biofilm: it represented over 50% of the biomass in the bottom 10 μm, and Flavobacterium was the dominant organism above 10 μm. The fraction of Pseudomonas gradually decreased until it was absent at a height of 27 μm in R1, and at 16 μm in R4 and R7. The maximum height of the Flavobacterium biofilm was 36 μm. In locations R2 and R5, Flavobacterium also existed as large tufts with pillars and towers of cells, while Pseudomonas occurred only in a thin and continuous layer at the base of the biofilm. In these regions, Flavobacterium reached maximum thicknesses of 50 μm at location R2 and 58 μm at location R5, while Pseudomonas disappeared at 19 μm (R2) and 15 μm (R5). Smoother biofilms formed in regions of faster local velocity. Region R8 had a faster velocity (9.78 cm min−1), leading to greater accumulation of Pseudomonas and Flavobacterium and the formation of some tower structures. Pseudomonas was the dominant organism to a height of 18 μm and disappeared at 25 μm, while Flavobacterium was found at a maximum height of 44 μm. Regions R6 and R9 had the highest velocities (10.1 cm min−1 and 10.4 cm min−1, respectively), and accumulated more Pseudomonas than other regions. Pseudomonas was the dominant organism to a height of 23 μm at R6 and 16 μm at R9, whereas Flavobacterium only achieved maximum heights of 36 μm at R6 and 29 μm at R9.
3.2 Single-species biofilms under flow gradients
We observed the behavior of single-species Pseudomonas and Flavobacterium biofilms to provide a basis for evaluating their interactions in mixed-species biofilms. Single-species Pseudomonas biofilms achieved steady state after four days and single-species Flavobacterium biofilms achieved steady state after six days. Figure 3 shows the morphologies of seven-day single-species biofilms of Pseudomonas and Flavobacterium under the two inflow rates.
Figure 3.
Representative seven-day-old single-species biofilms of Pseudomonas aeruginosa PAO1 and Flavobacterium sp. CDC-65 under inflow rates 0.16 mL min−1 and 0.80 mL min−1. Results are shown for each of the nine regions indicated in Figure 1C. Local velocities in each region are given in parentheses, and the grid unit is 37.6 μm.
Morphological gradients were observed in single-species Pseudomonas biofilms relative to the imposed flow gradient. Pseudomonas formed sporadic small colonies under the slower inflow condition. When the local velocity increased from 2.52 cm min−1 to 3.72 cm min−1, the number of colonies increased with local flow velocity, but there was no continuous biofilm detected under these conditions (Figure 3A). More continuous biofilms formed under the faster inflow condition (Figure 3C). Large clusters formed in region R1, where the local velocity was 5.76 cm min−1. In regions R2 and R4, which had higher velocities of 7.62 cm min−1 and 7.50 cm min−1, respectively, clusters connected together leading to higher surface coverage. Smooth, continuous biofilms developed in region R7, where the local velocity was 9.00 cm min−1. Additional roughness developed under higher velocities at R5 and R3, and then more clusters, towers and mushroom features appeared under the fastest velocities in regions R6, R8, and R9.
Single-species Flavobacterium biofilms developed different morphologies under the same flow conditions. Under the slow inflow rate, very thin and discontinuous biofilms formed, and there was no significant difference between regions R1-R9, as shown in Figure 3B. Loose and filamentous biofilms formed under the fast inflow rate, and biofilm structures changed with local velocities as shown in Figure 3D. Sparse biofilms formed in region R1 under the flow velocity 5.76 cm min−1. Rougher and thicker biofilms formed in regions R2, R3, R4, R5, and R7, which had higher velocities ranging from 7.50 cm min−1 to 9.36 cm min−1. When the local velocity exceeded 9.78 cm min−1, biofilms became looser and were unable to fully cover the substratum (regions R6, R8, and R9).
3.3 Quantitative description of biofilm morphology
Quantitative analysis of the confocal images supports more specific comparisons of differences in biofilm structure with flow conditions. We quantitatively compared the biofilm morphology using four parameters calculated from the confocal images: Biomass per unit area (μm3μm−2), average biofilm thickness (μm), roughness (dimensionless), and surface-area-to-volume ratio (As/V, μm2μm−3) (Heydorn, et al., 2000).. These parameters are plotted as functions of local velocity in Figure 4.
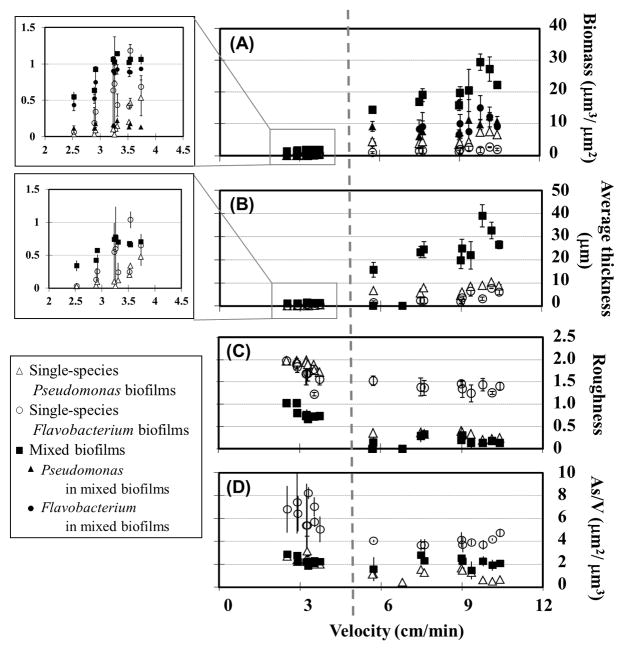
Figure 4.
Biofilm biomass and morphology as a function of local velocity in single- and mixed-species biofilms. Open symbols represent single-species biofilms of Pseudomonas aeruginosa PAO1 and Flavobacterium sp. CDC-65, and solid symbols represent mixed biofilms. A) Total biomass of single- and mixed-species biofilms, and biomass of each organism within mixed biofilms. B) Average thickness, C) Roughness and D) Surface-area-to-volume ratio (As/V) of single- and mixed-species biofilms. Plotted points and error bars represent the average and standard deviation of three replicate observations. Similar trends were observed in other experiments.
Under velocities of 2.52–3.72 cm min−1, Flavobacterium in mixed biofilms accumulated 0.4–0.9μm3μm−2 biomass, while Pseudomonas biomass only reached 0.11–0.22 μm3μm−2. Overall, Flavobacterium accumulated 4–7 times more biomass than Pseudomonas, and was the dominant member of the consortium in mixed biofilms (p < 0.01). The average thickness of the mixed biofilms increased from 0.34μm to 0.74μm under velocities of 2.52 cm min−1 to 3.24 cm min−1, and then reached a constant value of 0.73 μm under velocities of 3.24–3.72 cm min−1.
Under the fast flow conditions, the biomass of Pseudomonas in mixed cultures was around 7.0μm3μm−2 under velocities of 7.50–9.00 cm min−1, and rose above 10.0 μm3μm−2 when velocity exceeded 9.36 cm min−1. A positive relationship was observed between biomass accumulation and local velocity for Flavobacterium under low velocities, but this trend reversed under high local velocity. The Flavobacterium biomass increased from 3.8 μm3μm−2 at 5.76 cm min−1 to 15.1 μm3μm−2 at 9.78 cm min−1, but declined to 12.6 μm3μm−2 at 10.1 cm min−1 and even further to 9.2 μm3μm−2 at 10.4 cm min−1 (R9). Total biomass showed the same trend as Flavobacterium, with a positive relationship between biomass accumulation and velocity until the velocity exceeded 9.78 cm min−1. The average thickness of the mixed biofilms reached 38.9 μm under a velocity 9.78 cm min−1, and then decreased with velocity to 32.7 μm under 10.1 cm min−1 and 26.5 μm under 10.4 cm min−1 (Figure 4B).
The roughness of mixed biofilms showed an inverse relationship with velocity under slow inflow conditions, with roughness decreasing from 1.0 to 0.7 as velocity increased from 2.52 cm min−1 to 3.72 cm min−1. Under fast flow conditions, the roughness of mixed biofilms varied between 0.1 and 0.3 (Figure 4C). As/V values of mixed biofilms ranged from 1.5 to 2.8 μm2μm−3 under velocities of 2.52–10.4 cm min−1. In addition, mixed biofilms with more Pseudomonas biomass had less complex structures than those dominated by Flavobacterium. For instance, the lowest As/V observed in mixed biofilms, 1.5, occurred in locations R1 and R3 under velocities of 5.76 cm min−1 and 9.36 cm min−1, where Pseudomonas had significantly greater biomass than Flavobacterium (Figure 4A).
To compare the difference between single-species biofilms and mixed-species biofilms, characteristics of single-species biofilms are shown in Figure 4. Under slow inflow conditions, biomass of both single-species biofilms showed a positive relationship with local velocity (inserts in Figures 4A and 4B). Single-species Flavobacterium biofilms had greater biomass (0.06–1.18 μm3μm−2) than single-species Pseudomonas biofilms (biomass 0.03–0.46 μm3μm−2). Compared with mixed biofilms, Pseudomonas accumulated more biomass in single-species cultures (p < 0.01), but Flavobacterium did not show a significant difference between mixed and single-species cultures (p > 0.05).
Under fast inflow conditions, single-species Pseudomonas biofilms showed a slight increase from 4.4 μm3μm−2 to 7.5 μm3μm−2 over the velocity range of 5.76–10.4 cm min−1, and then maintained around 7.0 μm3μm−2 of biomass up to a velocity of 10.4 cm min−1. Single-species Flavobacterium biofilms accumulated less biomass than single-species Pseudomonas: 0.99 μm3μm−2 under 5.76 cm min−1, and 1.6–2.6 μm3μm−2 at velocities from 7.50 cm min−1 to 10.4 cm min−1. In addition, Flavobacterium accumulated significantly less biomass in single-species biofilms than it did in mixed biofilms (p < 0.001). Under higher local velocities, the average thickness of single-species Pseudomonas remained at 4.6–10.3 μm, while the thickness of single-species Flavobacterium increased slightly with velocity, though less than observed for single-species Pseudomonas. The single-species biofilms achieved significantly lower average thicknesses everywhere than mixed biofilms: single-species Pseudomonas biofilms were 2–4 times thinner and single-species Flavobacterium biofilms were 3–10 times thinner than the mixed-species biofilm (Figure 4B).
When local velocities increased from 2.52 cm min−1 to 3.72 cm min−1, the roughness of both single-species biofilms declined: from 2.0 to 1.7 with Pseudomonas and from 2.0 to 1.6 with Flavobacterium. Similar behavior was observed in mixed biofilms, but single-species biofilms were rougher under all conditions. Under higher velocities, 5.76–10.4 cm min−1, single-species Pseudomonas biofilms maintained a low roughness of 0.2–0.4, but Flavobacterium biofilms had a high roughness of 1.2–1.5 (Figure 4C).
In Figure 4D, it can be seen that As/V values of single-species Pseudomonas ranged between 2.0–3.1 μm2μm−3 under slow inflow conditions, but single-species Flavobacterium had much greater As/V values of 5.0–8.2 μm2μm−3. With the greater velocity under the fast inflow conditions, the As/V values of both organisms decreased. Single-species Pseudomonas biofilms had a small As/V of 0.5–1.5 μm2μm−3 under velocities of 5.76–10.4 cm min−1, and single-species Flavobacterium biofilms had As/V ranging from 3.7–4.8 μm2μm−3. As/V values of mixed biofilms didn’t significantly change from slow inflow conditions to fast inflow conditions. Moreover, they were similar with the values of single-species Pseudomonas biofilms under slow inflow conditions; while they were greater than that of single-species Pseudomonas biofilms and smaller than that of single-species Flavobacterium biofilms under fast inflow conditions.
3.4 Effects of solute transport on biofilm growth
Energy-yielding substrates and nutrients in growth media are consumed by metabolically active cells within biofilms. To test the effects of this depletion, we evaluated biofilm properties as a function of solute travel time and distance from the flow cell inlets. The travel distance to each location within the flow cell was calculated by integrating along the flow paths determined using the lattice Boltzmann model simulations, and the travel time was calculated similarly by integrating the local velocity along flow paths. Travel time and distance here are surrogate measures for the overall depletion of the growth medium during transport through the flow cell. We used these measures because we could calculate them directly for all locations in the flow cell, but we could not measure relevant chemical concentrations in situ. The solute travel time and distance from the flow cell inlets were calculated by the lattice Boltzmann simulations based on the velocity field shown in Figure 1B (Zhang, et al., 2011).The relationship between biomass accumulation and solute travel time is shown in Figure 5, and the associated relationship with travel distance is presented in Supplementary Figure 1.
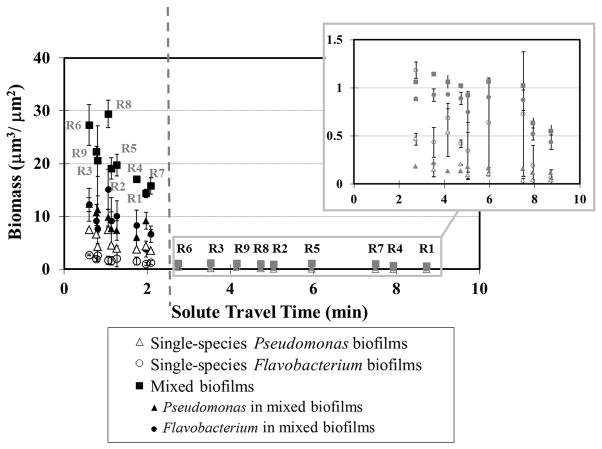
Figure 5.
Relationship between biofilm biomass and solute travel time from the flow-cell inlet to the observation locations. Open symbols represent single-species biofilms, and solid symbols represent mixed biofilms. Black icons show observations obtained under fast inflow rate (0.80 mL min−1), and gray icons in the inset show observations obtained under the slow inflow rate (0.16 mL min−1). Plotted points and error bars represent the average and standard deviation of three replicate observations. Similar trends were observed in other experiments.
In single-species Pseudomonas biofilms, an inverse relationship was found between the solute travel time and biomass: when solute travel time increased from 0.6 min to 8.7 min, Pseudomonas biomass decreased from 7.5 μm3μm−2 to 0.03 μm3μm−2. A similar but weaker trend was found for Pseudomonas in mixed biofilms, with accumulated biomass decreasing from 12.2μm3μm−2 at 0.6 min to 0.11μm3μm−2 at 8.7 min. Single-species Flavobacterium biofilms accumulated biomass of 0.06–2.7 μm3μm−2, but the amount of biomass did not decrease with solute travel time. Flavobacterium accumulated more biomass in mixed biofilms than in single-species culture, and interestingly, in mixed biofilms it also showed the inverse relationship between biomass and solute travel time as that observed for Pseudomonas. The biomass of Flavobacterium in mixed biofilms decreased from 12.3 μm3μm−2 to 0.43 μm3μm−2 as solute travel time increased from 0.6 min to 8.7 min. Total biomass of mixed biofilms also showed a significant inverse relationship over the same range of solute travel time, with observed total biomass decreasing from 27.3 μm3μm−2 to 1.27 μm3μm−2.
We will now consider the inverse relationship between biofilm biomass and travel time for the mixed culture in more detail. Under the fast inflow condition, regions R2 and R4 had similar velocities and shear stresses, but biomass abundance in R2 was 19.1 μm3μm−2, greater than that observed at R4 (17.0 μm3μm−2). This difference is explained by the difference in the solute travel time to these regions. The travel time to R2 was much faster than to R4 (1.1 min vs. 1.8 min). Regions R3, R5 and R7 also showed similar local velocities but inverse relationships between travel time and biomass (travel times: R3 = 0.8 min, R5 = 1.3 min, R7 = 2.1 min, biomass accumulation: R3 = 20.6 μm3μm−2, R5 =19.7 μm3μm−2 and R7 = 15.8 μm3μm−2). The only exception to this trend was region R8 under the fast flow condition, where the travel time was 1.1 min but the accumulated biomass was 29.4 μm3μm−2. No clear relationship was found between biomass accumulation and solute travel distance in either single- or mixed-species biofilms under either flow condition (Supplementary Figure 1). Note that the fluid velocity varies substantially within the flow cell (Figure 1), so the solute travel time is not linearly proportional to the solute travel distance. Therefore, these results indicate that the travel time, and not simply travel distance, provides the best measure of the consumption of growth medium from community metabolism along flow paths.
4 Discussion
We studied interactions between Pseudomonas and Flavobacterium in mixed-species biofilms under imposed flow gradients to evaluate relationships between environmental conditions, community structure (dominant organism), and biofilm morphology. We found that Pseudomonas and Flavobacterium showed different behavior in single- and mixed-species biofilms due to interactions between the two organisms. Further, the interactions between the two organisms varied between competitive and synergistic depending on external flow conditions.
Competitive interactions occurred under slow-flow conditions, which provide a lower supply of growth medium, including nutrients and substrates that support biomass synthesis and substrates that support catabolism. The delivery of nutrients and substrates to cells involves not only bulk transport into and through the flow cell, but also transport from the bulk fluid into and through the biofilm. Mathematical simulations and experimental results have shown that faster bulk fluid flow increases the delivery of nutrients and substrates into biofilms even under identical bulk influent concentration (Duddu, et al., 2009, Zhang, et al., 2011). Conversely, nutrients and substrates become depleted by cumulative metabolism along flow paths, leading to spatial patterns in biofilm development. We observed a positive correlation between biomass abundance and influx of growth medium, and a negative correlation between biomass abundance and solute travel time, suggesting that biofilm growth was limited by progressive depletion of nutrients and/or substrates along flow paths within the flow cell. Under competitive conditions, with nutritional limitations imposed by slow influx of growth medium, Pseudomonas accumulated less biomass in mixed biofilms than in single-species culture. Moreover, the spatial distributions of Pseudomonas and Flavobacterium also reflected their competition for growth medium in mixed biofilms. Flavobacterium secured greater opportunity for nutrient replenishment by colonizing the top layer of the biofilm and generating greater surface area. Pseudomonas was overgrown by Flavobacterium and was therefore restricted to the poorer nutritional environment at the base of the biofilm. Similar spatial structure had been observed previously in co-culture of P. aeruginosa and Agrobacterium tumefaciens, but in that consortium P. aeruginosa predominated and buried A. tumefaciens (An, et al., 2006). Here, the specific growth rate of Flavobacterium under the experimental temperature (25°C) was greater than that of Pseudomonas, suggesting that Flavobacterium was able to win the competition for access to fresh growth medium in the mixed biofilm simply by out-growing, and ultimately over-growing Pseudomonas. Liu et al. (2009) similarly found that trends in growth rate observed in batch do not always hold in more complex open systems. The absence of sensitivity to metabolic products from either organism suggests that metabolic inhibition did not influence growth patterns, but rather simple competition for resources caused the decreased growth of Pseudomonas in mixed biofilms under slow inflow conditions. Thus, Flavobacterium won the competition for nutrients and substrates under slow flow conditions and its existence inhibited the growth of Pseudomonas by limiting Pseudomonas to poorer-quality habitat.
Different patterns were observed under faster flow conditions that provided increased influx of growth medium. Under the fast-flow conditions, both organisms accumulated significantly more biomass in mixed biofilms than in single-species biofilms, and more biofilm biomass accumulated in regions having higher local velocity. However, single-species biofilms exhibited different behavior. Single-species Pseudomonas biofilms accumulated significantly greater biomass under the higher inflow condition, which indicated that increasing influx supported more growth of Pseudomonas. We previously observed a similar positive relationship between fluid velocity and Pseudomonas biofilm biomass with Luria-Bertani broth (Zhang, et al., 2011). Higher hydrodynamic shear eventually leads to biofilm detachment (Zhang, et al., 2011), but we didn’t observe significant detachment of Pseudomonas in the experiments reported here. Conversely, the growth of Flavobacterium biofilms in monoculture did not depend on local velocity, and single-species Flavobacterium biofilms accumulated less biomass than single-species Pseudomonas biofilms. The results indicated that trends of biomass accumulation observed under flow conditions didn’t follow growth rates observed in batch experiments. The growth rates observed in mixed-species biofilms also didn’t follow trends from single-species biofilms, indicating that spatial patterns of nutrients or substrate depletion substantially modified local growth behavior. In addition, Flavobacterium biofilms were more heterogeneous and had more complex surface morphology than Pseudomonas biofilms, as evidenced by their greater roughness and surface area. Rougher and more porous morphologies are more easily detached by hydrodynamic shear (Garny, et al., 2008), suggesting that the accumulation of Flavobacterium in single-species biofilms was limited by detachment induced by hydrodynamic shear stress. Mixed biofilms showed decreased roughness, which are more stable. Moreover, the competition relationship between Pseudomonas and Flavobacterium under slow flow rate indicated that they don’t produce molecules to help the other. Thus, increased Flavobacterium accumulation in mixed-species biofilms can potentially be explained by the changes in the biofilm morphology that occurred in co-culture with Pseudomonas.
While we did directly observe local velocities, we expect that biofilm development had a small effect on the flow field in these experiments. R2A medium was used here to be relevant to typical depleted environmental conditions, and only thin biofilms (average thickness < 40 μm) formed in this nutrient-poor environment. Although biofilms have been observed to influence fluid flow patterns (Stoodley, et al., 1994, Manz, et al., 2003), significant changes normally only occur with more extensive biofilm growth. The feedback between biofilm growth and the flow field should be considered when biofilms become thicker. This feedback is likely to be important over the long term and in more nutrient-rich environments.
In sum, our results indicate that inter-species interactions between Pseudomonas and Flavobacterium in co-cultured biofilms were significantly affected by external flow conditions. While organism-specific growth rates are important in the competition for securing space and providing favorable access to nutrients and substrates, the structural and morphological properties of the biofilm are also important because they mediate both solute delivery to cells within the biofilm and adherence of the community to the surface. We found that biofilm morphology and mechanical properties are particularly important to the accumulation of biomass under flow. This finding suggests that organisms that produce the most resilient biofilms are critical to the colonization of surfaces in flowing waters, as such stable biofilms can provide protected habitat that other organisms can colonize. Similarly, inter-species interactions that favor development of more resilient biofilms can support enhanced colonization of surfaces subject to high hydrodynamic shear. Further, our observation that both species showed greater biomass in mixed biofilms suggests that synergy arises from increased efficiency of overall community metabolism as a result of the spatial segregation of organisms within the biofilm. Such enhanced community metabolism depends not only on the metabolic capability of each organism, but also on the spatial configuration and mechanical properties of the biofilm because these properties determine the regions that can be colonized by each organism in the community. This was exemplified here by the structural changes to the biofilm that allowed Flavobacterium to accumulate more biomass in co-culture with Pseudomonas than it could on its own. These findings are important because they indicate that the behavior of multi-species biofilms cannot be understood purely by integrating information obtained from a sense of independent investigations of individual organisms. Instead, it is necessary to specifically identify ecological interactions between species that influence the exploitation of the environment by complex communities.
Supplementary Material
References
- An DD, Danhorn T, Fuqua C, Parsek MR. Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3828–3833. doi: 10.1073/pnas.0511323103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaissie EJ, Penzak SR, Dignani MC. The hospital water supply as a source of nosocomial infections. Archives of Internal Medicine. 2002;162:1483–1492. doi: 10.1001/archinte.162.13.1483. [DOI] [PubMed] [Google Scholar]
- Battin TJ, Kaplan LA, Newbold JD, Hansen CME. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature. 2003;426:439–442. doi: 10.1038/nature02152. [DOI] [PubMed] [Google Scholar]
- Beaufort S, Da Silva T, Lafforgue C, Alfenore S. Fluorescent proteins as in-vivo and in-situ reporters to study the development of a Saccharomyces cerevisiae yeast biofilm and its invasion by the bacteria Escherichia coli. Fems Microbiology Ecology. 2012;80:342–351. doi: 10.1111/j.1574-6941.2012.01301.x. [DOI] [PubMed] [Google Scholar]
- Brinkmeyer R, Knittel K, Jurgens J, Weyland H, Amann R, Helmke E. Diversity and structure of bacterial communities in arctic versus antarctic pack ice. Applied and Environmental Microbiology. 2003;69:6610–6619. doi: 10.1128/AEM.69.11.6610-6619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp DL, Kirisits MJ, Moran B, Parsek MR. A mathematical model of quorum sensing in a growing bacterial biofilm. Journal of Industrial Microbiology & Biotechnology. 2002;29:339–346. doi: 10.1038/sj.jim.7000316. [DOI] [PubMed] [Google Scholar]
- Davey ME, O’toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiology and Molecular Biology Reviews. 2000;64:847. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddu R, Chopp DL, Moran B. A Two-Dimensional Continuum Model of Biofilm Growth Incorporating Fluid Flow and Shear Stress Based Detachment. Biotechnology and Bioengineering. 2009;103:92–104. doi: 10.1002/bit.22233. [DOI] [PubMed] [Google Scholar]
- Elvers KT, Leeming K, Moore CP, Lappin-Scott HM. Bacterial-fungal biofilms in flowing water photo-processing tanks. Journal of Applied Microbiology. 1998;84:607–618. doi: 10.1046/j.1365-2672.1998.00388.x. [DOI] [PubMed] [Google Scholar]
- Ford TE. Microbiological safety of drinking water: United States and global perspectives. Environmental Health Perspectives. 1999;107:191–206. doi: 10.1289/ehp.99107s1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garny K, Horn H, Neu TR. Interaction between biofilm development, structure and detachment in rotating annular reactors. Bioprocess and Biosystems Engineering. 2008;31:619–629. doi: 10.1007/s00449-008-0212-x. [DOI] [PubMed] [Google Scholar]
- Hansen SK, Rainey PB, Haagensen JAJ, Molin S. Evolution of species interactions in a biofilm community. Nature. 2007;445:533–536. doi: 10.1038/nature05514. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Ersboll BK, Hentzer M, Parsek MR, Givskov M, Molin S. Experimental reproducibility in flow-chamber biofilms. Microbiology-Uk. 2000;146:2409–2415. doi: 10.1099/00221287-146-10-2409. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology-Uk. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE. Oral microbial communities: Biofilms, interactions, and genetic systems. Annual Review of Microbiology. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tay JH. The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Research. 2002;36:1653–1665. doi: 10.1016/s0043-1354(01)00379-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang W, Sileika T, Warta R, Cianciotto NP, Packman A. Role of bacterial adhesion in the microbial ecology of biofilms in cooling tower systems. Biofouling. 2009;25:241–253. doi: 10.1080/08927010802713414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang W, Sileika T, Warta R, Cianciotto NP, Packman AI. Disinfection of bacterial biofilms in pilot-scale cooling tower systems. Biofouling. 2011;27:393–402. doi: 10.1080/08927014.2011.577525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz B, Volke F, Goll D, Horn H. Measuring local flow velocities and biofilm structure in biofilm systems with magnetic resonance imaging (MRI) Biotechnology and Bioengineering. 2003;84:424–432. doi: 10.1002/bit.10782. [DOI] [PubMed] [Google Scholar]
- Manz W, Wendt-Potthoff K, Neu TR, Szewzyk U, Lawrence JR. Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microbial Ecology. 1999;37:225–237. doi: 10.1007/s002489900148. [DOI] [PubMed] [Google Scholar]
- Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology-Sgm. 2001;147:3121–3126. doi: 10.1099/00221287-147-11-3121. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen S, Salminen S, Bylund G, Ouwehand AC. Characterization of the properties of human- and dairy-derived probiotics for prevention of infectious diseases in fish. Applied and Environmental Microbiology. 2001;67:2430–2435. doi: 10.1128/AEM.67.6.2430-2435.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrison LH, Bibb WF, Cherry WB, Thacker L. Determination of Antigenic Relationships among Legionellae and Non-Legionellae by Direct Fluorescent-Antibody and Immunodiffusion Tests. Journal of Clinical Microbiology. 1983;17:332–337. doi: 10.1128/jcm.17.2.332-337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmann J, van Gemerden H. Microbial interactions involving sulfur bacteria: implications for the ecology and evolution of bacterial communities. Fems Microbiology Reviews. 2000;24:591–599. doi: 10.1111/j.1574-6976.2000.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Pearson ML, Farr BM, Garland JS, Mermel LA, Raad II, Sheretz RJ, Stover BH. Guideline for prevention of intravascular device-related infections.1. Intravascular device-related infections: An overview. American Journal of Infection Control. 1996;24:262–293. doi: 10.1016/s0196-6553(96)90058-9. [DOI] [PubMed] [Google Scholar]
- Purevdorj B, Costerton JW, Stoodley P. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology. 2002;68:4457–4464. doi: 10.1128/AEM.68.9.4457-4464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H. Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Applied and Environmental Microbiology. 1999;65:1280–1288. doi: 10.1128/aem.65.3.1280-1288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm WE, Colella JJ, Anderson RL, Dixon RE. Indwelling Arterial Catheters as a Source of Nosocomial Bacteremia - Outbreak Caused by Flavobacterium Species. New England Journal of Medicine. 1975;292:1099–1102. doi: 10.1056/NEJM197505222922105. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Debeer D, Lewandowski Z. Liquid Flow in Biofilm Systems. Applied and Environmental Microbiology. 1994;60:2711–2716. doi: 10.1128/aem.60.8.2711-2716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Ward DM, Ferris MJ, Nold SC, Bateson MM. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiology and Molecular Biology Reviews. 1998;62:1353. doi: 10.1128/mmbr.62.4.1353-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengler K. Central Role of the Cell in Microbial Ecology. Microbiology and Molecular Biology Reviews. 2009;73:712–729. doi: 10.1128/MMBR.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sileika TS, Chen C, Liu Y, Lee J, Packman AI. A novel planar flow cell for studies of biofilm heterogeneity and flow-biofilm interactions. Biotechnology and Bioengineering. 2011;108:2571–2582. doi: 10.1002/bit.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.