Abstract
Development of specific ligands for protein targets that help decode the complexities of protein-protein interaction networks is a key goal for the field of chemical biology. Despite the emergence of powerful in silico and experimental high-throughput screening strategies, the discovery of synthetic ligands that selectively modulate protein-protein interactions remains a challenge for bioorganic and medicinal chemists. This Perspective discusses emerging principles for the rational design of PPI inhibitors. Fundamentally, the approach seeks to adapt nature’s protein recognition principles for the design of suitable secondary structure mimetics.
Keywords: Protein-protein interactions, Secondary structure mimics, Computational design, Hot spot residues, Rational design
1. Introduction
Protein-protein interactions (PPIs) are attractive targets for therapeutic intervention because of their fundamental roles in vital biological processes including gene expression, cell growth, proliferation, nutrient uptake, morphology, motility, intercellular communication and apoptosis. The past decade has seen emerging methods to inhibit these complexes, which have traditionally been termed “undruggable”. Although it is early to say if inhibition of PPIs will become a routine strategy for drug design, preliminary success in the field provides guidelines for the types of interfaces that may be amenable to disruption by synthetic compounds.1–4 Pharmaceutical chemists often gravitate toward design of enzyme inhibitors as drug candidates – enzymes as a class constitute roughly half of drug targets.5 Enzymes are appealing targets for a list of reasons: (1) they serve as critical levers for biological functions, (2) nature offers small molecules that may be used as templates for further manipulation,6 (3) mechanism-based inhibitors may be rationally designed,7 and (4) enzyme pockets are often appropriately sized for small molecules.8, 9 The largely flat and pocket-less protein interfaces lack several of these features but are fascinating both because of basic challenges associated with molecular design and for the prospect of exploring relatively uncharted biology for therapeutic intervention.
2. A Secondary Structure-Centric View of Protein Interfaces
The Protein Data Bank, with roughly 10,000 entries of multiprotein complexes, provides a treasure of illustrations to unravel the rules nature employs to bring protein partners together (Figure 1). One approach for the design of synthetic inhibitors is based on rational design of protein subdomains to interfere with these complex formations.4, 10–13 A second successful method utilizes computational and experimental high-throughput and fragment-based screening strategies to locate small molecule fragments that stick to protein surfaces.14–19 Strategies that afford scaffolds for PPIs based on natural products and natural product like molecules,20 peptide macrocycles21 and phage display-based miniproteins22–24 have also led to significant success. Although, PPIs cover large surface areas, often, a small subset of residues (termed “hot spot residues”) contributes significantly to the binding free energy.25–28 An analysis of PPIs, which have been successfully inhibited by small molecules, suggests that a category of PPIs contains features that approximate enzyme active sites, i.e., they contain an array of hot spot residues clustered within relatively small radii.27–29 With this viewpoint, we and others have assessed the dataset in the PDB30 to identify the subset of PPIs that can be potential targets for small molecules, and those that would require larger molecules.4, 31–34 Several computational strategies to define pockets on protein-protein interfaces for drug design have been outlined.4, 35–39 Our approach has centered on the role of secondary structures in mediating protein-protein interactions.40 A key advantage of designing protein secondary structure mimetics is that the array of side chain residues along a conformationally defined backbone facilitates molecular design. A second advantage is that direct mimics of protein secondary structures provide medium-sized molecules, which may potentially target the chosen protein with high affinity and specificity.
Figure 1.
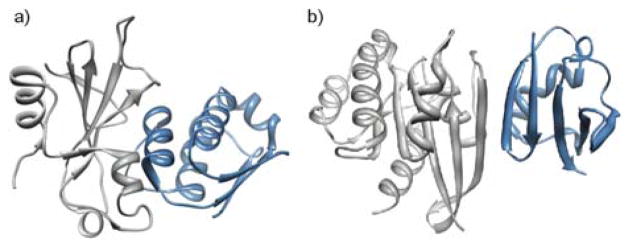
Protein-protein interactions are often mediated by secondary structures: (a) α-helical and (b) β-sheet interfaces from barnase_barstar (PDB code: 1BGS) and Raf-Rap (PDB code: 1GUA), respectively.
A basic challenge associated with this secondary structure-centric approach involves dissection of the energetic contribution of the specific secondary structure to the protein-protein complex. Three related questions include: (1) what is the minimum proportion of the free energy of binding that must reside on the secondary structure for its mimetic to retain inhibitory activity? (2) Are there interfaces that are naturally more suited for small mimics of target protein secondary structures and those that would require larger mimics? (3) How small can the mimetic be made to procure the “drug like” properties linked to small molecules while retaining specificity associated with larger molecules? We have undertaken computational efforts to probe these questions and have begun to experimentally evaluate hypotheses emerging from these studies.
Our initial analysis has focused on PPIs that feature helices at interfaces, although the approach can be extended to other motifs. Targeting of α-helical interfaces offers several basic advantages: α-helices constitute the largest class of protein secondary structures – roughly 60% of protein-protein interactions in the current PDB contain helices at interfaces.31 And, helices are often easier to mimic than other secondary structures such as β-strands, which tend to aggregate; although, there has been significant progress in the design of β-strand mimics.41–48 Importantly, stable mimics of interfacial helices have been shown to be useful as potential leads for drug design.11, 49–55
In this Perspective, we discuss structural attributes of PPIs that our group uses to initiate design of either small molecule helix mimetics56–60 or stabilized peptide helices.10, 13, 61–63 Some interfaces may be targeted by either strategy. Stabilized peptide helices utilize constraints to order the peptide backbone while small molecule or nonpeptidic helix mimetics array the critical peptide side chain functional groups on a synthetic scaffold.13 On the basis of spatial arrangement of hotspot residues at the interface, revealed by alanine scanning mutagenesis data,64, 65 we classified PPIs as binding clefts or extended interfaces (Figure 2).31 Receptors with clefts are targeted by helices with two or more hot spot residues within a 7Å radius, while the extended interfaces category features a distribution of hot spot residues over a larger distance of 7–30 Å. Camacho and coworkers recently suggested that a combination of computed change in solvent accessible surface areas (ΔSASA) and energy scores may be a better gauge of hot spot residues than alanine scans alone.33 For alanine mutagenesis scans, the ΔΔG value refers to the change in free energy when a residue is mutated to alanine, thus a positive value indicates that mutation to alanine decreases the affinity of PPI and wild type residue contributes to binding. For interfacial residues, the ΔSASA of a residue is calculated by subtracting the SASA of the residue in the PPI complex from the SASA of the individual residue without any partner protein chains, and a positive value indicates that the residue is buried in the PPI complex and less accessible to solvent. Rosetta29, 65 and PocketQuery66 offer easily accessible resources for such computational analyses.
Figure 2.
Helical interfaces can be divided between those that feature clefts for binding and those with extended interfaces. The cleft interfaces may be compared to enzyme pockets in that a high density of important contacts is concentrated in a small region. The Gleevek/tyrosine kinase (PDB code: 1XBB), p53/MDM2 (PDB code: 1YCR) and cyclin- dependent kinase6/D-type viral cyclin (PDB code: 1G3N) complexes are representative examples of enzyme pockets, binding cleft and extended interfaces, respectively.
We hypothesized that a single turn of the α-helix approximates the distance traversed by typical small drug candidates,39 i.e. Lipinski’s “rules of five” compliant molecules.67, 68 Indeed, the classical small molecule inhibitors of PPIs – notably the nutlin family compounds developed by Roche to target the p53/Hdm2 complex – are mimics of residues that span one helical turn.69, 70 The high density of hot spot residues in binding clefts evoke array of functionality in enzymatic pockets. Based on this analysis, we postulate that binding clefts may be targetable by small molecule or nonpeptidic helix mimetics; however, extended interfaces that feature hot spot residues spanning a much larger number of helical turns will likely require medium to large sized molecules such as stabilized peptide helices for specific inhibition.31 Although, these larger inhibitors will not fit the mold of canonical “drug-like” molecules, emerging evidence suggests that macrocycles and other discretely folded molecules may translocate to target intracellular interactions.71–73
We further classified interfacial interactions as potentially capable of leading to “high affinity” inhibitors if the average experimental/computed ΔΔGavg for alanine mutagenesis of 2–4 hot spot residues is ≪2 kcal/mol. These empirically derived values emerge from the analysis of known small molecule inhibitors of PPIs, as described previously.31 We predict that if the ΔΔG for alanine substitution of wild-type hot spot residues is ≪2 kcal/mol, inhibitors based on wild-type sequence may bind the target protein with a low affinity (i.e. with dissociation constants in the micromolar range). However, this analysis only considers natural interactions and wild-type residues; use of non-natural residues may provide higher affinity leads. Furthermore, these predictions are based on high-resolution structures of multi-protein complexes and do not account for protein dynamics. Nevertheless, use of these rubrics has provided a starting point for the rational design of new classes of protein-protein interaction inhibitors.52, 74 Below, we discuss specific examples of protein complexes where the secondary structure-centric approach has been or could be applied to discover inhibitors.
3. Classification of helical protein interfaces
3.1. Binding Clefts
This category of PPIs contains a cleft for helical residues, that we predict are candidates for high throughput screening efforts with small molecule libraries;38, 75 medium-sized molecules such as stabilized peptide helices may also effectively modulate these interactions. Several members in this class of PPIs have been successfully targeted.3, 19 The reason for this success could be due to the fact that the physical attributes shared by members of this class are reminiscent of traditional enzyme targets.
The interaction of the p53 activation domain with MDM2 is perhaps the most successfully targeted protein-protein interaction.76–80 A number of inhibitors are currently in clinical trials as potential cancer therapeutics.76 The p53 AD adopts a helical conformation to target MDM2 with three hydrophobic hot spot residues on successive helical turns.69 Successful synthetic analogs mimic two or all three of these residues.70, 77 The three hot spots lie close in space to each other with very large average alanine mutagenesis, ΔΔGavg ~3.5 kcal/mol, and ΔSASA values (Table 1). The interactions of Bcl-2 family anti-apoptotic proteins with the pro-apoptotic BH3 domains81 provide alanine scan and ΔSASA values of similarly high magnitude have also been successfully targeted with small molecules82–84 and stabilized helices.50, 85 Realization of potent inhibitors for these interactions suggests that complexes that feature a similar density of hot spot may also be targeted effectively. A full set of such complexes has been reported.31 Table 1 lists a few examples, with ΔΔG and ΔSASA values and relative positioning of hot spots, that fit our rubric for cleft interfaces.
Table 1.
Classification of helical protein interfaces.
| Types of PPIs | PDB code, helix sequence | Arrangement of hot spot residues on the target helix | ΔΔG (kcal/mol)1 | ΔSASA2 | Known Inhibitors | Protein names Function |
|---|---|---|---|---|---|---|
| Interfaces with Binding Clefts | ||||||
|
1YCR 19FSDLWKLLPE28 |
i | 3.8 | 131.1 | MDM2 (gray): coactivator p53 (green): transcriptional activator. Function:Apoptotic signaling, cell-cycle arrest |
|
| i + 4 | 4.4 | 136.7 | ||||
| i+ 7 | 2.0 | 73.4 | ||||
| (Avg) | (3.4) | (113.7) | ||||

|
1Y3A 5WYDFLM10 |
i | 4.2 | 168.3 | Giα 1 (gray): Guanine nucleotide-binding protein alpha-1 subunit KB752 (green): peptide Function: Signaling protein |
|
| i + 3 | 3.3 | 93.9 | ||||
| i +4 | 1.8 | 83.8 | ||||
| (Avg) | (3.1) | (115.3) | ||||
|
1BXL 578LAIIGDDI585 |
i | 2.7 | 102.2 | BCL-XL (gray): BAK peptide (green): Function: apoptosis |
|
| i + 3 | 2.2 | 87.3 | ||||
| i +7 | 1.2 | 79.3 | ||||
| (Avg) | (2.0) | (89.6) | ||||
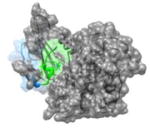
|
1JSU 85PEFYYR90 |
i | 2.9 | 122.1 | CDK2(gray): cyclin dependent kinase-2 P27(KIP1): kinase inhibitor (blue, helix in green) Function: Enzyme Kinase |
|
| i+1 | 4.9 | 116.8 | ||||
| i+3 | 1.1 | 64.6 | ||||
| (Avg) | (3.0) | (101.2) | ||||
| Extended Helical Interfaces | ||||||
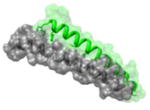
|
1AIK 628WMEWDREINNYTSLI642 |
i | 2.8 | 160.69 | HR1 (gray): N-terminal heptad repeat of HIV-1 GP41 HR2 (green): C-terminal heptad repeat of HIV-1 GP-41 Function: glycoprotein |
|
| i + 3 | 1.92 | 124.62 | ||||
| i+ 10 | 2.01 | 77.32 | ||||
| i+ 14 | 1.76 | 92.59 | ||||
| (Avg) | (2.12) | (113.8) | ||||
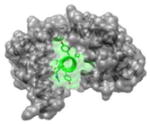
|
3BXK 1961IYAAMMIMEYYRQS1974 |
i | 1.9 | 130.49 | No known inhibitors | Ca2+-CaM (gray): calmodulin Cav 2.1 IQ (green): voltage gated calcium channels-Isoleucine-glutamine Function: Signaling protein |
| i + 1 | 1.6 | 105.38 | ||||
| i + 4 | 1.0 | 104.8 | ||||
| i + 6 | 3.1 | 111.97 | ||||
| i+ 10 | 2.7 | 103.28 | ||||
| i+11 | 2.0 | 107.88 | ||||
| (Avg) | (2.05) | (110.6) | ||||
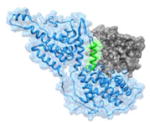
|
1NVW 930FGIYLTNILKTEEGN944 |
i | 1.64 | 43.73 | Ras (gray): guanine nucleotide binding protein Sos (blue, helix in green): Ras-specific guanine nucleotide exchange factor Function: signaling protein |
|
| i+6 | 1.11 | 57.75 | ||||
| i+13 | 1.10 | 57.98 | ||||
| i + 15 | 2.35 | 67.70 | ||||
| (Avg) | (1.55) | (56.79) | ||||
w
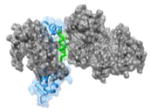
|
1K90 82EEEIREAFRVFDK94 |
i | 0.71 | 78.71 | No known inhibitors | EF(gray): Edema factor Calmodulin (blue, helix in green): Function: Anthrax Toxin |
| i+3 | 1.99 | 90.67 | ||||
| i+6 | 1.84 | 105.7 | ||||
| i+7 | 1.26 | 81.14 | ||||
| i+10 | 1.53 | 64.99 | ||||
| (Avg) | (1.47) | (84.24) | ||||
3.2. Extended Helical Interfaces
Protein-protein interactions that involve discontinuous interaction surfaces with hot spot residues distributed over large contact areas are classified in this category. These PPIs are especially challenging for small molecule inhibitors but may be amenable to disruption by larger molecules such as stabilized helices and peptide foldamers. A full list of extended helical interfaces in the PDB has also been reported and select examples are shown in Table 1.31
The entry of HIV-1 into host cells requires formation of a coiled-coil bundle consisting of gp41 N-terminal and C-terminal helices.86–89 Design of small molecules that target this interaction with high affinity and specificity has been challenging;90 however, potent inhibitors derived from peptide and β-peptide foldamers have been reported,53, 55, 91–94 in keeping with the broad distribution of hot spot residues at this interface.95 Similarly, attempts to target the interaction of Ras and SOS with small molecules had been largely unsuccessful because two key residues on a Sos helix that contacts Ras are located four helical turns away from each other.96, 97 A stabilized helix that spans this length and encompasses key hot spot residues was recently developed as the first direct inhibitor of this complex formation,52 and regulator of the Ras signaling pathway.98 Recent efforts in fragment screening suggest that small molecules that target an allosteric site on Ras may be designed to modulate activity of this therapeutically important target.99, 100
4. Array of Hot Spots on Interfacial α-Helices
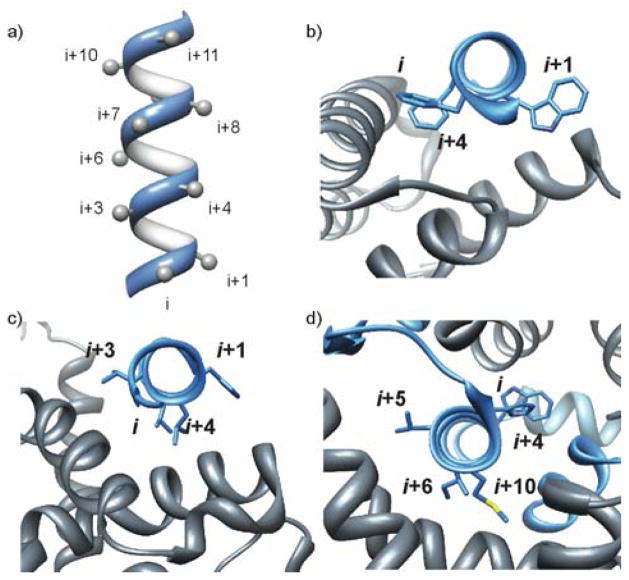
Based on the relative density of hot spots, the helical interfaces may be classified as cleft or extended as discussed above, allowing judicious design of inhibitors or screening strategies. As a next step, rational design of general classes of helix mimics as inhibitors requires mimicry of hot spot positioning on different helical faces. Examination of 480 interfacial helices with high alanine mutagenesis values, i.e. ΔΔGavg ≥ 2 kcal/mol, provides an indication of the distribution of hot spots are arrayed on interfacial helices.32 Roughly 60% of helical interfaces in this dataset feature helices with hot spot residues on one face of the helix, a third of the complexes utilize helices with hot spots on two faces and roughly 10% require all three faces for interaction with the target protein partner.32 Examples of protein complexes with hot spot residues on one face, two faces and three faces are shown in Figure 3.
Figure 3.
(a) Positioning of side-chain residues on a canonical α-helix. (b–d) examples of protein complexes with hot spot residues on one face, two faces, and three faces. (b) Yersinia pestis YopN/TyeA complex, PDB code 1XL3; (c) ligand-binding domain (LBD) of the retinoid X receptor/coactivator complex (PDB code 1XIU); (d) E. coli sigma factor/anti-sigma RseA complex, PDB code 1OR7.
Several different synthetic strategies for helix mimicry have been reported.10, 11, 13 These include design of non-peptidic helix mimetics, constrained helices and foldamers that display protein-like functionality. Each of these strategies offers different advantages. The small molecules potentially excel at bioavailability, while the larger molecules offer additional contacts for enhanced specificity. Helical peptides and foldamers also provide ready access to scaffolds capable of displaying multiple hot spots on all three faces.32 We hypothesize that interfaces that contain a cluster of hot spot residues on the same face of the helix are well suited for small molecules. There are >200 such complexes in the current PDB, providing an attractive opportunity for design of new therapeutics.
5. Conclusions
Mimicry of protein secondary structures has been a long-standing goal of bioorganic and medicinal chemists. Evaluation of protein complexes suggests that interfaces where the hot spot functionality is localized on secondary structures may be inhibited through rational design of peptidic and nonpeptidic scaffolds that feature key functional groups in the desired conformation. We have undertaken a comprehensive study to locate every helical protein-protein interaction in the Protein Data Bank. Computational analysis of these complexes allows prediction of interfaces that may be inhibited by small molecules because the hot spot residues are clustered in a small region or by larger molecules because the hot spots are distributed over a bigger area. We expect similar studies to facilitate future design of PPI inhibitors as new classes of therapeutics.
Acknowledgments
We thank the National Institutes of Health (GM073943) and the National Science Foundation (CHE-1151554) for financial support. B.N.B. thanks New York University for the Dean’s Dissertation Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Morelli X, Bourgeas R, Roche P. Curr Opin Chem Biol. 2011;15:475. doi: 10.1016/j.cbpa.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Thangudu RR, Bryant SH, Panchenko AR, Madej T. J Mol Biol. 2012;415:443. doi: 10.1016/j.jmb.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells JA, McClendon CL. Nature. 2007;450:1001. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 4.Wanner J, Fry DC, Peng Z, Roberts J. Future Med Chem. 2011;3:2021. doi: 10.4155/fmc.11.156. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins AL, Groom CR. Nature reviews Drug discovery. 2002;1:727. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 6.Drahl C, Cravatt BF, Sorensen EJ. Angew Chem Int Ed. 2005;44:5788. doi: 10.1002/anie.200500900. [DOI] [PubMed] [Google Scholar]
- 7.Babine RE, Bender SL. Chemical reviews. 1997;97:1359. doi: 10.1021/cr960370z. [DOI] [PubMed] [Google Scholar]
- 8.Laskowski RA, Luscombe NM, Swindells MB, Thornton JM. Protein science : a publication of the Protein Society. 1996;5:2438. doi: 10.1002/pro.5560051206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dundas J, Adamian L, Liang J. J Mol Biol. 2011;406:713. doi: 10.1016/j.jmb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner J, Harding MM. Organic & biomolecular chemistry. 2007;5:3577. doi: 10.1039/b710425a. [DOI] [PubMed] [Google Scholar]
- 11.Edwards TA, Wilson AJ. Amino Acids. 2011;41:743. doi: 10.1007/s00726-011-0880-8. [DOI] [PubMed] [Google Scholar]
- 12.Goodman CM, Choi S, Shandler S, DeGrado WF. Nat Chem Biol. 2007;3:252. doi: 10.1038/nchembio876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henchey LK, Jochim AL, Arora PS. Curr Opin Chem Biol. 2008;12:692. doi: 10.1016/j.cbpa.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toogood PL. J Med Chem. 2002;45:1543. doi: 10.1021/jm010468s. [DOI] [PubMed] [Google Scholar]
- 15.Erlanson DA, Wells JA, Braisted AC. Annu Rev Biophys Biomol Struct. 2004;33:199. doi: 10.1146/annurev.biophys.33.110502.140409. [DOI] [PubMed] [Google Scholar]
- 16.Arkin MR, Wells JA. Nature reviews Drug discovery. 2004;3:301. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 17.Meireles LM, Mustata G. Current topics in medicinal chemistry. 2011;11:248. doi: 10.2174/156802611794072632. [DOI] [PubMed] [Google Scholar]
- 18.Congreve M, Chessari G, Tisi D, Woodhead AJ. J Med Chem. 2008;51:3661. doi: 10.1021/jm8000373. [DOI] [PubMed] [Google Scholar]
- 19.Hajduk PJ, Greer J. Nature reviews Drug discovery. 2007;6:211. doi: 10.1038/nrd2220. [DOI] [PubMed] [Google Scholar]
- 20.Tan DS. Nat Chem Biol. 2005;1:74. doi: 10.1038/nchembio0705-74. [DOI] [PubMed] [Google Scholar]
- 21.Terrett NK. Drug Discovery Today: Technologies. 2010;7:e97. [Google Scholar]
- 22.Herman RE, Badders D, Fuller M, Makienko EG, Houston ME, Quay SC, Johnson PH. J Biol Chem. 2007;282:9813. doi: 10.1074/jbc.M610722200. [DOI] [PubMed] [Google Scholar]
- 23.Hu B, Gilkes DM, Chen J. Cancer Research. 2007;67:8810. doi: 10.1158/0008-5472.CAN-07-1140. [DOI] [PubMed] [Google Scholar]
- 24.Gemperli AC, Rutledge SE, Maranda A, Schepartz A. J Am Chem Soc. 2005;127:1596. doi: 10.1021/ja0441211. [DOI] [PubMed] [Google Scholar]
- 25.Clackson T, Wells JA. Science. 1995;267:383. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- 26.Keskin O, Gursoy A, Ma B, Nussinov R. Chemical reviews. 2008;108:1225. doi: 10.1021/cr040409x. [DOI] [PubMed] [Google Scholar]
- 27.Bogan AA, Thorn KS. J Mol Biol. 1998;280:1. doi: 10.1006/jmbi.1998.1843. [DOI] [PubMed] [Google Scholar]
- 28.Moreira IS, Fernandes PA, Ramos MJ. Proteins. 2007;68:803. doi: 10.1002/prot.21396. [DOI] [PubMed] [Google Scholar]
- 29.Kortemme T, Baker D. Proc Natl Acad Sci USA. 2002;99:14116. doi: 10.1073/pnas.202485799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. Nucleic Acids Res. 2000;28:235. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jochim AL, Arora PS. ACS Chem Biol. 2010;5:919. doi: 10.1021/cb1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullock BN, Jochim AL, Arora PS. J Am Chem Soc. 2011;133:14220. doi: 10.1021/ja206074j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koes DR, Camacho CJ. Bioinformatics. 2012;28:784. doi: 10.1093/bioinformatics/btr717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourgeas R, Basse MJ, Morelli X, Roche P. PLoS One. 2010;5:e9598. doi: 10.1371/journal.pone.0009598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henrich S, Salo-Ahen OM, Huang B, Rippmann FF, Cruciani G, Wade RC. Journal of molecular recognition : JMR. 2010;23:209. doi: 10.1002/jmr.984. [DOI] [PubMed] [Google Scholar]
- 36.Reynes C, Host H, Camproux AC, Laconde G, Leroux F, Mazars A, Deprez B, Fahraeus R, Villoutreix BO, Sperandio O. PLoS Comput Biol. 2010;6:e1000695. doi: 10.1371/journal.pcbi.1000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayal M, Honig B. Proteins. 2006;63:892. doi: 10.1002/prot.20897. [DOI] [PubMed] [Google Scholar]
- 38.Higueruelo AP, Schreyer A, Bickerton GR, Pitt WR, Groom CR, Blundell TL. Chem Biol Drug Des. 2009;74:457. doi: 10.1111/j.1747-0285.2009.00889.x. [DOI] [PubMed] [Google Scholar]
- 39.Davis FP, Sali A. PLoS Comput Biol. 2010;6:e1000668. doi: 10.1371/journal.pcbi.1000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jochim AL, Arora PS. Mol Biosyst. 2009;5:924. doi: 10.1039/b903202a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammond MC, Harris BZ, Lim WA, Bartlett PA. Chem Biol. 2006;13:1247. doi: 10.1016/j.chembiol.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Khasanova TV, Khakshoor O, Nowick JS. Org Lett. 2008;10:5293. doi: 10.1021/ol8021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loughlin WA, Tyndall JDA, Glenn MP, Fairlie DP. Chemical reviews. 2004;104:6085. doi: 10.1021/cr040648k. [DOI] [PubMed] [Google Scholar]
- 44.Smith AB, Keenan TP, Holcomb RC, Sprengeler PA, Guzman MC, Wood JL, Carroll PJ, Hirschmann R. J Am Chem Soc. 1992;114:10672. [Google Scholar]
- 45.Angelo NG, Arora PS. J Am Chem Soc. 2005;127:17134. doi: 10.1021/ja056406z. [DOI] [PubMed] [Google Scholar]
- 46.Angelo NG, Arora PS. J Org Chem. 2007;72:7963. doi: 10.1021/jo701292h. [DOI] [PubMed] [Google Scholar]
- 47.Ko E, Liu J, Burgess K. Chemical Society reviews. 2011;40:4411. doi: 10.1039/c0cs00218f. [DOI] [PubMed] [Google Scholar]
- 48.Ko E, Liu J, Perez LM, Lu G, Schaefer A, Burgess K. J Am Chem Soc. 2010;133:462. doi: 10.1021/ja1071916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Nature. 2009;462:182. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart ML, Fire E, Keating AE, Walensky LD. Nat Chem Biol. 2010;6:595. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patgiri A, Yadav KK, Arora PS, Bar-Sagi D. Nat Chem Biol. 2011;7:585. doi: 10.1038/nchembio.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horne WS, Johnson LM, Ketas TJ, Klasse PJ, Lu M, Moore JP, Gellman SH. Proc Natl Acad Sci USA. 2009;106:14751. doi: 10.1073/pnas.0902663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison RS, Shepherd NE, Hoang HN, Ruiz-Gomez G, Hill TA, Driver RW, Desai VS, Young PR, Abbenante G, Fairlie DP. Proc Natl Acad Sci U S A. 2010;107:11686. doi: 10.1073/pnas.1002498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephens OM, Kim S, Welch BD, Hodsdon ME, Kay MS, Schepartz A. J Am Chem Soc. 2005;127:13126. doi: 10.1021/ja053444+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cummings CG, Hamilton AD. Curr Opin Chem Biol. 2010;14:341. doi: 10.1016/j.cbpa.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Tosovska P, Arora PS. Org Lett. 2010;12:1588. doi: 10.1021/ol1003143. [DOI] [PubMed] [Google Scholar]
- 58.Lee JH, Zhang Q, Jo S, Chai SC, Oh M, Im W, Lu H, Lim HS. J Am Chem Soc. 2011;133:676. doi: 10.1021/ja108230s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plante JP, Burnley T, Malkova B, Webb ME, Warriner SL, Edwards TA, Wilson AJ. Chem Commun. 2009:5091. doi: 10.1039/b908207g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaginian A, Whitby LR, Hong S, Hwang I, Farooqi B, Searcey M, Chen J, Vogt PK, Boger DL. J Am Chem Soc. 2009;131:5564. doi: 10.1021/ja810025g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patgiri A, Jochim AL, Arora PS. Acc Chem Res. 2008;41:1289. doi: 10.1021/ar700264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schafmeister CE, Po J, Verdine GL. J Am Chem Soc. 2000;122:5891. [Google Scholar]
- 63.Shepherd NE, Hoang HN, Abbenante G, Fairlie DP. J Am Chem Soc. 2005;127:2974. doi: 10.1021/ja0456003. [DOI] [PubMed] [Google Scholar]
- 64.Cunningham BC, Wells JA. Science. 1989;244:1081. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 65.Kortemme T, Kim DE, Baker D. Science’s STKE : signal transduction knowledge environment. 2004;2004:pl2. doi: 10.1126/stke.2192004pl2. [DOI] [PubMed] [Google Scholar]
- 66.Koes DR, Camacho CJ. Nucleic Acids Res. 2012;40:W387. doi: 10.1093/nar/gks336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Advanced Drug Delivery Reviews. 1997;23:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 68.Hann MM, Keseru GM. Nature reviews Drug discovery. 2012;11:355. doi: 10.1038/nrd3701. [DOI] [PubMed] [Google Scholar]
- 69.Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Science. 1996;274:948. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- 70.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. Science. 2004;303:844. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 71.Alex A, Millan DS, Perez M, Wakenhut F, Whitlock GA. Medchemcomm. 2011;2:669. [Google Scholar]
- 72.White TR, Renzelman CM, Rand AC, Rezai T, McEwen CM, Gelev VM, Turner RA, Linington RG, Leung SSF, Kalgutkar AS, Bauman JN, Zhang Y, Liras S, Price DA, Mathiowetz AM, Jacobson MP, Lokey RS. Nat Chem Biol. 2011;7:810. doi: 10.1038/nchembio.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arora PS, Ansari AZ. Nature. 2009;462:171. doi: 10.1038/462171a. [DOI] [PubMed] [Google Scholar]
- 74.Henchey LK, Kushal S, Dubey R, Chapman RN, Olenyuk BZ, Arora PS. J Am Chem Soc. 2010;132:941. doi: 10.1021/ja9082864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welsch ME, Snyder SA, Stockwell BR. Curr Opin Chem Biol. 2010;14:347. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheok CF, Verma CS, Baselga J, Lane DP. Nat Rev Clin Oncol. 2011;8:25. doi: 10.1038/nrclinonc.2010.174. [DOI] [PubMed] [Google Scholar]
- 77.Shangary S, Wang S. Annu Rev Pharmacol Toxicol. 2009;49:223. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vassilev LT. Trends Mol Med. 2007;13:23. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Chene P. Nat Rev Cancer. 2003;3:102. doi: 10.1038/nrc991. [DOI] [PubMed] [Google Scholar]
- 80.Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL. J Am Chem Soc. 2007;129:2456. doi: 10.1021/ja0693587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Science. 1997;275:983. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 82.Gavathiotis E, Reyna DE, Bellairs JA, Leshchiner ES, Walensky LD. Nat Chem Biol. 2012;8:639. doi: 10.1038/nchembio.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. Nature. 2005;435:677. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 84.Vogler M, Dinsdale D, Dyer MJS, Cohen GM. Cell Death Differ. 2008;16:360. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 85.Stewart ML, Fire E, Keating AE, Walensky LD. Nat Chem Biol. 2010;6:595. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eckert DM, Kim PS. Annu Rev Biochem. 2001;70:777. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 87.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Nature. 1997;387:426. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 88.Tan K, Liu J, Wang J, Shen S, Lu M. Proc Natl Acad Sci U S A. 1997;94:12303. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan DC, Fass D, Berger JM, Kim PS. Cell. 1997;89:263. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 90.Pan C, Liu S, Jiang S. J Formos Med Assoc. 2010;109:94. doi: 10.1016/S0929-6646(10)60029-0. [DOI] [PubMed] [Google Scholar]
- 91.Matthews T, Salgo M, Greenberg M, Chung J, DeMasi R, Bolognesi D. Nature reviews Drug discovery. 2004;3:215. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 92.Wang D, Lu M, Arora PS. Angew Chem Int Ed. 2008;47:1879. doi: 10.1002/anie.200704227. [DOI] [PubMed] [Google Scholar]
- 93.Sia SK, Carr PA, Cochran AG, Malashkevich VN, Kim PS. Proc Natl Acad Sci USA. 2002;99:14664. doi: 10.1073/pnas.232566599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ernst JT, Kutzki O, Debnath AK, Jiang S, Lu H, Hamilton AD. Angew Chem Int Ed. 2002;41:278. doi: 10.1002/1521-3773(20020118)41:2<278::aid-anie278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 95.Johnson LM, Horne WS, Gellman SH. J Am Chem Soc. 2011;133:10038. doi: 10.1021/ja203358t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. Nature. 1998;394:337. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- 97.Hall BE, Yang SS, Boriack-Sjodin PA, Kuriyan J, Bar-Sagi D. J Biol Chem. 2001;276:27629. doi: 10.1074/jbc.M101727200. [DOI] [PubMed] [Google Scholar]
- 98.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. Nat Rev Cancer. 2011;11:761. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, Fauber BP, Pan B, Malek S, Stokoe D, Ludlam MJ, Bowman KK, Wu J, Giannetti AM, Starovasnik MA, Mellman I, Jackson PK, Rudolph J, Wang W, Fang G. Proc Natl Acad Sci USA. 2012;109:5299. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun Q, Burke JP, Phan J, Burns MC, Olejniczak ET, Waterson AG, Lee T, Rossanese OW, Fesik SW. Angew Chem Int Ed. 2012;51:6140. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henchey LK, Porter JR, Ghosh I, Arora PS. ChemBiochem. 2010;11:2104. doi: 10.1002/cbic.201000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kritzer JA, Lear JD, Hodsdon ME, Schepartz A. J Am Chem Soc. 2004;126:9468. doi: 10.1021/ja031625a. [DOI] [PubMed] [Google Scholar]
- 103.Murray JK, Gellman SH. Biopolymers. 2007;88:657. doi: 10.1002/bip.20741. [DOI] [PubMed] [Google Scholar]
- 104.Fasan R, Dias RLA, Moehle K, Zerbe O, Vrijbloed JW, Obrecht D, Robinson JA. Angew Chem Int Ed. 2004;43:2109. doi: 10.1002/anie.200353242. [DOI] [PubMed] [Google Scholar]
- 105.Yin H, Lee G-i, Park HS, Payne GA, Rodriguez JM, Sebti SM, Hamilton AD. Angew Chem Int Ed. 2005;44:2704. doi: 10.1002/anie.200462316. [DOI] [PubMed] [Google Scholar]
- 106.Austin RJ, Ja WW, Roberts RW. J Mol Biol. 2008;377:1406. doi: 10.1016/j.jmb.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Millward SW, Fiacco S, Austin RJ, Roberts RW. ACS Chem Biol. 2007;2:625. doi: 10.1021/cb7001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bruncko M, Oost TK, Belli BA, Ding H, Joseph MK, Kunzer A, Martineau D, McClellan WJ, Mitten M, Ng SC, Nimmer PM, Oltersdorf T, Park CM, Petros AM, Shoemaker AR, Song X, Wang X, Wendt MD, Zhang H, Fesik SW, Rosenberg SH, Elmore SW. J Med Chem. 2007;50:641. doi: 10.1021/jm061152t. [DOI] [PubMed] [Google Scholar]
- 109.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. Cancer Res. 2008;68:3421. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 110.Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, Yuan J. Nat Cell Biol. 2001;3:173. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 111.Yang B, Liu D, Huang Z. Bioorg Med Chem Lett. 2004;14:1403. doi: 10.1016/j.bmcl.2003.09.101. [DOI] [PubMed] [Google Scholar]
- 112.Wang D, Liao W, Arora PS. Angew Chem Int Ed. 2005;44:6525. doi: 10.1002/anie.200501603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kutzki O, Park HS, Ernst JT, Orner BP, Yin H, Hamilton AD. J Am Chem Soc. 2002;124:11838. doi: 10.1021/ja026861k. [DOI] [PubMed] [Google Scholar]
- 114.Lee J, Kim K-H, Jeong S. Bioorganic & Medicinal Chemistry Letters. 2011;21:4203. doi: 10.1016/j.bmcl.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 115.Bramson HN, Corona J, Davis ST, Dickerson SH, Edelstein M, Frye SV, Gampe RT, Jr, Harris PA, Hassell A, Holmes WD, Hunter RN, Lackey KE, Lovejoy B, Luzzio MJ, Montana V, Rocque WJ, Rusnak D, Shewchuk L, Veal JM, Walker DH, Kuyper LF. J Med Chem. 2001;44:4339. doi: 10.1021/jm010117d. [DOI] [PubMed] [Google Scholar]
- 116.Frey G, Rits-Volloch S, Zhang XQ, Schooley RT, Chen B, Harrison SC. Proc Natl Acad Sci USA. 2006;103:13938. doi: 10.1073/pnas.0601036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferrer M, Kapoor TM, Strassmaier T, Weissenhorn W, Skehel JJ, Oprian D, Schreiber SL, Wiley DC, Harrison SC. Nat Struct Biol. 1999;6:953. doi: 10.1038/13324. [DOI] [PubMed] [Google Scholar]