Abstract
Purpose
Although there is substantial evidence that physical activity reduces a person's risk of cardiovascular disease (CVD), few of these studies have included African Americans. The studies that have included African Americans offer inconclusive evidence on the association and none studied heart failure separately. We used data from the Atherosclerosis Risk in Communities study cohort to examine, in African Americans, the association of physical activity with the incidence of CVD and its major components – stroke, heart failure, and coronary heart disease.
Methods
Participants aged 45 to 64 years (3,707 African Americans and, for comparison, 10,018 Caucasians) had physical activity assessed via questionnaire in 1987 and were followed for incident CVD (n=1,039) through 2008.
Results
After adjustment for potential confounders, physical activity was inversely related to CVD, heart failure, and coronary heart disease incidence in both races (p-values for trend <.0001), and with stroke in African Americans. Hazard ratios (95% confidence intervals) for CVD for each higher physical activity category were similar by race: 1.0, 0.65 (0.56, 0.75), and 0.59 (0.49, 0.71) for African Americans and 1.0, 0.74 (0.66, 0.83), and 0.67 (0.59, 0.75) for Caucasians (p-value for interaction = 0.38).
Conclusions
Our findings reinforce recommendations that regular physical activity is important for CVD risk reduction in African Americans as well as Caucasians and support the idea that some physical activity is better than none.
Keywords: exercise, stroke, coronary heart disease, heart failure, race
Introduction
There is substantial evidence that physical activity reduces a person's risk of cardiovascular disease (CVD) (30). However, the vast majority of U.S. studies on this topic have been in Caucasian populations. A quote from the 2008 Physical Activity Guidelines Advisory Committee Report (30) indicated that “few studies conducted in the United States have had an adequate sample size and clinical outcomes to evaluate the association between physical activity and CVD clinical events in race or ethnic groups other than non-Hispanic whites.”
The relation between physical activity and CVD in African Americans is among the associations not well documented: Only seven (6; 8; 9; 11; 15; 19; 33) reports that employed a cohort study design had >10% African Americans in their study populations. Four of these seven (8; 11; 15; 33) focused on mortality from total CVD and not incidence (morbidity); only two (9; 19) studied coronary heart disease (CHD) as a separate endpoint, one (6) studied stroke, and none studied heart failure (HF). Two studies indicated that the benefits of physical activity on CVD risk do not vary by race, and, therefore, extend to African Americans (11; 19). However, one (11) was done in a diabetic population, so it cannot be generalized to the population at large. Three other studies with a large number of African American participants found a strong inverse association between physical activity and CVD in their populations, but did not stratify or look for interaction by race (8; 15; 33). The remaining two reports (6; 9) were from the Atherosclerosis Risk in Communities (ARIC) study. The first (6) found a weak inverse association between physical activity and incident ischemic stroke. It did not test for race interaction, but when the cohort was stratified by race, stroke was (qualitatively) inversely related to physical activity in African Americans, while the pattern was less consistent among Caucasians. The second report (9) from ARIC suggested that the benefits of physical activity on CVD risk do vary by race and may not extend to African Americans. It examined the association between physical activity at baseline and CHD incidence in African Americans over 4–7 years. It reported no association, but was limited by few CHD events (n=89) over a relatively short follow-up. Additional data would help solidify conclusions about physical activity and CVD in African Americans. The cardiorespiratory health section of the 2008 Physical Activity Guidelines Advisory Committee Report (30) concludes with research needs: “In the course of reviewing the literature…several significant deficiencies in the published literature became apparent. More information addressing the following issues would have significantly improved the information base used to formulate physical activity recommendations.” Of the 9 items that were listed, one was “Are there responses that differ by ethnic and racial minority differences?”
We currently have the opportunity to reevaluate this association in ARIC, except with longer follow-up, a greater number of events, and, therefore, greater statistical power. This report details, in African Americans in ARIC, the association of physical activity, measured at baseline and visit 3, with CVD incidence and its major components – stroke, HF, and CHD – over a follow-up period of 21 years. For comparison purposes, we also examine the association of physical activity with CVD in ARIC Caucasians. We hypothesize that there will be an inverse association between physical activity and CVD in African Americans and that there will be no differences by race.
Methods
Study design and population
The ARIC study is a large, population-based cohort with the primary aim of identifying the causes of atherosclerosis and its clinical outcomes. Detailed descriptions of the ARIC study design and objectives have been published elsewhere (27). Briefly, the study enrolled a biracial cohort of 15,792 adults aged 45 to 64 years at baseline from four US communities: Forsyth County, NC; Jackson, MS (African Americans only); suburban Minneapolis, MN; and Washington County, MD. Cardiovascular risk factors were collected at the baseline clinic examination, conducted between 1987–89. Approximately 46% of eligible participants in Jackson and 65% in the other three communities completed the clinic visit, yielding a total of 15,792 participants. A previous report described the non-participants (13). The cohort underwent reexamination visits at roughly 3-year intervals, with a 93% return rate for visit 2 (1990 – 92), 86% for visit 3 (1993 – 95), and 81% for visit 4 (1996 – 98). Follow-up also occurs yearly by telephone to maintain contact and to assess the health status of the cohort. Written informed consent was obtained from participants, as approved by the institutional review boards at each study center. ARIC protocols have been described elsewhere (27).
Physical activity measure
We assessed physical activity using the Baecke questionnaire (4), which was modified as described elsewhere (9). ARIC chose this questionnaire because it is brief, measures usual (habitual) activity, and was deemed appropriate for the middle-aged ARIC study population. Baecke et al. defined 3 semi-continuous indices ranging from 1 (low) to 5 (high) for physical activity in sports, during leisure time, and at work. The sports questions itemize sports (or exercise) participation in up to 4 sports in the past year and their frequency and duration. The “sports score” is a function of 1) the frequency, the duration, and an assigned intensity of the reported sports and 2) three additional questions on the frequency of sweating, the general frequency of playing sports, and a self-rating of the amount of leisure time physical activity compared with others of the same age. In addition, for this analysis, the Baecke sports questions were converted to “minutes per week” of moderate or vigorous exercise. This conversion was based on metabolic equivalent of task (MET) values, using an updated version (14) of the Compendium of Physical Activities as a guide, and incorporating number of months annually a participant partook in the activity. The four questions that constitute the “leisure score” asked about frequency of watching television (scored inversely), walking, bicycling, and walking/biking to work or shopping. The eight questions related to work asked about the participant's main occupation; his/her self-rating of the work's vigor (in comparison with others of the same age); the frequency of sitting, standing, walking, lifting, and sweating at work; and the frequency of fatigue after work. Those not working were assigned the lowest value of physical activity in the “work score”.
The reliability and validity of the Baecke questionnaire were evaluated in several other populations and summarized elsewhere (1; 2; 4; 14; 21; 23). In ARIC, physical activity was measured at baseline and visit 3. To increase precision, physical activity was analyzed using a cumulative average approach (12). For all participants who had an event between baseline and visit 3, physical activity was modeled using baseline physical activity information. For all participants who had an event after visit 3, if a visit 3 value for physical activity existed, an average of baseline and visit 3's physical activity values was used. If no visit 3 value for physical activity existed, then just the baseline value for physical activity was used.
Other baseline measures
Fasting blood samples were drawn from an antecubital vein for measurement of lipids and glucose. Laboratory assays for triglycerides, high-density lipoprotein cholesterol, and calculated low-density lipoprotein cholesterol were performed in standardized research laboratories (10). Plasma total cholesterol was measured by enzymatic methods (26). Use of the following, within the 2 weeks prior to the baseline interview, was self-reported or identified from prescription bottles: antihypertensive medication, cholesterol-lowering medication, and/or hormone therapy. Dyslipidemia was defined as cholesterol-lowering medication use or lipid levels beyond ATP III (7) cut-points (mg/dL): high-density lipoprotein cholesterol < 40, low-density lipoprotein cholesterol ≥ 160, triglycerides ≥ 200, or total cholesterol ≥ 240. ARIC assayed serum glucose by the hexokinase method. We defined prevalent diabetes mellitus as a fasting glucose level ≥126 mg/dL, non-fasting glucose level ≥200 mg/dL, and/or a history of or treatment for diabetes. Cigarette smoking status (current, former, or never smokers), participant demographics, education level, and alcohol intake were derived from interviews. Alcohol intake (grams/week) was skewed and, therefore, categorized (grams/week): 0, 1–100, and >100. Sitting blood pressure was measured 3 times using a random-zero sphygmomanometer after a 5-minute rest. The mean of the last two measurements was used for analysis. Hypertension was defined as 1) diastolic blood pressure ≥ 90 mmHg, 2) systolic blood pressure ≥ 140 mmHg, or 3) taking medication for high blood pressure. Body mass index (BMI) (kg/m2) was computed from weight in a scrub suit and standing height. We defined obesity as BMI ≥ 30 kg/m2. Usual dietary intake was assessed by a 66-item, interviewer-administered, semi-quantitative food-frequency questionnaire (FFQ), which was a modified version of the 61-item instrument developed by Willett (32). Food and beverages from the FFQ were categorized into 29 food sub-groups, which were used to derive `Western' and `Prudent' dietary patterns via principal components analysis, as described elsewhere (18).
Pre-existing HF at baseline was defined as 1) an affirmative response to “Were any of the medications you took during the last 2 weeks for heart failure?” or 2) stage 3 or “manifest heart failure” by Gothenburg criteria (5; 17). Pre-existing CHD at baseline was defined as 1) a self-reported previous physician diagnosis of myocardial infarction (MI) or coronary revascularization or 2) as prevalent MI by 12-lead electrocardiography. Pre-existing stroke was defined by any self-reported previous physician diagnosis of stroke.
Ascertainment of incident events
An incident CVD event, our main outcome, was defined as the first occurrence of 1) HF, 2) a definite or probable stroke, or 3) CHD, defined as a definite or probable MI or definite fatal CHD.
Incident HF in the ARIC study was defined as the first occurrence of either a 1) hospitalization that included a primary or secondary diagnosis of International Classification of Diseases-9th Revision (ICD-9) discharge code of 428 (428.0 to 428.9) or 2) a death certificate with an ICD-9 code of 428 or an ICD-10 code of I50 among the listed underlying causes of death (17). Validation studies (24) using physician review of the ARIC study hospital records beginning in 2005 indicated the positive predictive value of ICD-9 428 to be 93% for acute decompensated HF and 97% for chronic HF.
For patients hospitalized with a potential MI, trained abstractors recorded the presenting symptoms and related clinical information, including cardiac biomarkers, and photocopied up to three 12-lead electrocardiograms for Minnesota coding (22). Out-of-hospital deaths were investigated by means of death certificates and, in most cases, by an interview with ≥1 next of kin and a questionnaire completed by the patient's physician. Coroner reports or autopsy reports, when available, were abstracted for use in validation. A CHD event was defined as a validated hospitalization for definite or probable MI or a definite CHD death. The criteria for definite or probable MI were based on combinations of chest pain symptoms, electrocardiographic changes, and cardiac biomarker levels. The criteria for definite fatal CHD were based on chest pain symptoms, history of CHD, underlying cause of death from the death certificate, and any other associated hospital information or medical history, including that from an ARIC study clinic visit (31).
The diagnostic classification of stroke has been described previously (25). In brief, for patients hospitalized for potential strokes (any type), the abstractors recorded signs and symptoms and photocopied neuroimaging (computed tomography or magnetic resonance imaging) and other diagnostic reports. Using criteria adopted from the National Survey of Stroke (28), definite or probable strokes were classified by computer algorithm and separate review by a physician, with disagreements resolved by a second physician.
Statistical Analyses
Of 15,792 ARIC participants at baseline, we excluded, due to small numbers, 55 African Americans from Washington County and Minneapolis suburbs. We further excluded participants who were neither Caucasian nor African American (n=48); had missing physical activity data at baseline (n=474); or had prevalent CVD at baseline (n=1,490), defined as CHD, stroke, or HF, leaving 13,725 participants. For each outcome (CVD, stroke, HF, or CHD), follow-up time was calculated as the time elapsed from the baseline examination in 1987 to 1989 to the date of the specific outcome of interest, death, loss to follow-up, or, otherwise, through December 31, 2008. Thus, for CVD, the first of any of the three outcomes was the incidence date.
Due to the skewness of the physical activity minutes per week variable, we categorized minutes per week of exercise by the American Heart Association's (AHA) ideal CVD health guidelines (16). “Recommended” physical activity was defined as meeting AHA's physical activity recommendation for ideal cardiovascular health status: ≥150 min/wk of moderate activity or ≥75 min/wk of vigorous activity or ≥150 min/wk of moderate + vigorous activity. “Intermediate” physical activity was defined as 1–149 min/wk of moderate activity or 1–74 min/wk of vigorous activity or 1–149 min/wk of moderate + vigorous activity. “Poor” physical activity was defined as 0 min/wk of physical activity. We additionally categorized 1) the sports score, as previously done (9), for comparison purposes, and 2) physical activity by quintiles for each score (sports, leisure, and work).
Race-specific means and counts of participant characteristics by AHA physical activity category (poor, intermediate, recommended) were computed. Using Cox proportional hazards regression, we computed adjusted hazard ratios (HRs) of the CVD outcomes in relation to physical activity groups. We stratified by race and sometimes sex. Models were adjusted for potential confounders to the association between physical activity and CVD: age (continuous), smoking (current, former, never), alcohol intake (0 grams/week, 1–100 grams/week, >100 grams/week), sex/hormone therapy use (male, female using hormone therapy, female not using hormone therapy), education (grade school or 0 years education; high school, but no degree; high school graduate; vocational school; college; graduate school or professional school), and `Western' and `Prudent' dietary pattern scores (continuous). By including cross-product terms in models, we tested for interactions of physical activity with race, sex and several clinical characteristics (obesity, diabetes, hypertension, and dyslipidemia). The latter four variables were not considered confounders because they may be a consequence of physical inactivity and, therefore, part of the causal pathway. We tested for statistical trend across physical activity categories by additionally running each model with physical activity categories treated as continuous variables. The proportional hazards assumption was not violated, confirmed by qualitatively verifying that ln(-ln) survival curves for incident CVD were parallel by physical activity categories. Poisson regression was used to compute adjusted CVD incidence rates by physical activity categories in African Americans.
Results
The ARIC cohort free of CVD in 1987–1989 included 3,707 African Americans and 10,018 Caucasians. As indicated in Table 1, African Americans, on average, were less likely to engage in physical activity than Caucasians. The percentages of African Americans and Caucasians, respectively, in each AHA physical activity category were 43.6% and 20.3% in the poor category, 34.2% and 37.5% in the intermediate category, and 22.2% and 42.3% in the recommended category. A qualitative examination of Table 1 shows that most CVD risk factors - current smoking, no alcohol intake, hypertension, obesity, diabetes, higher `Western' dietary pattern score, and lower `Prudent' dietary score – were lower in both African Americans and Caucasians for each higher physical activity category. Hormone therapy use and attained education were positively associated with physical activity level. Age and sex did not substantially differ by physical activity level in African Americans or Caucasians. Within physical activity levels, obesity, hypertension, diabetes, and no alcohol intake were more common in African Americans compared to Caucasians and dyslipidemia was less common.
Table 1.
Baseline characteristics of study participants free of cardiovascular disease according to American Heart Association physical activity categories‖, ARIC, 1987–89.
| Poor physical activity | Intermediate physical activity | Recommended physical activity | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| African Americans (n = 1,617) | Caucasians (n = 2,030) | African Americans (n = 1,268) | Caucasians (n = 3,754) | African Americans (n = 822) | Caucasians (n = 4,234) | |
|
|
||||||
| Age, years (SD) | 53.6 (5.8) | 53.8 (5.6) | 53.4 (5.8) | 54.0 (5.7) | 52.8 (5.9) | 54.5 (5.7) |
| Male, n (%) | 586 (36.2) | 939 (46.3) | 445 (35.1) | 1,493 (39.8) | 380 (46.2) | 2,145 (50.7) |
| Current smoker, n (%) | 562 (34.9) | 735 (36.3) | 310 (24.5) | 903 (24.1) | 216 (26.3) | 832 (19.7) |
| Alcohol nondrinker, n (%) | 1,174 (74.1) | 1,290 (63.8) | 936 (75.0) | 2,243 (59.9) | 549 (67.9) | 2,113 (50.0) |
| Hormone therapy use in women, n (%) | 132 (13.1) | 205 (19.0) | 124 (15.4) | 478 (21.3) | 88 (20.2) | 501 (24.2) |
| More than a high school education, n (%) | 427 (26.5) | 664 (32.8) | 532 (42.1) | 1,653 (44.1) | 445 (54.1) | 2,378 (56.3) |
| Dietary pattern scores, n (%) in highest quintile | ||||||
| `Western' | 394 (24.6) | 573 (28.3) | 247 (19.6) | 716 (19.1) | 147 (17.9) | 657 (15.6) |
| `Prudent' | 205 (12.8) | 286 (14.1) | 247 (19.6) | 664 (17.7) | 241 (29.4) | 1,091 (25.8) |
| Hypertension, n (%) | 917 (57.1) | 570 (28.3) | 648 (51.3) | 903 (24.2) | 375 (45.9) | 964 (22.8) |
| Obesity, n (%) | 697 (43.4) | 588 (29.1) | 482 (38.1) | 882 (23.5) | 276 (33.6) | 718 (17.0) |
| Diabetes, n (%) | 321 (20.6) | 201 (10.0) | 199 (16.0) | 297 (7.9) | 117 (14.6) | 306 (7.2) |
| Dyslipidemia, n (%) | 671 (44.4) | 1,150 (56.7) | 574 (47.1) | 1,896 (50.6) | 337 (42.6) | 2,056 (48.7) |
Recommended physical activity is defined per reference (15) as ≥150 min/wk moderate or ≥75 min/wk vigorous or ≥150 min/wk moderate + vigorous activity. Intermediate physical activity is defined as 1–149 min/wk moderate or 1–74 min/wk vigorous or 1–149 min/wk moderate + vigorous activity. Poor physical activity is defined as 0 min/wk of activity.
As Table 2 shows, among African Americans, there were 1,039 CVD, 350 stroke, 633 HF, and 465 CHD incident events. Among Caucasians, 1,992 CVD, 480 stroke, 1,115 HF, and 971 CHD incident events occurred. Total follow-up time was 235,276 person-years and mean was 17. The association of CVD with AHA physical activity categories did not differ by sex (interaction p-values for African Americans and Caucasians =0.92 and 0.33, respectively), so men and women were analyzed together. After adjustment for age, sex, cigarette smoking, alcohol intake, hormone therapy use, education, and `Western' and `Prudent' dietary pattern scores, AHA physical activity categories were inversely related to CVD, HF, and CHD incidence in African Americans and Caucasians (p-values for trend tests <.0001). AHA physical activity categories were also inversely related to stroke incidence, but the trend was not statistically significant among Caucasians. The HRs for CVD for each higher physical activity category were similar by race: 1.0, 0.65, and 0.59 for African Americans and 1.0, 0.74, and 0.67 for Caucasians (p-value for interaction = 0.38). Similar statistically significant trends were seen in African Americans and Caucasians for the individual CVD outcomes (CHD, HF, and stroke), with an approximate one-third reduction in risk for intermediate and recommended physical activity versus poor physical activity.
Table 2.
Adjusted1 hazard ratios (95% confidence intervals) for incident cardiovascular disease by American Heart Association physical activity categories2, ARIC, 1987–2008.
| Poor physical activity | Intermediate physical activity | Recommended physical activity | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| # of events | Hazard ratios | # of events | Hazard ratios | # of events | Hazard ratios | Ptrend* | |
|
|
|||||||
| Cardiovascular disease incidence | |||||||
| African Americans | 560 | 1.00 | 304 | 0.65 (0.56, 0.75) | 175 | 0.59 (0.49, 0.71) | <.0001 |
| Caucasians | 525 | 1.00 | 721 | 0.74 (0.66, 0.83) | 746 | 0.67 (0.59, 0.75) | <.0001 |
| Stroke incidence | |||||||
| African Americans | 188 | 1.00 | 96 | 0.65 (0.50, 0.83) | 66 | 0.72 (0.53, 0.98) | 0.006 |
| Caucasians | 116 | 1.00 | 168 | 0.76 (0.60, 0.97) | 196 | 0.78 (0.61, 0.99) | 0.08 |
| Heart failure incidence | |||||||
| African Americans | 350 | 1.00 | 178 | 0.62 (0.51, 0.75) | 105 | 0.59 (0.47, 0.74) | <.0001 |
| Caucasians | 303 | 1.00 | 414 | 0.76 (0.65, 0.88) | 398 | 0.64 (0.54, 0.75) | <.0001 |
| Coronary heart disease incidence | |||||||
| African Americans | 271 | 1.00 | 114 | 0.59 (0.47, 0.75) | 80 | 0.66 (0.50, 0.86) | <.0001 |
| Caucasians | 248 | 1.00 | 357 | 0.76 (0.65, 0.90) | 366 | 0.68 (0.58, 0.80) | <.0001 |
Adjusted for age, sex, smoking, alcohol intake, hormone therapy use, education, and `Western' and `Prudent' dietary pattern scores.
See table 1 for physical activity category definitions.
Trend across physical activity categories.
In African Americans only, AHA physical activity categories were inversely related to CVD incidence similarly depending on obesity (p-value for interaction = 0.26), diabetes status (p-value for interaction = 0.45), and hypertension (p-value for interaction = .38). The physical activity categories were inversely related to CVD differently for those with dyslipidemia versus those without (p-value for interaction = .0005) - HRs for participants without dyslipidemia were closer to 1 than those with dyslipidemia. In light of the difference in the prevalence of obesity between African Americans and Caucasians, we conducted further stratification and examined effect-modification by BMI (< 25, 25–29.9, ≥ 30 kg/m2): The p-value for 3-way interaction between BMI, physical activity, and race was 0.77 and, qualitatively, the association between physical activity and CVD did not look different by race or BMI category.
There also was an inverse relation between physical activity categories, represented as the Baecke sports score, and both CVD and CHD in African Americans and Caucasians (Table 3). Despite no interaction between sex and physical activity, we stratified by sex and looked at CHD separately in order to compare with previous research (8). Comparing the highest category of sports to the lowest, the HRs for CVD ranged from 0.56 to 0.70 and for CHD ranged from 0.54 to 0.81. There was significant interaction between sports score categories and race for CVD in females (p-value = 0.007), CHD in females (p-value = 0.005), and CHD in both sexes combined (p-value = 0.007); African Americans had HRs further below 1 than Caucasians.
Table 3.
Adjusted* hazard ratios (95% confidence intervals) for incident cardiovascular disease and coronary heart disease by sports index categories†, ARIC, 1987–2008.
| Category 1 (Range: 0–1.74) | Category 2 (Range: 1.75–2.24) | Category 3 (Range: 2.25–2.74) | Category 4 (Range: ≥ 2.75) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| # of events/# of participants | Hazard ratios | # of events/# of participants | Hazard ratios | # of events/# of participants | Hazard ratios | # of events/# of participants | Hazard ratios | Ptrend‡ | |
|
|
|||||||||
| Cardiovascular Disease Incidence | |||||||||
| African Americans | |||||||||
| Overall | 229/672 | 1.00 | 401/1351 | 0.74 (0.63, 0.88) | 231/909 | 0.61 (0.51, 0.74) | 172/764 | 0.58 (0.47, 0.72) | <.0001 |
| Male | 88/234 | 1.00 | 160/456 | 0.90 (0.69, 1.18) | 117/358 | 0.75 (0.56, 1.00) | 79/358 | 0.56 (0.41, 0.77) | <.0001 |
| Female | 141/438 | 1.00 | 241/895 | 0.64 (0.51, 0.79) | 114/551 | 0.51 (0.40, 0.66) | 93/406 | 0.61 (0.46, 0.80) | <.0001 |
| Caucasians | |||||||||
| Overall | 259/1148 | 1.00 | 531/2432 | 0.88 (0.76, 1.03) | 501/2501 | 0.80 (0.68, 0.93) | 699/3930 | 0.70 (0.61, 0.82) | <.0001 |
| Male | 117/376 | 1.00 | 281/980 | 0.85 (0.69, 1.06) | 275/1099 | 0.73 (0.59, 0.91) | 470/2118 | 0.69 (0.56, 0.85) | 0.0002 |
| Female | 142/772 | 1.00 | 250/1452 | 0.91 (0.74, 1.12) | 226/1402 | 0.88 (0.71, 1.09) | 229/1812 | 0.69 (0.55, 0.85) | 0.0003 |
| Coronary Heart Disease Incidence | |||||||||
| African Americans | |||||||||
| Overall | 105/672 | 1.00 | 178/1351 | 0.77 (0.60, .99) | 81/909 | 0.49 (0.37, 0.67) | 75/764 | 0.61 (0.44, 0.83) | <.0001 |
| Male | 48/234 | 1.00 | 86/456 | 0.95 (0.65, 1.37) | 46/358 | 0.58 (0.38, 0.88) | 38/358 | 0.54 (0.35, 0.85) | 0.0005 |
| Female | 57/438 | 1.00 | 92/895 | 0.63 (0.45, 0.89) | 35/551 | 0.42 (0.27, 0.64) | 37/406 | 0.68 (0.44, 1.05) | 0.02 |
| Caucasians | |||||||||
| Overall | 115/1148 | 1.00 | 279/2432 | 1.06 (0.85, 1.32) | 254/2501 | 0.94 (0.75, 1.17) | 346/3930 | 0.81 (0.65, 1.01) | 0.003 |
| Male | 57/376 | 1.00 | 166/980 | 1.03 (0.77, 1.40) | 157/1099 | 0.89 (0.66, 1.21) | 254/2118 | 0.79 (0.59, 1.06) | 0.01 |
| Female | 58/772 | 1.00 | 113/1452 | 1.11 (0.80, 1.53) | 97/1402 | 1.05 (0.75, 1.47) | 92/1812 | 0.81 (0.57, 1.13) | 0.09 |
Adjusted for age, smoking, alcohol intake, education, `Western' and `Prudent' dietary pattern scores, and hormone therapy use in females. Overall model also adjusted for sex.
Physical activity categories were defined per reference (8) for comparison purposes.
Trend across physical activity categories.
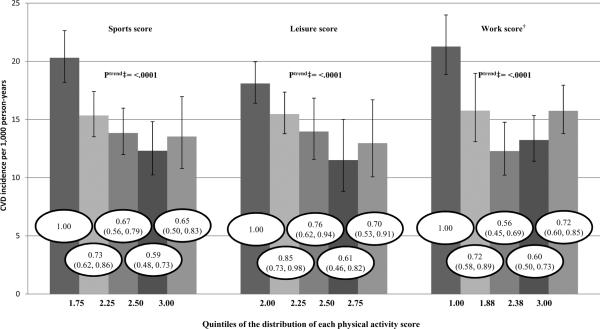
As Figure 1 depicts in African Americans only, the incidence rates of CVD, adjusted for age, sex, smoking, alcohol intake, hormone therapy use, education, and dietary pattern scores, were approximately 30% lower in the highest quintile versus the lowest quintile of the sports score [HR (95% confidence interval (CI)) = 0.65 (.50, .83)], leisure score [HR (95% CI) = 0.70 (.53, .91)], and work score [HR (95% CI) = 0.72 (.60, .85)]. Thus, all three scores showed a significant inverse trend with CVD incidence in African Americans.
Figure 1. Adjusted* incidence of cardiovascular disease (CVD) by physical activity quintiles in African Americans, ARIC, 1987–2008.
Bar height depicts CVD per 1,000 person-years (95% confidence intervals) according to quintiles of the distribution of each physical activity score quintile. Values within the bars represent the relative risk of CVD (95% confidence intervals) for that quintile referenced to the first quintile.
*Adjusted for age, sex, smoking, alcohol intake, hormone therapy use, education, and western and prudent dietary pattern scores.
†Those not working (24.7%) were assigned the lowest value of physical activity.
‡Trend of hazard ratios across physical activity categories.
Discussion
Key findings
The goal of this report was to examine, in African Americans in ARIC, the relation of physical activity with CVD incidence and its major components – stroke, HF, and CHD – over a follow-up period of 21 years. This is the first cohort study to report the association of physical activity with HF incidence in African Americans. We found that physical activity had a strong inverse relation with CVD, HF, and CHD incidence in both African Americans and Caucasians. Physical activity also had a strong inverse association with stroke incidence in African Americans. These associations were present in African Americans regardless of obesity, diabetes, hypertension, and dyslipidemia status, though somewhat stronger in those with dyslipidemia than those without. Thus, long-term follow-up of ARIC participants suggest that physical activity may benefit African Americans at least as much as Caucasians.
Possible mechanisms, explanations, and comparisons with relevant findings
For both African Americans and Caucasians, while there was a monotonic decrease in CVD incidence across physical activity categories, HRs were significantly less than 1 with just intermediate physical activity. This corroborates previous conclusions (30) that, while more physical activity is better, even modest physical activity is potentially beneficial. The sports, leisure, and work scores were all inversely related to CVD incidence in African Americans, although not all monotonically, lending further support that some activity is better than none and should be encouraged.
Much research has shown that higher levels of physical activity are associated with fewer CVD events (30). Many potential biological mechanisms exist for this association. For example, physical activity may reduce CHD by augmenting myocardial oxygen supply, reducing myocardial work and oxygen demand, improving myocardial function, and increasing the electrical stability of the myocardium (3). Physical activity is known to decrease certain CVD risk factors, such as hypertension, dyslipidemia, obesity and diabetes (30), yet these do not fully explain the inverse association between physical activity and CVD incidence (20).
Limitations
Limitations of this report warrant consideration. Firstly, most ARIC African Americans live in Jackson, MS, so they may not be representative of the entire U.S. African American population. Yet, in the absence of national data, our findings do represent the most complete evidence on physical activity and CVD in African Americans to date.
Secondly, misclassification of self-reported physical activity due to imprecision and self-report bias must be considered - as with any self-reported data collection - even though we averaged two measures of physical activity in an attempt to add precision. People are likely to over-report activity due to self-report bias. However, because the physical activity measure was collected before the CVD outcome, any misclassification was most likely non-differential and would typically attenuate the results towards the null. Therefore, the strong inverse association between physical activity and CVD incidence that was observed in African Americans may even be stronger than reported.
Thirdly, although we controlled for many potentially confounding variables in our analyses, there may have been residual confounding from unmeasured or poorly measured confounders.
Conclusions
Our findings reinforce public health recommendations (29) that regular physical activity is important for CVD risk reduction, including reductions in stroke and HF. They provide strong new evidence that this risk reduction applies to African Americans as well as Caucasians and support the idea that some physical activity is better than none.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions over many years.
The ARIC Study is supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
Elizabeth J. Bell was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL007779. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
No conflicts of interest.
The results of the present study do not constitute endorsement by ACSM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainsworth BE, Jacobs DR, Leon AS, Richardson MT, Montoye HJ. Assessment of the accuracy of physical activity questionnaire occupational data. Journal of occupational medicine. 1993 Oct;35(10):1017–27. [PubMed] [Google Scholar]
- 2.Albanes D, Conway JM, Taylor PR, Moe PW, Judd J. Validation and comparison of eight physical activity questionnaires. Epidemiology. 1990 Jan;1(1):65–71. doi: 10.1097/00001648-199001000-00014. [DOI] [PubMed] [Google Scholar]
- 3.American Heart Association Primer in Preventive Cardiology. 1994:183. [Google Scholar]
- 4.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. The American journal of clinical nutrition. 1982 Nov;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svärdsudd K. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. European heart journal. 1987 Sep;8(9):1007–14. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 6.Evenson KR, Rosamond WD, Cai J, Toole JF, Hutchinson RG, Shahar E, Folsom a.R. Physical Activity and Ischemic Stroke Risk : The Atherosclerosis Risk in Communities Study. Stroke. 1999 Jul;30(7):1333–1339. doi: 10.1161/01.str.30.7.1333. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection, Evaluation and T of HBC in A Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001 May;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Fang J, Wylie-Rosett J, Cohen HW, Kaplan RC, Alderman MH. Exercise, body mass index, caloric intake, and cardiovascular mortality. American journal of preventive medicine. 2003 Nov;25(4):283–9. doi: 10.1016/s0749-3797(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Medicine and science in sports and exercise. 1997 Jul;29(7):901–9. doi: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 11.Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KMV. Relationship of walking to mortality among US adults with diabetes. Archives of internal medicine. 2003 Jun;163(12):1440–7. doi: 10.1001/archinte.163.12.1440. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. American journal of epidemiology. 1999 Mar;149(6):531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 13.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, Shahar E, Kalsbeek W. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Journal of clinical epidemiology. 1996 Dec;49(12):1441–46. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs DR, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine and science in sports and exercise. 1993 Jan;25(1):81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan G a, Strawbridge WJ, Cohen RD, Hungerford LR. Natural history of leisure-time physical activity and its correlates: associations with mortality from all causes and cardiovascular disease over 28 years. American journal of epidemiology. 1996 Oct;144(8):793–7. doi: 10.1093/oxfordjournals.aje.a009003. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010 Mar;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 17.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) The American journal of cardiology. 2008 Apr;101(7):1016–22. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008 Mar;117(6):754–61. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. The New England journal of medicine. 2002 Sep;347(10):716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 20.Mora S, Cook N, Buring JE, Ridker PM, Lee I-M. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007 Nov;116(19):2110–8. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pols MA, Peeters PH, Bueno-De-Mesquita HB, Ocké MC, Wentink CA, Kemper HC, Collette HJ. Validity and repeatability of a modified Baecke questionnaire on physical activity. International journal of epidemiology. 1995 Apr;24(2):381–8. doi: 10.1093/ije/24.2.381. [DOI] [PubMed] [Google Scholar]
- 22.Prineas RJ, Crow RSBH. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. John Wright-PSG Inc.; Littleton, Mass: 1982. p. 10. [Google Scholar]
- 23.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. International journal of epidemiology. 1995 Aug;24(4):685–93. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 24.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circulation - Heart failure. 2012 Mar;5(2):152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999 Apr;30(4):736–43. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 26.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clinical chemistry. 1983 Jun;29(6):1075–80. [PubMed] [Google Scholar]
- 27.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989 Apr;129(4):687–702. [PubMed] [Google Scholar]
- 28.The National Survey of Stroke The National Survey of Stroke. National Institute of Neurological and Communicative Disorders and Stroke. Stroke. 1981;12(2 Pt 2 Suppl 1):I1–91. [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services . 2008 Physical Activity Guidelines for Americans. Washington, DC: 2008. p. vii. [Google Scholar]
- 30.U.S. Department of Health and Human Services . Physical Activity Guidelines Advisory Committee Report. Washington, DC: 2008. pp. G2-1–G2-57. [Google Scholar]
- 31.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. Journal of clinical epidemiology. 1996 Mar;49(2):223–33. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. American journal of epidemiology. 1985 Jul;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 33.Win S, Parakh K, Eze-Nliam CM, Gottdiener JS, Kop WJ, Ziegelstein RC. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the Cardiovascular Health Study. Heart. 2011 Mar;97(6):500–5. doi: 10.1136/hrt.2010.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]