Abstract
Our work suggests that heteromer formation, mainly involves linear motifs found in disordered regions of proteins. Local disorder imparts plasticity to linear motifs. Many molecular recognition of proteins occur between short linear segments, known as LMs. Interaction of short continuous epitopes are not constrained by sequence and have the advantage of resulting in interactions with micromolar affinities which suites transient, reversible complexes such as receptor heteromers. Electrostatic Interactions between epitopes of the GPCR involved, is the Key step in driving heteromer formation forward. The first step in heteromerization, involves phosphorylating the Ser/Thr in an epitope containing a casein kinase 1/2 (CK1/2)-consensus site. Our data suggests that dopaminergic neurotransmission, through cAMP dependent PKA slows down heteromerization. The negative charge, acquired by the phosphorylation of a Ser/Thr in a PKA consensus site in the Arg rich epitope, affects the activity of the receptors involved in heteromerization by causing allosteric conformational changes, due to the repulsive effect generated by the negatively charged phosphate. In addition to modulating heteromerization, it affects the stability of the heteromers’ interactions and their binding affinity. So here we have an instance where phosphorylation is not just an on/off switch, instead by weakening the noncovalent bond, heteromerization acts like a rheostat that controls the stability of the heteromer through activation or inhibition of adenylate cyclase by the neurotransmitter Dopamine depending on which Dopamine receptor it docks at.
Keywords: Dopamine Receptors, Adenosine Receptor, Receptor heteromers, Noncovalent Interactions, Disordered Proteins, phosphorylation
Introduction
Agnati and Fuxe first pointed to the fact that G protein coupled receptors (GPCR) could interact in the cell plasma membrane resulting in receptor heteromers4,10. Woods previously demonstrated that epitopes containing two or more adjacent Arg residues will form a noncovalent complex (NCX) with ones containing a phosphorylated amino acid residue through an electrostatic bond3. GPCR such as the D2R/D4R and A2AR do form heteromers through this type of Coulombic interaction24-27 between the guanidinium groups of two adjacent arginine (Arg) residues and the phosphate group of a phosphorylated Ser/Thr (Figure 1a and b). These finding were confirmed by Łukasiewicz13 (Lukasiewicz et al., 2009). Heteromers involving other receptors have also been demonstrated12,13
Figure 1.
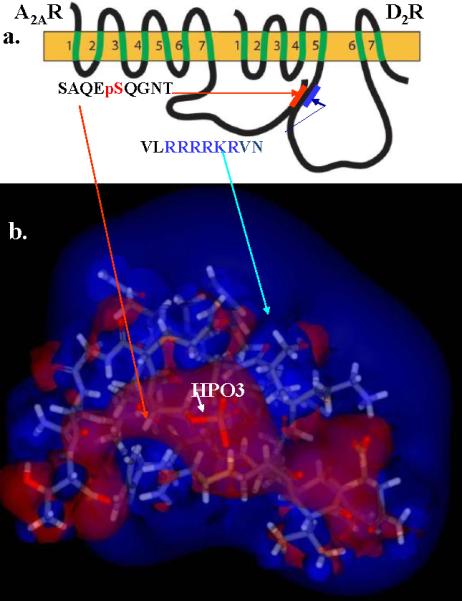
a) Epitopes location within D2R and A2A. b) The Arginine positive charge, the blue blob, engulfing the negatively charged (red) phosphate.
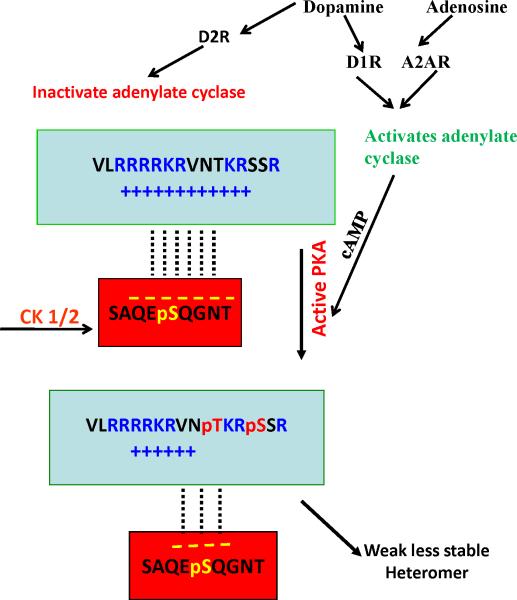
It was argued that if heteromerization was driven by Coulombic interactions (unlike charges attract and like charges repel), it would be non-specific, as any phosphorylated Ser/Thr residue (negatively charged) would interact with epitopes containing multiple Arg (positively charged). Thus these interactions would take place indiscriminately between any proteins containing these motifs. However, we found that the first step in heteromerization, involves phosphorylating the Ser/Thr contained in a casein kinase 1/2 (CK1/2)-consensus site16,26.27 in the A2AR in the case of the D2R/D4R-A2AR, and in D1R in the case of the D1R-NMDAR NR1 subunit heteromers. Phosphorylation drives the interaction forward25-27, and when we used CK1/2 inhibitors the interaction failed16. Tobin demonstrated that kinases could phosphorylate and regulate GPCR and other receptors23. We also noted that in all cases of heteromer formation, the GPCR domain containing the Arg rich epitope has downstream and less often upstream from the adjacent Arg residues, Ser/Thr that are located in a protein kinase A (PKA)-consensus site, i.e. they are phosphorylated by PKA25,26 (Figure 2).
Figure 2.
Schematic of the mechanism of the Adenosine A2A Dopamine D2 receptor interaction, showing how kinases control heteromer formation, Casein kinase as an on/off switch and Protein kinase A as a way to slow down the attraction.
In addition proteins or at least protein domains that get phosphorylated are intrinsically disordered1,3,6,7,27, i.e. their amino acid composition as well as their sequence complexity, hydrophobicity, charge and other properties of the regions surrounding the residues to be phosphorylated are very similar to those of intrinsically disordered proteins. These regions contain a high percentage of disorder promoting amino acids (A, R, G, Q, S, P, E, D and K)3,6,7, mainly hydrophilic residues and proline, which also promotes the localization of these sequences at the surface of the protein, thus making phosphorylation sites accessible to kinases and phosphatases. This fact provides strong support for the hypothesis that phosphorylation predominantly occurs within proteins’ intrinsically disordered regions11. These disordered regions also contain PEST motifs, 15-16 rich in proline (P), glutamic acid (E), serine (S), and threonine (T). PEST sequences are associated with proteins that have a short intracellular half-life; hence, it is hypothesized that it acts as a signal peptide for protein degradation 20-22. According to Fuxreiter et al., local disorder imparts plasticity to linear motifs [LM] 5. At the structural level, Protein-Protein Interactions can follow one of two mechanisms; globular proteins with their well-defined three-dimensional conformation make high-affinity complexes with their partners. However, it was noted that many molecular recognition of proteins occur between short linear segments, known as LMs, such as in the case of the SH3 domain17-20. Interaction through such linear motifs (LMs) gives an alternative, more versatile way for protein-protein interaction. Short continuous epitopes are not constrained by sequence and have the advantage of resulting in interactions with micromolar affinities which suites transient, reversible complexes such as receptor heteromers; thus explaining why LMs are primarily found in signaling pathways18,19. In general, these short segments (referred to as epitopes in this manuscript) are characterized by local flexibility, and are found in disordered regions of the parent protein1,27. This is a good description of most epitopes involved in heteromer formation.
Protein phosphorylation, a major regulatory mechanism in eukaryotic cells, is a reversible event and is influenced by proteins or peptides composition and environment11,14,18. At least one-third of all eukaryotic proteins are estimated to undergo reversible phosphorylation11,14. Phosphorylation modulates the activity of numerous proteins involved in signal transduction17,18 and regulates the binding affinity of transcription factors to their coactivators and DNA thereby altering gene expression, cell growth and differentiation6,7. Phosphorylation sites frequently cluster within functionally important protein domains, as seen in the case of the Dopamine D2, D3 and D4 receptors where the phosphorylation sites are located in their 3rd intracellular loop25-27 and in the carboxy terminus of the Adenosine A2A receptor. The phosphorylation of PEST motifs influences ubiquitin10 mediated protein degradation, which explains the short half-life of PEST rich proteins. With regard to the structural consequences of phosphorylation, both disorder to order and order to disorder transitions have been observed to follow the phosphorylation event16,25,27. Conformational changes upon phosphorylation often affect protein function. For example, serine phosphorylation of the peptide corresponding to the calmodulin binding domain of human protein p4.1 influences the ability of the peptide to adopt an alpha-helical conformation and thereby impairs the calmodulin-peptide interaction11.
Missale et al. showed that the second messenger Cyclic AMP (cAMP) is generated when the Dopamine D1 receptor (D1R) or the Adenosine A2A receptor (A2AR) are stimulated by their respective ligands, Dopamine and Adenosine, resulting in the activation of the enzyme adenylate cyclase, which generates cAMP15; while activation of the Dopamine D2R or D4R receptors inhibits adenylate cyclase reducing the cAMP levels2. cAMP is known to activate Protein kinase A (a cAMP-dependent protein kinase), which goes on to phosphorylate proteins amongst which are many receptor proteins26-27. In the case of receptor heteromers, PKA is the kinase that phosphorylates the Ser/Thr adjacent to the Arg residues in the Arg rich epitope of the third intracellular loops of the D2, D3 and D4 Dopamine receptors.
It is our contention that in the case of the A2AR-D2R/D4R heteromerization, phosphorylation of the Ser/Thr adjacent to the Arg in the 3rd IL of the D2, D3 or D4 receptors, a PKA consensus site, adds a huge negative charge next to the positively charged arginines, which slows down the interaction by 40% or more25-27. In this particular case, instead of phosphorylation acting as an On/Off switch, we observed that when the Arg rich epitope is phosphorylated, it results in a rheostat like effect. This rheostat is driven by charge rather than resistance, resulting in the weakening of the interaction rather than the switching off of the interaction. The rheostat effect could be a fine tuning mechanism that might have a role in the changes brought up by heteromerization. We propose that the key molecule that drives the “rheostat effect” is cAMP.
Material and Methods
Epitopes
The peptides RRRRKRVNTKRSSR/ RRRRKRVNpTKRpSSR (D2R epitope), PQTRRRRRAKITG/ PQpTRRRRRAKIpTG (D4R epitope), SFKRRRSSK/ SFKRRRpSSK (NMDA NR1 subunit epitope), GpSSEDLKKEE (D1R epitope) and SAQEpSQGNT A2AR epitope), where p denotes a phosphorylated residue, were synthesized at the John Hopkins School of Medicine Peptide Synthesis Core Facility. Stock solutions were prepared in water at a concentration of 1 nmol/mL.
Sample mixtures: were prepared for mass analysis as follows: SFKRRRSSK (10pmol/μL)+ GpSSEDLKKEE (50pmol/μL), SFKRRRpSSK (10pmol/μL)+ GpSSEDLKKEE (50pmol/μL), PQTRRRRRAKITG (20pmol/μL)+ SAQEpSQGNT (100pmol/μL), PQpTRRRRRAKIpTG (20pmol/μL)+ SAQEpSQGNT (100pmol/μL), RRRRKRVNTKRSSR (20pmol/μL)+ SAQEpSQGNT (100pmol/μL), RRRRKRVNpTKRpSSR, (20pmol/μL)+ SAQEpSQGNT (100pmol/μL) in 50% ethanol.
Instrument
An Orbitrap-Velos mass spectrometer (Thermo Fisher, San Jose, CA) with a static nanospray source was used in positive ion mode for mass analysis. Spray voltages between 1.1-1.5 kV were employed and 4 μm nanospray tips (New Objective, Woburn, MA) were used. The resolving power of the instrument was selected at 60,000 FWHM for both MS and MS2 experiments. For MS experiments, one microscan at 100 ms was used for data acquisition. MS2 experiments utilized one microscan at 500 ms and HCD collision energies between 0 – 30 V. For stability/disassociation curves, the relative ion intensities of each complex ion are plotted as a function of HCD collision energy, using the average of 25 scans/point. The ion intensities of each complex were normalized by the total ion current in each spectra.
Results
In order to probe the effect of phosphorylation on epitope- epitope interaction, the following epitope mixtures were analyzed: A2AR epitope (SAQEpSQGNT) with the nonphosphorylated/phosphorylated D2R epitope (RRRRKRVNTKRSSR/RRRRKRVNpTKRpSSR); A2AR epitope (SAQEpSQGNT) with the nonphosphorylated/phosphorylated D4R epitope (PQTRRRRRAKITG/PQpTRRRRRAKIpTG); and D1R epitope (GpSSEDLKKEE) with nonphosphorylated/phosphorylated NMDAR-NR1 (SFKRRRSSK/ SFKRRRpSSK). Initial MS analysis showed that all epitope mixtures readily produced several NCX ions (3+, 4+, 5+, 6+) (data not shown).
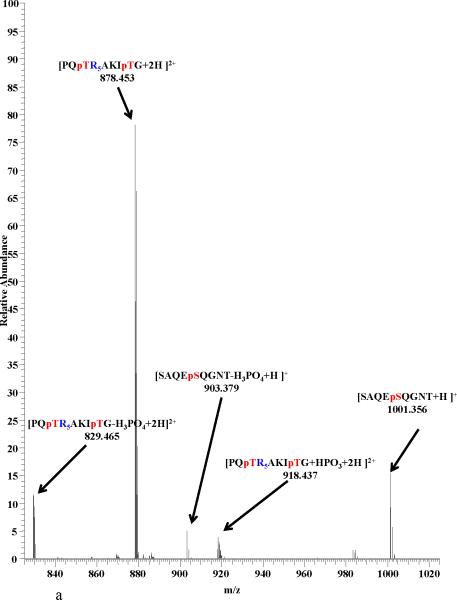
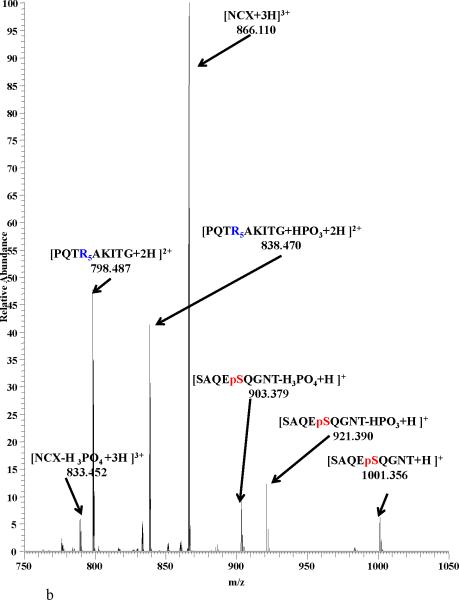
In order to better understand epitope- epitope interactions involving the presence or absence of phosphorylated amino acid residues, MSn analysis were conducted on the NCX mass peaks formed between the epitope pairs listed above. In all cases, the major dissociation pathway for the NCX is the disruption of the noncovalent bond formed by the interaction of the phosphate group with the Arg residues guanidinium group, resulting in the generation of ions corresponding to the intact epitopes25-27 (figure 3a). An alternative pathway was observed for the noncovalent complexes between the A2AR and D2R/D4R epitopes in which the NCX complex is fragmented along the covalent bond between the oxygen from Ser and the phosphorus of the phosphate from the A2AR epitope8,9 (figure 3b). Similar pathways have been previously observed for NCXs formed by Arg and phosphorylated amino acid residues8,9,25-27, In addition, this pathway was favored by 40% more in the nonphosphorylated epitopes compared to the phosphorylated ones26,27. The absence of the observation of this pathway in the NCXs between D1R and NMDAR-NR1 epitopes suggests that the electrostatic attraction between them are weaker compared to the other epitopes in this study. Figure 3 shows product ion spectra for the NCX3+ between A2AR epitope and the nonphosphorylated/phosphorylated D4R epitope. We observed, that the [PQTRRRRRAKITG + HPO3 +2H]2+ peak in Fig 3a is more abundant when compared to the [PQpTRRRRRAKIpTG + HPO3 +2H]2+ peak in Fig 3b. This suggests that the electrostatic interaction is stronger in the A2AR + nonphosphorylated D4R complex compared to the A2AR+ phosphorylated D4R complex.
Figure 3.
Product-ion spectra of NCX3+ from a peptide mixture of SAQEpSQGNT [A2AR] and (a) PQTRRRRRAKIT [D4R] and (b) PQpTRRRRRAKIpT [D4R] with HCD at a collision energy of 21 V.
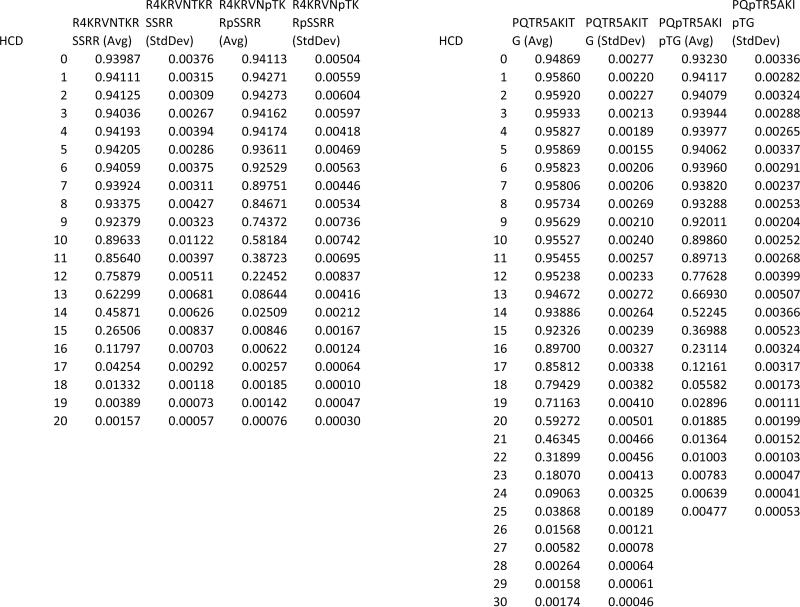
To prove the effect of phosphorylation we tested the stability of the noncovalent complex between the A2AR epitope and the non-phosphorylated and phosphorylated D2R/D4R epitopes, as well as between D1R epitope and the NMDAR NR1 non-phosphorylated and phosphorylated epitopes. This was done as follow: The molecular ions of the NCX were fragmented with increasing collision energies and the results for all NCX ions are summarized in Figure 4 and table 1. The relative ion intensities of each complex ion in Figure 4 are plotted as a function of collision energy. This also shows that the presence of phosphorylated residues in the D2R epitope RRRRKRVNpTKRpSSR (Figure 4a) or the D4R epitope PQpTRRRRRAKIpTG, (Figure 4b) greatly decreases its stability in the gas phase, suggesting a much weaker electrostatic interaction when the D2R/D4R epitope is phosphorylated, as according to Coulomb's law, like charges (in this case the addition of the negatively charged phosphate next to the positively charged Arg), weakens the attraction for the A2AR phosphate. Then in both cases, the A2AR and the D2R/D4R phosphorylated epitopes repel, giving less stable NCX than when the A2AR and the non-phosphorylated D2R/D4R epitopes are involved in heteromer formation, as unlike charges (the positively charged Arginines and the negatively charged phosphate) form more stable complexes in the absence of phosphorylation on the D2R/D4R epitopes. In the D1R-NMDAR NR1 subunit case, the NCX GpSSEDLKKEE+ SFKRRRSSK and GpSSEDLKKEE + SFKRRRpSSK show only a slight difference, as the presence of a pair of positively charged lysine residues (KK) on the D1R epitope, already weakens the interaction with the NMDAR NR1subunit epitope's Arg motif, further strengthening our hypothesis, that the stability of the interaction is governed by the electrostatic bond strength.
Figure 4.
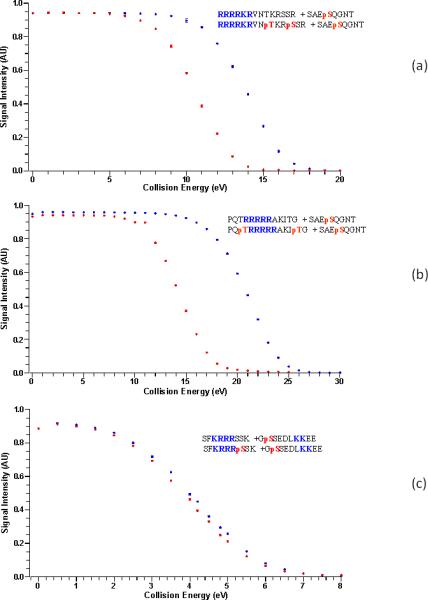
A study of the stability of the NCX of (a) RRRRKRVNTKRSSR ( ) and RRRRKRVNpTKRpSSR(
) and RRRRKRVNpTKRpSSR( )[D2R] with SAQEpSQGNT [A2AR] , NCX5+ (b) PQpTRRRRRAKIpT (
)[D2R] with SAQEpSQGNT [A2AR] , NCX5+ (b) PQpTRRRRRAKIpT ( ) PQTRRRRRAKIT (
) PQTRRRRRAKIT ( ) [D4R] with SAQEpSQGNT [A2AR], NCX3+ (c) SFKRRRSSK (
) [D4R] with SAQEpSQGNT [A2AR], NCX3+ (c) SFKRRRSSK ( ) and SFKRRRpSSK (
) and SFKRRRpSSK ( )[NMDAR NR1 subunit] with GpSSEDLKKEE [D1R], NCX3+, when exposed to HCD of the NCX at varying collision energies.
)[NMDAR NR1 subunit] with GpSSEDLKKEE [D1R], NCX3+, when exposed to HCD of the NCX at varying collision energies.
Table 1.
Collision Energy (V) at signal intensity 4.5 AU, at which NCX starts dissociating
| Receptor | non-phos | Phos |
|---|---|---|
| D2R-A2AR | 15 | 12 |
| D4R-A2AR | 21 | 15 |
| NMDAR-D1R | 4 | 4 |
Discussion
We previously demonstrated that reversible protein phosphorylation25-27 is a major regulatory mechanism in receptor heteromerization, and that the region's environment, i.e. amino acid composition, sequence complexity, hydrophobicity, charge and other sequence attributes of regions adjacent to phosphorylation sites, are very similar to those of intrinsically disordered protein motifs1,27. This provides strong support for the hypothesis that protein phosphorylation occurs predominantly within intrinsically disordered protein regions. At least one-third of all eukaryotic proteins are estimated to undergo reversible phosphorylation6,7,27. Numerous proteins involved in signal transduction, use Phosphorylation to modulate their activity, it also regulates the binding affinity of transcription factors to their coactivators and DNA thereby altering gene expression, cell growth and differentiation20. Phosphorylation sites frequently cluster within functionally important protein domains, i.e. the majority of phosphorylation sites of Mdm2 are located in its p53- and p14-ARF-binding regions19,20. With regard to the structural consequences of phosphorylation, both disorder to order and order to disorder transitions have been observed to follow the phosphorylation event. Conformational changes upon phosphorylation often affect protein function. For example, serine phosphorylation of the peptide corresponding to the calmodulin binding domain of human protein p4.1 influences the ability of the peptide to adopt an alpha-helical conformation and thereby impairs the calmodulin-peptide interaction20. The high disorder and secondary structure patterns detected in all the receptors examined, suggest the presence of molecular recognition elements (MoREs)5,19,20,27 that may mediate phosphorylation-regulated intra- and inter-domain interactions. Dunker and Fuxreiter proposed that proteins have such structural elements or features that mediate the binding events of disordered regions and that this element consists of a short linear region (LM) that undergoes coupled binding and folding within a longer region of disorder and called these features “molecular recognition elements” (MoREs)17-19,20,27. We believe that the positively and negatively charged epitopes in many receptors are the molecular recognition elements involved in heteromerization25-27.
These functional interactions depend on heteromerization, between the D1R and a specific subunit of the NMDA receptor, the NR1 subunit. When the neurotransmitter Dopamine docks at the D1R; it activates adenylate cyclase (AC) generating the second messenger cyclic AMP (cAMP), which in turn activates the kinase PKA [AC → cAMP → PKA cascade] in dynorphin neurons, which modulates neuronal excitability and glutamatergic neurotransmission and induces PKA-mediated phosphorylation of a Ser downstream from the Arg motif in the NMDA NR1 subunit epitope25-27. On the other hand when Dopamine docks at the D2R/D4R it inhibits the [AC → cAMP → PKA cascade]. While, when the neurotransmitter Adenosine docks at the Adenosine A2AR, as in the case of Dopamine at the D1R, it also activates the AC-cAMP-PKA cascade resulting in the phosphorylation of the Ser/Thr adjacent to the D2R/D4R 3rd IL Arg-rich epitopes, which are the positively charged motifs involved in heteromer formation with the A2AR Ser374, located in A2AR C-terminus, which becomes negatively charged when phosphorylated by Casein kinase 1 (CK1). As the electrostatic interaction between these charged epitopes is the first and essential step that drives receptors to form heteromers, addition of a phosphate next to the Arg rich motifs decreases the positive charge of the epitope, resulting in the weakening of the electrostatic interaction that leads to heteromer formation (figure 2). This is confirmed by the data in figure 4a and b and table 1 which suggests that the phosphorylated epitope decreases heteromerization by 40-50%
In conclusion, docking of Adenosine at the A2AR decreases the interaction with D2R/D4R, while docking of Dopamine at the D2R/D4R enhances it, as it inhibits [AC → cAMP → PKA cascade] 2,11,15 and prevents phosphorylation of the D2R/ D4R epitope (Figure 2). Thus when Dopamine is the ligand at D2R/D4R, the electrostatic interaction is unaffected, and the heteromer formed is stable, while when Adenosine is the ligand at A2AR activation of the [AC → cAMP → PKA Cascade] leads to phosphorylation of the D2R/D4R epitope and weakening of the interaction. So what we have here is a physiological event, where phosphorylation does not act as an on/off switch. Instead phosphorylation of the Ser/Thr adjacent to the positively charged motif, acts as a rheostat, as it decreases the interaction without disrupting the heteromers. Our data suggests that receptor heteromer formation between D2R/D4R-A2AR is influenced by their respective ligands, the neurotransmitters Dopamine and Adenosine, through activation or inhibition of the [AC → cAMP → PKA cascade]. When Adenosine docks at A2AR or Dopamine at D1R, adenylate cyclase is activated and cAMP is generated, which in turn activates the kinase PKA, which then phosphorylates Ser/Thr adjacent to the Arg rich sequence in the positively charged epitope. When this event occurs, the Coulombic interaction between this epitope and the negatively charged epitope on the opposing receptor is weakened. However the heteromer still exists. So in this system, instead of a phosphorylation event turning a switch on/off; the stability of the interaction is modulated by phosphorylation of the positively charged epitope. Addition of this negative charge diminishes the positive epitope likelihood of interacting with the negatively charged epitope on its partner receptor, by weakening the stability of the noncovalent electrostatic bond between receptors. It also suggests that ligands (Dopamine and Adenosine) have a say in heteromerization and can alter the stability of heteromers, once they are formed. In other words, the phosphorylation state sets the “gain” of the system (figure 2). It determines whether a neurotransmitter molecule, mainly Dopamine or Adenosine will be effective or not in activating a receptor, if this receptor is involved in the formation of a heteromer, as these neurotransmitters, don't just act as receptors’ ligands. When Adenosine docks at the A2AR it also activates adenylate cyclase, which then generates cAMP, which activates PKA, which then phosphorylates the Ser/Thr adjacent to the Arg rich motif, which weakens the interaction. While when Dopamine docks at the D2R or D4R, it also inhibits adenylate cyclase. Thus no additional phosphorylation occurs on the D2R or D4R, thus preventing the disruption of the Coulombic interaction. So we could say that when Dopamine is the ligand, the D2R or D4R lead the dance, and likes to keep its partner the A2AR receptor in a tight electrostatic embrace. While when Adenosine is the ligand, A2AR leads the dance. While not quitting the dance floor, by adding a phosphate, it forces its partner the D2R or D4R to keep a respectful distance. Hence when Dopamine is around D2R or D4R are in charge, while when Adenosine is, A2AR has the upper hand, thus suggesting why the pharmaceutical properties of compounds are altered when receptors form heteromers or a mosaic.
Heteromers form through electrostatic interactions of linear motifs located in disordered regions of receptors’ proteins
Activation/inhibition of adenylate cyclase by Adenosine/Dopamine controls production of cAMP which activates PKA
Phosphorylation of Ser/Thr by cAMP activated PKA in the Arginine Rich epitope controls the stability of heteromers
Dopaminergic neurotransmission, through cAMP dependent PKA slows down heteromerization
In heteromerization, phosphorylation is not just an on/off switch, by weakening noncovalent bonds, it acts like a rheostat
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse. The authors thank Drs. Marisela Morales and Aurelie Roux for their intellectual input, the Office of National Drug Control Policy (ONDCP) for instrumentation funding, without which this and other projects could not have been accomplished.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agnati LF, Fuxe K, Woods AS, Genedani S, Guidolin D. Theoretical Considerations on the Topological Organization of Receptor Mosaics. Current Protein and Peptide Science. 2009;10:559–569. doi: 10.2174/138920309789630606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arslan G, Kull B, Fredholm BB. Signaling via A2A Adenosine receptor in four PC12 cell clones. Naunyn-Schmiedeberg's Arch Pharmacol. 1999;359:28–32. doi: 10.1007/pl00005319. [DOI] [PubMed] [Google Scholar]
- 3.Dyson HJ, Wright PE. Coupling of folding and binding for unstructured proteins. Curr. Opin. Struct. Biol. 2002;12:54–60. doi: 10.1016/s0959-440x(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 4.Fuxe K, agnati LF. Receptor-receptor interactions in the central nervous system. A new integrative mechanism in synapses. Med Res Rev. 1985;5:441–482. doi: 10.1002/med.2610050404. [DOI] [PubMed] [Google Scholar]
- 5.Fuxreiter M, Tompa P, Simon I. Local structural disorder imparts plasticity to linear motifs. Bioinformatics. 2007;23:950–956. doi: 10.1093/bioinformatics/btm035. [DOI] [PubMed] [Google Scholar]
- 6.Garza AMS, Khan SH, Kumar R. Site-Specific Phosphorylation Induces Functionally Active Conformation in the Intrinsically Disordered N-Terminal Activation Function (AF1) Domain of the Glucocorticoid Receptor. Mol. and Cell.Biol. 2010;30:220–230. doi: 10.1128/MCB.00552-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iakoucheva LM, Radivojac P, Brown CJ, O'Connor TR, Sikes JG, Obradovic Z, Dunker AK. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32:1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson SN, Wang HYJ, Yergey A, Woods AS. Phosphate stabilization of intermolecular interactions. J. Proteome Res. 2006;5:122–126. doi: 10.1021/pr0503578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson SN, Moyer SC, Woods AS. The Role of Phosphorylated Residues in Peptide-Peptide Noncovalent Complexes. JASMS. 2008;19:1535–1541. doi: 10.1016/j.jasms.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenakin T, Agnati LF, Caron M, Fredholm B, Guidolin D, Kobilka B, Lefkowitz RW, Lohse M, Woods A, Fuxe K. International Workshop at the Nobel Forum, Karolinska Institutet on G protein-coupled receptors: finding the words to describe monomers, oligomers, and their molecular mechanisms and defining their meaning. Can a consensus be reached? J Recept Signal Transduct Res. 2010;30:284–286. doi: 10.3109/10799893.2010.512438. [DOI] [PubMed] [Google Scholar]
- 11.Kull B, et al. Reciprocal interactions between Adenosine A2A and Dopamine D2 receptors in Chinese hamster ovary cells co-transfected with the two receptors. Biochemical Pharmacology. 1999;58:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee FJ, et al. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the Dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- 13.Łukasiewicz S, Faron-Gorecka A, Dobrucki J, Agnieszka Polit A, Dziedzicka-Wasylewska M. Studies on the role of the receptor protein motifs possibly involved in electrostatic interactions on the Dopamine D1 and D2 receptor oligomerization. FEBS J. 276:760–775. doi: 10.1111/j.1742-4658.2008.06822.x. [DOI] [PubMed] [Google Scholar]
- 14.Mann M, Ong SE, Grønborg M, Steen H, Jensen ON, Pandey A. Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends Biotechnol. 2000;20:261–268. doi: 10.1016/s0167-7799(02)01944-3. [DOI] [PubMed] [Google Scholar]
- 15.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine Receptors: From Structure to Function. Physiological Reviews. 1998;78:190–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 16.Navarro G, Ferré S, Cordomi A, Moreno E, Mallol J, Casadó V, Cortés A, Hoffmann H, Ortiz J, Canela EI, Lluís C, Pardo L, Franco R, Woods AS. Electrostatic Interactions as Key Determinants of the Quaternary Structure of Receptor Heteromers. JBC. 2010;285:27346–27359. doi: 10.1074/jbc.M110.115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neduva V, et al. Linear motifs: evolutionary interaction switches. FEBS Lett. 2005;579:3342–3345. doi: 10.1016/j.febslet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine Receptor Signaling. J of Recept and Signl Transduction. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield CJ, Cheng Y, Cortese MS, Romero P, Uversky VN, Dunker AK. Coupled Folding and Binding with R-Helix-Forming Molecular Recognition Elements. Biochemistry. 2005;44:12454–12470. doi: 10.1021/bi050736e. [DOI] [PubMed] [Google Scholar]
- 20.Ren R, et al. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 21.Rogers S, Wells R, Rechsteiner M. “Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis”. Science. 1986;234(4774):364–768. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 22.Singh GP, Ganapathi M, Sandhu KS, Dash D. Intrinsic Unstructuredness and Abundance of PEST Motifs in Eukaryotic Proteomes. PROTEINS: Structure, Function, and Bioinformatics. 2006;62:309–315. doi: 10.1002/prot.20746. [DOI] [PubMed] [Google Scholar]
- 23.Torrecilla I, Spragg EJ, Poulin B, McWilliams PJ, Mistry SC, Blaukat A, Tobin AB. Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2. J Cell Biol. 2007;177:127–137. doi: 10.1083/jcb.200610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods AS. The Mighty Arginine, the Stable Quaternary Amines, the Powerful Aromatics and the Aggressive Phosphate: Their Role in the Noncovalent Minuet. J. of Proteome Research. 2004;3:478–484. doi: 10.1021/pr034091l. [DOI] [PubMed] [Google Scholar]
- 25.Woods AS, Ciruela F, Fuxe K, Agnati LF, Lluis C, Franco R, Ferré S. The role of electrostatic interaction in receptor-receptor heteromerization. J. of Mol Neurosci. 2005;26:125–132. doi: 10.1385/JMN:26:2-3:125. [DOI] [PubMed] [Google Scholar]
- 26.Woods AS, Ferre S. The Amazing Stability of the Arginine-Phosphate Electrostatic Interaction. J. Proteome Res. 2005;4:1397–1402. doi: 10.1021/pr050077s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woods AS. The Dopamine D4 Receptor, the Ultimate Disordered Protein. Journal of Receptors and Signal Transduction. 2010;30:331–336. doi: 10.3109/10799893.2010.513842. [DOI] [PMC free article] [PubMed] [Google Scholar]