Abstract
Background
Increasing evidence shows that excessive alcohol consumption during adolescence increases vulnerability to alcohol use disorders in adulthood. The aim of this study was to examine differences between adolescent and adult C57BL/6 mice in drinking behavior and blood ethanol concentrations (BECs) after chronic ethanol exposure and withdrawal.
Methods
Male adolescent (PND=28–30) and adult (PND=70) C57BL/6J mice were allowed to consume ethanol in a two-bottle choice paradigm (15% ethanol vs. water) for three weeks (Baseline Drinking, Test 1, and Test 2), which were interspersed with 2 cycles (Cycles I and II) of chronic ethanol vapor or air inhalation (16 h) and withdrawal (8 h). Blood ethanol concentrations (BECs) were determined during both cycles.
Results
Chronic ethanol exposure led to increased ethanol intake during Test 1 and Test 2 in both adolescent and adult mice compared to air exposed controls, and no differences between age groups were observed. During Cycle I adult mice showed higher BECs compared to adolescents. During Cycle II, BECs were lower in adult mice as compared to Cycle I, and BECs in adolescent mice did not change between the two cycles.
Conclusions
Chronic ethanol exposure followed by withdrawal periods increases ethanol consumption similarly in both adolescent and adult mice, despite differences in BECs.
Keywords: ethanol drinking, chronic ethanol exposure, adolescent, withdrawal, C57BL/6J mice
INTRODUCTION
Evidence shows that alcohol use during adolescence increases vulnerability towards the development of alcohol use disorders in adulthood, and that age of initial alcohol use is predictive of abuse and dependence in adults (De Wit et al., 2000; Ehlers et al., 2006; Grant and Dawson, 1997). Similar observations have been reported using rodent models (Hayashi and Takodoro, 1985; Walker and Ehlers, 2009). Additionally, some characteristics of adolescence (impulsivity, risk taking behavior, response to novelty) have been linked to high vulnerability towards drug experimentation and drug abuse (Chambers et al., 2003; Spear, 2000).
Other than age, a previous history of alcohol exposure may also promote an increase in alcohol drinking (Camarini and Hodge, 2004), and chronic alcohol intoxication interspersed with repeated periods of withdrawal provides motivation for alcohol seeking to alleviate the negative symptoms of withdrawal (Griffin et al., 2009a,b; Heilig et al., 2010; Koob and Le Moal, 2001). Alcohol dependent individuals invariably experience repeated periods of abstinence and, thus, have repeated opportunities to associate alcohol consumption with the alleviation of withdrawal symptoms. Using rodent models of alcohol dependence, studies in mice (Becker and Lopez, 2004; Chu et al., 2007; Finn et al., 2007) and rats (Gilpin et al., 2008; Roberts et al., 2000; Sommer et al., 2008) have demonstrated escalation of alcohol consumption following chronic alcohol exposure and repeated withdrawal experiences. Further, increased alcohol self-administration was shown to be facilitated when the chronic alcohol exposure was delivered in an intermittent fashion (with intervening periods of withdrawal) rather than a continuous fashion (Lopez and Becker, 2005; O’Dell et al., 2004).
In mouse models of alcohol dependence and relapse drinking, previous studies have demonstrated that repeated cycles of chronic ethanol exposure and withdrawal experience produced escalation of voluntary ethanol consumption in the home cage using a limited access paradigm in C57BL/6J adult mice (Becker and Lopez, 2004; Griffin et al., 2009a,b; Lopez and Becker, 2005). This increased alcohol intake produces significant elevation in both blood and brain ethanol concentrations (Griffin et al., 2009a). This model may be compared to the chronic episodic nature of alcohol dependence in humans, where individuals experience prolonged periods of intoxication due to heavy drinking followed by multiple attempts of abstinence.
In general, adolescents are less sensitive than adults to ethanol-induced sedation, motor impairment and withdrawal effects (Spear and Varlinskaya, 2005). Adolescent mice (Hefner and Holmes, 2007) and rats (Silveri and Spear, 1998) recover faster from ethanol intoxication than adults, although they are more sensitive to its deleterious effects (De Bellis et al., 2005; Monti et al., 2005), including brain damage and ethanol-induced cognitive impairment (Crews et al., 2000; White and Swartzwelder, 2004). The apparent low sensitivity of adolescents to the aversive consequences of ethanol may allow them to engage in more frequent bouts of excessive consumption (i.e., binge drinking) and consequent intoxication. Moreover, repeated or even a brief ethanol exposure during adolescence can enhance later ethanol intake (Acevedo et al., 2010; Pascual et al., 2009).
The purpose of the present study was to evaluate ethanol drinking patterns in adolescent and adult mice exposed to repeated cycles of chronic ethanol exposure and withdrawal. Previous studies have shown that in order to observe an increase in voluntary ethanol intake after chronic ethanol exposure, a previous experience with the positive reinforcing effects of ethanol is required (Meisch, 1983). This presented a problem for the design of the present study since it was intended to evaluate ethanol intake after chronic ethanol exposure and withdrawal within the restricted age range for adolescence (Spear, 2000). In order to condense in time and maximize the ethanol drinking experience before mice received chronic intermittent ethanol exposure, a 24 h drinking procedure was used. Previous studies in our laboratory (Becker and Lopez, 2004; Griffin et al., 2009a,b; Lopez and Becker, 2005) have used a limited access procedure (2 h/day), more recently, it was also observed that chronic intermittent ethanol exposure also induces elevated ethanol intake when mice have access to ethanol (vs. water) for 24 h/day (unpublished observations). We evaluated the effect of repeated cycles of chronic intermittent ethanol exposure in adult and adolescent mice with continuous free-choice access to ethanol in their home cage. In addition, potential age-related differences in blood ethanol concentrations after ethanol exposure were also assessed.
MATERIALS AND METHODS
Subjects
Adult (postnatal day 70; PND 70) and adolescent (PND 30) at the start of the study) male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were used as subjects. Adolescent mice were shipped after weaning at PND 21. After a period of acclimation (4 days), during which mice were housed in groups of 5 animals/cage, mice were then transferred to individual housing under a 12 h light/dark cycle (lights on at 4:00 AM) in a temperature and humidity controlled AAALAC-accredited animal facility. The transfer to individual housing occurred 3 days prior to initial ethanol exposure. Animals were provided free access to food and water throughout the experiment. All procedures were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996).
Study Design
The overall study design involved five phases that lasted five weeks, as shown in Table 1. Mice were first given the opportunity to drink ethanol under a free-choice continuous access paradigm to establish baseline drinking. After this Baseline phase, one group of mice (EtOH group) received chronic intermittent exposure to ethanol vapor in inhalation chambers (16 h/day for 4 days), while the remaining mice (CTL group) were similarly handled, but maintained in control air inhalation chambers. Access to free-choice drinking was suspended during the inhalation exposure (Exposure Cycle I). After a 72 h abstinence period (when physical signs of acute withdrawal have resolved; Becker, 1999; Becker and Lopez, 2004), all mice were given the opportunity to drink ethanol for 5 consecutive days under the same access conditions as during the Baseline phase of the study. This pattern of chronic intermittent ethanol (or air) exposure followed by a 72 h forced abstinence period and then 5 days of testing voluntary ethanol drinking was repeated for a second cycle (Exposure Cycle II). Thus, after the Baseline drinking phase, adolescent and adult mice received 2 weekly cycles of inhalation treatment (Cycle I and Cycle II), each followed by 72 h of forced abstinence prior to 5-day drinking test sessions (Test 1 and Test 2).
Table 1.
Experimental phases of ethanol drinking in a two-bottle choice paradigm (15% ethanol vs. water) for three weeks (Baseline, Test 1 and Test 2) interspersed with 2 cycles of chronic ethanol vapor or air inhalation and withdrawal (Cycles I and II). Each Cycle consisted of 16 h of ethanol or air inhalation in chambers followed by an 8 h period of withdrawal. EtOH: ethanol; CTL: control.
| Phases and Age | DAY 1 | DAY 2 | DAY 3 | DAY 4 | DAY 5 | DAY 6 | DAY 7 |
|---|---|---|---|---|---|---|---|
|
BASELINE Ado (30) Adu (70) |
Bottles offered | Daily quantification of volume drank in each bottle | Quantification and removal of bottles | Regular water bottles | |||
|
CYCLE I Ado (37) Adu (77) |
EtOH or CTL injection + 16h EtOH (or air)/8h withdrawal Blood sample collected from the EtOH groups | Regular water bottles | |||||
|
TEST 1 Ado (44) Adu (84) |
Bottles offered | Daily quantification of volume drank in each bottle | Quantification and removal of bottles | Regular water bottles | |||
|
CYCLE II Ado (51) Adu (91) |
EtOH or CTL injection + 16h EtOH (or air)/8h withdrawal Blood sample collected from the EtOH groups | Regular water bottles | |||||
|
TEST 2 Ado (58) Adu (98) |
Bottles offered | Daily quantification of volume drank in each bottle | Quantification and removal of bottles* | ||||
Ado = Adolescent, Adu = Adult. The numbers in parenthesis indicate the postnatal day of the animals in each experimental phase.
Blood ethanol concentrations (BEC) during chronic ethanol exposure allowed comparison of age related differences in BECs produced by chronic intermittent ethanol vapor exposure.
Two-bottle choice procedure
During two-bottle choice procedures, individually housed animals were provided two 15 ml (±0.1ml) graduated tubes in the home cage for 5 consecutive days. One bottle contained 15% v/v ethanol (diluted in water from a 95% v/v stock solution), and the other contained only tap water. The amount of water and ethanol solution consumed was recorded daily, as well as the animal’s body weight. Solutions were prepared and changed daily and provided at room temperature. Bottles were changed late in the light period of the light/dark cycle, at which time they were also weighed. The position of the ethanol and water tubes was alternated daily to prevent the development of side preferences.
Ethanol Vapor Exposure
After quantification of baseline ethanol intake during the first 5 days of the two bottle choice paradigm, adolescent and adult mice were distributed into ethanol (EtOH) and control (CTL) groups. Mice were distributed in the two groups based on their baseline ethanol intake values to avoid differences in ethanol intake prior to commencement of chronic intermittent ethanol or air exposure. Mice in the EtOH group were subjected to 16 h of continuous exposure to ethanol vapor in inhalation chambers followed by an 8 h period of withdrawal, for a total of 4 consecutive days. CTL mice were treated in a similar way, except that they were placed in control air chambers.
Ethanol or air was delivered in Plexiglas inhalation chambers (60 × 36 × 60 cm). Ethanol was volatilized by passing air through an air stone submerged in ethanol (95% v/v). The ethanol vapor was mixed with fresh air and delivered to the chambers at a rate that provides stable blood ethanol levels in the range of 175–225 mg/dl ethanol in adult C57BL/6J mice (Griffin et al. 2009a,b; Lopez and Becker, 2005). Before placement into the chambers for each 16 h exposure period, intoxication was initiated by administration of ethanol (1.6 g/kg, i.p.; 8% w/v in saline). Ethanol is readily absorbed in the respiratory tract during inhalation, and the metabolism and clearance are greater after inhalation than after intraperitoneal administration. Therefore, we administered ethanol via the intraperitoneal route to produce high blood concentrations during the initiation of vapor inhalation. We routinely use this procedure and have previously found it to be critical for maintaining stable ethanol intoxication during vapor inhalation procedures (Griffin et al., 2009a; Badanich et al., 2011). Blood ethanol concentrations were stabilized by administration of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg, i.p.). Both injections were given in a volume of 0.2 ml/10 g body weight. Control mice received similar handling in identical chambers but were exposed to air only, and received injections of saline (rather than ethanol) and pyrazole at the same dose.
Assessment of Blood Ethanol Concentration
Blood samples (approximately 40 μl) were obtained from EtOH groups twice a week during ethanol Exposure Cycles I and II. Immediately upon removal from the inhalation chambers, blood samples were obtained from the retro-orbital sinus in heparinized capillary tubes and then centrifuged at 14,000 rpm for 15 min. A 10 μl sample of the resulting plasma supernatant was assessed for ethanol content using an Analox® AM-1 analyzer (Lunenburg, MA, USA). Data are represented as an average of BECs obtained within each cycle.
Data Analysis
Ethanol consumption (g/kg) and preference during each drinking period (Baseline, Test 1, and Test 2) were analyzed by 3-way repeated measures ANOVA, with Age (adolescent, adult) and Treatment (EtOH vs. CTL groups) as between-subjects variables and Days as a repeated measure. Mean baseline consumption values for ethanol and air exposed groups were used to analyze changes in intake during the 10 test drinking sessions. In addition, separate analyses of ethanol consumption (g/kg) and preference with each group’s drinking period means (collapsed across days) was conducted by a 3-way repeated measures ANOVA (Age x Treatment x Period), with Period (Baseline, Test 1, Test 2) considered as a repeated measure. A 3-way repeated measures ANOVA (Age x Treatment x Days) was used to analyze body weight variation during the experiment, with Days as repeated measure. ANOVAs were followed by Bonferroni’s post-hoc tests where appropriate.
Blood ethanol concentration (BEC) values obtained from adolescent and adult animals exposed to ethanol inhalation (EtOH group) were analyzed by a 2-way ANOVA (Age x Exposure Cycle), followed by Bonferroni-corrected post-hoc tests. Differences were considered significant when p< 0.05.
RESULTS
Ethanol intake/preference after chronic intermittent ethanol or air vapor exposure in adolescent and adult mice
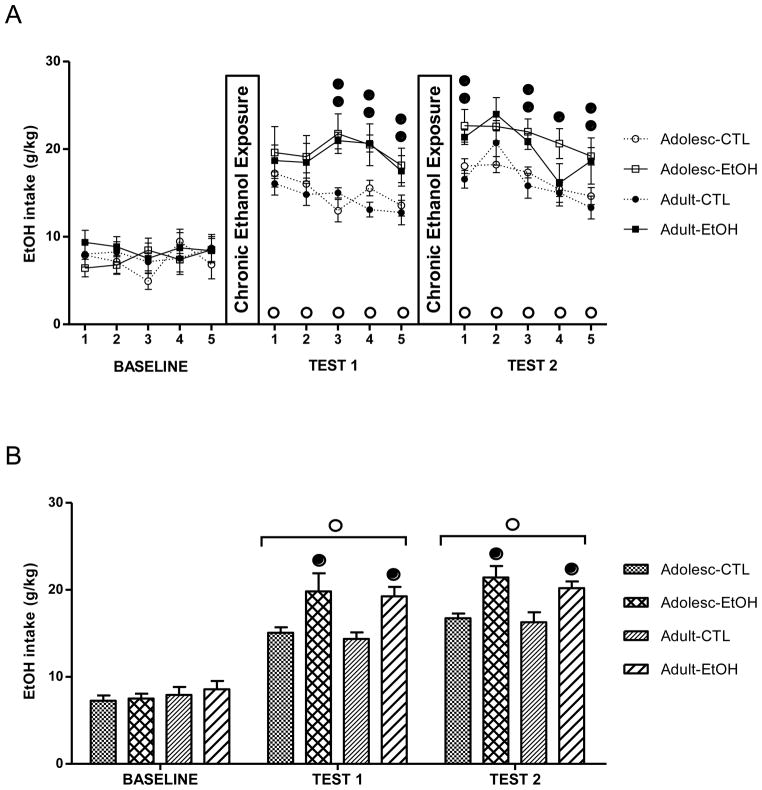
Figures 1A and 1B show ethanol consumption (g/kg) during daily drinking sessions and averaged over the five days within each period of the study, respectively. There were no group differences during baseline drinking [F(1,36) = 0.07; p = 0.79]. A significant increase in consumption (g/kg) in EtOH groups (adolescent-EtOH and adult-EtOH) and CTL groups (Adolescent-CTL and Adult-CTL) was observed during test periods compared with the Baseline drinking period, as indicated by significant main effect of Days [F(10,360)= 30.48; p< 0.01] and Period [F(2,72)= 202.67; p< 0.01] (Fig. 1A and 1B, respectively). ANOVA also revealed a significant Treatment x Days interaction (Fig. 1A) [F(10,360)= 2.22; p< 0.01] and Treatment x Period interaction (Fig. 1B) [F(2,72)= 8.39; p< 0.01]. Post-hoc analyses indicated that while there were no group differences during baseline drinking, chronic ethanol exposed groups (EtOH) showed increased drinking in both age groups compared to their respective CTL groups during Test 1 and Test 2 phases of the study. A main effect of Age did not achieve statistical significance when data were analyzed for daily intake (Fig. 1A) [F(1,36)= 0.39; p= 0.536] or averaged intake for the different test periods (Fig. 1B) [F(1,36) = 0.79; p =0.79]. Also, increased ethanol consumption in chronic ethanol exposed mice compared to controls during Test 1 and Test 2 did not differ between the two age groups tested.
Figure 1.
Mean ethanol consumption (in g/kg), (mean ± SEM) during Baseline, Test 1 and Test 2 in ethanol (EtOH) and control (CTL) groups of adolescent (Adolesc) and adult mice. (A) Ethanol consumption during daily sessions. (B) Ethanol consumption collapsed across the five consecutive daily sessions. (●) indicates a significant difference between EtOH and the respective control groups (p<0.05); (○) indicates a significant difference from average baseline intake (p<0.05). The number of mice in each experimental group was as follows: n=12 (Adolescent-control), n=10 (Adolescent-EtOH), n=9 (Adult-control), n=9 (Adult-EtOH).
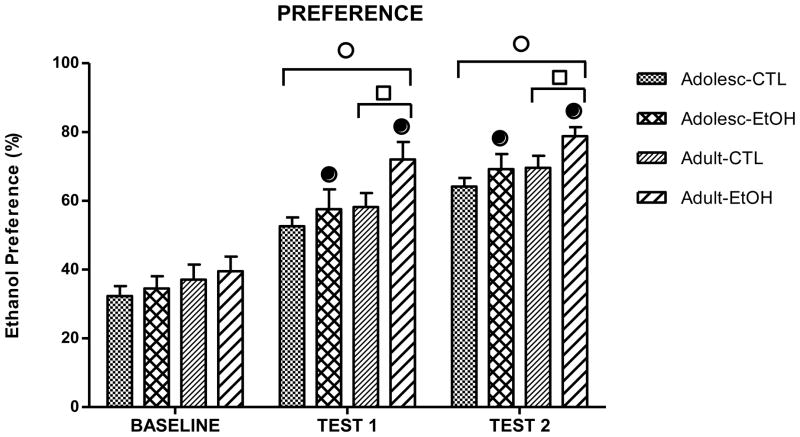
Ethanol preference data are shown in Fig. 2. The 3-way repeated measures ANOVA revealed significant main effects of Age [F(1,36)= 7.4; p< 0.01], Treatment [F(1,36)= 5.23; p< 0.05] and Period [F(2,72)= 115.44; p< 0.01]. This latter effect reflects a gradual increase in preference for ethanol exhibited during the test periods as compared to Baseline. The 2-way ANOVA performed for each period revealed similar ethanol preferences for all mice during baseline [F(1,36)= 0.0009; p= 0.97]. However, chronic ethanol exposed groups showed a significantly higher ethanol preference compared to the CTL groups during Test 1 [F(1,36)= 4.52; p< 0.05] and Test 2 [F(1,36)= 4.58; p< 0.05]. Further, adult mice displayed higher ethanol preference compared to the adolescents during Test 1 [F(1,36)= 5.15; p< 0.05] and Test 2 [F(1,36)= 5.1; p< 0.05] phases of the study, as revealed by a Bonferroni post-hoc test.
Figure 2.
Ethanol preference (ethanol volume/total volume consumed) during Baseline, Test 1 and Test 2 in ethanol (EtOH) and control (CTL) groups of adolescent (Adolesc) and adult mice. Ethanol preference averaged across the five consecutive daily sessions. (●) indicates a significant difference between EtOH and the respective control groups (p<0.05); (○) indicates a significant difference from average baseline intake (p<0.05); (□) indicates a significant difference from adolescent animals (p<0.05).
Blood ethanol concentrations in adolescent and adult mice
Figure 3 shows mean BEC values collected during each exposure cycle for each age group. ANOVA (Exposure Cycle x Age) revealed a significant Age x Exposure Cycle interaction [F(1,17)= 18.73; p=0.00046]. Post-hoc analysis revealed that adult mice exhibited higher BECs compared to adolescents during the first exposure cycle but lower BECs compared to adolescents during the second exposure cycle. BEC values were lower during Exposure Cycle II compared to Exposure Cycle I in adult mice while BECs were similar across both cycles in adolescent mice.
Figure 3.
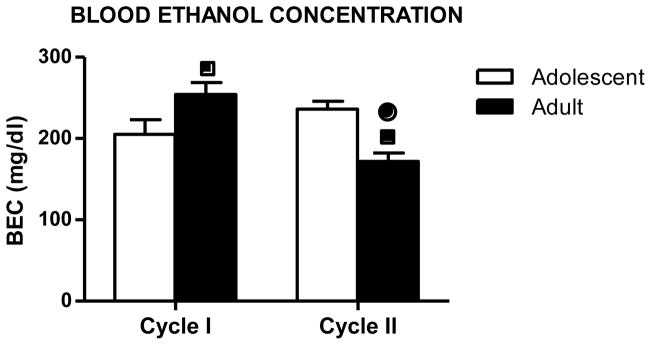
Blood ethanol concentrations (mean ± SEM) of adolescent (white bar) and adult (black bar) mice exposed to two cycles of ethanol vapor inhalation (Cycle I and Cycle II). (■) indicates a significant difference compared to adolescent animals in the same cycle. (●) indicates a significant difference compared to the same age group in Cycle I (p < 0.05). The number of mice in each experimental group was as follows: n=10 (Adolescent-EtOH), n=9 (Adult-EtOH).
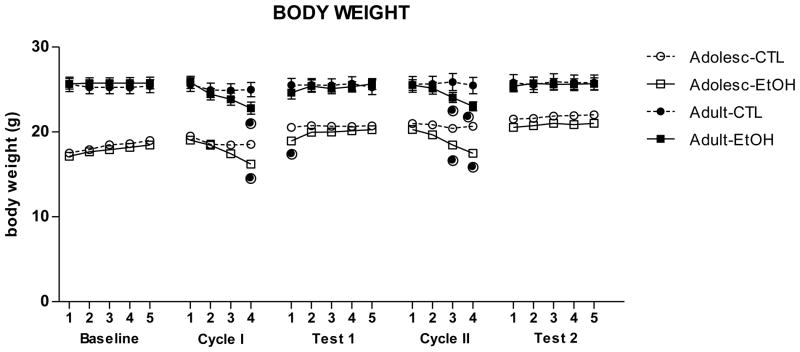
Body weight variation during chronic intermittent ethanol exposure
With regards to body weights, a significant main effect of Days [F(22,792)= 65.81; p< 0.01], an interaction of Age x Days [F(22,792)= 31.94; p< 0.01], and an interaction of Treatment x Days [F(22,792)= 12.99; p< 0.01] were observed. Adolescent and adult mice showed a decrease in body weight at the end of each Exposure Cycle compared to their respective CTL groups. However, at the beginning of the following week, weight loss was recovered, as the differences between ethanol-exposed and air-exposed mice were no longer detected. Thus, mild body weight decreases appeared to be related to ethanol vapor exposure (Fig. 4).
Figure 4.
Body weight (mean ± SEM) of all groups during the experiment. (●) indicates a significant difference compared to the respective control group (p < 0.05).
DISCUSSION
The main findings of the present study are that chronic intermittent ethanol vapor exposure enhanced subsequent voluntary ethanol self-administration in both adolescent and adult mice. Previous studies by our group have demonstrated that this model of chronic intermittent ethanol exposure produces increased ethanol consumption in adult C57BL/6 mice (Becker and Lopez, 2004; Griffin et al., 2009a,b; Lopez and Becker, 2005), and the present study corroborates these findings. In addition, whereas our previous work involved using a limited access (2 h/day) drinking model, results presented here also indicated that augmented alcohol intake following chronic ethanol exposure and withdrawal can be observed in mice that have unlimited access (24 h/day) to ethanol in the home cage.
Our results suggest that repeated chronic alcohol exposure and withdrawal may increase the propensity to consume ethanol during future drinking episodes. Using two-bottle drinking models, studies have demonstrated that adolescent and adult animals increase consumption after a deprivation period (Siegmund et al., 2005; Tambour et al., 2008), which is also in agreement with the present results. In the present study, an increase in ethanol intake and preference was observed in CTL groups after deprivation periods. These animals were ethanol deprived since access to ethanol bottles was suspended during the time they received inhalation (air exposure) treatment. Moreover, chronic intermittent ethanol vapor exposure promoted higher ethanol drinking compared to control animals that were not exposed to ethanol in the inhalation chambers. This phenomenon observed in CTL mice has been described as an alcohol deprivation effect (ADE) and this effect has been demonstrated in rats, mice and monkeys (Salimov and Salimova, 1993; Sinclair and Senter, 1968).
Recent studies have described progressive increases in the amount of ethanol consumed by C57BL/6J adult mice after periods of deprivation (Hwa et al., 2011; Melendez et al., 2006). The detection of such drinking behavior may vary according to the length and number of deprivations as well as the consumption regimen (limited, continuous, or intermittent access). In fact, animals that experience ethanol drinking intermittently develop escalation of ethanol drinking and more severe withdrawal symptoms compared to animals exposed to a continuous access regimen. Longer periods of deprivation (e.g., 2 weeks) followed by continuous access may not reveal an ADE and may even result in decreased consumption. Repeated sessions of shorter deprivation periods (6 days) followed by ethanol access (1 day) resulted in escalation in drinking (Melendez et al., 2006). In comparison, our experimental design for CTL groups involved three weeks of consumption intercalated by one-week deprivation periods. Additionally, studies have indicated that animal strains with high propensity for alcohol drinking are also more prone to exhibit increases in ethanol consumption after deprivation (Sinclair and Tiihonen, 1988; Vengeliene et al., 2003). This is in general agreement with our findings in C57BL/6J mice and, thus, it could be argued that the increase in voluntary ethanol intake observed in CTL mice in the present study is due to deprivation periods. It is interesting to note that previous studies conducted with limited access procedures (Becker and Lopez, 2004; Griffin et al., 2009a,b; Lopez and Becker, 2005) have not shown this effect of alcohol deprivation in control mice. It may be that ADE is observed when mice have unlimited access to ethanol in their home cage, but not when mice are offered access to ethanol on a limited basis. Nevertheless, and most importantly, in the present study adolescent and adult mice that experienced repeated cycles of chronic ethanol exposure and withdrawal (EtOH groups) showed even higher levels of voluntary ethanol intake compared to controls. Surprisingly, this increase in ethanol intake resulted in a significant shift in preference for ethanol only in adult mice. Both, adult and adolescent mice that were exposed to ethanol vapor (EtOH groups) showed an increase in the total volume of intake (ethanol plus water) during the test cycles (data not shown). However, given the observed increase in ethanol preference in adult mice but not in adolescents, it is clear that the increase in ethanol intake in adolescent mice in the EtOH group was accompanied by a proportionally greater intake of water. Future studies should evaluate more closely this effect that can indicate important differences in ethanol intake and preference across development.
Although there are several studies reporting elevated ethanol consumption in adolescent rodents as compared to adults (Doremus et al., 2005; Vetter et al., 2007; Hargreaves et al., 2009; Holstein et al., 2011), adolescent rats in other studies showed less alcohol consumption or preference than adults at the onset of ethanol drinking (Siegmund et al., 2005 and Hefner and Holmes, 2007, respectively). For instance, Siegmund et al (2005) demonstrated that a deprivation phase equalized ethanol consumption in both adolescent and adult rodents and stress induced increases in alcohol intake in the group that initiated alcohol consumption during adolescence. These data are in agreement with the present study, in which age of initiation of ethanol drinking may not be the main factor for subsequent increase in consumption after periods of intoxication and deprivation. The alcohol consumption pattern models should also be considered to explain these discrepancies. Some studies have used limited or intermittent access sessions (Hargreaves et al., 2009; Holstein et al., 2011), while in the present study, mice had free-choice continuous access to the bottles. Moreover, one should be note that forced administration of ethanol via vapor chambers during adolescence did not result in increased consumption in adult Sprague-Dawley rats (Slawecki and Betancourt, 2002; Tolliver and Samson, 1991).
An interesting observation was that adult but not adolescent mice developed tolerance to ethanol vapor induced increases in BECs during ethanol intoxication, since adult BECs in Cycle II were lower than those in Cycle I, while adolescent BECs did not change between the cycles. Moreover, adults BECs were lower than adolescents BECs in Cycle II. In other words, a second cycle of ethanol inhalation and withdrawal may produce increased ethanol metabolism in adult animals as compared to adolescents. Elimination of alcohol is enhanced after frequent exposure to ethanol due to induction of metabolizing enzymes promoted by ethanol (Zuba et al., 2002; Yadav et al., 2006). Interestingly, adolescent mice did not present a pharmacokinetic tolerance, although studies of ethanol intoxication (Morris et al., 2010) and loss of righting reflex (Linsenbardt et al., 2009) suggest that juvenile animals eliminate ethanol faster than adults. This is not what has been observed in this study. Differences in BEC were not especially large and it could be the results of small variations in ethanol concentration in the inhalation chambers. Ethanol concentrations were very similar across chambers (16–18 mg/l of air). However, it is important to note that ethanol concentrations in the inhalation chambers were set based on evidence from previous studies that indicate ethanol chamber concentrations that induce BEC within a range of 175–225 mg/dl are necessary to induce significant increases in voluntary ethanol intake in adult C57BL/6J mice (Griffin et al., 2009b). Future studies should evaluate more closely which are the optimal parameters for ethanol vapor exposure to induce BEC that result in significant increases in voluntary ethanol intake in adolescent mice.
The catalytic efficiency of liver alcohol dehydrogenase for alcohol varies with age, with a maximum efficiency observed during adolescence (Hollstedt et al., 1977). The activity of ADH-2 and ADH-3 are higher in juvenile rats as compared to their adult counterparts (Chung et al., 2008). However, possibility of adolescent mice being more sensitive to the hepatotoxic effects of ethanol exists, which might explain the observed BEC differences. Accordingly, pyrazole might also differently affect the catalytic efficiency of liver alcohol dehydrogenase during adolescence versus adulthood. Thus, these factors need to be considered carefully when interpreting alcohol consumption data from adolescent subjects.
Chronic exposure to ethanol vapor (EtOH-adolescent and EtOH-adult) induced loss of weight at the end of the exposure cycles. These animals recovered their body weight at the beginning of the following week. Thus, our results clearly indicate that the relatively modest loss of body weight was due to the chronic intermittent ethanol exposure, which is in agreement with the findings of Diaz-Granados and Graham (2007).
In summary, we have shown that repeated cycles of chronic intermittent ethanol exposure, which mimics characteristics of alcohol dependence in humans, increases ethanol consumption in both adolescent and adult mice. While age differences in resulting BECs were apparent, this difference did not appear to have an influence on ethanol consumption.
Acknowledgments
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and NIH grant AA013852 (MFO), AA014095 (HCB and MFL). PFCN was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors thank Kay Fernandes for technical support.
References
- Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Dev Psychobiol. 2010;52:424–440. doi: 10.1002/dev.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Becker HC, Woodward JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci. 2011;125:879–891. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Alcohol withdrawal: Neuroadaptation and sensitization. CNS Spectr. 1999;4:38–65. [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Camarini R, Hodge CW. Ethanol preexposure increases ethanol self-administration in C57BL/6J and DBA/2J mice. Pharmacol Biochem Behav. 2004;79:623–632. doi: 10.1016/j.pbb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivationin adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CS, Wang J, Wehman M, Rhoads DE. Severity of alcohol withdrawal symptoms depends on developmental stage of Long–Evans rats. Pharmacol Biochem Behav. 2008;89:137–144. doi: 10.1016/j.pbb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- De Wit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolescence of the aversive properties of ethanol during adulthood. Alcohol Clin Exp Res. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased Drinking During Withdrawal From Intermittent Ethanol Exposure Is Blocked by the CRF Receptor Antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM–IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and Duration of Chronic Ethanol Exposure Is Critical for Subsequent Escalation of Voluntary Ethanol Drinking in Mice. Alcohol Clin Exp Res. 2009a;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009b;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves GA, Monds L, Gunasekaran N, Dawson B, McGregor IS. Intermittent access to beer promotes binge-like drinking in adolescent but not adult Wistar rats. Alcohol. 2009;43:305–314. doi: 10.1016/j.alcohol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Takodoro S. Learning retardation and enhanced ethanol preference produced by postnatal pretreatments with ethanol in adult rats. Jpn J Pharmacol. 1985;37:269–276. doi: 10.1254/jjp.37.269. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. The effect of alcohol on the developing organism. Genetical, teratological and physiological aspects. Med Biol. 1977;55:1–14. [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent Escalation of Alcohol Drinking in C57BL/6J Mice With Intermittent Access to 20% Ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Moal ML. Drug Addiction, Dysregulation of Reward, and Allostasis. Neuropsychopharmacology. 2001;24:98–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL. Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Meisch R. Relationship between physical dependence on ethanol and reinforcing properties of ethanol in animals. NIAAA Res Monogr. 1983;13:27–32. [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, Miranda R, Jr, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, White A, Crews FT. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. 2010;44:89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Salimov RM, Salimova NB. The alcohol-deprivation effect in hybrid mice. Drug Alcohol Depend. 1993;32:187–191. doi: 10.1016/0376-8716(93)80012-4. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Tiihonen K. Lack of alcohol-deprivation effect in AA rats. Alcohol. 1988;5:85–87. doi: 10.1016/0741-8329(88)90048-1. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosc Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence: Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and Age at Drinking Onset Affect Voluntary Alcohol Consumption but Neither the Alcohol Deprivation Effect nor the Response to Stress in Mice. Alcohol Clin Exp Res. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive Motivational Experience During Adolescence Results in Enhanced Alcohol Consumption During Adulthood. Behav Neurosci. 2009;123:926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Yadav S, Dhawan A, Singh RL, Seth PK, Parmar D. Expression of constitutive and inducible cytochrome P450 2E1 in rat brain. Mol Cell Biochem. 2006;286:171–180. doi: 10.1007/s11010-005-9109-z. [DOI] [PubMed] [Google Scholar]
- Zuba D, Piekoszewski W, Pach J, Winnik L, Parczewski A. Concentration of ethanol and other volatile compounds in the blood of acutely poisoned alcoholics. Alcohol. 2002;26:17–22. doi: 10.1016/s0741-8329(01)00186-0. [DOI] [PubMed] [Google Scholar]