Abstract
Mouse models carrying Disc1 mutations may provide insights into how Disc1 genetic variations contribute to schizophrenia (SZ) susceptibility. Disc1 mutant mice show behavioral and cognitive disturbances reminiscent of SZ. To dissect the synaptic mechanisms underlying these phenotypes, we examined electrophysiological properties of cortical neurons from two mouse models, the first expressing a truncated mouse Disc1 (mDisc1) protein throughout the entire brain, and the second expressing a truncated human Disc1 (hDisc1) protein in forebrain regions. We obtained whole-cell patch voltage clamp recordings to examine how altered expression of Disc1 protein changes excitatory and inhibitory synaptic transmission onto cortical pyramidal neurons in the medial prefrontal cortex in 4–7 month-old mDisc1 and hDisc1 mice. In both mDisc1 and hDisc1 mice, the frequency of spontaneous EPSCs was greater than in wild-type littermate controls. Male mice from both lines were more affected by the Disc1 mutation than were females, exhibiting increases in the ratio of excitatory to inhibitory events. Changes in spontaneous IPSCs were only observed in the mDisc1 model and were sex-specific, with diminished cortical GABAergic neurotransmission, a well-documented characteristic of SZ, occurring only in male mDisc1 mice. In contrast, female mDisc1 mice showed an increase in the frequency of small-amplitude sIPSCs. These findings indicate that truncations of Disc1 alter glutamatergic and GABAergic neurotransmission both commonly and differently in the models and some of the effects are sex-specific, revealing how altered Disc1 expression may contribute to behavioral disruptions and cognitive deficits of SZ.
Keywords: schizophrenia, genetic mouse models, Disc1, electrophysiology, synaptic activity, patch clamp, EPSCs, glutamate
1. Introduction
Schizophrenia (SZ) is a multifactorial psychiatric condition characterized by both positive and negative symptoms (Tamminga and Holcomb, 2005). Although the etiology of SZ remains poorly understood, the disease has a clear genetic component, with a heritability thought to exceed 75% (Wexler and Geschwind, 2011). Major efforts have identified genetic risk factors, but few have demonstrated strong biological support (Moens et al., 2011; Sanders et al., 2008). One notable exception is the gene Disrupted-in-Schizophrenia-1 (Disc1). A positive association between SZ and a chromosomal translocation (1:11) disrupting the Disc1 gene on chromosome 1 was reported in a Scottish family with a high rate of SZ, depression, and bipolar disorder (Millar et al., 2000). Since this discovery, studies have supported a central role for common Disc1 genetic variation in conferring susceptibility to psychiatric disease (Cannon et al., 2005; Hashimoto et al., 2006; Moens et al., 2011; Thomson et al., 2005)
Disc1 encodes the Disc1 protein, which acts as a cytosolic scaffold protein required for processes such as neurogenesis, neuronal migration, dendritic growth, and synaptic maintenance (Brandon et al., 2009; Brandon and Sawa, 2011; Hayashi-Takagi et al., 2010; Jaaro-Peled et al., 2009; Porteous et al., 2011). In mutation carriers, either haploinsufficiency (conferring reduced functional Disc1 expression) or dominant-negative effects of the mutated Disc1 may form the basis for susceptibility to psychiatric disorders (Hikida et al., 2007; Porteous et al., 2006; Sawa and Snyder, 2005).
Genetic animal models have been designed to elucidate how Disc1 mutations produce the pathophysiology of psychiatric illnesses. These models include mice with abolished expression of the full-length mouse Disc1 (mDisc1) protein (Koike et al., 2006), and mice expressing a truncated human Disc1 (hDisc1) protein (Hikida et al., 2007; Li et al., 2007; Pletnikov et al.,2008). These mice exhibit disturbances in sensorimotor gating, hyperactivity, cognitive deficits, depression, and altered social interactions (Kvajo et al., 2008; Pletnikov et al., 2008).
At present, the effects of mutant Disc1 expression on the functional properties of cortical neurons remain unknown. We used electrophysiological methods to examine how expression of truncated Disc1 protein alters membrane properties and synaptic transmission in cortical pyramidal neurons from the medial prefrontal cortex, a brain region crucial to most pathophysiological hypotheses of SZ, in mDisc1 and hDisc1 mouse models.
2. Methods
2.1. Animals
Two genetic mouse models were used. In the first model, a 129S6/SvEv mDisc1 allele with a 25-bp deletion variant in exon 6 that resulted in the introduction of a premature termination codon in exon 7 was transferred into the C57BL/6J background, resulting in a strain that expresses truncated mDisc1 (Koike et al., 2006; Kvajo et al., 2008). The second model, hDisc1, expresses truncated human Disc1 protein and was created by breeding B6;SJL-Tg(TRE-CMV-hDISC1) founders with single transgenic (control) B6;CBA-Tg(Camk2a-tTA)1Mmay/j mice (The Jackson Laboratory, Bar Harbor, ME, USA) to generate double transgenic mutant mice of the hybrid B6;SJL;CBA background (Pletnikov et al., 2008). Expression of mutant hDisc1 was limited to forebrain regions including the cerebral cortex, hippocampus, and striatum. mDisc1 mice and wildtype (WT) littermates were obtained from breeding colonies at the University of California, Los Angeles (UCLA). Transgenic hDisc1 mice and single transgenic CAMKII tTA littermates used as controls were obtained from breeding colonies at the Johns Hopkins University School of Medicine in Baltimore, Maryland and shipped to UCLA. All mice were examined at 4–7 months of age and compared with age-matched WT littermates (mDisc1) or controls (hDisc1). All procedures were performed in accordance with the Public Health Service's Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at UCLA. Mice were individually housed on a 12 h light-dark cycle, and food and water were available ad libitum. Mice were genotyped twice, once at weaning and again after experimentation. There were no significant differences in body weight between WT and mutant mice of the same sex.
2.2. Slice preparation
Mice were anaesthetized using isoflurane, decapitated, and the brain rapidly removed to ice-cold dissection artificial cerebrospinal fluid (ACSF), containing 130 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 1.25 mM NaHPO4, 10 mM glucose, 5 mM MgCl2 and 1 mM CaCl2; aerated with 95% O2/5% CO2 (pH = 7.2; 290–300 mOsm/L). The brain was trimmed and glued to the stage of a vibrating microtome (model VT1000S; Leica), and 300 µm coronal slices of the cerebral cortex were cut. Slices were stored and allowed to recover at room temperature in a chamber submerged in oxygenated standard ACSF (same composition as dissection ACSF, except that MgCl2 and CaCl2 were 2 mM) for at least 1 h before experimentation.
2.3. Electrophysiology
We performed whole-cell patch clamp recordings from layer II/III pyramidal cells of the medial prefrontal cortex. Neurons in layers II/III of the prefrontal cortex are critically involved in working memory and in SZ models a reduction of gamma oscillations along with a loss of parvalbumin interneurons occurs selectively in superficial but not deep cortical layers (Cunningham et al., 2006). Furthermore, the inputs to and the morphology of cortical layer II/III pyramidal cells have been shown to undergo pathophysiological alterations in SZ (Hill et al., 2006; Lewis and Gonzalez-Burgos, 2000; Pierri et al., 2001). Pyramidal neurons were visualized with infrared videomicroscopy and differential interference contrast optics and identified by somatic size and typical basic membrane properties (Cummings et al., 2009). Recordings were made with borosilicate glass micropipettes filled with a cesium methanesulfonate (CsMeth)-based internal solution containing 125 mM cesium methanesulfonate, 4 mM NaCl, 3 mM KCl, 1 mM MgCl2, 9 mM EGTA, 8 mM Hepes, 5 mM MgATP, 1 mM Tris/GTP, 10 mM di sodium phosphocreatine and 0.1 mM leupeptin (pH = 7.2; 270–280 mOsm/L). In some experiments QX-314 (4 mM) was included in the internal solution to prevent Na+ channel activation when holding at depolarized membrane potentials. Voltage clamp recordings were performed using a Multiclamp 700B patch-clamp amplifer (Molecular Devices, Sunnyvale, CA). During electrophysiological recordings, slices were continuously perfused in 95% O2/5%CO2 ACSF at a flow rate of ~2 ml/min at room temperature.
Spontaneous excitatory and inhibitory postsynaptic currents (sEPSCs and sIPSCs, respectively) were recorded, filtered at 1 kHz and digitized at 100–200 µs using Clampex 10.2 in gap-free mode (Molecular Devices). To assess basic membrane properties and sEPSCs, cells were voltage-clamped at −70 mV. Cell membrane capacitance was determined from a depolarizing step voltage command (10 mV) using the membrane test function integrated in the pClamp10 software.
To more completely isolate glutamate receptor-mediated sEPSCs, bicuculline methobromide (BIC, 5 µM) was applied in order to block y-aminobutryric acid type A (GABAA) receptor-mediated currents. This concentration of BIC completely abolishes sIPSCs in cortical pyramidal neurons (Cummings et al., 2009). To isolate sIPSCs membranes were stepped to a holding potential of+10 mV and currents were recorded in regular ACSF. No glutamate receptor antagonists were used for this experiment as this would have precluded estimation of glutamate-GABA ratios and also because quinoxaline derivatives such as CNQX and NBQX alter sIPSC frequency (Brickley et al., 2001; McBain et al., 1992). Nevertheless, after addition of BIC no spontaneous synaptic activity was observed at +10 mV indicating that glutamate synaptic events did not contribute to sIPSCs at this holding potential.
Spontaneous EPSCs and IPSCs were analyzed offline using the automatic detection protocol within the Mini Analysis program (Justin Lee, Synaptosoft, version 6.0) and subsequently checked manually for accuracy. The threshold amplitude for the detection of an event (5 pA for EPSCs; 10 pA for IPSCs) was set above the root mean square noise (<2 pA at Vhold = −70 mV and <4 pA at Vhold = +10 mV). Event kinetic analysis used the Mini Analysis Program, and EPSCs and IPSCs with peak amplitudes between 10–50 pA and 10–100 pA, respectively, were grouped, aligned by half-rise time, and normalized by peak amplitude. In each cell, grouped events were averaged to obtain rise times, decay times, and half-amplitude durations. We calculated the ratio of excitatory to inhibitory events by dividing the frequency of sEPSCs by the frequency of sIPSCs for each cell.
2.4. Statistical analyses
Values in figures and text are means ± SEMs. Differences between group means were assessed with appropriate Student's t-tests (unpaired), or Mann-Whitney Rank Sum Test when distributions were not normal, and two-way analyses of variance (ANOVA) with one repeated measure (RM), followed by Bonferroni post hoc tests. Differences were considered statistically significant if p<0.05. Microsoft Excel and Sigma Stat 3.5 were used to perform all statistical analyses.
3. Results
3.1. mDisc1 mice
We first examined electrophysiological properties of cortical prefrontal pyramidal neurons from WT (n=39 mice, 22 male and 17 female) and mutant (n=29 mice, 22 male and 7 female) mice (average age was 159±3 and 162±4 days, respectively). Layer II/III pyramidal neurons of the infralimbic region of the medial prefrontal cortex were voltage clamped using CsMeth as the internal solution (n=55 cells from WT and n=58 cells from mutant mDiscl mice). There were no consistent differences in average cell membrane capacitance, input resistance, and time constant either when cells from males and females were combined or when they were separated by sex.
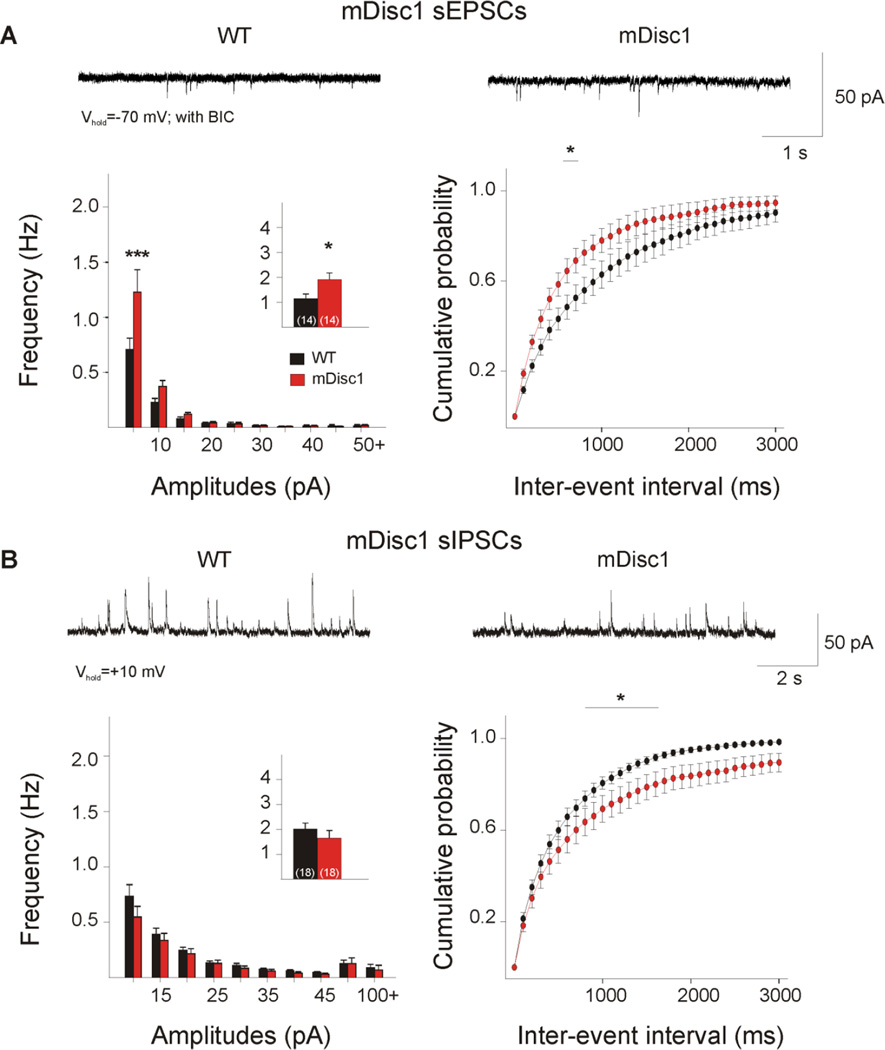
Glutamate receptor-mediated synaptic currents were significantly altered. At Vhold = −70 mV, the majority of spontaneous synaptic events are mediated by glutamatergic a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and the contribution of inhibitory currents is negligible, as this holding potential is very close to the Cl− reversal potential (where Vrev for Cl− is ~−60 mV with CsMeth). Cells from mDiscl mice showed a significant increase in mean frequency of sEPSCs (p=0.034, Table 1, Figure 1A, inset). Amplitude-frequency histograms were significantly different between the genotype groups (F(9,234)=3.18, p=0.001). Post hoc comparisons showed a significant increase in the frequency of sEPSCs at the 5–10 pA amplitude bin (p<0.001). A significant leftward shift occurred in the cumulative distributions of inter-event intervals (IEIs) (F(29,725)=2.26, p<0.001), and post hoc comparisons demonstrated significant differences in the 700–800 ms interval bins (p=0.042–0.047), suggesting that excitatory events occur more frequently in mutant cortical pyramidal cells. Analyses of sEPSC kinetics (e.g., rise time, decay time, and half-amplitude duration) showed no significant group differences (not shown).
Table 1.
| sEPSCs | sIPSCs | |||
|---|---|---|---|---|
| mDisc1 | Frequency (Hz) | Amplitude (pA) | Frequency (Hz) | Amplitude (pA) |
| WT ♂ | 1.1 ± 0.4 | 16.1 ± 3.7 | 2.4 ± 0.4 | 35.3 ± 5.6 |
| Mutant ♂ | 1.8 ± 0.3 | 12.5 ± 1.0 | 1.3 ± 0.3* | 26.3 ± 4.1 |
| WT ♀ | 1.2 ± 0.2 | 11.5 ± 0.8 | 1.8 ± 0.3 | 26.6 ± 3.2 |
| Mutant♀ | 2.0 ± 0.7 | 11.1 ± 1.3 | 2.9 ± 0.6 | 26.8 ± 1.8 |
| WT ♂+♀ | 1.2 ± 0.2 | 13.0 ± 1.4 | 2.0 ± 0.2 | 29.7 ± 2.9 |
| Mutant ♂+♀ | 1.9 ± 0.3* | 12.2 ± 0.8 | 1.6 ± 0.3 | 26.4 ± 3.2 |
| hDisc1 | ||||
| WT ♂ | 1.0 ± 0.4 | 12.2 ± 1.3 | 2.7 ± 0.4 | 26.5 ± 1.6 |
| Mutant ♂ | 3.9 ± 1.7* | 13.2 ± 1.8 | 4.1 ± 0.7 | 28.0 ± 2.7 |
| WT ♀ | 2.0 ± 0.9 | 12.9 ± 1.2 | 5.6 ± 0.7 | 30.8 ± 4.7 |
| Mutant ♀ | 2.9 ± 0.6 | 14.6 ± 1.6 | 5.0 ± 0.9 | 32.8 ± 3.9 |
| WT ♂+♀ | 1.4 ± 0.4 | 12.5 ± 0.9 | 4.1 ± 0.5 | 28.6 ± 2.5 |
| Mutant ♂+♀ | 3.3 ± 0.7* | 14.0 ± 1.2 | 4.4 ± 0.6 | 29.7 ± 2.2 |
Denotes p<0.05,
denotes male, and
denotes female.
Figure 1. Glutamatergic and GABAergic synaptic activity in mDisc1 mice.
A. Typical voltage-clamp traces of sEPSCs recorded from cortical pyramidal neurons at −70 mV in the presence of 5 µM BIC. mDisc1 mice showed a significant increase in mean sEPSC frequency (inset) compared to WT mice. Amplitude-frequency histograms indicated a significant increase in small-amplitude events 5–10 pA (left panel). A significant difference was observed in the cumulative distributions of IEIs from 700–800 ms (right panel). B. Typical traces of sIPSCs recorded in ACSF at a holding potential of + 10mV. No significant differences were observed in mean sIPSC frequency or amplitude-frequency histograms. Significant differences occurred in the cumulative distributions of IEIs in the 900–1700 ms intervals. For this figure and subsequent figures, * signifies p<0.05; ** signifies p<0.01; and *** signifies p<0.001.
Spontaneous IPSCs were recorded at +10 mV (Table 1, Figure 1B). No statistically significant differences occurred in the mean frequency of sIPSCs or the amplitude-frequency histograms. The difference between mDisc1 and WT cumulative distributions of IEIs approached statistical significance (F(l,34)=3.70,p=0.063). Post hoc analysis of IEI cumulative distributions demonstrated significant differences in the 900–1700 ms intervals (p=0.038–0.050), suggesting that inhibitory events occur less frequently in mutant cortical cells. Table 1 summarizes sEPSC and sIPSC average frequencies and amplitudes.
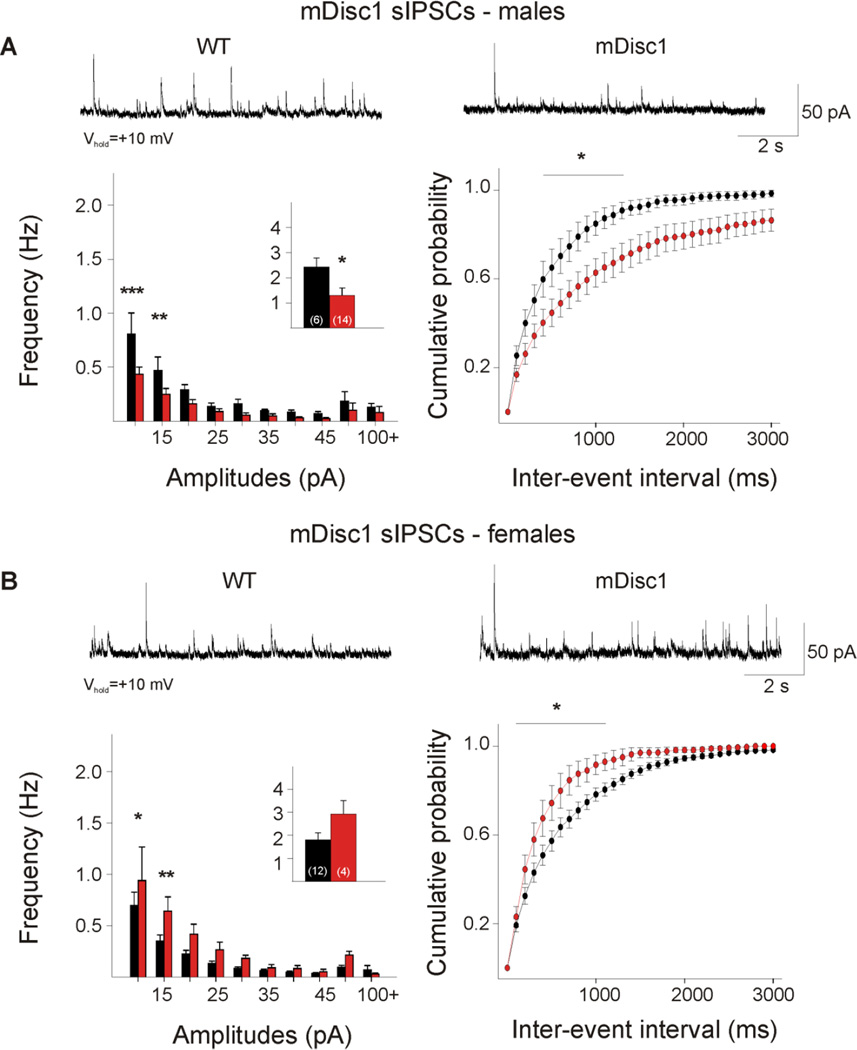
When analyzed for potential sex differences, cells from male mutants demonstrated a significant decrease in the frequency of sIPSCs (Figure 2A, p=0.039). Amplitude-frequency histograms were significantly different (F(9,162)=2.26,p=0.021), and post hoc comparisons revealed that mutant cells had a significantly lower frequency of events at the 10 and 15 pA bins (p<0.001, p=0.01). The difference between male mDisc1 and WT cumulative distributions of IEIs approached statistical significance (F(l,18)=3.80,p=0.067). Post hoc analysis demonstrated significant differences in the 500–1400 ms intervals (p=0.024–0.043), suggesting that cortical inhibitory events occur less frequently in mutant males. In contrast, a trend towards an increase in the frequency of sIPSCs occurred in cells from female mutants (Figure 2B, p=0.095). Amplitude-frequency histograms approached statistical significance (F(1,14)=3.20,p=0.095). Post hoc analysis demonstrated that female mutants have a significantly higher frequency of sIPSCs at the 10and 15 pA bins (p=0.031, p=0.009). A significant leftward shift occurred in the cumulative distributions of IEIs (F(29,406)=3.24, p<0.001), and post hoc comparisons demonstrated significant differences in the 200–1200 ms interval bins (p=0.002–0.038). This suggests that less frequent GABAergic input onto cortical pyramidal cells occurs in male mutants, but more frequent GABAergic input onto cells occurs in female mutants. The kinetics of spontaneous inhibitory events also were examined. No differences were found when both sexes were combined. However, when the sexes were separated, a significant decrease in the decay time occurred in cells from mutant females (WT females: 21.0±1.7 ms, mDisc1 females: 15.8±1.0 ms, p=0.039).
Figure 2. Sex differences in mDisc1 GABAergic synaptic activity.
A. Typical traces of sIPSCs recorded at +10 mV from male mDisc1 cortical pyramidal neurons. Note the significant decrease in mean sIPSC frequency (inset). Amplitude-frequency histograms indicated a significant decrease in small-amplitude events at the 10 and 15 pA bins. A significant difference was observed in the cumulative distributions of IEIs from 500–1400 ms. B. Typical traces of sIPSCs recorded at +10 mV from female mDisc1 cortical pyramidal neurons, showing a trend towards an increase in mean sIPSC frequency (inset). Note the significant increase in events at the 10 and 15 pA bins. A significant difference occurred in the cumulative distributions of IEIs from 200–1200 ms intervals.
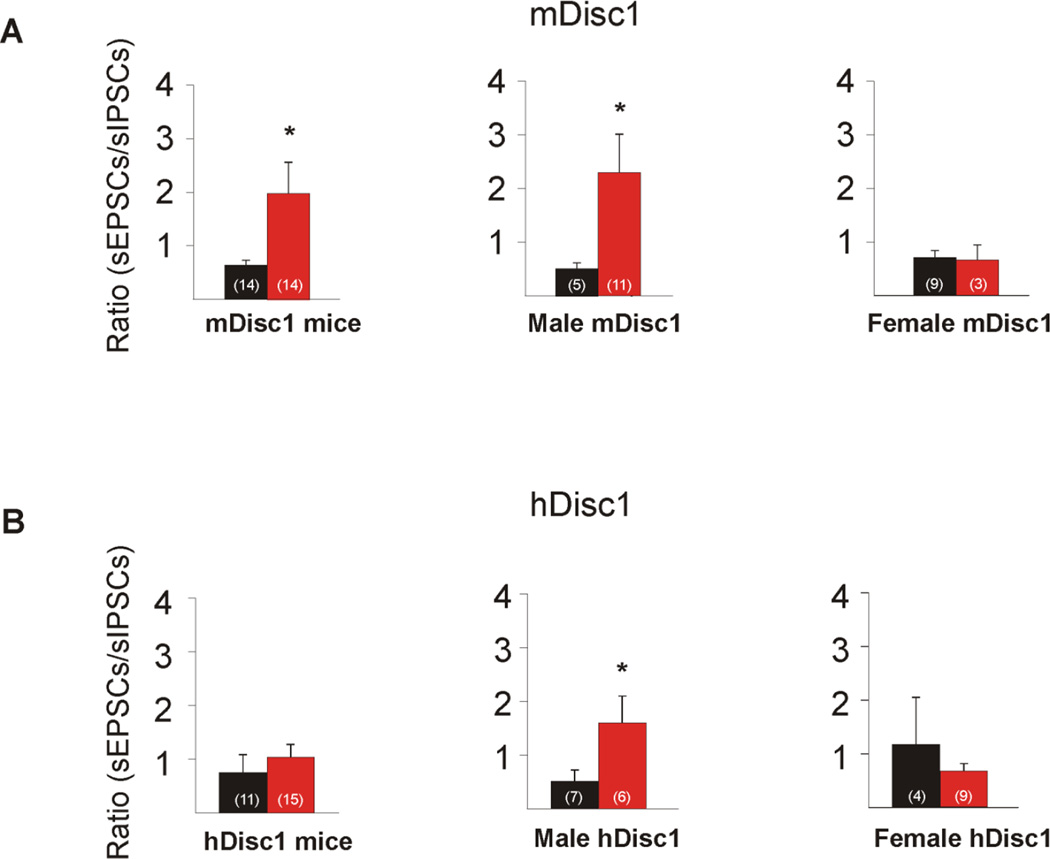
We also calculated the ratio of excitatory to inhibitory events by dividing the frequency of sEPSCs by the frequency of sIPSCs (frequency of events recorded at −70 mV in BIC/frequency of events recorded at +10 mV ACSF) for each cell. Overall, the mean excitation/inhibition ratio was significantly higher in mutant cells (p=0.036, Figure 3A). We then compared cells from WT and mutant littermates of the same sex. Mutant cells from males displayed a significantly higher excitation/inhibition ratio (p= 0.013, Mann-Whitney Rank Sum Test), suggesting an increase in the relative contribution of excitatory inputs to pyramidal neurons.
Figure 3. mDisc1 and hDisc1 excitation/inhibition ratios.
A. mDisc1 mice showed a significant increase in the excitation/inhibition ratio, suggesting an increase in the relative contribution of excitatory inputs to pyramidal neurons in mutant animals. When the ratios were separated by sex, mDisc1 males displayed a significant increase, while mDisc1 females did not. B. No difference occurred in the excitation/inhibition ratio in hDisc1 mice. When the ratios were separated by sex, hDisc1 males showed a significant increase in the excitation/inhibition ratio, while female hDisc1 mice did not.
3.2. hDisc1 mice
In this model, 9 control (4 male and 5 female) and 14 mutant (8 male and 6 female) mice were examined. Average age was 160±5 and 165±4 days respectively. The membrane properties of cortical pyramidal neurons (layers II/III) in slices from mutant mice (n=46 cells) and control littermates (n=33 cells) were measured in voltage clamp mode. There were no consistent significant differences in capacitance, input resistance, and time constant between control and mutant mice.
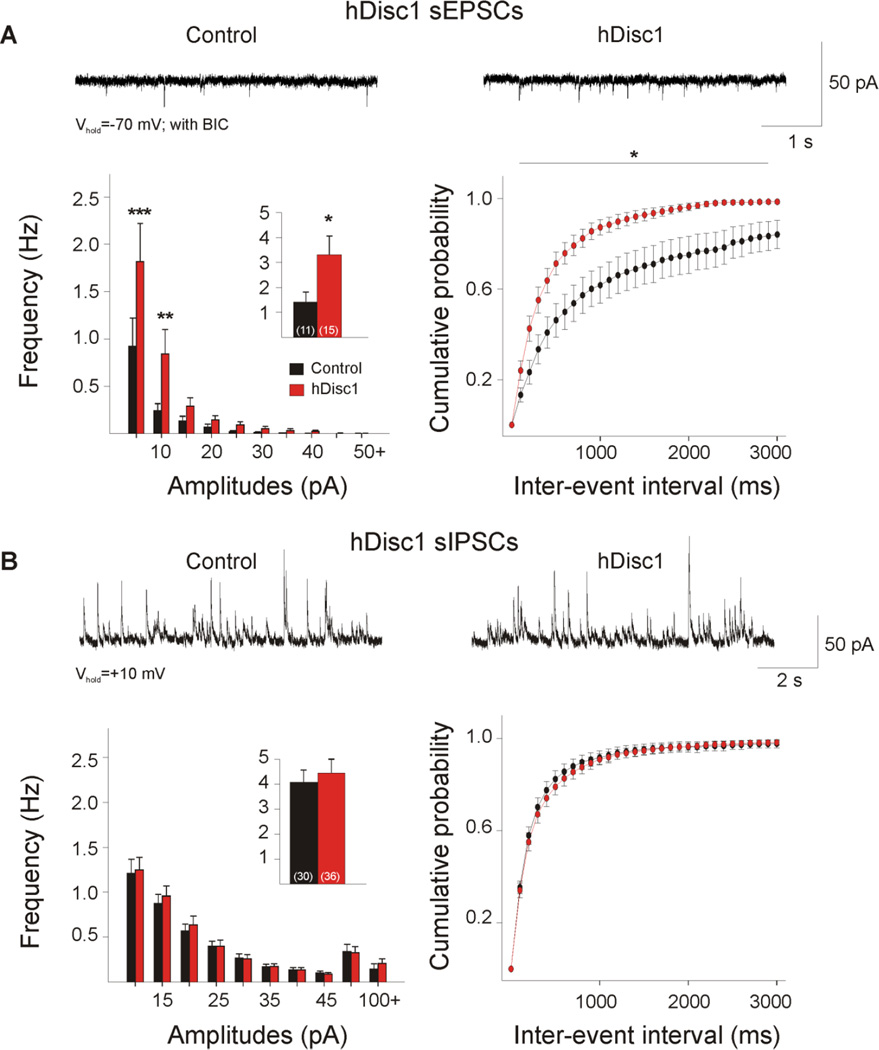
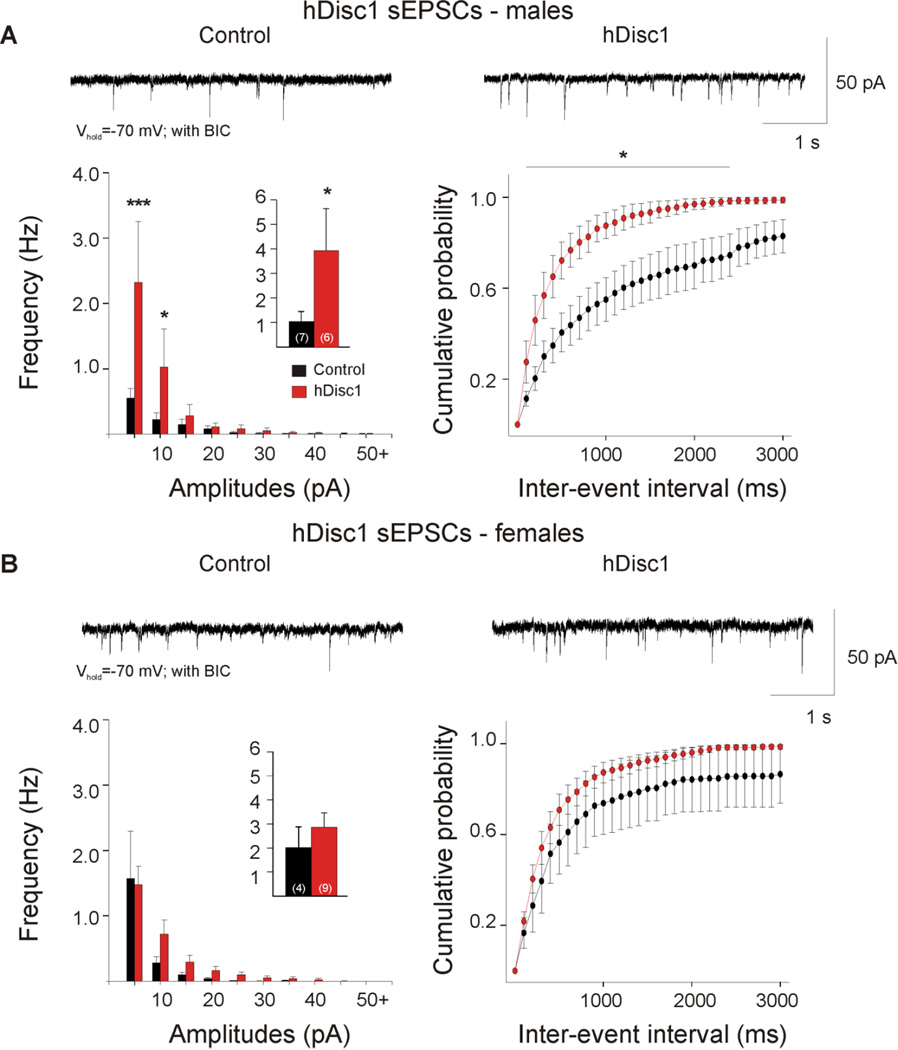
Mutant cells exhibited a significant increase in mean frequency of sEPSCs (Table 1, Figure 4A, p=0.036). Amplitude-frequency histograms were significantly different (F(9,216)=2.65,p=0.006), and post hoc comparisons revealed a significant increase in the frequency of events between 5–15 pA in amplitude in mutant cells (p<0.001,p=0.003). A significant leftward shift occurred in the cumulative distributions of IEIs (F(29,696)=1.60, p=0.024), andpost hoc comparisons demonstrated significant differences in the 200–2900 ms interval bins (p=0.001–0.045). In contrast, event kinetics were similar between mutant and control groups.
Figure 4. Glutamatergic and GABAergic synaptic activity in hDisc1 mice.
A. Typical traces of sEPSCs (−70 mV, with 5 µMBIC) from cortical pyramidal neurons. hDisc1 mice showed a significant increase in mean sEPSC frequency (inset) and frequency of events 5–15 pA. A significant difference occurred in the cumulative distributions of IEIs from 200–2900 ms. B. Typical traces of sIPSCs recorded in ACSF at a holding potential of+10 mV. No significant differences were observed in mean sIPSC frequency, amplitude-frequency histograms, or IEI cumulative distributions.
The data were then analyzed for sex differences. In cells from hDiscl males, a significant increase in sEPSCs (Table 1, Figure 5A, p=0.035, Mann-Whitney Rank Sum Test) and a significant difference in the amplitude-frequency histogram occurred (F(9,99)=3.59, p<0.001). Post hoc comparisons demonstrated a significant increase in the frequency of events between 5–15 pA in mutants (p<0.001, p=0.017). A significant difference occurred in the cumulative distributions of IEIs (F(l,l 1)=7.14, p=0.022), and post hoc analysis demonstrated significant differences in the 200–2400 ms interval bins (p=0.007–0.042). No significant differences were observed between female hDisc1 and control cells in the mean frequency of sEPSCs, amplitude-frequency histograms, IEI distributions, or event kinetics (Table 1, Figure 5B).
Figure 5. Sex differences in hDisc1 glutamatergic synaptic activity.
A. Typical traces of sEPSCs recorded from male hDisc1 cortical pyramidal neurons. Note the significant increase in mean sEPSC frequency (inset) and frequency of small-amplitude events at 5 and 10 pA bins. A significant difference was observed in the cumulative distributions of IEIs from 200–2400 ms. B. Typical traces of sEPSCs recorded from female hDisc1 cortical pyramidal neurons. No significant differences were observed in mean sIPSC frequency, amplitude histograms, or IEI cumulative distributions.
No significant differences were observed between mutant and control cells in the mean frequency of sIPSCs, amplitude-frequency histograms, IEI distributions, or event kinetics (Table 1, Figure 4B). Spontaneous IPSC frequencies did not differ in the presence of glutamate receptor antagonists (WT: 4.3±0.7 Hz, n=16. hDisc1: 5.3±0.9 Hz, n=20) and no significant sex differences were observed.
The excitation/inhibition ratio was similar between mutant and control cells (Figure 3B, p=0.484). However, when cells from male mutants were compared with those from control littermates, a significantly higher ratio occurred in the mutant group (Figure 3B, p= 0.035, Mann-Whitney Rank Sum Test).
4. Discussion
The main finding of the present study was significantly elevated frequency of sEPSCs in pyramidal neurons from superficial layers of the medial prefrontal cortex from mutants expressing either truncated mouse or human Disc1 protein. In particular, increased excitatory input to pyramidal neurons of mutant males appeared to be a consistent feature of both models. In contrast, when both sexes were considered, neither model demonstrated differences in the mean frequency of sIPSCs. As these characteristics were not unique to either model, our data suggest that an increase in cortical excitation is one factor contributing to functional alterations caused by truncated Disc1 expression. The similarity between the hDisc1 and mDisc1 data is consistent with the idea that the truncated Disc1 protein acts as a dominant negative, resulting in loss-of-function consequences (Ross et al., 2006; Sawa and Snyder, 2005).
4.1. Cortical Excitation
Elevated excitatory drive was observed in both models. Male mutants appeared to be more severely affected by Disc1 truncation, as both male mDisc1 and hDisc1 mice showed a significant increase in the excitation/inhibition ratio, and hDisc1 mice exhibited a significant increase in the frequency of AMPAR-mediated sEPSCs. An increase in spine density may contribute to the increased frequency of EPSCs seen in both models, as the great majority of excitatory input occurs on spines. However, an increase in membrane area generally results in greater cell membrane capacitance and reduced input resistance, changes that we did not observe based on electrophysiological measures. This, in conjunction with lack of changes in average sEPSC amplitude and kinetics, suggest that presynaptic mechanisms could play a principal role.
Although we did not examine the morphology of individual neurons, previous studies in the mDiscl model demonstrated morphological alterations in dentate granular cells but not in medial prefrontal cortical neurons (Kvajo et al., 2008). In the hDiscl model, prenatal expression of the mutant protein was associated with increased spine density in the temporo-parietal cortex (Ayhan et al., 2011). Similarly, Disci knockdown induced increased spine density in newborn neurons of the dentate gyrus in the adult hippocampus (Duan et al., 2007). However, in other models carrying missense mutations a significant reduction in spine density in both frontal cortex and hippocampus were reported (Lee et al., 2011), consistent with observations in postmortem human SZ tissue (Glantz and Lewis, 2000). Interestingly, in primary cortical neuronal cultures, short-term reduction of Disci expression through RNA interference leads to a transient outgrowth of spines, but long-term suppression leads to spine shrinkage (Hayashi-Takagi et al., 2010). Overall, these studies suggest that changes in spine density and morphology after mutant Disci expression are region-specific and time-dependent. Thus, it can be speculated that initial increases in spine density and sEPSC frequency may lead, after long-term expression of mutant Disci, to excitotoxic effects of glutamate and consequent reductions in spine density.
A major hypothesis of SZ proposes that hypofunction of the N-methyl-D-aspartate receptor (NMDAR) underlies the pathophysiology of SZ (Coyle, 2006; Jentsch and Roth, 1999). Such hypofunction may generate alterations in other glutamate receptors as a compensatory mechanism, and vice versa (Zavitsanou et al., 2002). AMPARs are the primary mediators of fast EPSCs in the central nervous system and studies have documented increased ligand binding to AMPARs in the cortex of schizophrenics, possibly due to increased glutamate release and/or activation of non-NMDA postsynaptie glutamate receptors to counter the proposed hypofunetion of the NMDAR (Dracheva et al., 2005). Other studies performed in postmortem tissue from SZ patients have documented an increase in proteins involved in the forward trafficking of AMPARs, leading to an upregulation of AMPARs in frontal regions (Hammond et al., 2010). Our finding that AMPAR-mediated glutamatergie neurotransmission is increased in the Disci mouse models is consistent with other major findings suggesting increased glutamatergie activity in SZ and in the presence of the Disci mutation. For instance, an earlier report correlated spine enlargement in cortical neurons with an increase in surface expression of GluRl subunits and in EPSCs following Disci knockdown (Hayashi-Takagi et al., 2010). Another electrophysiological study of the effects of truncated Disc1 expression similarly found enhanced EPSC frequency in mutant animals (Maher and LoTurco, 2012). In these studies, this increase occurred mostly in miniature EPSCs, suggesting presynaptic influences.
If NMDAR hypofunetion occurs in the Disci models, it is possible that AMPAR function is increased in the prefrontal cortex as a compensatory mechanism. We have previously demonstrated that mice with NRl knockdown display an increase in AMPAR-mediated currents in striatal cells (Jocoy et al., 2011). In an electrophysiological study of deep-layer pyramidal neurons from mice with insufficient levels of dystrobrevin binding protein-1 (dysbindin or DTNBP1), NMDA-evoked currents and NMD A receptor subunit 1 (NRl) expression were reduced, while no differences were found in AMPA-evoked currents (Karlsgodt et al., 2011). In contrast to the present study, these cells also demonstrated reductions in spontaneous EPSCs (Jentsch et al., 2009). Thus, it is possible that alterations in glutamatergic transmission depend on the gene risk factor expressed, as well as methodological differences.
4.2. Cortical Inhibition
Although inhibitory neurotransmission displayed unique characteristics in each model, neither model demonstrated significant changes in the mean frequency of sIPSCs when sexes were combined. In the mDisc1 model, there was a significant decrease in the number of short- to medium-interval events, suggesting a decrease in the probability of GAB A release, but the mean frequency of sIPSCs was unaffected. The hDisc1 model failed to reveal changes in inhibitory neurotransmission. This difference between both models may relate to the fact that in mDisc1 mice the mutant protein is expressed in projection neurons and interneurons, whereas in the hDisc1 mice the expression is restricted only to pyramidal neurons.
When the data were parsed by sex, several differences were noted. mDisc1 males demonstrated a significant decrease in sIPSC frequency, especially in the smaller amplitude bins. mDisc1 females displayed an increase in sIPSC frequency at the smaller amplitude bins, with a close to significant increase in mean sIPSC frequency, and a significantly shorter decay time, suggesting that sex-specific postsynaptic influences may be involved in mediating sIPSC changes.
Neuroanatomical studies of postmortem human brain tissue have indeed provided consistent evidence that deficient GABAergic transmission may play a role in SZ (Benes and Berretta, 2001). In rodents, deficits in GAB A transmission have been linked to a reduction in parvalbumin GABAergic interneurons in the hippocampus and medial prefrontal cortex (Shen et al., 2008). These interneurons are vital to corticolimbic circuitry, providing both inhibitory and disinhibitory modulation of cortical and hippocampal neuronal circuits (Benes and Berretta, 2001). The characteristics of male mDiscl inhibitory neurotransmission are thus consistent with these reports. The divergent trends of female mDiscl mice may form one basis for the known differential sex-dependent effects of SZ and highlight postsynaptic influences in mediating these effects. Although still speculative, this increase may be compensatory, preventing the increase in excitation seen predominantly in males.
Clinical investigations have indicated significant sex differences in SZ. Male schizophrenic patients show lower premorbid functioning, more serious cognitive deficits, an earlier age at onset, and a poorer course of illness (Canuso and Pandina, 2007; Han et al., 2012). The present study demonstrates that, in general, male mutants were more affected than females by Disci truncation. Our observations that mDiscl and hDiscl males displayed an increase in excitation, and male mDiscl mice showed a decrease in inhibition provide additional evidence for sex differences in SZ, and are consistent with previous reports of sexually dimorphic behavioral deficits in Disci mice (Ayhan et al., 2011; Pletnikov et al., 2008).
The primary focus of the present study was on cortical pyramidal neurons. Future studies will have to address the role of cortical interneurons to better understand the source of reduced inhibition in mDiscl mice. Identification of cortical interneurons is difficult unless a fluorescent reporter gene is used. Electrophysiological experiments using Disc1 mutant mice crossed with animals expressing a fluorescent reporter gene will help identify and characterize alterations in cortical interneurons.
4.3. Conclusions
In summary, the present findings demonstrate alterations in the physiology of cortical pyramidal neurons in two genetic mouse models of SZ produced by Disc1 mutations, which are specific to males, and emphasize increases in excitation in the presence of truncated Disc1 expression. The differences between the mDisc1 and hDisc1 models may relate to regional and cell-type specific differences in Disc1 expression. Taken together, our data suggest that alterations in glutamatergic and GABAergic neurotransmission may be sex-specific and contribute to behavioral disruptions and cognitive deficits seen in SZ.
Acknowledgements
This work was supported by USPHS Grants NS33538, MH083728, MH084018, and MH083269.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copy editing, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of the funding source
USPHS provided the financial support for these studies.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Contributors
S.M.H. and E.A.W. performed experiments, analyzed data, and wrote the first draft of the manuscript. C.C. contributed to the electrophysiology experiments and provided critical intellectual contributions to carry out experiments and write the manuscript. J.D.J., M.V.P., C.A.R., and M.S.L. designed the experiments and led the overall project.
References
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, Sawa A, Margolis RL, Cadet JL, Mori S, Vogel MW, Ross CA, Pletnikov MV. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2011;16(3):293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A. Understanding the role of DISC1 in psychiatric disease and during normal development. J Neurosci. 2009;29(41):12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12(12):707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Farrant M, Swanson GT, Cull-Candy SG. CNQX increases GABA-mediated synaptic transmission in the cerebellum by an AMPA/kainate receptor-independent mechanism. Neuropharmacology. 2001;41(6):730–736. doi: 10.1016/s0028-3908(01)00135-6. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, Gasperoni T, Tuulio-Henriksson A, Pirkola T, Toga AW, Kaprio J, Mazziotta J, Peltonen L. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62(11):1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- Canuso CM, Pandina G. Gender and schizophrenia. Psychopharmacol Bull. 2007;40(4):178–190. [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4–6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Andre VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington's disease. J Neurosci. 2009;29(33):10371–10386. doi: 10.1523/JNEUROSCI.1592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26(10):2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, McGurk SR, Haroutunian V. mRNA expression of AMP A receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J Neurosci Res. 2005;79(6):868–878. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMP A receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. 2010;35(10):2110–2119. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Huang XF, Chen DC, Xiu MH, Hui L, Liu H, Kosten TR, Zhang XY. Gender differences in cognitive function of patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, Mori T, Nemoto K, Adachi N, Izumi A, Chiba S, Noguchi H, Suzuki T, Iwata N, Ozaki N, Taguchi T, Kamiya A, Kosuga A, Tatsumi M, Kamijima K, Weinberger DR, Sawa A, Kunugi H. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15(20):3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A. Disrupted-in-Schizophrenia 1-DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13(3):327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104(36):14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11(6):557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32(9):485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jocoy EL, Andre VM, Cummings DM, Rao SP, Wu N, Ramsey AJ, Caron MG, Cepeda C, Levine MS. Dissecting the contribution of individual receptor subunits to the enhancement of N-methyl-d-aspartate currents by dopamine D1 receptor activation in striatum. Front SystNeurosci. 2011;5:28. doi: 10.3389/fnsys.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A, Jentsch JD. Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance. Biol Psychiatry. 2011;69(1):28–34. doi: 10.1016/j.biopsych.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci U S A. 2006;103(10):3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105(19):7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FH, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ, Roder JC, Wong AH. Disc1 point mutations in mice affect development of the cerebral cortex. J Neurosci. 2011;31(9):3197–3206. doi: 10.1523/JNEUROSCI.4219-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Intrinsic excitatory connections in the prefrontal cortex and the pathophysiology of schizophrenia. Brain Res Bull. 2000;52(5):309–317. doi: 10.1016/s0361-9230(99)00243-9. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, Hennah W, Peltonen L, Lonnqvist J, Huttunen MO, Kaprio J, Trachtenberg JT, Silva AJ, Cannon TD. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A. 2007;104(46):18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BJ, LoTurco JJ. Disrupted-in-schizophrenia DISC1) functions presynaptically at glutamatergic synapses. PLoS One. 2012;7(3):e34053. doi: 10.1371/journal.pone.0034053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Eaton JV, Brown T, Dingledine R. CNQX increases spontaneous inhibitory input to CA3 pyramidal neurones in neonatal rat hippocampal slices. Brain Res. 1992;592(1–2):255–260. doi: 10.1016/0006-8993(92)91683-6. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9(9):1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Moens LN, De Rijk P, Reumers J, Van den Bossche MJ, Glassee W, De Zutter S, Lenaerts AS, Nordin A, Nilsson LG, Medina Castello I, Norrback KF, Goossens D, Van Steen K, Adolfsson R, Del-Favero J. Sequencing of DISC1 pathway genes reveals increased burden of rare missense variants in schizophrenia patients from a northern Swedish population. PLoS One. 2011;6(8):e23450. doi: 10.1371/journal.pone.0023450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58(5):466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13(2):173–186. doi: 10.1038/sj.mp.4002079. 115. [DOI] [PubMed] [Google Scholar]
- Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol Med. 2011;17(12):699–706. doi: 10.1016/j.molmed.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of DISC1--an emerging role in psychosis and cognition. Biol Psychiatry. 2006;60(2):123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52(1):139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, Burrell GJ, Rice JP, Nertney DA, Olincy A, Rozic P, Vinogradov S, Buccola NG, Mowry BJ, Freedman R, Amin F, Black DW, Silverman JM, Byerley WF, Crowe RR, Cloninger CR, Martinez M, Gejman PV. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165(4):497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Genetics. Two genes link two distinct psychoses. Science. 2005;310(5751):1128–1129. doi: 10.1126/science.1121114. [DOI] [PubMed] [Google Scholar]
- Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, Chatzi C, He S, Mackie I, Brandon NJ, Marquis KL, Day M, Hurko O, McCaig CD, Riedel G, St Clair D. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28(43):10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Holcomb HH. Phenotype of schizophrenia: a review and formulation. Mol Psychiatry. 2005;10(1):27–39. doi: 10.1038/sj.mp.4001563. [DOI] [PubMed] [Google Scholar]
- Thomson PA, Wray NR, Millar JK, Evans KL, Hellard SL, Condie A, Muir WJ, Blackwood DH, Porteous DJ. Association between the TRAX/DISC locus and both bipolar disorder and schizophrenia in the Scottish population. Mol Psychiatry. 2005;(7):657–668. doi: 10.1038/sj.mp.4001669. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Geschwind DH. DISC1: a schizophrenia gene with multiple personalities. Neuron. 2011;72(4):501–503. doi: 10.1016/j.neuron.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Ward PB, Huang XF. Selective alterations in ionotropic glutamate receptors in the anterior cingulate cortex in schizophrenia. Neuropsychopharmacology. 2002;27(5):826–833. doi: 10.1016/S0893-133X(02)00347-0. [DOI] [PubMed] [Google Scholar]