Abstract
Preclinical studies in aged, surgically-menopausal rhesus monkeys have revealed powerful benefits of intermittent estrogen injections on prefrontal cortex-dependent working memory, together with corresponding effects on dendritic spine morphology in the prefrontal cortex. This contrasts with the inconsistent effects of hormone therapy (HT) reported in clinical studies in women. Factors contributing to this discrepancy could include differences in the formulation and sequence of HT regimens, resulting in different neurobiological outcomes. The current study evaluated, in aging surgically menopausal rhesus monkeys, the cognitive effects of four HT regimens modeled directly on human clinical practice, including continuous estrogen treatment opposed by progesterone. None of the regimens tested produced any cognitive effect, despite yielding physiologically relevant serum hormone levels, as intended. These findings have implications for the design of regimens that might optimize the benefits of hormone treatment for healthy aging, and suggest that common HT protocols used by women may fail to result in substantial cognitive benefit, at least via direct effects on the prefrontal cortex.
Keywords: ovarian hormones, aging, macaque, learning, memory, prefrontal, temporal
1. Introduction
The effects of ovarian hormones on cognition are age-dependent. That is, loss of circulating ovarian hormones early in the lifespan has relatively little effect on cognitive function, but produces more dramatic and widespread impairment later in life, around the time of natural menopause (e.g., Hao, et al., 2007, Markowska and Savonenko, 2002, P.R. Rapp, et al., 2003, Voytko, 2000, Voytko, 2002). These studies, as well as at least some observational investigations in women, support the notion that hormone therapy (HT) after menopause benefits cognitive function. However, the Women's Health Initiative Memory Study (WHIMS) found that treatment of older women (aged 65 to 79 at study onset) with conjugated equine estrogen (CEE) alone, or CEE plus progestin (medroxyprogesterone acetate, MPA) had no beneficial effect on global cognitive function, was associated with decline in global cognitive function in some women, and increased the risk of mild cognitive impairment (MCI) and Alzheimer's disease (Espeland, et al., 2004, S.R. Rapp, et al., 2003, Shumaker, et al., 2004,Shumaker, et al., 2003). Considerable discussion around these findings has centered on the limitations of the WHIMS design (Craig, et al., 2005, Maki, 2004, Sherwin and Henry, 2008), including a “healthy user” bias in women that elect HT, confounds associated with other age-related health problems, such as obesity and hypertension, the specific hormone formulations tested (Zhao and Brinton, 2006; Nilsen and Brinton 2003), and the fact that women in the WHIMS began HT after a prolonged period of ovarian hormone deprivation (the window of opportunity hypothesis).
Studies in animal models mitigate some of the challenges associated with studying the relationship between ovarian hormones and cognition in aging humans. Nonhuman primates, specifically rhesus monkeys, are well-suited for modeling the relationship between neuroendocrine and cognitive aging in humans. Many aspects of reproductive physiology are similar between rhesus monkeys and women, notably including the periodicity of the normal menstrual cycle and the late-life onset of menopause (Gilardi, et al., 1997, Walker, 1995). Impairment in cognitive function accompanies menopause in rhesus monkeys (Roberts, et al., 1997), which as in humans includes prefrontal cortex dysfunction (e.g., Weber, et al., 2012).
Our goal in the present study was to investigate whether HT strategies that incorporate continuous E dosing, as well as progesterone, benefit cognition in aged, ovariectomized (OVX) rhesus monkeys. An initial study in aged OVX rhesus monkeys (mean age 22 years) found strong cognitive benefits of cyclic estradiol therapy, consisting of an injection of 100 μg estradiol cypionate (E) every 21 days (P.R. Rapp, et al., 2003). Although this regimen is FDA-approved for use in women, continuous E supplementation is more common because it avoids symptoms associated with fluctuating E levels, such as hot flashes. Thus, one goal was to determine whether continuous E administration provided behavioral benefits similar to those previous seen for cyclic E therapy. A second issue relates to the modulatory effect of progesterone (P). Most women who receive HT after menopause take a combination of E and P to counter the proliferative effects of E on the uterine endometrium. Whereas data from rodents on the memory effects of P are conflicting (reviewed in Frick, 2009), a recent study in aged rhesus monkeys (mean age 19.7 years) found that cyclic treatment with P did not modify the cognitive effects of a continuous E treatment regimen supplemented by E injections (Voytko, et al., 2008, Voytko, et al., 2009). Thus, we also determined the effects of combining continuous or cyclic P with E treatment. These studies used the same model, modified to better mimic natural ovarian cyclicity, and testing procedures that were sensitive to a beneficial effect of cyclic E treatment on cognition (P.R. Rapp, et al., 2003).
If continuous E or E treatments combined with P fail to modulate cognition in older OVX monkeys, this would strongly suggest that the effectiveness of HT is determined by the specific hormones given (E alone versus E and P together) as well as the timing of dosing (cyclic versus continuous). In this way, we aimed to determine how the specific characteristics of clinically relevant hormone replacement regimens contribute to key cognitive outcomes, independent of other factors that might also influence the effects of HT.
2. Methods
2.1 Overview of experimental timeline
Aged female behaviorally-naive rhesus monkeys (late teens through mid-20's) were screened for inclusion in the study according to several criteria: the absence of previous experimental surgical manipulations making them ineligible for inclusion in a subsequent research protocol involving survival surgery, per United States Department of Agriculture regulations; no previous memory testing, long-term neuropharmacological or dietary intervention, pre- or perimenopausal reproductive status, based on ovarian hormone profiles (determined by urinary hormone metabolite assays); and satisfactory physical health including an absence of opthalmic impairments (including cataracts or macular degeneration), severe arthritis, or gross physical abnormalities. Thus, monkeys that began the study were in excellent physical health and had intact ovarian function at the beginning of the experimental protocol.
Monkeys selected for inclusion in the study were trained to criterion on the delayed response (DR) task at 0 and 1 sec delays, ovariectomized, and recovered from ovariectomy for between 6 and 12 weeks. During this time, serum hormone assays were conducted to verify effectiveness of ovariectomy. Each monkey was then assigned to one of five treatment groups balanced for their preoperative performance on DR: vehicle (VEH, N=7), continuous estrogen by silastic implant (ECONT, N=9), continuous estrogen and daily oral progesterone (ECONT+PCONT, N=9), continuous estrogen and intermittent (cyclic) progesterone (ECONT+PCYC, N=8), or intermittent injections of estrogen and intermittent progesterone (ECYC+PCYC, N=9). Beginning one month after initiation of hormone treatment, behavioral testing resumed, consisting of re-acquisition of DR at a 1 sec delay, then proceeding through DR testing with increasing delays, DR with distraction, delayed nonmatching-to-sample (DNMS) training to criterion at a 10 sec delay, DNMS with increasing delays, DNMS with distraction, object discrimination (OD) training, and OD testing with distraction. Two of these tasks (DR and DNMS) have documented sensitivity to ovarian hormone treatment (injection of estrogen every 21 days) in aged, ovariectomized monkeys (P.R. Rapp, et al., 2003). Versions of all three tasks with distraction were also included to test the hypothesis that beneficial effects of hormone treatment might be mediated by a general effect of ovarian hormones on a cognitive process engaged by multiple memory tests, in this case reduced distractibility. The median duration of treatment (time from initiation of hormone treatment post-OVX to perfusion) was 533 days (range 209 to 757 days), including monkeys that did not complete all phases of behavioral testing (see below).
2.2 Subjects
The experiments were performed at the California National Primate Research Center (CNPRC) under protocols approved by the University of California, Davis Institutional Animal Care and Use Committee. Forty-two aged (17.7–25.7 years old at ovariectomy; mean age ± SD = 20.9 ± 1.9 years) female behaviorally-naive rhesus monkeys (Macaca mulatta) were studied. Thirty-four monkeys completed all phases of behavioral testing. Completion of the entire postoperative testing battery took about 16 months on average (mean 15.8 months, range 12.6–21.8). The other 8 did not finish the entire protocol. Three completed DR testing through the distraction phrase of the task (see below) and were euthanized during DNMS testing, one for suspected endometriosis, one because of a rapidly-growing tumor on the mandible, and one because of poor recovery following treatment of an infection at the implant site. One monkey completed DR testing as well as DNMS (delays only), and then died due to a ruptured kidney. One completed delay and distraction testing phases of both DR and DNMS, and was then euthanized because of weight loss. Two completed all phases of testing except object discrimination with distraction, and were euthanised for poor physical condition or for diarrhea and poor appetite. The eighth monkey completed all behavioral testing in good health but exhibited motivational problems during object discrimination testing; because she would not reliably complete daily test sessions on this task, her data for this task were excluded. Because behavioral testing was suspended when health problems became apparent (in the seven monkeys that were euthanized) and responding in the test apparatus was prompt until that time (and until OD testing for the eighth monkey), their behavioral data were included in analyses for tasks for which complete data were available. The pattern of results does not change if all data are excluded from monkeys that failed to complete the entire behavioral protocol because of health reasons. The monkeys that were euthanized were in the ECONT group (N=4), ECONT+PCONT group (N=2), or ECYC+PCYC group (N=1). Thus, at the end of testing, the number of subjects in each group were: vehicle (N=7), ECONT (N=5), ECONT+PCONT (N=7), ECONT+PCYC (N=8), ECYC+PCYC (N=8). Most monkeys (36/42) were pair-housed during the day and separated at night. Night time separation facilitated monitoring of food intake and allowed for collection of individual animal urine samples for hormone assays.
Average longevity in captive rhesus macaques is less than 25 years (Peters, et al., 1996, Tigges, et al., 1988), and previous studies demonstrate that prominent behavioral, neurobiological and endocrine signs of aging are not observed in monkeys younger than 19 or 20 (Bachevalier, et al., 1991). The range of ages for the monkeys was therefore selected as the most appropriate for addressing the specific questions under investigation, given that our aim was to define the effects of HT manipulations when cognitive function is vulnerable to decline, but before the onset of more robust impairment that could prove refractory to treatment. Four monkeys were younger than 19 years at the time of ovariectomy (17.7, 18.4, 18.5, 18.8 years), were evenly distributed between the treatment groups (one in each group except ECYC+PCYC), and performed similarly to other monkeys in their respective groups. Most monkeys in the study (26/42) were 20 years or older at the time of ovariectomy.
2.3 Ovariectomy and hormone treatment
For ovariectomy (OVX) surgery, monkeys were sedated with 10 mg/kg ketamine i.m., given 0.04 mg/kg atropine s.c., then intubated, placed on isoflurane anesthesia to effect, and positioned in dorsal recumbency. A ventral caudal midline abdominal incision visualized the body of the uterus and both ovaries. Ovarian vessels and the Fallopian tubes were isolated, ligated, and severed. The abdominal wall was then closed in three layers with two layers of 2/0 absorbable suture and the final subcuticular layer with 3/0 absorbable suture. The animals were recovered in the CNPRC surgical recovery unit and given three days of post-operative analgesia, 1.5 mg/kg oxymorphone i.m. three times daily.
Hormone treatments were delivered as follows. All monkeys received silastic capsule implants that were changed every three months, placed subcutaneously between the shoulder blades under ketamine (10 mg/kg) and medetomidine (0.2–0.4 mg/kg) sedation, with atipamezole (0.2–0.4 mg/kg) given at the end of the procedure to reverse the medetomidine. Two silastic implants (3 cm long and 0.46 cm inner diameter containing estradiol) were used to provide continuous E treatment to monkeys in groups ECONT, ECONT+PCYC, and ECONT+PCONT. These implants were designed to achieve mean serum levels of 150 pg/ml. The implants maintain detectable circulating levels of E2 for at least 3 months, and were replaced every 3 months (or sooner if they were prematurely extruded, which was a rare event). Monkeys in the other two groups (VEH, ECYC+PCYC) received implants of empty capsules on the same time schedule. Monkeys in group ECYC+PCYC received an intramuscular injection of estradiol cypionate (100 μg) in 1 ml vehicle (peanut oil) every 28 days; monkeys in group VEH received intramuscular injections of 1 ml peanut oil on the same schedule. Monkeys in the other three groups did not receive injections. In addition, daily fruit treats were given to all monkeys. For monkeys in group ECONT+PCONT these always contained 100 mg micronized progesterone. This dose replicated luteal phase circulating levels. For monkeys in groups ECONT+PCYC and ECYC+PCYC the fruit contained micronized progesterone for 10 days out of every 28, beginning 10 days after estradiol (ECYC+PCYC) or vehicle (ECONT+PCYC) injection. Fruit given to monkeys in groups VEH and ECONT never contained progesterone. These procedures were designed to ensure that technical staff, who administered treatments as well as conducted behavioral testing, remained blind to hormone treatment status, and to equate frequency of implant surgeries across all the monkeys regardless of their specific treatment condition.
2.4 Hormone assays
Urinary metabolites of estrogen (E1C) and progesterone (PdG) were analyzed by enzyme immunoassay as previously described by Shideler and colleagues (2001) to ensure intact ovarian activity before inclusion in the study and the success of the OVX surgery. For urine collection, cage pans were placed in the late afternoon for overnight sampling, and 3 ml of urine was collected by 0900 the following morning. Samples were collected daily for 6 weeks. Urine was centrifuged and decanted to remove any solid material and then frozen until analysis by enzyme immunoassay. Hormone concentrations were indexed to creatinine (Cr) to adjust for differences in urine concentration.
Serum hormone assays were conducted quarterly in all monkeys to verify the effectiveness of the hormone treatment regimens using chemiluminescent immunoassays. For serum assays, four blood samples were collected quarterly by venipuncture, on the same schedule for all monkeys. These were timed to occur immediately before and 48 hours after E injection (or implant change) in monkeys receiving cyclic injections of E (or that had silastic implants of E), and immediately before and 7 days after initiation of oral P administration in monkeys receiving oral P. Monkeys in all groups received blood draws on the same schedule, so taking Day 1 as the first day of a monthly treatment cycle when injections were given to monkeys in groups ECYC+PCYC and VEH, samples were taken on Day 1 (before injection/implant change), Day 3, Day 11 (before oral P/fruit vehicle was given), and Day 18. This provided measures of steady-state E levels in groups receiving continuous E (days 1, 11 and 18) as well as the post-implant peak in E (day 3), a measure of serum E immediately before (day 1) and 48 hours after injection (day 3) in group ECYC+PCYC, steady-state P levels in groups receiving continuous P (all samples), and P levels during (day 18) and off (days 1, 3, 11) active P treatment in groups receiving cyclic P.
Circulating estradiol (E2) and progesterone (P) were evaluated utilizing the ADVIA Centaur CP (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). Briefly, both assays use competitive immunoassay and direct chemilumescent technology. In the estradiol assay, binding proteins are released via pre-treatment with a releasing agent. Both assays utilize a steroid specific monoclonal antibody labelled with acridinium ester to bind the estradiol or progesterone in the sample. A steroid specific derivative capture reagent covalently coupled to paramagnetic particles is then added to compete with estradiol or progesterone for binding of the acrdinium-labeled antibody. Bound and unbound components are separated via a wash step. The addition of acid and base initiates the chemilumniscent reaction and is measured in the system's luminometer. Raw data is expressed as relative light units (RLUs) and an inverse relationship exists between the amount of steroid in the sample and the amount of RLUs detected by the system. The inter- and intra-assay coefficients of variation (CV) for the estradiol assay are 4% and 5%, respectively. For progesterone, the inter- and intra-assay coefficients of variation are 7% and 6%, respectively.
2.5 Behavioral testing
Testing took place in a manual test apparatus, identical to previous descriptions (O'Donnell, et al., 1999,Rapp, et al., 1997, P.R. Rapp, et al., 2003). A white noise generator was used throughout training to mask extraneous sound. Acclimation and familiarization to the apparatus at the beginning of testing included offering the monkey food rewards in the test tray, as well as the opportunity to displace objects and plaques covering food wells in order to obtain reward.
2.5.1. Delayed Response (DR) training and testing
DR training was conducted in phases, with and without delays. Trials were initiated by raising the opaque barrier of the apparatus, and the monkey watched through a Plexiglas screen while one of the lateral wells of the stimulus tray was baited with a food reward (e.g., raisin or peanut). The lateral wells were subsequently covered with identical plaques and, during the initial phase of training, the clear barrier was raised immediately to permit a response (0 s delay). After the monkey displaced one of the plaques, the opaque barrier was lowered to impose a 20 s intertrial interval (ITI). Daily test sessions consisted of 30 trials, with the left and right food wells baited equally often according to a pseudorandom sequence. Monkeys were trained until they achieve a criterion of 90% correct (9 errors or less in 9 consecutive blocks of 10 trials). Testing subsequently continued in an identical fashion except that a 1 s delay was imposed between the baiting and response phase of each trial, and continued until criterion performance was re-achieved. At this point, monkeys were scheduled for ovariectomy (OVX) surgery. DR was reacquired to criterion at the 1 s delay postoperatively. In the next phase the memory demands of the DR task were made progressively more challenging by introducing delays of 5, 10, 15, 30 and 60 s; testing was otherwise conducted as before (i.e. 30 trials/day, 20 s ITI). Monkeys were tested for a total of 90 trials (3 days of 30 trials per day) at each retention interval.
Once testing with delays was complete, DR was tested at a fixed 30 sec delay, with other procedural details as described above (e.g., 30 trials/day, 20 s ITI), with and without distraction. First, performance was re-established on DR with a 1 second delay, to a 90% criterion across 4 days of testing (with 20 trials/day). Then they were tested on DR with a 30 s delay until they performed at 65% correct or better for 2 consecutive days (also 20 trials/day). After this point, sessions including distraction were alternated with standard, no-distraction sessions for 30 days. These sessions were 20 trials/day at a nominal 30 s delay. Distraction consisted of a salient but non-contingent event imposed during the 30 s delay interval of the DR trials: a single junk object (different on each trial) covered the central food well of the stimulus tray, with reward randomized across the distracter events (50% were rewarded). This procedure was intended to encourage a sustained focus on the salient task events in the face of distraction. The monkey was allowed up to 20 s to displace the object; after the object was displaced or 20 s elapsed, the screen was lowered between the monkey and the test tray, the plaques and reward were replaced for the DR trial, and testing continued. Because this could result in delay intervals longer than 30 s, average delay intervals during distraction sessions were calculated across 2 sessions of distraction testing and used in subsequent sessions without distraction, to equate delays across these conditions.
2.5.2 Delayed Nonmatching-to-Sample (DNMS) training and testing
Trials in this task consisted of two phases, initiated when the opaque barrier of the WGTA was raised to reveal an object covering the baited central well of the stimulus tray. After the reward was retrieved, the opaque screen was lowered, and the sample item positioned over one of the lateral wells. The other lateral well was baited and covered with a novel object. During training, a 10 s delay was imposed and recognition memory was tested by allowing monkeys to choose between the sample and the rewarded novel object. The discriminative stimuli were drawn from a pool of 800 objects according to a pre-determined sequence, ensuring that new pairs of objects were presented on every trial. Twenty trials per day were given using a 30 s ITI, counterbalancing the left/right position of the novel items. Subjects were tested until they reached a 90% correct criterion by committing no more than 10 errors in 5 consecutive sessions (100 trials). The memory demands of the DNMS task were then made progressively more challenging by introducing successively longer retention intervals of 15, 30, 60, 120 s (total = 100 trials each, 20/day), and 600 s (total = 50 trials, 5/day). Monkeys remained in the test chamber for all delays.
As for DR, once testing with delays was complete, DNMS was tested with and without distraction using a 120 s retention interval, with other procedural aspects of the task as described above (e.g., 20 trials/day, 30 s ITI). Baseline performance was re-established on DNMS at a 10 s delay to a 90% criterion across 3 days (i.e., 6 or fewer errors in 3 consecutive sessions). Then, each monkey was trained to a criterion performance at a 120 s delay equal to its accuracy at that delay during initial testing. That is, if a monkey performed at 80% correct during 120 s delays during the delay testing phase, sessions with a 120 s delay were given until scores were at or above 80% correct across 3 consecutive days. At this point, sessions including distraction were alternated with regular, no-distraction sessions for 30 days. Distraction was manipulated by presenting 3 distinct objects successively, one at a time, over the central well of the stimulus tray during the 120 s retention interval on each trial, at 30 s intervals (beginning 30 s into the delay). One of the 3 objects, chosen randomly, was rewarded. Monkeys were allowed 20 s to displace (or not) each distracter object. The sample and choice objects were then positioned over the lateral wells for the choice phase, which occurred 120 s after the sample was displaced.
2.5.3 Object discrimination (OD) training and testing
For each problem, two objects were positioned over the lateral wells of the apparatus, with their left/right locations varied pseudorandomly across trials. For each discrimination pair, one object was consistently associated with reward. A single “pre-trial” was run on the first session of each problem in which both objects were presented unbaited; the object the monkey did not pick on the “pre-trial” (which was not scored) was baited on the remainder of the trials for that problem. Test sessions consisted of 30 trials per day, using a 15 second intertrial interval. The first two test sessions were separated by 24 hours, and the third occurred 48 hours after the second test session.
OD testing occurred in two phases. In the “training” phase, four discrimination problems were tested in this manner with the order of problems presented fixed across animals. In the “testing” phase, eight problems were given in which the presence of distraction during training was alternated across problems, and the ITI was increased to 30 sec. During distraction problems, one randomly chosen distracter object was shown and rewarded 50% of the time during the intertrial interval between discrimination problem trials. Non-distraction problems were presented in the same manner as during training.
Measures of performance and data analysis
Trials to criterion (excluding the criterion run) were analyzed for acquisition of DR (at 0 sec delay pre-OVX, 1 sec delay pre-OVX, and 1 sec delay post-OVX) and DNMS (at a 10 sec delay) using one-way ANOVA. Percent correct across increasing delays was analyzed for DR and DNMS using delay as a repeated measure. For distraction testing of DR and DNMS, performance was analyzed separately for this phase using distraction (sessions with or without distracter presentation) as a repeated measure. Percent correct for OD was analyzed in blocks of 10 trials averaged across the 4 training problems, using block as a repeated measure. Performance during OD with distraction was analyzed in the same manner, with block and distraction (problems with/without distracter presentation during the ITI) as repeated measures. Hormone treatment group was a between-subject factor in all analyses.
3. Results
3.1 Verification of hormone treatment effectiveness
To summarize validation of hormone treatments conducted by serum E and P assays, median values were calculated for each monkey across relevant sampling periods. This minimizes the influence of extreme values, and adjusts for variations in duration of sampling across monkeys (e.g., when individual monkeys completed the protocol at different times) and occasional missing values due to assay failure or rare lost samples (less than 1%). These data are summarized in Table 1, as means (and ranges) of median values for individual monkeys. In the VEH group, median serum E ranged between 7.09 and 35.95 pg/ml (mean 20.03) and median serum P ranged from 0.12 to 0.59 ng/ml (mean 0.29) across the post-OVX study period, consistent with cessation of ovarian activity (P.R. Rapp, et al., 2003,Shideler, et al., 2003). E levels were low in group ECYC+PCYC pre-E injection, P levels were low in group ECONT, and in groups ECONT+PCYC and ECYC+PCYC when P was not being given orally, as expected. Steady-state E levels were similar in groups receiving continuous E by silastic implant, and these groups had similar post-implant change peaks in serum E levels. Monkeys receiving oral P treatment reached similar serum P levels during treatment, whether cyclic or continuous.
Table 1.
Serum estradiol (E) and progesterone (P) values in the treatment groups. Values are the mean, across monkeys in each group, of median values calculated for each individual monkey across samples collected in each treatment phase.
| Serum E (Pg/ml) | Serum P (ng/ml) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Group | Mean | Range | Mean | Range | ||
| VEH | 20.03 | 7.09–35.95 | 0.29 | 0.12–0.59 | ||
| ECONT | steady-state | 185.27 | 99.45–337.55 | 0.39 | 0.09–0.84 | |
| post-implant peak | 551.55 | 253.54–1193.02 | ||||
| ECONT+PCONT | steady-state | 219.77 | 80.17–449.28 | 3.04 | 2.84–3.40 | |
| post-implant peak | 692.25 | 158.55–1508.13 | ||||
| ECONT+PCYC | steady-state | 165.06 | 91.08–248.96 | on oral P | 3.61 | 1.45–7.00 |
| post-implant peak | 513.24 | 234.34–872.38 | off oral P | 0.50 | 0.11–1.35 | |
| ECYC+PCYC | 48 hr post-injection | 94.37 | 50.46–155.82 | on oral P | 4.12 | 2.61–5.08 |
| pre-injection | 16.87 | 4.93–31.46 | off oral P | 0.53 | 0.12–1.46 | |
With regard to the targets for the different treatments, the silastic implants were designed to target a mean serum level of 150 pg E/ml, which was achieved. The peak E levels 48 hours after cyclic injections (mean of median value 94.36 pg/ml) were lower than those in our previous study (P.R. Rapp, et al., 2003), where mean levels were 156.71 pg/ml 48 hours after injection. Samples collected prior to perfusion in monkeys on the current study, 24 hours post-E injection, revealed higher serum E levels (as expected), with a mean of 152.83 pg/ml, range 76.52 to 212.99. Thus, peak post-injection E levels in monkeys treated with cyclic E in the present study were consistently higher than 150 pg/ml, as intended, in both studies. Oral P administration approximated luteal serum P levels, 2 to 6 ng/ml, as intended.
3.2 Delayed response
Monkeys did not differ in pre-OVX acquisition of DR at 0 or 1 sec delays based on their subsequent assignment to hormone treatment groups, 0 sec F(4, 37) = 1.310, p = .284; 1 sec F(4, 37) = .516, p = .725. Reacquisition of the DR task at a 1 sec delay post-OVX was not affected by hormone treatment, F(4, 37) = 1.027, p = .406 (Figure 1). Performance across delays decreased as delay increased, main effect of delay F(4, 148) = 69.325, p < .0005, but there was no main effect of hormone treatment F(4, 37) = .178, p = .948, or treatment × delay interaction, F(16, 148) = .728, p = .763. Thus, none of the hormone treatments had any effect on performance across delays in the DR task. This is the task that shows the greatest effect of cyclic E treatment, demonstrated in a previous study (P.R. Rapp, et al., 2003).
Figure 1.
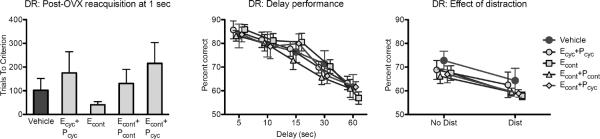
Delayed response (DR) performance is unaffected by hormone treatment. Reacquisition of the DR task at a 1-second delay did not vary across treatment groups. When memory is challenged by increasing the delay between baiting and test, all groups showed an equivalent decline across longer delays. Distraction during a 30-second delay interval impaired memory in all monkeys, an effect that did not interact with treatment condition.
Effects of hormone treatment were also compared in DR testing at a single (30 sec) delay, with and without distraction during the delay interval. This revealed an expected effect of distraction, which impaired memory performance in the DR task, F(1, 37) = 43.76, p < .0005, but no effect of hormone treatment, main effect of treatment F(4, 37) = .653, p = .628, treatment × distraction interaction F(4, 37) = .261, p = .901. Thus, there was no indication of a differential effect of hormone treatments in conditions of heightened distractability that impaired memory.
3.3 Delayed nonmatching-to-sample
Acquisition of the DNMS task at a 10 sec delay was not affected by hormone treatment, F(4, 35) = 1.224, p = .319 (Figure 2). DNMS accuracy deteriorated across delays as expected, main effect of delay F(4, 136) = 94.562, p < .0005, but there was no effect of hormone treatment, F(4, 34) = .838, p = .511, or treatment × delay interaction, F(16,136) = .462, p = .961. Thus, none of the hormone treatments had any effect on acquisition or performance across delays in the DNMS task. Like DR, DNMS also showed benefit from cyclic E treatment in our previous study (P.R. Rapp, et al., 2003).
Figure 2.

Delayed nonmatching-to-sample (DNMS) performance is unaffected by hormone treatment. Acquisition of the DNMS task at a 10-second delay did not vary across treatment groups. When memory is challenged by increasing the delay between sample and choice, all groups showed an equivalent decline across longer delays. Distraction during a 2-minute delay interval impaired memory in all monkeys, an effect that did not interact with treatment condition.
Effects of hormone treatment were also compared in DNMS testing at a single (120 sec) delay, with and without distraction during the delay interval, as for DR. This revealed an expected effect of distraction, F(1, 33) = 41.302, p < .0005, but no effect of hormone treatment, main effect F(4, 33) = 1.876, p = .138, treatment × distraction interaction F(4, 33) = 1.087, p = .379. Thus, although distraction reliably impaired DNMS performance (as it did delayed response), there is no evidence that any of the hormone treatments moderate this effect.
3.4 Object discrimination
Monkeys first encountered four “training” problems, given sequentially across 3 days of testing with 30 trials per day (the same protocol used in Rapp et al., 2003a). They then were given a further eight “testing” problems, four with distracting events occurring during the intertrial intervals and four without, again with each problem presented across 3 days of testing for 30 trials per day. Data from the four “training” problems were collapsed across problems and analyzed in blocks of 10 trials. This analysis revealed an expected effect of block, indicating acquisition of the discriminations, F(8, 248) = 65.551, p < .0005, but no main effect of hormone treatment, F(4, 32) = .273, p = .893, or treatment × block interaction, F(32, 248) = .630, p = .941 (Figure 3). Data from the eight “testing” problems were analyzed the same way, with distraction as an additional within-subject variable. This analysis revealed a main effect of block, F(8, 232) = 100.872, p < .0005, and a distraction × block interaction F(8, 232) = 2.839, p = .005, but no main effect of distraction, F(1, 29) = .745, p = .395. The distraction × block interaction appears to reflect mildly impaired learning in the first two blocks of problems with interpolated distraction, but slightly better performance on distraction problems during later blocks. None of these effects interacted with treatment group: distraction × treatment F(4, 29) = 1.970, p = .126; block × treatment F(32, 232) = .896, p = .633; distraction × block × treatment F(32, 232) = .722, p = .865. There was a main effect of hormone treatment across all 8 “testing” problems, F(4, 29) = 3.641, p = .016. This was decomposed with pairwise ANOVAs comparing each hormone treatment to the vehicle condition. These revealed a main effect of hormone treatment when the vehicle and ECONT+PCYC groups were compared, F(1, 13) = 4.882, p = .046; this effect did not interact with distraction, F(1, 13) = 2.073, p = .174, block (F < 1), or distraction × block (F < 1). Thus, there is indication that monkeys in the ECONT+PCYC group are impaired relative to vehicle controls.
Figure 3.
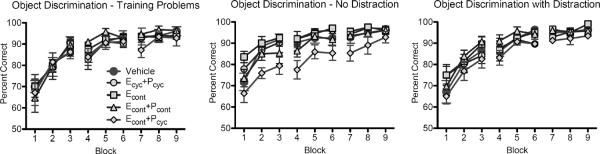
Object discrimination performance is not improved by hormone treatment. Acquisition of 4 object discrimination training problems did not vary across treatment groups. During a test phase where 8 additional problems were learned, 4 with distraction and 4 without, group ECONT+PCYC was significantly impaired relative to vehicle-treated monkeys. This effect is most pronounced in the no-distraction problems, but it did not interact with distraction condition. There was no overall effect of distraction on acquisition of object discrimination problems (although there was an interaction between distraction and block), perhaps because at this point in testing monkeys had become acclimated to distracting events during testing.
3.5 Comparison of all treatment groups versus vehicle
Finally, we conducted parallel analyses comparing the data from monkeys that received any hormone treatment to monkeys given vehicle. From a translational point of view, women take different approaches to HT, so it may be meaningful to ascertain whether the presence of any hormone supplementation, regardless of specific formulation, is associated with a change in cognition. Also, this approach increases statistical power to detect even very small effects of HT relative to vehicle-treated monkeys. All of the preceding ANOVAs were repeated using only two levels of group for the treatment factor: vehicle, or any hormone treatment. These analyses revealed (again) expected effects of procedural variables such as delay and distraction, as seen in the main analyses, but failed to show any significant effects of hormone treatment or interactions of hormone treatment with procedural manipulations, p-values > .215. There were also no significant group differences if monkeys that received progesterone (groups ECONT+PCONT, ECONT+PCYC, and ECYC+PCYC) were compared as a single group to monkeys that did not receive progesterone as part of their treatment (group VEH+ECONT), p-values > .089.
4. Discussion
The goal of the present study was to evaluate multiple, clinically-relevant HT regimens for their ability to improve cognition in aged, ovariectomized female rhesus monkeys. Previous work in this model has established that a cyclic regimen of E injections (one injection every 21 days) improves cognition and potently modulates dendritic spine morphology in prefrontal cortex of aged monkeys (Hao, et al., 2007,P.R. Rapp, et al., 2003). In contrast, none of the hormone regimens evaluated in the present study improved cognition relative to vehicle-treated monkeys, even under conditions of increased cognitive demand due to longer retention intervals or the presence of distraction during testing. This was despite documented treatment efficacy in achieving target levels of circulating ovarian hormone levels. Thus, the specific formulation of hormone regimen (E alone versus E and P together), and sequence of administration (continuous versus cyclic) appear to be key factors determining whether or not beneficial cognitive effects of HT are observed.
The procedures in the current study were substantially similar to those that demonstrated a positive effect of cyclic E alone (P.R. Rapp, et al., 2003). Monkeys were extensively health-screened before entering the protocol, and all had intact ovarian function as determined by urinary hormone metabolite assays despite variation in age between individual subjects. Behavioral analyses were extensive and included neuropsychological assessments that proved sensitive to cyclic E alone in our earlier investigation. There were, however, certain minor procedural differences that merit discussion. Notably, monkeys in the present study were pre-trained on DR prior to OVX and subsequent cognitive testing, and on average they were about a year younger, chronologically, than in our earlier study. There were no statistical differences in DR acquisition between vehicle-treated monkeys in the prior study (P.R. Rapp, et al., 2003), trained on DR after OVX, and those in the present study that were trained on DR prior to OVX: trials to criterion at 0 sec F(1, 12) = 1.316, p = .274; trials to criterion at 1 sec F(1, 12) = .201, p = .662. Thus, from the standpoint of cognitive aging, monkeys in the vehicle group were comparable to the vehicle-treated monkeys in the earlier study at the point they began behavioral testing. Delay performance in DR, although numerically better overall in the vehicle group in the current study, did not differ significantly from delay performance of monkeys in the previous study, main effect of study F(1, 12) = 4.081, p = .066; and importantly, forgetting curves did not differ between the two groups, delay × study interaction F(4, 48) = .361, p = .835. Thus, we do not think the procedural variation of pre-OVX DR training, or the slightly younger mean age of monkeys in the current study, prevented us from observing potential beneficial cognitive effects of the hormone regimens tested. Nonetheless, a recent study did report a beneficial effect of hormone treatment (E+P) on acquisition of a spatial working memory task in middle-aged rats with no effect on delay performance (Chisholm and Juraska, 2012), raising the possibility that DR acquisition might have been more sensitive to hormone treatments examined in the present study if treatment had begun prior to training. Our studies in monkeys also varied the schedule of cyclic E delivery, from every 21 days in our previous report (Rapp et al., 2003), to every 28 days in the current ECYC+PCYC group. Although a 28-day interval was used to better approximate the normal menstrual cycle while accommodating 10-day bouts of oral P treatment, we cannot discount the possibility that changing the timing of E treatment, rather than the combined administration of P, eliminated estrogen's beneficial effects. Moreover, most of the monkeys in the current study were pair-housed, rather than singly housed, raising the possibility of an interaction between stress or some other factor with cognitive aging and/or the effects of hormone therapy on cognition. A final possibility that cannot be fully excluded is that other aspects of somatic aging such as adrenal hormone levels differed across the studies and may have had an influence, as these do not necessarily closely match chronological age (Crawford, et al., 2009).
The absence of a concurrently-tested ECYC-alone comparison group is a limitation of the present study. This aspect of the design balanced animal resource constraints against other considerations; the inclusion of a 5th hormone treatment group would have reduced the number of aged monkeys available to test other hormone regimens or would have required the omission of one of the treatments tested.
As surprising as our finding was that none of the hormone treatments we tested produced significant benefits in cognition in aged ovariectomized rhesus monkeys, what is perhaps more surprising is the finding that monkeys receiving the ECONT+PCYC regimen actually showed cognitive impairment on the object discrimination task, the last one given in the testing schedule. A similar treatment regimen is in clinical use in postmenopausal women (Premphase). Although the specifics of hormone administration protocols differ between our study and the WHIMS study, this may mirror the deleterious effects of HT on cognition that were observed in the WHIMS study (Espeland, et al., 2004,S.R. Rapp, et al., 2003). It is unclear whether impaired performance on the object discrimination task in the ECONT+PCYC group is related to the length of treatment with ECONT+PCYC or the specific cognitive demands of the object discrimination task. This unexpected finding requires confirmation from additional studies. Caution is warranted in interpreting this finding given other studies that have reported benefits of E+P treatment (Chisholm and Juraska, 2012) or no deleterious effects of combined E and P (Voytko, et al., 2008,Voytko, et al., 2009).
Why would continuous administration of E, or co-administration of E and P, fail to improve cognition in aged ovariectomized rhesus monkeys, where cyclic administration of E had a documented beneficial effect? Some neurobiological effects of E may habituate or desensitize with chronic administration: for example, dendritic spine proliferation (Miranda, et al., 1999) or increases in cholinergic markers (Gibbs, 2000). Although some studies have found that continuous administration of E is as effective in improving memory in aged rats as intermittent injection (Bimonte-Nelson, et al., 2006), others report either no effect (Gresack and Frick, 2006) or a dramatic enhancement of chronic E's effect when primed with E injections (Markowska and Savonenko, 2002). Frick (2009) notes that no studies in rats have found that continuous E treatment is more effective than cyclic treatment. However, it may be important to exercise caution in extrapolating results from rodent studies to the primate, because treatment with E alone versus E+P has different effects on adrenal steroid production (Moran, et al., 2012). The steroids induced by E+P intervention in the primate adrenal are not produced by the rodent adrenal (Bielinska, et al., 2005).
The influence of the specific regimen of E may also vary by cognitive domain. A recent study in aged, OVX rhesus monkeys found that, whereas a novel therapy of continuous E (by implant) supplemented with E injections improved visual recognition memory at 12 weeks post-OVX, and executive function and attention at 2 and 12 weeks post-OVX, improvement was no longer apparent by 24 weeks (Voytko, et al., 2008,Voytko, et al., 2009). Furthermore, no effects of hormone treatment on spatial working memory were observed at any stage, consistent with findings for the ECONT group in the present study. Thus, cyclic administration of E that includes periods of both high and low circulating E may be particularly critical to observing beneficial effects on working memory, and other beneficial effects of continuous E may diminish with chronic treatment (see also Chisholm and Juraska, 2012). Habituation of hormone treatment effects cannot explain the lack of effect of any of the treatments on DR in this study, as monkeys began reacquisition of DR one month after hormone treatment was initiated. This may, however, explain the absence of treatment effects on DNMS in the ECONT group in the present study, because training on DNMS in this group typically started ~26 weeks after the beginning of hormone treatment. Our study (like others) cannot address whether very long-term administration of hormones might exert beneficial effects, perhaps by antiinflammatory, antioxidant, or bioenergetic effects (Benedusi, et al., 2012,Henderson and Brinton, 2010,Stice, et al., 2009,Yao, et al., 2012).
Cyclic E (unopposed by P) also stimulates spinogenesis in neurons in area 46 of prefrontal cortex (Hao, et al., 2007, Hao, et al., 2006). This is thought to be the morphological substrate of improved spatial working memory in aged, ovariectomized monkeys treated with cyclic E (Morrison and Baxter, 2012). Consistent with the negative behavioral findings reported here, none of the hormone treatments tested in the present study stimulated spinogenesis in the prefrontal cortex of these monkeys (Ohm, et al., 2012). This supports the hypothesis that beneficial effects of HT, when observed, are mediated (at least in part) by promoting the formation of dendritic spines in the prefrontal cortex.
These observations, in concert with earlier, positive behavioral results (P.R. Rapp, et al., 2003), support the conclusion that the specific regimen of HT, both in terms of timing and composition, may critically dictate whether it successfully ameliorates cognitive impairment associated with menopause. Our findings suggest that a cyclic regimen of unopposed estrogen may be optimal for this purpose. This provides an additional possible explanation for the failure of HT regimens that include continuous estrogen treatment, or progesterone, to improve cognition in postmenopausal women. The current data from the monkey model suggest that standard HT, as practiced clinically in the US, is not likely to provide optimal behavioral or neurobiological benefits to women. Thus, the challenge is to exploit the potential for beneficial effects of HT on cognition and the biology of the aging brain while minimizing negative impacts on other organ systems and preventing antagonism or habituation with continued HT. Coordinated research on the behavioral and neurobiological outcomes of different hormone regimens, and the further exploration of mechanisms of HT-induced behavioral improvements, will valuably inform this effort.
Acknowledgments
We thank Carmel Stanko, Tracy Ojakangas, and Lisa Novik for technical assistance, and Dr. Kari Christe for veterinary advice and support. This research was supported by NIA grant P01-AG016765, in part by the Intramural Research Program of the NIA, and was carried out at the California National Primate Research Center, supported by NCRR grant P51-RR000169.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: There are no actual or potential conflicts of interest.
6. References
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging. 1991;12(2):99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- Benedusi V, Meda C, Della Torre S, Monteleone G, Vegeto E, Maggi A. A Lack of Ovarian Function Increases Neuroinflammation in Aged Mice. Endocrinology. 2012;153(6):2777–88. doi: 10.1210/en.2011-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M, Genova E, Boime I, Parviainen H, Kiiveri S, Leppaluoto J, Rahman N, Heikinheimo M, Wilson DB. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinology. 2005;146(9):3975–84. doi: 10.1210/en.2004-1643. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24(1):229–42. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Chisholm NC, Juraska JM. Long-term replacement of estrogen in combination with medroxyprogesterone acetate improves acquisition of an alternation task in middle-aged female rats. Behav Neurosci. 2012;126(1):128–36. doi: 10.1037/a0026461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DG. The Women's Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol. 2005;4(3):190–4. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. The Journal of clinical endocrinology and metabolism. 2009;94(8):2945–51. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55(1):2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101(4):931–8. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57(2):335–40. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006;1115(1):135–47. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104(27):11465–70. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26(9):2571–8. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: Windows into Estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and risk for dementia: where do we go from here? Gynecol Endocrinol. 2004;19(6):354–9. doi: 10.1080/09513590400018207. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22(24):10985–95. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. J Neurosci. 1999;19(9):3316–25. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran FM, Chen J, Gee NA, Lohstroh P, Lasley BL. Dehydroepiandrosterone sulfate (DHEAS) levels reflect endogenous LH production and response to human chorionic gonadotropin (hCG) challenge in the older female macaque (Macaca fascicularis) Menopause. 2012 doi: 10.1097/GME.0b013e3182698f80. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13(4):240–50. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KA, Rapp PR, Hof PR. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Exp Neurol. 1999;160:300–10. doi: 10.1006/exnr.1999.7192. [DOI] [PubMed] [Google Scholar]
- Ohm DT, Bloss EB, Janssen WG, Dietz KC, Wadsworth S, Lou W, Gee NA, Lasley BL, Rapp PR, Morrison JH. Clinically relevant hormone treatments fail to induce spinogenesis in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2012 doi: 10.1523/JNEUROSCI.1881-12.2012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861–74. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. NeuroReport. 1997;8:1923–8. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–14. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KV, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. NeuroReport. 1997;8:2047–51. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29(1):88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod. 2001;65(6):1718–25. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Laughlin LS, Rapp PR, Morrison JH, Roberts JA, Moran FM, Lasley BL. Contribution of ovarian steroid production to urinary estrone conjugate concentrations in Macaca mulatta. Am J Primatol. 2003;61(3):111–21. doi: 10.1002/ajp.10114. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Stice JP, Lee JS, Pechenino AS, Knowlton AA. Estrogen, aging and the cardiovascular system. Future Cardiology. 2009;5(1):93–103. doi: 10.2217/14796678.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. Am J Primatol. 1988;15:263–73. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- Voytko ML. The effects of long-term ovariectomy and estrogen replacement therapy on learning and memory in monkeys (Macaca fascicularis) Behav Neurosci. 2000;114:1078–87. [PubMed] [Google Scholar]
- Voytko ML. Estrogen and the cholinergic system modulate visuospatial attention in monkeys (Macaca fascicularis) Behav Neurosci. 2002;116(2):187–97. doi: 10.1037//0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Higgs CJ, Murray R. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience. 2008;154(4):1205–17. doi: 10.1016/j.neuroscience.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci. 2009;29(33):10362–70. doi: 10.1523/JNEUROSCI.1591-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML. Menopause in Female Rhesus Monkeys. Am J Primatol. 1995;35(1):59–71. doi: 10.1002/ajp.1350350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MT, Mapstone M, Staskiewicz J, Maki PM. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause. 2012 doi: 10.1097/gme.0b013e318241fd22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial β-amyloid. Neurobiol Aging. 2012;33(8):1507–21. doi: 10.1016/j.neurobiolaging.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


