Abstract
Background
In women with chronic schizophrenia, higher levels of peripheral oxytocin have been associated with lower levels of positive but not negative symptoms. Sex-specific associations between endogenous levels of oxytocin (OT) and arginine-vasopressin (AVP) with clinical symptoms and cognition in untreated early course patients have not been examined.
Method
Clinical ratings and neuropsychological testing were performed in thirty-eight acutely ill, unmedicated first-episode schizophrenia patients (14 women, 24 men). Serum hormone assays were obtained in patients and thirty-eight demographically similar healthy controls.
Results
Patients demonstrated increased AVP levels compared to controls (p=0.01). Higher AVP levels were associated with greater positive symptoms (r=0.58, p=0.03) and worse verbal learning (r=−0.63, p=0.02) in female, but not male, patients. OT levels did not statistically differ between patients and controls, and were unrelated to clinical symptoms or cognition in patients.
Conclusion
Results suggest an association of endogenous AVP with increased positive symptom severity and worse cognition in untreated female, but not male, schizophrenia patients. Findings support the role of neuroendocrine alterations in acute psychosis and the importance of examining sex-specific neuroendocrine alterations early in the course of schizophrenia.
Keywords: oxytocin, vasopressin, symptoms, verbal learning, schizophrenia, sex differences
1. Introduction
Sex differences in neuroendocrine factors may contribute to the clinical presentation and course of schizophrenia. Two sexually dimorphic neuropeptides, oxytocin (OT) and arginine-vasopressin (AVP) – may particularly influence cognitive function and behavioral manifestations of schizophrenia. Previously, we reported sex-specific associations between peripheral OT, clinical symptoms, and emotion perception in chronic, medicated patients with schizophrenia (Rubin et al., 2011; 2010). In female, but not male, patients, higher peripheral OT levels were associated with less severe positive symptoms, less severe general psychopathology, and better emotion perception. It is unclear whether this reflects disease effects or the impact of antipsychotic medications on both OT and symptoms (Kiss et al., 2009; Uvnas-Moberg et al., 1992). Examining untreated, acutely ill, first-episode patients eliminates the potential confounding influence of chronic antipsychotic use, treatment responsiveness, and duration of illness effects.
OT is classically known for its role as a hormone involved in parturition and lactation. However, OT has wide reaching effects beyond reproductive behavior, and influences behaviors that are typically impaired in schizophrenia, including attachment, trust, stress management, social cognition, and memory (Carter et al., 2008; Fehm-Wolfsdorf and Born, 1991; Heinrichs et al., 2009). Animal and human studies suggest that abnormalities in OT may contribute to the clinical presentation of schizophrenia (for reviews, Feifel, 2011, 2012). In animal models of schizophrenia, haplo-insufficient reeler mice show reductions in OT receptors in regions of the hippocampus and retrosplenial and piriform cortex (Liu et al., 2005); which are critical areas for emotion, cognition, and positive symptoms in schizophrenia (Jardri et al., 2011; Kuperberg and Heckers, 2000). Although the direction is not always consistent, patients with schizophrenia have been shown to have altered central (Beckmann et al., 1985; Linkowski et al., 1984) and peripheral levels (Goldman et al., 2008; Keri et al., 2009) of OT in most but not all studies (Glovinsky et al., 1994; Rubin et al., 2011; 2010; Sasayama et al., 2012).
Pharmacological studies administering exogenous OT to predominantly chronic male patients also suggest short-term benefits for positive and negative symptoms and social cognition (Averbeck et al., 2011; Feifel et al., 2010; Goldman et al., 2011; Pedersen et al., 2011). Mechanisms by which OT may have therapeutic effects on clinical symptoms could involve modulation of emotional regulation and the autonomic nervous system (Porges, 2001) and neurochemical systems dependent on dopamine and/or glutamate (Caldwell et al., 2009; Feifel and Reza, 1999; Qi et al., 2008; Rosenfeld et al., 2011; Sarnyai and Kovacs, 1994; Shahrokh et al., 2010).
OT interacts dynamically with a related neuropeptide, AVP. Both OT and AVP act within the central nervous system with effects on behavior and physiology (Landgraf and Neumann, 2004). Similar to OT, AVP is found in high concentrations in the paraventricular nucleus and the supraoptic nucleus of the hypothalamus and is transported to the posterior pituitary where it is released peripherally. AVP is classically known for its role in the kidney as a potent antidiuretic hormone (Weitzman and Kleeman, 1979). AVP is also released centrally during stressful experiences (Landgraf et al., 1998) and is implicated in the regulation of the hypothalamic-pituitary adrenal (HPA) axis, including cortisol secretion (Meyer-Lindenberg et al., 2011).
Changes in stress-related hormones such as AVP may be associated with the clinical symptoms and cognition observed in schizophrenia. Whereas OT influences stress management, cardiovascular regulation, and under some conditions may have amnestic effects on verbal learning and memory; AVP is more typically associated with vigilance, mobilization, increased reactivity to stressors, and improvements in verbal learning and memory (Carter, 1998; Carter et al., 2008; Fehm-Wolfsdorf and Born, 1991; Ferris, 2008; Gutkowska and Jankowski, 2012; Heinrichs et al., 2009; Strupp et al., 1983). Disruptions and interactions among these hormones may regulate physiology, behavior, and cognition allowing shifts between positive social behaviors and defensive states that are associated with the clinical symptoms of schizophrenia.
The purpose of the present study was two-fold. First, we aimed to compare the concentration of peripheral OT and AVP levels in unmedicated, acutely-ill first-episode schizophrenia patients to healthy controls. Second, we aimed to evaluate whether peripheral OT and AVP levels are associated with positive symptom severity and verbal learning in these patients. We hypothesized that OT levels would be decreased and AVP levels would be increased in patients compared to controls. We also predicted that OT would be associated with less severe positive symptoms and worse verbal learning, whereas AVP would be associated with more severe positive symptoms and possibly better verbal learning. Based on our earlier findings, we predicted that hormone associations with symptoms would be most pronounced in female patients.
2. Methods
2.1. Participants
The sample included 38 patients (14 women, 24 men; Table 1) recruited from the University of Illinois at Chicago (UIC) First-Episode Psychosis Program. Patients were between 16 and 50 years of age and had no known systemic, endocrine, or neurological disease. Of the 38 patients, 26 (68%) had no prior lifetime exposure to antipsychotic medications. The 12 patients with prior lifetime exposure to antipsychotic medications had prior treatment limited to less than eight weeks of cumulative exposure (median = 3.07 weeks). These antipsychotic medications were typically prescribed in the course of their illness prodrome or earlier during their first episode when they were initially seen by an emergency room or a community mental health center. These patients were antipsychotic free for at least 3 days (median time of washout = 5 days) at the time of the initial study assessments. Diagnoses were assigned according to Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV) criteria using the Structured Clinical Interview (SCID; First et al., 1995), along with collateral clinical data which were reviewed at consensus diagnosis meetings. Patients were diagnosed with either schizophrenia (n=34; 90%) or schizoaffective disorder depressed type (n=4; 10%).
Table 1.
Baseline and clinical characteristics as a function of sex.
| Patients | Healthy controls | |||
|---|---|---|---|---|
| Variables | Women (n=14) |
Men (n=24) | Women (n=14) |
Men (n=24) |
| Demographics | ||||
| Age (years), M (SD)G | 26.93 (9.20) | 21.83 (5.30) | 28.43 (9.40) | 27.58 (3.26) |
| IQ, M (SD) | 97.00 (11.44) | 92.21 (13.69) | 99.00 (10.73) | 94.96 (30.96) |
| Race/Ethnicity (%) | ||||
| African-American non-Hispanic | 57% | 54% | 43% | 48% |
| Caucasian, non-Hispanic | 14% | 21% | 29% | 26% |
| Hispanic | 14% | 21% | 21% | 13% |
| Other | 14% | 4% | 7% | 13% |
| Clinical variables | ||||
| Consensus diagnosis (%) | ||||
| Schizophrenia | 79% | 96% | ||
| Schizoaffective depressed type | 21% | 4% | ||
| No prior antipsychotic treatment (%) | 86% | 58% | ||
| Antipsychotic free at initial testing (%) | 100% | 100% | ||
| Treatment 48 hours prior to testing (%) | 7% | 0% | ||
| Antidepressants | 0% | 0% | ||
| Mood stabilizer or stimulants | 7% | 8% | ||
| Benzodiazepines | ||||
| PANSS, M (SD) | ||||
| Positive subscale | 23.93 (5.59) | 24.50 (3.79) | ||
| Negative subscale | 19.50 (7.86) | 20.58 (4.97) | ||
| Hormone Values, M (SD) | ||||
| Oxytocin (pg/ml) | 349.09 (227.41) | 364.52 (257.03) | 342.98 (217.30) | 427.28 (259.01) |
| Vasopressin (pg/ml)G | 108.39 (89.85) | 109.63 (72.97) | 69.18 (25.86) | 74.10 (46.50) |
Note.
Main effect of group is p<0.05. PANSS = Positive and Negative Syndrome Scale.
A sample of 38 healthy controls (14 women, 24 men) were recruited from the community with no history of Axis I disorders based on the SCID or history among first-degree relatives of psychotic or mood disorders. Controls were comparable to patients on race and IQ but were slightly older than patients (28.0 vs. 24.4 years of age, p<0.05). No participants had history of head trauma with loss of consciousness for more than 10 minutes, neurological disorder, or lifetime history of alcohol or drug dependence. Two female patients and one female control were receiving oral contraceptives; including these women did not change the pattern of results.
2.2. Measures
2.2.1. Clinical and Neuropsychological Evaluation
The Positive and Negative Symptom Scale (PANSS; Kay et al., 1987) was administered to patients to assess positive and negative symptoms. The neuropsychological battery included our primary outcome measure, the California Verbal Learning Test (CVLT), and a number of secondary outcome measures to determine the sensitivity and specificity of our findings. The CVLT (Delis et al., 1987) is a measure of verbal learning; outcome = total learning across trials 1–5. Secondary neuropsychological outcomes included: 1) WAIS-R Digit Span (Wechsler, 1981) a measure of attention (Forward) and working memory (Backward); outcome = total correct; 3) Trail Making Test (Reitan and Wolfson, 1985) a measure of processing speed (Part A) and mental flexibility (Part B); outcome = time to complete; and 4) WMS-III Spatial Span Test (Wechsler, 1997) a measure of short term non-verbal working memory; outcome = total correct.
2.2.2. Serum Hormone Assays
Blood samples were drawn at the same time of day for patients (95% blood draws ≤ noon). At UIC, samples were stored in plain tubes, spun at 4°C, and placed into freezers set at −80°C. All samples were batched by assays, diluted in an assay buffer to give reliable results within the linear portion of the standard curve (OT 1:4; AVP 1:2), and completed in duplicate on unextracted serum. OT and AVP were quantified with an EIA kit (Enzo Life Sciences/Assay Designs); as validated for OT and AVP (Carter et al., 2007). These EIAs are highly sensitive (minimal detection levels <12 pg/ml OT; 4 pg/ml AVP) and specific with cross-reactivity between OT and AVP of <0.04%). All samples were run at the same time and intra-assay coefficients of variation were less than 11.5% for both assays.
2.3. Statistical Analyses
To compare concentrations of peripheral hormone levels between male and female patients and controls, we conducted a series of between subjects ANOVAs with Group (patient vs. control) and Sex as the between-subjects factors. Pearson correlations were conducted to evaluate associations between hormone levels, clinical symptoms, and cognition among patients. Given the small sample sizes, when significant correlations were observed (when the 95% confidence interval (CI) does not include 0) Baysian Bootstrapping of the correlation coefficient (r̂; 1000 iterations) was conducted to determine the strength of the associations and to ensure that findings were not driven by outliers. Correlations were compared between male and female patients using Fisher’s r-to z transformation. All p-values are two sided and the statistical significance level was set at p<0.05.
3. Results
3.1. Do peripheral concentrations of OT or AVP differ between unmedicated patients and controls?
Patients (M=109.01, SE=10.60) demonstrated increased AVP levels compared to healthy controls (M=71.64, SE=10.60, p<0.05; Table 1). The two subject groups showed similar levels of OT. No hormone levels differed as a function of sex and there were no sex by group interactions. OT and AVP were not associated in patients, but there was a trend for higher levels of AVP to relate to OT in controls (r=0.38, p=0.02; r̂=0.35, 95%CI −0.11, 0.70).
3.2. Are peripheral levels of OT or AVP associated with clinical symptoms or cognition in unmedicated schizophrenia patients?
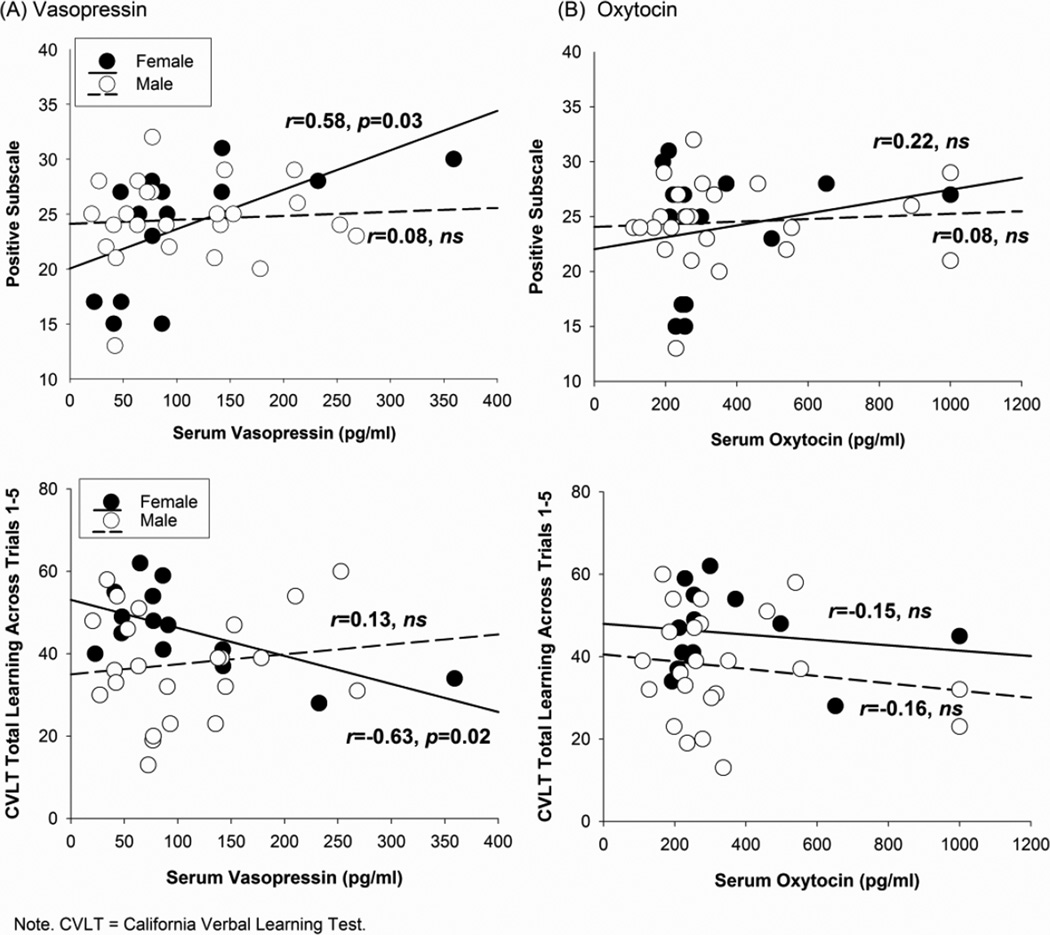
As predicted, higher AVP levels were associated with more severe positive symptoms (r=0.58, p<0.05; r̂ = 0.62, 95%CI 0.30, 0.89; Figure 1) and lower verbal learning scores (r=−0.63, p=0.02; r̂=−0.63, 95%CI −0.92, −0.09) in female, but not in male, patients. These differences in the correlation coefficients between male and female patients were significant for verbal learning (Z=−2.34, p=0.02) and marginally significant for positive symptoms (Z=1.73, p=0.08). This association was also not significant in female (r=0.33, p=0.52) or male controls (r=−0.14, p=0.75). Exploratory analyses on individual PANSS items on the positive subscale indicated that in female patients, higher levels of AVP were significantly associated with greater grandiosity (r=0.72, p<0.01; r̂=0.69, 95%CI 0.09, 0.93). There was also a trend for higher levels of AVP to relate to suspiciousness (r=0.52, p=0.059; r̂=0.56, 95%CI −0.01, 0.90). AVP was not associated with any of the secondary cognitive outcomes. OT was not associated with clinical symptoms or cognitive functioning in female or male patients. OT was also not associated with verbal learning in female (r=−0.14, p=0.79) or male controls (r=0.08, p=0.85).
Figure 1.
In unmedicated patients, serum vasopressin is associated with more prominent positive symptoms and poorer verbal learning in female but not male patients (A). Serum oxytocin (B) levels were not associated with positive symptoms or verbal learning in male or female patients.
4. Discussion
The primary finding was that AVP levels were higher in patients compared to healthy controls; no group differences were evident in OT levels. Moreover, AVP was associated with more prominent positive symptoms and poorer verbal learning prior to treatment in female, but not male, patients. These findings in unmedicated patients early in the course of illness complement our previous work in chronic patients (Rubin et al., 2011; 2010), highlighting the potential importance of neuroendocrine alterations in acute psychosis, and underscoring the importance of investigating sex-specific alterations of AVP/OT in relation to symptom severity and cognition in schizophrenia.
Consistent with previous studies (de Leon et al., 1994; Goldman, 2009), our findings implicate possible AVP dysregulation in unmedicated first-episode patients with schizophrenia. Here we found AVP levels to be higher in patients compared to controls. This finding is consistent with Raskind (1978), but inconsistent with other studies reporting lower peripheral (Legros et al., 1992; Ryan et al., 2004) and central AVP levels (Linkowski et al., 1984) in schizophrenia compared to controls (cf., Beckmann et al., 1985; Walsh et al., 2005). However, inconsistencies exist across these studies including illness severity (acute vs. chronic), diagnosis, treatment effect differences, sample sizes, and the sex of participants.
One possible explanation for higher levels of AVP in unmedicated first-episode patients is that they may experience dysregulation of biological stress response systems (e.g., hyperactive or reactive HPA axis or autonomic nervous system) and may be more sensitive to stressful experiences (for review, Aiello et al., 2012). AVP is part of the neuroendocrine system activated during stressful experience and dysregulation of this system might result in AVP being continuously synthesized and/or released in response to the emotional experiences or emotion reactivity that accompanies psychosis (Phillips and Seidman, 2008). Animal studies suggest various forms of stress may increase the synthesis and release of AVP (Ma and Lightman, 1998; Neumann et al., 2006; Ruscio et al., 2007; Todeschin et al., 2009). Peripheral AVP levels also increase in response to intense exercise stress in women (Altemus et al., 1995; 2001). Higher levels of AVP might be the result of stressors precipitating the psychotic episode or alternatively could result from stress associated with the psychotic episode itself. Alternatively excessive dopamine release, which is thought to represent an important component in the neurobiology of psychosis (Abi-Dargham et al. 1998; Laruelle et al., 1996), may increase the synthesis or release of AVP or cause a cascade of catecholamines, influencing AVP release. For example, apomorphine, a non-selective D1 and D2 dopamine receptor agonist, results in a two-fold increase in basal AVP levels in patients with schizophrenia compared to controls (Legros et al., 1992).
Despite increases in AVP levels in patients, we did not find OT levels to be significantly altered in first episode patients. This finding is not consistent with our previous observations in chronic patients with schizophrenia (Rubin et al., 2011; 2010) or with a previous report that cerebrospinal fluid OT levels are high in chronic unmedicated male patients compared to controls (Beckmann et al., 1985). Differences across studies could reflect different characteristics of our patient populations (first episode vs. chronic) as well as our focus on peripheral rather than central OT levels. Comparisons are difficult since little is known about the endocrine characteristics of first-episode patients, either during a period of psychosis or in periods of remission. It is possible that a psychotic experience might have overwhelmed the body’s capacity to regulate OT, leaving only the effects of AVP detectable and proportional to the intensity of the symptoms experienced. OT may have been unable to attenuate the stress response system, thus resulting in peripheral OT levels in patients that appeared similar to those in controls. There are previous reports that the beneficial or “anti-stress” effect of OT is most apparent in response to chronic stressors such as isolation or adversity (Grippo et al., 2009; Heim et al., 2009; Penza et al., 2003). The present study may be more consistent with an acute stress model resulting from either the psychotic episode itself or a result of an acute environmental or emotional experience precipitating the psychotic episode. It is also important to note that not all stressors stimulate both OT and AVP release in animals or humans (Altemus et al., 1995; 2001; Gibbs, 1984; Jezova et al., 1995).
Another notable result was that higher levels of AVP were associated with more positive symptoms and lower verbal learning scores in female, but not in male, patients. The present findings are consistent with a previous small study of predominately female patients in which higher AVP levels were associated with higher levels of positive symptoms (Raskind, 1978). These findings are also consistent with the literature in healthy individuals (Fehm-Wolfsdorf and Born, 1991; Weingartner et al., 1981), in which higher AVP levels were related to worse verbal learning.
Although peripheral AVP levels do not typically differ between the sexes (Sanders et al., 1990), animal and human studies suggest that the effects of central AVP on behavior are sexually dimorphic (Carter, 2007; Thompson et al., 2006). Sex differences in central AVP are most prominent in the amygdala, bed nucleus of the stria terminalis and lateral septum (de Vries, 2008; van Leeuwen et al., 1985). This system is particularly important for determining reactions to emotional stimuli, shows sex differences (Domes et al., 2010; Veenema et al., 2012), and is impaired in schizophrenia (Kalkstein et al., 2010). Thus, it is possible that central AVP may play an important but different role in modulating positive symptoms and impairing verbal learning in male and female patients, especially early in the course of illness.
Results of our study should be considered preliminary. Limitations of the present study include a small sample size, cross-sectional study design, and single measurement of AVP/OT levels. Findings need to be replicated in larger samples and longitudinal studies or more experimental approaches, possibly using agents that block the AVP receptor. Multiple measurements, perhaps in the presence of a known stressor, would provide more precise estimates of AVP and OT secretion, and might serve to explain the observed differences between unmedicated patients and controls.
Despite limitations, the present data highlight the role of possible neuroendocrine alterations in acute psychosis and suggest that these neuroendocrine profiles are different in men and women with first-episode psychosis. If altered AVP levels can be confirmed to occur as a correlate or a consequence of psychosis, and if antipsychotic treatment alters these levels differently in male and female patients, then these findings may have potential clinical and therapeutic implications. Future studies also should consider variations in AVP and AVP receptor genes as potential moderators of the relationship between hormone levels, clinical symptoms, and cognition. Polymorphisms in genes in the AVP pathway have been associated with schizophrenia (Feifel and Priebe, 2001; Levin et al., 2009; Teltsh et al., 2011), possibly accounting for some of the observed variations in outcomes among the studies in this field.
Acknowledgement
This publication was made possible by funds from NIH (MH080066, MH077862, MHRR013987) as well as services provided by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050. Dr. Rubin’s effort was supported by grant number K12HD055892 from the National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Office of Research on Women's Health (ORWH). Dr. Bishop’s effort was supported by NIMHK08MH083888. Dr. Reilly’s effort was supported by NIMHK08MH083126. Dr. Bishop’s effort was supported by NIMHMH072767. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Dr. Bishop has received research grant support from Ortho-Mcneil Janssen. Dr. Sweeney has consulted with Takeda, Lilly, BMS, Roche, and Pfizer.
Role of funding source
The funding agencies had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The other authors have nothing to disclose.
Contributors
Dr. Rubin conceived the idea for the study of oxytocin and vasopressin in this first episode study sample as part of her K12 award, conducted the data analysis, interpreted results and wrote the manuscript. The study was a secondary analysis of data collected as part of Dr. Sweeney’s First-Episode Psychosis Program at the University of Illinois at Chicago (UIC). Dr. Sweeney provided mentorship to Dr. Rubin, supported the infrastructure for the First Episode Psychosis program, and provided oversight as senior author for all activities related to subject recruitment, assessment, diagnoses, data interpretation, and manuscript preparation Dr. Carter developed the methodology to examine oxytocin and vasopressin, served as an advisor and resource on this project for understanding these hormones, and assisted in data interpretation and manuscript preparation. Dr. Bishop collected and managed the biological samples, assisted with the laboratory analyses, data interpretation, and manuscript preparation. Dr. Pournajafi-Nazarloo ran the all of the oxytocin and vasopressin assays. Drs. Reilly and Harris provided subject recruitment, clinical and neuropsychological assessments, consensus diagnosis evaluations, data interpretation, and assisted with manuscript preparation. Dr. Hill provided analysis and interpretation of neuropsychological data and assisted with manuscript preparation. All authors contributed to the writing of the manuscript and approval of the final version.
References
- Aiello G, Horowitz M, Hepgul N, Pariante CM, Mondelli V. Stress abnormalities in individuals at risk for psychosis: A review of studies in subjects with familial risk or with “at risk” mental state. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endocrinol Metab. 1995;80(10):2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J Clin Endocrinol Metab. 2001;86(6):2525–2530. doi: 10.1210/jcem.86.6.7596. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2011:1–8. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann H, Lang RE, Gattaz WF. Vasopressin--oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10(2):187–191. doi: 10.1016/0306-4530(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Stephens SL, Young WS., 3rd Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14(2):190–196. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176(1):170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- de Leon J, Verghese C, Tracy JI, Josiassen RC, Simpson GM. Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol Psychiatry. 1994;35(6):408–419. doi: 10.1016/0006-3223(94)90008-6. [DOI] [PubMed] [Google Scholar]
- de Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Ober BA. California Verbal Learning Test: Adult version manual. San Antonio: The Psychological Coorporation; 1987. [Google Scholar]
- Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Human brain mapping. 2010;31(5):758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm-Wolfsdorf G, Born J. Behavioral effects of neurohypophyseal peptides in healthy volunteers 10 years of research. Peptides. 1991;12(6):1399–1406. doi: 10.1016/0196-9781(91)90226-f. [DOI] [PubMed] [Google Scholar]
- Feifel D. Is oxytocin a promising treatment for schizophrenia? Expert Rev Neurother. 2011;11(2):157–159. doi: 10.1586/ern.10.199. [DOI] [PubMed] [Google Scholar]
- Feifel D. Oxytocin as a potential therapeutic target for schizophrenia and other neuropsychiatric conditions. Neuropsychopharmacology. 2012;37(1):304–305. doi: 10.1038/npp.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68(7):678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Feifel D, Priebe K. Vasopressin-deficient rats exhibit sensorimotor gating deficits that are reversed by subchronic haloperidol. Biol Psychiatry. 2001;50(6):425–433. doi: 10.1016/s0006-3223(01)01100-3. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology (Berl) 1999;141(1):93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- Ferris CF. Functional magnetic resonance imaging and the neurobiology of vasopressin and oxytocin. Prog Brain Res. 2008;170:305–320. doi: 10.1016/S0079-6123(08)00425-1. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Gibbs DM. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sci. 1984;35(5):487–491. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- Glovinsky D, Kalogeras KT, Kirch DG, Suddath R, Wyatt RJ. Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophr Res. 1994;11(3):273–276. doi: 10.1016/0920-9964(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Goldman M, Marlow-O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res. 2008;98(1–3):247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB. The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Res Rev. 2009;61(2):210–220. doi: 10.1016/j.brainresrev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, 2nd, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34(10):1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M. Oxytocin revisited: its role in cardiovascular regulation. J Neuroendocrinol. 2012;24(4):599–608. doi: 10.1111/j.1365-2826.2011.02235.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14(10):954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Ann N Y Acad Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Current topics in behavioral neurosciences. 2010;4:373–390. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Soc Neurosci. 2009;4(4):287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kiss A, Bundzikova J, Pirnik Z, Mikkelsen JD. Different antipsychotics elicit different effects on magnocellular oxytocinergic and vasopressinergic neurons as revealed by Fos immunohistochemistry. J Neurosci Res. 2009 doi: 10.1002/jnr.22226. [DOI] [PubMed] [Google Scholar]
- Kuperberg G, Heckers S. Schizophrenia and cognitive function. Curr Opin Neurobiol. 2000;10(2):205–210. doi: 10.1016/s0959-4388(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3–4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wotjak CT, Neumann ID, Engelmann M. Release of vasopressin within the brain contributes to neuroendocrine and behavioral regulation. Prog Brain Res. 1998;119:201–220. doi: 10.1016/s0079-6123(08)61571-x. [DOI] [PubMed] [Google Scholar]
- Legros JJ, Gazzotti C, Carvelli T, Franchimont P, Timsit-Berthier M, von Frenckell R, Ansseau M. Apomorphine stimulation of vasopressin- and oxytocin-neurophysins. Evidence for increased oxytocinergic and decreased vasopressinergic function in schizophrenics. Psychoneuroendocrinology. 1992;17(6):611–617. doi: 10.1016/0306-4530(92)90019-4. [DOI] [PubMed] [Google Scholar]
- Levin R, Heresco-Levy U, Bachner-Melman R, Israel S, Shalev I, Ebstein RP. Association between arginine vasopressin 1a receptor (AVPR1a) promoter region polymorphisms and prepulse inhibition. Psychoneuroendocrinology. 2009;34(6):901–908. doi: 10.1016/j.psyneuen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Geenen V, Kerkhofs M, Mendlewicz J, Legros JJ. Cerebrospinal fluid neurophysins in affective illness and in schizophrenia. Eur Arch Psychiatry Neurol Sci. 1984;234(3):162–165. doi: 10.1007/BF00461555. [DOI] [PubMed] [Google Scholar]
- Liu W, Pappas GD, Carter CS. Oxytocin receptors in brain cortical regions are reduced in haploinsufficient (+/−) reeler mice. Neurol Res. 2005;27(4):339–345. doi: 10.1179/016164105X35602. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL. The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. J Physiol. 1998;510(Pt 2):605–614. doi: 10.1111/j.1469-7793.1998.605bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Toschi N, Veenema AH. Oxytocin actions within the supraoptic and paraventricular nuclei: differential effects on peripheral and intranuclear vasopressin release. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;291(1):R29–R36. doi: 10.1152/ajpregu.00763.2005. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. 2011;132(1):50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Arch Womens Ment Health. 2003;6(1):15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophr Bull. 2008;34(5):888–903. doi: 10.1093/schbul/sbn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol. 2008;376(6):441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- Raskind MA. Is antidiuretic hormone elevated in psychosis? A pilot study. Biol Psychiatry. 1978;13(3):385–390. [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- Rosenfeld AJ, Lieberman JA, Jarskog LF. Oxytocin, dopamine, and the amygdala: a neurofunctional model of social cognitive deficits in schizophrenia. Schizophr Bull. 2011;37(5):1077–1087. doi: 10.1093/schbul/sbq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res. 2011;130(1–3):266–270. doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res. 2010;124(1–3):13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Sue Carter C. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51(1):54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Sanders G, Freilicher J, Lightman SL. Psychological stress of exposure to uncontrollable noise increases plasma oxytocin in high emotionality women. Psychoneuroendocrinology. 1990;15(1):47–58. doi: 10.1016/0306-4530(90)90046-c. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology. 1994;19(1):85–117. doi: 10.1016/0306-4530(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sasayama D, Hattori K, Teraishi T, Hori H, Ota M, Yoshida S, Arima K, Higuchi T, Amano N, Kunugi H. Negative correlation between cerebrospinal fluid oxytocin levels and negative symptoms of male patients with schizophrenia. Schizophrenia Research; 2012. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp B, Weingartner H, Goodwin FK, Gold PW. Neurohypophyseal hormones and cognition. Pharmacol Ther. 1983;23(2):267–279. doi: 10.1016/0163-7258(83)90016-5. [DOI] [PubMed] [Google Scholar]
- Teltsh O, Kanyas-Sarner K, Rigbi A, Greenbaum L, Lerer B, Kohn Y. Oxytocin and vasopressin genes are significantly associated with schizophrenia in a large Arab-Israeli pedigree. Int J Neuropsychopharmacol. 2011:1–11. doi: 10.1017/S1461145712001162. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103(20):7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todeschin AS, Winkelmann-Duarte EC, Jacob MH, Aranda BC, Jacobs S, Fernandes MC, Ribeiro MF, Sanvitto GL, Lucion AB. Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Horm Behav. 2009;56(1):93–100. doi: 10.1016/j.yhbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl) 1992;109(4):473–476. doi: 10.1007/BF02247726. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325(1–2):391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav. 2012;61(1):50–56. doi: 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P, Spelman L, Sharifi N, Thakore JH. Male patients with paranoid schizophrenia have greater ACTH and cortisol secretion in response to metoclopramide-induced AVP release. Psychoneuroendocrinology. 2005;30(5):431–437. doi: 10.1016/j.psyneuen.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised manual. New York: The Psychological Coorporation; 1981. [Google Scholar]
- Wechsler D. The Wechsler Memory Scale. 3rd ed. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- Weingartner H, Gold P, Ballenger JC, Smallberg SA, Summers R, Rubinow DR, Post RM, Goodwin FK. Effects of vasopressin on human memory functions. Science. 1981;211(4482):601–603. doi: 10.1126/science.7455701. [DOI] [PubMed] [Google Scholar]
- Weitzman RE, Kleeman CR. The clinical physiology of water metabolism. Part I: The physiologic regulation of arginine vasopressin secretion and thirst. The Western journal of medicine. 1979;131(5):373–400. [PMC free article] [PubMed] [Google Scholar]