Abstract
Central to macro-connectomics and much of systems neuroscience is the idea that we can summarise macroscopic brain connectivity using a network of “nodes” and “edges” – functionally distinct brain regions and the connections between them. This is an approach that allows a deep understanding of brain dynamics and how they relate to brain circuitry. This approach, however, ignores key features of anatomical connections, such as spatial arrangement and topographic mappings. In this article, we suggest an alternative to this paradigm. We propose that connection topographies can inform us about brain networks in ways that are complementary to the concepts of “nodes” and “edges”. We also show that current neuroimaging technology is capable of revealing details of connection topographies in vivo. These advances, we hope, will allow us to explore brain connectivity in novel ways in the immediate future.
Introduction
The current paradigm in macro-connectomics, the science of measuring brain networks at a systems level [1], rests upon the notions of functional segregation and integration. The brain is viewed as a set of discrete, functionally specialised areas that interact within networks and sub-networks to produce coherent thoughts and behaviour [2]. This view highlights the principle that brain function is determined by the properties of brain circuitry, and is a powerful approach to studying relationships between structure and function [3]. However, the concept of segregated brain regions that form the nodes of the macro-connectome remains elusive, and at best loosely defined. As a consequence, despite a century of effort using a variety of techniques [4–7], there is still no consensus on the most reliable, reproducible, and meaningful approach to delineating functional subdivisions in the brain.
There are several reasons why it has been difficult to reach an agreement on brain segregation. Specialised regions are usually defined according to spatial transitions in aspects of cytoarchitecture, myeloarchitecture, density of transmitter receptors, or anatomical connections. However, transitions between grey matter areas may be subtle and may occur in some but not all dimensions [8, 9]. For example, differences in the arrangement of cells may reveal transitions between areas detectable using cytoarchitecture measurements, but with little changes in the large-scale connection patterns, or vice versa. Also, sharp transitions may be detected along the cortex when considering some subsets of the connections, but not others. Perhaps more importantly, transitions may not necessarily be abrupt but smooth and gradual, as discussed in detail below.
These anatomical realities challenge the concept of confined, discrete and segregated brain regions. In defining the nodes of the connectome as functional units, subtleties in the internal organisation of these units are often ignored.
In this article, we concentrate on one particular aspect of this detailed anatomy. In describing large-scale connections between grey matter regions, we would like to consider the organization of connections within the ‘edges’ of the connectome, and in particular the topographic, or spatial pattern of these connections. Axons that connect a region to its target often follow some sort of spatial arrangement, and connections to different target regions may have different arrangements. As we will see below, topographic connections are ubiquitous in the brain. They are thought to play a role not only in representing sensory information, but also in complex cognitive processes that involve more abstract representations. Yet, the spatial organisation of connections has thus far been largely ignored in network models for macro-connectomics. Current systems-level models often summarise brain networks using a simplified picture such as the binary presence or absence of connections, or some gradation in the “strength” of connectivity, with little attention to how the connections are spatially organised.
In the remainder of this paper, we discuss the relevance of connection topographies to studying brain function at a systems level. We also show that we can have access to these detailed patterns of connections with state-of-the-art in vivo imaging technology. The availability of these techniques is a tremendous opportunity for the field of macro-connectomics. However, we also note that analysis methods that can reveal these complex patterns of connections are unfortunately lacking.
Topographic connections in the brain
In neuroscience, topographic connections are generally understood as point-to-point mappings that preserve spatial arrangements: nearby locations in a source region connect to nearby locations in the target [10]. In this article, we will follow the same definition for the term topography. For lack of a better terminology, the more generic meaning of topography, i.e that of a spatial pattern of connectivity between areas, will be referred to simply as connection pattern. This includes point-to-point topology-preserving connections as well as connection maps that break topology and/or form clusters in the target region.
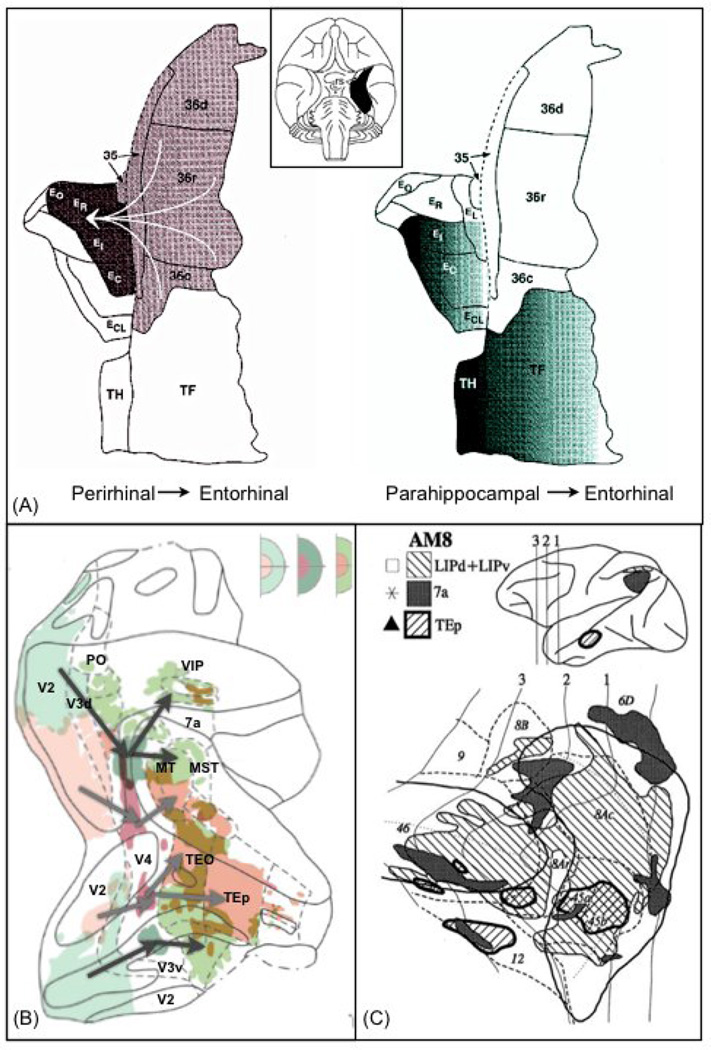
Figure 1 shows examples of different patterns of cortico-cortical connections observed in the monkey brain. In Figure 1A, the connections from perirhinal to entorhinal cortices do indeed appear to be uniform such that any point in one region is equally likely to connect to any point in the other. Such a connection pattern could be well approximated by current macro-connectomic strategies. By contrast, the connections from parahippocampal cortex (Pc) to entorhinal cortex (Ec) respect a spatial gradient, such that the most medial part of Pc projects to the most medial part of Ec. Figure 1B shows another example of topographically organised connections. Visual area V4 receives input from and sends output to lower and higher visual areas respectively. These connections follow a retinotopic organisation. Finally, figure 1C shows a different type of connectivity pattern that may break the nodes and edges assumption. Parietal area 7a in the macaque sends connections to the prefrontal cortex that are not topographic, but form clusters. These clusters only partially overlap with various prefrontal areas, and some of these target areas contain more than one cluster. By contrast, connections from area LIP conform more to an all-to-all connectivity pattern.
Figure 1.
Examples of spatial connection patterns in the monkey brain, measured using invasive chemical tracers. (A) Connections between Entorhinal cortex (Eo, Er, Et, Ec) and perirhinal (36, 35) and parahippocampal (TH, TF) cortices. Entorhinal cortex contains two different mappings: connections from perirhinal are all-to-all, connections from parahippocampal are organised into a medial-lateral gradient. (B) Flat representation of visual areas and their connections to area V4 in the macaque brain. Retrograde and anterograde injections into V4 show that the tracers follow a route that respects retinotopy. Foveal areas (green) and peripheral areas (pink) are mapped onto each other throughout the cortical hierarchy. The top-right inserts show the retinotopic mapping associated with lower (left), and higher (right) visual areas. The middle panel indicates retinotopy of area V4. (C) Patterns of connections between parietal/temporal regions and prefrontal cortex. Connections from area 7a form patches that partially overlap several prefrontal areas. Connections from LIP cover a larger part of prefrontal cortex. Connections from area TEp in the temporal lobe overlaps with parietal projections in a few localised patches. Figures were modified from the following sources: [32] (A), [70] (B) and [71] (C).
Topographic maps are ubiquitous in the vertebrate brain [11]. Most sensory inputs to the brain are topographically organised, and this topography is continued across several relay nuclei through to the cortex. For instance, retinofugal projections are retinotopically organised; the auditory projections connect with the cochlear nucleus according to a tonotopic arrangement, and sensory projections from the skin innervate the primary sensory cortex somatotopically. In fact, topographies in the visual cortex have been pivotal in guiding detailed cortical sub-divisions of the occipital lobes [4, 12]. Topographic connections are also found between cortical regions. The clearer examples of such cortico-cortical topographies are callosal projections. Every cortical site that projects through the main commissure preferentially connects to the contra-lateral homologous zone [13–15]. Topographies are also found in cortico-basal ganglia loops [16–18] and cortico-cerebellar loops [19].
A key feature of spatial patterns of connections is that they can overlap. The same piece of cortex may contain different topographic organisations, depending on the target under consideration. Furthermore, different types of patterns may simultaneously be present. While connections to some targets may be arranged in gradients, others may appear more clustered or intermingled. Figure 1A shows an example of such superposition. Connections between the entorhinal and parahippocampal cortices are arranged along a gradient, but connections from the perirhinal gyrus follow an “all-to-all” organisation when they reach the entorhinal cortex.
The superposition of different connection patterns within the same target region may form the basis for specific types of information coding [20, 21]. For example, overlapping topographic maps may constitute an efficient way of computing transformations between spatial representations in the brain, using short-range circuitry [22].
Topographies can be measured in vivo
The most common approach for measuring topographic maps in the brain is to probe primary sensory systems. Vision, touch, audition and taste have all been mapped onto the cortex using simultaneous stimulation of the sensors and recording of brain activity [4, 23–25]. These maps are indicative of topographically organised connections: as the cortex does not contain the primary sensory receptors, finding a map in the cortex indicates that the connections are topographically arranged. Modern neuroimaging technology, such as functional magnetic resonance imaging (FMRI), can be used to visualise the spatial arrangements of these maps in vivo. For example, retinotopic maps in the visual cortex can simply be obtained by stimulating the retina using a periodic stimulus that sweeps the visual field in an orderly fashion [4, 12]. By finding the FMRI signals that are in phase with the stimulus, it is possible to obtain a map of the visual field directly on the cortex.
Secondary maps have also been found throughout the cortex. These maps of sensory space have been found outside primary sensory cortices, and therefore indicate topographies between cortical areas. For instance, human and macaque in vivo experiments using FMRI have shown spatial retinotopic maps in the parietal [26–28], temporal [29] and prefrontal [30, 31] cortices. Many of these secondary maps cannot be revealed by simply probing the primary sensors. Instead, it is common to use more elaborate tasks that involve memory and/or attention components.
It is easy to imagine the existence of topographic connections between areas of the cortex that transmit non-sensory information. Indeed, figure 1A shows evidence for such type of organisation [32]. It is less easy to imagine what type of information these topographies might be transmitting, nor why they are topographically organised in the first place [10, 20]. As a consequence, task-based experiments cannot easily be used to find these types of cortico-cortical topographies.
How can we detect topographic maps without using a task? We may draw from the principles used in task-based studies. Experimentally, in order to find a map (a continuous representation of function) in the brain, some continuous variable is changed (e.g. spatial position of stimulus), and the response in the brain is monitored. If the response is a gradual shift in the position of activation, the experimenter has found a map. In the same way, using some measure of brain connectivity, we can consider a seed region whose position changes continuously, and monitor for continuous changes in the position of the target regions.
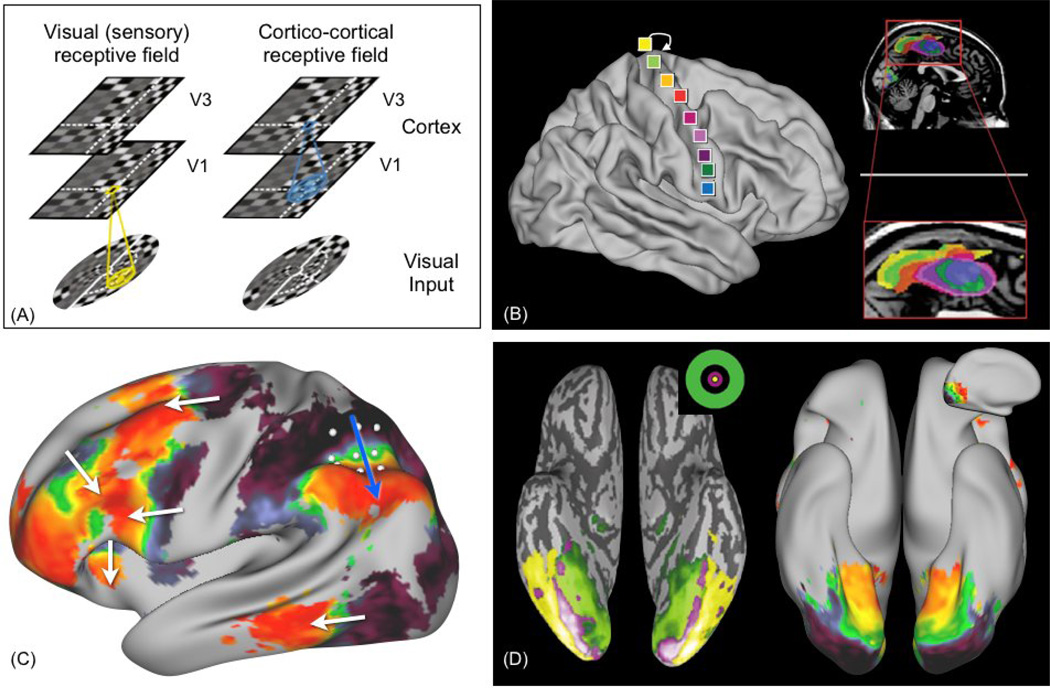
A handful of neuroimaging studies have recently used this principle to measure topographic connections without a task [33–38]. In [37], Heinzle et al. used in vivo measurements of resting-state FMRI (rsFMRI) to reveal topographic projections between V1 and V3 in the visual cortex of humans. rsFMRI measures spontaneous brain activity at rest, i.e. in the absence of an explicit task. Temporal covariance of the rsFMRI signals between brain areas, or functional connectivity, is thought to reflect direct or indirect anatomical connections [39–44]. Heinzle et al. used a clever method to demonstrate that functional connectivity between V1 and V3 is topographically organised. For each location in V3, they calculated a statistical map within V1 that encodes how well V1 voxels can predict the signal in V3. These maps (one for each voxel in V3) were then aligned according to retinotopy. Their results showed that the V1 map was replicated in V3, via spontaneous fluctuations of brain function, even in the absence of a visual stimulus. They coined the term cortico-cortical receptive field (figure 2A,D), in analogy to the receptive fields of neurons in V1 that reflect retinotopy.
Figure 2.
Evidence for topographic connections from human in vivo rsFMRI experiments. (A) analogy between receptive fields in the visual cortex (retinotopy) and cortico-cortical receptive fields resulting from ordered connections between V1 and V3 (source: [37]). The cortico-cortical receptive field (blue) is defined as the spatial filter within a lower area (V1) that best predicts activity in a higher area (V3). (B) Functional connectivity between supplementary motor area (SMA) in the medial frontal lobe and the primary motor cortex reveals somatotopy in the SMA (modified from: [35]). (C) Resting state FMRI connectivity between parietal cortex (inferior parietal sulcus - IPS) and the rest of the cortex. A grid is drawn on the IPS, then partial correlation with cortex vertices is calculated, and vertices are colour-coded according to the grid point that they correlate the most with. This analysis reveals a surprising pattern: gradients are detected in several areas of prefrontal and temporal cortices, where the directions of the white arrows correspond to the direction on the original grid (blue arrow). This pattern does not hold along another orientations on the grid. (D) Qualitative comparison between eccentricity maps in the ventral temporal cortex (left, source=[72]), and functional connectivity maps from resting FMRI (right). The pattern on the right is obtained using the same data and method as in (C), by positioning the grid along the medial surface of the occipital lobe. The data shown in (C) and (D) (right-hand side) have been acquired by members of the Human Connectome Project [48].
Another recent study showed topographic connections between occipital and parietal cortex [36]. This study used a measurement that relates to structural connectivity. The authors used diffusion MRI (dMRI) tractography [45–47], a technique that measures water diffusion in the brain, and that is capable of revealing white matter fibre orientations and trajectories as the directions of least hindrance to diffusion. They observed that fibres connecting V1 to the intra-parietal sulcus were topographically organised. Their findings may explain the multiple retinotopic maps that have been observed, using task FMRI, in the parietal cortex [26].
The approach of finding topographic maps using non-task data has also been applied to the motor system. Cauda et al. [35], using rsFMRI, showed somatotopy in the medial frontal cortex by mapping functional connectivity with the primary motor cortex (figure 2B). Buckner et al. found somatotopic connections between the motor cortex and the cerebellum using rsFMRI functional connectivity [34].
The above studies demonstrate that measurement technology is already available to study topographic connections in the living brain, either using structural (dMRI) or functional (rsFMRI) measurements. Although these studies have all considered topographies that relate to sensory cortex, the same techniques can be used to measure topographic connections between higher order association cortices. Figure 2C shows an example using data from the Human Connectome Project [1, 48] (unpublished, very high quality rsFMRI data). The figure shows multiple topographic cortico-cortical connections between parietal cortex and frontal and temporal cortices using rsFMRI in humans. The role of these topographic connections between association cortices remains to be explored.
Relation to brain function
The role of topographic connections in early relay nuclei and primary sensory areas of the cortex is relatively clear (although see [49] for an epiphenomenal argument). For instance, topographic maps allow for efficient local computations by grouping together neurons that interact the most [50], thus reducing wiring costs, and dealing with allocation of processing resources. It is also thought that topographic maps arise naturally from the need to project the 3D (or more) external world onto the 2D cortical surface in an optimal fashion [51].
Some brain areas contain neural maps that receive converging inputs from different sensory modalities. These maps have been described in many brain areas, such as the superior colliculus, parietal cortex and premotor cortex, and are thought to contribute to an integrated representation of surrounding events [52]. The presence of many topographic maps between higher order association areas is somewhat more puzzling [10]. If these topographic connections are not transferring sensory maps, then what type of information do they convey, and why do they have spatial regularity?
It has been argued that cortico-cortical topographic maps may contribute to more abstract representations, and that they may be useful for implementing simple mechanisms of coding, learning and reasoning [10, 20]. Of particular interest is the idea of convergence of connection patterns, including topographies, onto a single cortical region. Neuroscientists are starting to propose models of brain function that make use of a “principle of superposition” of connection patterns, whereby superposed local connectivity patterns interact locally to encode complex computations using relatively simple spatial rules. For example, a cortical layer that contains a superposition of topographically organised sensory projections and top-down projections can lead to simple mechanisms for implementing coincidence detection [20, 53].
Predicting brain activity
One interesting use of cortico-cortical connectivity patterns is to make predictions of brain activity [54–56]. If we can predict brain activity using brain connections, this tells us not only which connections are being used to perform a given function, but also how regions may interact during a task.
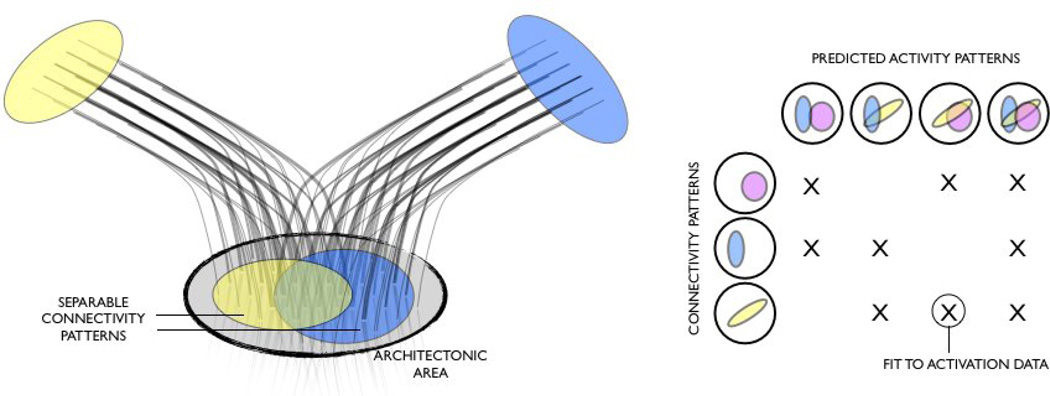
Consider the simplistic example in figure 3. A cyto-architectonic region contains distinct but overlapping connection patterns with two other regions. If a task involves all three areas, then we can hypothesise that the spatial pattern of activity in the overlap area may be a function of both connection patterns. The right hand-side figure shows the different brain activities that we can predict with different linear combinations of connection patterns in the case of 3 target areas.
Figure 3.
Illustration of an example use of connection maps to predict brain activation. Left: Cartoon illustration of two overlapping connection patterns (non-topographical) between a confined cyto-architectonic area and two targets. Right: Linear (or non-linear) combinations of spatial connectivity patterns can be used to predict the spatial pattern of activity. The weights from this model fitting can then be used to predict a new subject's activation data using the subject's own connectivity patterns.
A recent study by Saygin et al. [55, 57] tested this idea explicitly. The authors built a model that uses anatomical connection patterns (as measured with dMRI tractography) to predict brain activation measured with task FMRI. They considered an extended region in the ventral occipito-temporal cortex (OT), and calculated connections to a collection of cortical areas, summarised as maps of connection probabilities from OT locations. These maps were then used to predict brain activity in single subjects: First, the coefficients of a linear regression model were learned from training data in a group of subjects. Then the learned coefficients were used on a new subject to predict brain activity using that subject’ s own anatomical connectivity patterns. The authors used a task where participants were instructed to identify faces versus scenes. They were able to predict the shape of brain activity with a high degree of accuracy. Furthermore, each subject’s anatomy was a better predictor of its own brain activity than the average subject’s anatomical connectivity pattern.
The topographic connectome, challenges and opportunities
Neuroimaging techniques have made significant progress in the past two decades. Improvements in spatial resolution and signal-to-noise have been extremely important in driving the recent discoveries of spatial maps throughout the cortex. As we have seen, the same technologies can be used to measure connection topographies without using explicit tasks. We can use these methods to interrogate connectivity data in new ways, and switch from the reductionist view of nodes and edges, to incorporating spatial and overlapping connection patterns. These exciting new possibilities also open new challenges.
Firstly, how can we reconcile the idea of graded connection patterns with the concept of functional segregation? Are the two mutually exclusive? In recent years, neuroimaging methods have been used to segregate the brain into sub-networks or parcels using differences in connections [7, 58–67]. But was this the best way to view connectomes? Should the concept of functional segregation be applied to pieces of cortex, or should we consider the segregation of connection patterns?
Secondly, the discovery of overlapping patterns of connections invites the idea of overlapping (and interacting) systems. However, we are also immediately faced with the question of how many superposed connection patterns, including superposed topographies, are present within a given brain area? We can perhaps hypothesise that regions that perform complex computations, using converging inputs, may have more superposed connection patterns than regions in primary cortices. Methods to quantify, classify, and test for these overlapping connections remain to be developed.
Another important hurdle is the measurement techniques themselves. We can only measure anatomical connections in vivo using diffusion MRI tractography [46]. However, this technique is indirect and error-prone. While it can reveal the relative position of white matter tracts with accuracy, it is not so accurate for revealing the precise site-to-site connectivities [68], a requirement if we want to measure topographies. On the other hand, rsFMRI does not have the same spatial ambiguities, as data is recorded directly at each voxel, and need not be integrated over long distances. However, the measurement is one of function. Functional connectivity may be direct or indirect, it may also reflect a common driving input, which hinders the interpretation of functional topographies as being driven by connectivity. Furthermore, traces of topographic connectivity in the FMRI signal may be transient, and may not be easily detected using conventional time-averaged analysis techniques [69].
Conclusions
To conclude, we would like to emphasise that this opinion piece does not suggest that we should discard current approaches based on the nodes and edges concepts. Rather, we aim to propose an alternative approach that may be carried out in parallel to other approaches. We do not present new ideas either; topographies have been recognized for a long time in neuroscience. Our purpose is to encourage researchers to use the concept of spatial arrangements in systems level network models. This alternative view may allow us to not only glean more information from current connectivity data, but could also potentially tell us something new about the functioning of the brain. Additionally, we highlight the fact that current state-of-the-art data acquisitions allow us to begin to explore this new route, but exploiting this wealth of high quality data requires further advances in the sophistication of current network models.
Finally, in this article, we mainly discussed topographic connections. Some of the points we made, however, hold generally for any type of connection pattern. The key message in this article is not simply that connections follow spatial patterns, but that we should consider using these patterns in network analyses of the macro-connectome.
Highlights.
Connections in the brain are often topographically organised
Topographies have important functional consequences
Current macro-connectomic methods do not account for topographies
Modern neuroimaging technologies allow us to measure topographic connections
These advances will allow us to explore brain connectivity in novel ways in the immediate future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Of special interest:
Of outstanding interest
- 1.Van Essen DC, Ugurbil K. The future of the human connectome. Neuroimage. 2012;62(2):1299–1310. doi: 10.1016/j.neuroimage.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens TE, Sporns O. Human connectomics. Curr Opin Neurobiol. 2012;22(1):144–153. doi: 10.1016/j.conb.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988;335(6188):311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]
- 4.Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vision Res. 2011;51(7):718–737. doi: 10.1016/j.visres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. A comprehensive study of the functional organisation of the cerebral cortex in humans using rsFMRI.
- 6.Zilles K, Amunts K. Centenary of Brodmann's map--conception and fate. Nat Rev Neurosci. 2010;11(2):139–145. doi: 10.1038/nrn2776. [DOI] [PubMed] [Google Scholar]
- 7.Behrens TE, Johansen-Berg H. Relating connectional architecture to grey matter function using diffusion imaging. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):903–911. doi: 10.1098/rstb.2005.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zilles K, Palomero-Gallagher N, Schleicher A. Transmitter receptors and functional anatomy of the cerebral cortex. J Anat. 2004;205(6):417–432. doi: 10.1111/j.0021-8782.2004.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheperjans F, et al. Transmitter receptors reveal segregation of cortical areas in the human superior parietal cortex: relations to visual and somatosensory regions. Neuroimage. 2005;28(2):362–379. doi: 10.1016/j.neuroimage.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Thivierge JP, Marcus GF. The topographic brain: from neural connectivity to cognition. Trends Neurosci. 2007;30(6):251–259. doi: 10.1016/j.tins.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Udin SB, Fawcett JW. Formation of topographic maps. Annu Rev Neurosci. 1988;11:289–327. doi: 10.1146/annurev.ne.11.030188.001445. [DOI] [PubMed] [Google Scholar]
- 12.Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56(2):366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Hofer S, et al. Rhesus monkey and human share a similar topography of the corpus callosum as revealed by diffusion tensor MRI in vivo. Cereb Cortex. 2008;18(5):1079–1084. doi: 10.1093/cercor/bhm141. [DOI] [PubMed] [Google Scholar]
- 14.Wahl M, et al. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27(45):12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saenz M, Fine I. Topographic organization of V1 projections through the corpus callosum in humans. Neuroimage. 2010;52(4):1224–1229. doi: 10.1016/j.neuroimage.2010.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draganski B, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28(28):7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haber S, et al. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. Journal of Comparative Neurology. 1990;293:282–298. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- 18.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89(1):634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- 20.Tinsley CJ. Creating abstract topographic representations: implications for coding, learning and reasoning. Biosystems. 2009;96(3):251–258. doi: 10.1016/j.biosystems.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Op de Beeck HP, Haushofer J, Kanwisher NG. Interpreting fMRI data: maps, modules and dimensions. Nat Rev Neurosci. 2008;9(2):123–135. doi: 10.1038/nrn2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci. 2006;9(10):1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, et al. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333(6047):1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinds O, et al. Locating the functional and anatomical boundaries of human primary visual cortex. Neuroimage. 2009;46(4):915–922. doi: 10.1016/j.neuroimage.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphries C, Liebenthal E, Binder JR. Tonotopic organization of human auditory cortex. Neuroimage. 2010;50(3):1202–1211. doi: 10.1016/j.neuroimage.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saygin AP, Sereno MI. Retinotopy and attention in human occipital, temporal, parietal, and frontal cortex. Cereb Cortex. 2008;18(9):2158–2168. doi: 10.1093/cercor/bhm242. [DOI] [PubMed] [Google Scholar]
- 27.Arcaro MJ, et al. Visuotopic organization of macaque posterior parietal cortex: a functional magnetic resonance imaging study. J Neurosci. 2011;31(6):2064–2078. doi: 10.1523/JNEUROSCI.3334-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94(2):1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arcaro MJ, et al. Retinotopic organization of human ventral visual cortex. J Neurosci. 2009;29(34):10638–10652. doi: 10.1523/JNEUROSCI.2807-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13(11):488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagler DJ, Jr, Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29(2):567–577. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994;14(3 Pt 2):1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson JS, et al. Topographic maps of multisensory attention. Proc Natl Acad Sci U S A. 2010;107(46):20110–20114. doi: 10.1073/pnas.1011616107. A demonstration of topographic organisation of the intra-parietal sulcus connections to several resting-state networks.
- 34.Buckner RL, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cauda F, et al. Discovering the somatotopic organization of the motor areas of the medial wall using low-frequency BOLD fluctuations. Hum Brain Mapp. 2011;32(10):1566–1579. doi: 10.1002/hbm.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenberg AS, et al. Visuotopic cortical connectivity underlying attention revealed with white-matter tractography. J Neurosci. 2012;32(8):2773–2782. doi: 10.1523/JNEUROSCI.5419-11.2012. In vivo demonstration of topographic structural connections between early visual cortex and parietal cortex using diffusion MRI tractography.
- 37. Heinzle J, Kahnt T, Haynes JD. Topographically specific functional connectivity between visual field maps in the human brain. Neuroimage. 2011;56(3):1426–1436. doi: 10.1016/j.neuroimage.2011.02.077. In vivo demonstration of functional topography between V1 and V3 using resting-state FMRI.
- 38. Taren AA, Venkatraman V, Huettel SA. A parallel functional topography between medial and lateral prefrontal cortex: evidence and implications for cognitive control. J Neurosci. 2011;31(13):5026–5031. doi: 10.1523/JNEUROSCI.5762-10.2011. Resting state FMRI reveals topographic connectivity between medial and lateral prefrontal cortices.
- 39.Adachi Y, et al. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex. 2012;22(7):1586–1592. doi: 10.1093/cercor/bhr234. [DOI] [PubMed] [Google Scholar]
- 40.Greicius MD, et al. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106(6):2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? Neuroimage. 2010;52(3):766–776. doi: 10.1016/j.neuroimage.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 43.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, et al. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20(5):1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basser P, et al. In Vivo fiber tractography using DT-MRI data. MRM. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 46.Behrens TE, Jbabdi S. Diffusion MRI. San Diego: Academic Press; 2009. MR Diffusion Tractography; pp. 333–351. [Google Scholar]
- 47.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Van Essen DC, et al. The Human Connectome Project: A data acquisition perspective. Neuroimage. 2012;62(4):2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberg RJ. Are topographic maps fundamental to sensory processing? Brain Res Bull. 1997;44(2):113–116. doi: 10.1016/s0361-9230(97)00095-6. [DOI] [PubMed] [Google Scholar]
- 50.Kaas JH. Topographic maps are fundamental to sensory processing. Brain Res Bull. 1997;44(2):107–112. doi: 10.1016/s0361-9230(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 51.Graziano MS, Aflalo TN. Mapping behavioral repertoire onto the cortex. Neuron. 2007;56(2):239–251. doi: 10.1016/j.neuron.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Stein BE, Meredith MA. Multisensory integration. Neural and behavioral solutions for dealing with stimuli from different sensory modalities. Ann N Y Acad Sci. 1990;608:51–65. doi: 10.1111/j.1749-6632.1990.tb48891.x. discussion 65–70. [DOI] [PubMed] [Google Scholar]
- 53.Tinsley CJ. Using topographic networks to build a representation of consciousness. Biosystems. 2008;92(1):29–41. doi: 10.1016/j.biosystems.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Homola GA, et al. A Brain Network Processing the Age of Faces. PLoS ONE. 2012;7(11):e49451. doi: 10.1371/journal.pone.0049451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jbabdi S, Behrens TE. Specialization: the connections have it. Nat Neurosci. 2012;15(2):171–172. doi: 10.1038/nn.3031. This study is a nice demonstration that functional specialisation is intimately related to large-scale integration. The authors use structural connectivity patterns to predict the spatial pattern of functional activation in the fusiform face area. The shape of brain activity is accurately predicted by combinations of structural connectivity patterns at the level of individuals.
- 56.Jbabdi S, Homola G, Bartsch AJ. Structural connectivity between functional activations, beyond simple “blob” location. 16th Organization for Human Brain Mapping; Barcelona. 2010. [Google Scholar]
- 57.Saygin ZM, et al. Wired for function: Anatomical connectivity patterns predict face-selectivity in the fusiform gyrus. Nat Neurosci. 2012;15(2) doi: 10.1038/nn.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anwander A, et al. Connectivity-Based Parcellation of Broca's Area. Cereb Cortex. 2007;17(4):816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- 59.Behrens TE, et al. A consistent relationship between local white matter architecture and functional specialisation in medial frontal cortex. Neuroimage. 2006;30(1):220–227. doi: 10.1016/j.neuroimage.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 60.Behrens TE, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6(7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 61.Johansen-Berg H, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A. 2004;101(36):13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mars RB, et al. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci. 2011;31(11):4087–4100. doi: 10.1523/JNEUROSCI.5102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomassini V, et al. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci. 2007;27(38):10259–10269. doi: 10.1523/JNEUROSCI.2144-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mars RB, et al. Connectivity-based subdivisions of the human right "temporoparietal junction area": evidence for different areas participating in different cortical networks. Cereb Cortex. 2012;22(8):1894–1903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- 65.Bellec P, et al. Identification of large-scale networks in the brain using fMRI. Neuroimage. 2006;29(4):1231–1243. doi: 10.1016/j.neuroimage.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 66.Bellec P, et al. Multi-level bootstrap analysis of stable clusters in resting-state fMRI. Neuroimage. 2010;51(3):1126–1139. doi: 10.1016/j.neuroimage.2010.02.082. [DOI] [PubMed] [Google Scholar]
- 67. Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. Non-stationary analysis of rsFMRI data reveal overlapping "functional modes" that co-exist within the same cortical areas but appear as transient phenomena in the data.
- 68.Jbabdi S, Johansen-Berg H. Tractography: where do we go from here? Brain Connect. 2011;1(3):169–183. doi: 10.1089/brain.2011.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith SM, et al. Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci U S A. 2012;109(8):3131–3136. doi: 10.1073/pnas.1121329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ungerleider LG, et al. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18(3):477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- 71.Schall JD, et al. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15(6):4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levy I, et al. Center-periphery organization of human object areas. Nat Neurosci. 2001;4(5):533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]