Abstract
Objective
Better understanding of the temporal development of cardiovascular risk will permit more targeted prevention of premature cardiovascular mortality in schizophrenia.
Methods
The sample for this analysis was drawn from referrals (between 2006-‘11) to an early psychosis clinic based in a U.S. urban community mental health center. 76 individuals with schizophrenia who were young (mean 22.4 years, SD 4.8), early course (median duration of illness 31 weeks) and with minimal prior antipsychotic exposure (median 2 weeks) were compared to age-, gender-, and race-matched peers drawn from the National Health and Nutrition Survey (2007-’08). Measures of cardiovascular risk at baseline, 6 months, and 1 year are reported.
Results
While indistinguishable from peers at entry, patients suffered pervasive adverse trajectories of cardiovascular risk factors over the subsequent year. 16 of 44 initial non-smokers became nicotine dependent and none of 32 entering smokers quit. 17 patients transitioned to overweight (BMI 25–29.9, n=3) or obese (BMI>30, n=14) categories, while only 24 of 38 (63%) sustained normal weight over one year. Similar adverse trends in blood pressure, lipids, and fasting glucose led to an increase in prevalence of metabolic syndrome (1.31% to 5.26%). 10-year cardiovascular risk estimates showed a small and significant increase although remaining in the low risk (<10%) category.
Conclusions
The early emergence of obesity and smoking in younger schizophrenia samples provides a rational focus for primary prevention of premature cardiovascular mortality. The first year of treatment constitutes the beginning of a critical period for such preventive efforts.
Keywords: First episode psychosis, Cardiovascular risk, Cardiovascular mortality, Critical period, Early intervention, schizophrenia, knowledge translation
Introduction
Individuals with chronic psychotic disorders die on average twenty years before their peers and predominantly (>80%) of cardiovascular diseases (Brown et al. 2010). Patients with chronic schizophrenia are known to have elevated rates of smoking, hypertension, and diabetes (Goff et al. 2005). Furthermore, growing evidence implicates antipsychotic medications in worsening cardiovascular risk (Foley and Morley 2011). Finally, problems of access, application, and adherence to routine preventative and chronic illness care (Druss et al. 2008) likely contribute to these patients failing to benefit from the significant improvements in cardiovascular outcomes amongst their non mentally-ill peers. Given evidence of a widening in this mortality gap over 3 prior decades (Saha et al. 2007), patients with schizophrenia now deserve attention as a health disparities group (Chwastiak and Tek 2009).
Despite growing awareness of premature mortality, little is known about the development of cardiac risk in schizophrenia. Our previous analysis of a sample of 56 first-episode patients entering treatment at a mean age of 22.5 (4.4) years, found no elevation in 10-year cardiovascular risk estimates (less than 1%) compared to peers matched for race and gender(Phutane et al. 2011). In contrast, analysis of the largest available sample of chronic schizophrenia-spectrum patients reported significant elevations (in comparison to peers) for males (9.4% vs. 7.0%) and females (6.3% vs. 4.2%)(Goff et al. 2005). The mean age of this sample was 41.4 years (SD 10.7). This suggests that persons with schizophrenia, compared to their non-mentally ill peers, accumulate significant cardiovascular risk between the 2nd to 4th decades of life. The early course of psychotic illness may thus represent a critical period for primary prevention of cardiovascular mortality(Phutane et al. 2011).
Understanding the trajectories of specific cardiovascular risk factors over the early course of schizophrenia spectrum disorders will permit more targeted and effective prevention. We followed measures of cardiovascular risk through the first year of treatment for schizophrenia-spectrum illnesses.
Methods
Subjects
Subjects for this analysis were drawn from a trial of an early intervention service titled STEP (Specialized Treatment Early in Psychosis) (Srihari et al. 2009). The trial seeks to determine the effectiveness and costs of a package of empirically supported approaches to reduce psychotic relapse and improve functional outcomes for schizophrenia spectrum disorders. Individuals between the ages 16 and 45 years, who were in the first five years since psychosis onset, had received less than 12 weeks lifetime exposure to antipsychotic medication and were willing to travel to the outpatient EI service for care were randomized to receive either EI or referral to usual sources of community care. Subjects with co-morbid intellectual disability or clear substance-induced psychosis were excluded from trial participation, but those with diagnostic uncertainty with respect to affective, substance-induced, or medical etiologies were enrolled until this was clarified over longitudinal follow-up. Subjects for this analysis, drawn from both treatment arms, included only those who had a primary non-affective psychotic disorder confirmed at one-year follow-up. Recruitment occurred primarily by referral from local psychiatric hospital units and emergency rooms to the Connecticut Mental Health Center (CMHC), where the EI service is located. CMHC is an urban, state-owned community mental health center that serves uninsured and publicly insured individuals although the EI service also admits individuals with private insurance.
Study Design
We first conducted a cross-sectional analysis of baseline data from 76 subjects enrolled in the trial between April 2006 and April 2011. This replicated, with an enlarged sample, a previous analysis(Phutane et al. 2011) that compared our subjects to an age-, race-, and gender-matched sample from the U.S. National Health and Nutrition Examination Survey (NHANES) (2007–2008) database. NHANES is a probability sample of a civilian, non-institutionalized U.S. population and was designed to assess the nutrition and health status of children and adults in the United States. The strength of this survey is that it combines data from detailed interviews and physical and laboratory examinations. Along with medical morbidities, it also screens for the presence of anxiety, depression, eating disorders, and panic disorders. Although the survey does not specifically screen out psychotic disorders, it does query for the use of any psychotropic medications. The controls for this analysis denied having any of these psychiatric disorders or receiving psychotropic prescriptions.
Second, we compared established cardiovascular risk factors at baseline, 6 months, and 1 year to determine trajectories over the first year of follow-up. 10-year cardiovascular risk was also calculated for each subject. There are two commonly used methods to estimate 10-year risk of developing coronary heart disease. The Framingham coronary heart disease risk score estimates the risk for persons of age 30 years and above(Wilson et al. 1998); while a tool developed by National Cholesterol Education Program estimates the risk for age 20 and above. We used the latter because most of our patients were between the ages of 17 and 30 years. The NCEP-ATP III tool uses age, gender, total-and HDL-cholesterol, smoking status, systolic blood pressure and status of antihypertensive medications for the estimation of 10-year risk of developing coronary heart disease (myocardial infarction and coronary death). Risks, based on the final score, have been previously characterized as: ‘very high’ (20% or more), ‘moderate’ (10–19%) or ‘low’ (below 10%)(Grundy et al. 2004).
Of 129 total enrollees to STEP in this period, 53 were excluded from this analysis, either because relevant cardiovascular measures were not yet available at the 6 month and 1 year time points (n=36), or because they were diagnosed with non-schizophrenia spectrum disorders by 6 months follow-up (n=17). The 36 excluded patients with schizophrenia did not differ from those included with respect to demographic and clinical variables.
Assessments
Socio-demographic data was collected with a semi-structured questionnaire. The Structured Clinical Interview for DSM-IV Axis-I disorders (SCID) was used for the assessment of the diagnosis at baseline and at one-year follow-up(First et al. 1995). Nicotine use was assessed with the Alcohol/ Drug Use scale(Drake et al. 1996) and patients were classified as smokers if they had used more than 5 cigarettes in the previous week(Goff et al. 2005). A structured medical history included questions about previous diagnoses of hypertension or diabetes, other medical illnesses, and current medications. Physical examination including vital signs and laboratory evaluation was used to screen for common medical illnesses. Patients were asked to return, when necessary, for fasting blood draws (8 hours post-prandial). Patients were categorized as having diabetes in NHANES according to Expert committee guidelines published by American Diabetes Association in 2003 criteria (Anonymous 2004) with one caveat detailed below. These criteria included symptomatic hyperglycemia with a random plasma glucose level of ≥200 mg/ dL, or a previous diagnosis confirmed by current prescription of oral hypoglycemics or insulin. Additional ADA criteria includes the use of fasting plasma glucose levels (FPG≥126 mg/ dL) or an oral glucose tolerance test (2 hour post load glucose of ≥200 mg/ dL) which have to be confirmed by repeat testing on a different day, but in NHANES this repeat measurement was not required to classify patients as diabetic. This classification is thus referred to as NHANES-defined diabetes in Table 1. The full ADA 2003 criteria were used for follow-up assessments with the STEP cohort (Table 2). Patients were categorized as having impaired fasting glucose for FPG between 100 and 125 mg/ dL. Additions to these criteria in 2012 include values for glycosylated hemoglobin proposed as both a cutoff for the diagnosis of diabetes (HbA1c ≥ 6.5%) and a range for elevated risk (5.7–6.4%)(American Diabetes Association 2012). While HbA1c criteria are acknowledged to be less sensitive than traditional glucose measures, they are easier to obtain and are intended to expand access to screening. We collected HbA1c levels every six months in our early psychosis sample, but did not use these to revise categorizations based on glucose measurements. Hypertension was defined as mean systolic blood pressure of 140 mm of Hg or greater, mean diastolic blood pressure of 90 mm of Hg or greater or both, or a previous prescription for antihypertensive medications. Unlike standard clinical criteria (Chobanian et al. 2003a) repeat confirmatory measurements within 2 months were not required in NHANES but were used in follow-up assessments of our sample. Weight was measured using a standard calibrated floor scale without requiring the subject to undress (as in the NHANES protocol) and classified with standard Body Mass Index or BMI criteria: Underweight (BMI < 18.5); Normal (BMI 18.5–24.9); Overweight (BMI 25–29.9) and Obese (BMI>30) (Centers for Disease Control and Prevention (CDC) 2011). We also transformed the BMI of patients to waist circumference estimates in order to use the most common criteria to calculate the prevalence of metabolic syndrome (De Hert et al. 2008)
Table 1.
Baseline comparison of cardiovascular risk
| Variables | STEP Patients (n=76) |
NHANES Controls (n=156) |
|---|---|---|
| Smokers3 | 32 (42%) | 51 (33%) |
| Body Mass Index (kg/m2)2 | 25.0 (4.0) | 26.1 (5.2) |
| Systolic blood pressure, mm of Hg 1, 2 | 122.5 (10.4) | 115.8 (9.9) |
| Diastolic blood pressure, mm of Hg 1, 2 | 72.3 (10.3) | 66.0 (9.4) |
| Fasting serum glucose2 | 88.8 (7.7) | 91.2 (9.1) |
| NHANES-defined Diabetes3 | 0 (0%) | 1 (0.6%) |
| Impaired fasting glucose (FPG: 100–125 mg /dL)3 | 7 (9%) | 16 (10%) |
| Total Cholesterol, mg /dL2 | 169.8 (25.4) | 171.7 (32.0) |
| HDL Cholesterol, mg /dL2 | 50.9 (10.7) | 51.9 (13.2) |
| LDL Cholesterol, mg /dL2 | 91.8 (10.2) | 96.2 (16.3) |
| Triglyceride, mg /dL2 | 89.5 (16.7) | 92.9 (20.2) |
| HbA1C | 5.46 (0.3) | 5.25 (0.6) |
| Prevalence of Metabolic Syndrome, n(%) | 1 (1.31%) | 2 (1.28%) |
| 10-year risk for developing coronary heart disease, % Mean (SD) Median (Range) |
0.70 (1.02) 0 (0–5) |
0.74 (1.03) 0 (0–5) |
p<0.05,
mean (SD),
n (%)
Table 2.
Trajectory of cardiovascular risk in early psychosis
| Baseline (n=76) |
6-months (n=76) |
1-year (n=76) |
|
|---|---|---|---|
| Smokers, n (%) 1 | 32 (42%) | 38 (50%) | 48 (63%) |
| Weight (kg) 1,2 | 78.3 (12.1) | 83.4 (13.8) | 86.5 (14.6) |
| Body Mass Index (kg/m2) 1,2 | 25.0 (4.0) | 26.6 (4.8) | 27.2 (4.8) |
| Weight Classification Underweight (BMI < 18.5) Normal (BMI 18.5–24.9) Overweight (BMI 25–29.9) Obese (BMI>30) |
2 39 29 6 |
1 31 30 14 |
0 24 32 20 |
| Systolic blood pressure, mm of Hg 2 | 122.5(10.4) | 123.4 (9.7) | 124.3 (9.8) |
| Diastolic blood pressure, mm of Hg 1,2 | 72.2 (10.3) | 72.8 (8.6) | 74.6 (8.7) |
| Fasting serum glucose 1,2 | 88.8 (7.7) | 89.7 (6.8) | 91.1 (6.9) |
| Diabetes (by standard ADA def) | 0 | 0 | 0 |
| Impaired fasting glucose 1 | 7 (9.2%) | 9 (11.8%) | 12 (15.8%) |
| HbA1C 1,2 | 5.46 (0.3) | 5.50 (0.3) | 5.62 (0.3) |
| Total Cholesterol 1,2 | 169.8(25.4) | 178.5 (19.6) | 185.9(22.5) |
| HDL Cholesterol 1,2 | 50.6 (10.7) | 47.9 (9.1) | 45.7 (7.8) |
| LDL Cholesterol 1,2 | 91.8 (10.2) | 99.8 (14.1) | 110.6(15.8) |
| Triglycerides level 1,2 | 89.5 (16.6) | 107.2 (17.2) | 126.2(23.6) |
| Prevalence of metabolic syndrome, n (%) 1 | 1 (1.31%) | 3 (3.95%) | 4 (5.26%) |
| 10-year cardiovascular risk, % Mean (SD) 1 Median (range) |
0.70 (1.02) 0 (0–5) |
0.92 (1.22) 1 (0–6) |
1.22 (1.67) 1 (0–10) |
p<0.05,
mean (SD)
Statistical Analyses
Computation was done by SPSS 17.0 software for windows (SPSS Inc., Chicago, IL). Independent samples' t –test were used for continuous variables and chi-square test were used for categorical variables to compare demographic variables and cardiovascular risk factors between the STEP and NHANES groups. We used the Wilcoxon rank-sum test to compare 10-year risk of developing coronary heart disease in the two groups. For analyzing changes in cardiovascular risk over time, we used repeated measures analysis of variance. A sensitivity analysis excluding all females was conducted to query if the findings were robust to the gender imbalance in the study sample.
Results
Description of Sample
The sample for this analysis includes young (baseline mean of 22.4 years, SD 4.8), predominantly male (68 of 76 or 89.5%) early psychosis patients who one year after entry were confirmed to have a non-affective, primary psychotic disorder, including: schizophrenia (46%); schizoaffective disorder (17%); and psychotic disorder not otherwise specified (37%). The sample was ethnically and racially diverse with 34 (44.2 %) African-Americans, 29 (38.2%) Caucasians, 12 (15.8%) Hispanics, and 1 (1.3%) Asian. Most were prescribed risperidone (n= 27), several were not on any medications (n=16), and others were on olanzapine (n=8), perphenazine (n=8), haloperidol (n= 7), aripiprazole (n=4), ziprasidone (n=4), and fluphenazine (n=2). The median duration of illness was 31 weeks, with a range from <1 week to 101 weeks. The median duration of treatment with antipsychotic medication was 2 weeks, with a range from 0 to 6 weeks. No patients were prescribed medications for diabetes, hypertension, or hyperlipidemia (including propranolol for akathisia).
Baseline comparison of cardiovascular risk
All available subjects from the NHANES database who matched our patients for race, gender, and age (n=156) served as the control sample (‘NHANES’) for the baseline analysis depicted in Table 1.
The prevalence of smoking at treatment entry was high among STEP patients (42%) but statistically indistinguishable from their peers (33%). Elevations in mean diastolic and systolic blood pressure amongst STEP participants were small and none of the patients met current criteria for hypertension (Chobanian et al. 2003b). There was no significant difference in weight, body mass index, blood pressure, or total- or HDL-cholesterol. The two groups were not different with respect to the prevalence of diabetes (0% of STEP patients vs. 0.6% of controls), impaired fasting glucose (9% of STEP patients vs. 10% of controls), or mean [SD] glycosylated hemoglobin (HbA1C) levels (5.46% [0.3] vs. 5.2% [0.6]).
We detected no elevations in the prevalence of metabolic syndrome (1.31% vs. 1.28%) or 10-year risk of developing coronary heart disease (0.70% vs. 0.74%) at treatment entry compared to non-mentally ill peers. Both groups were well within the ‘low risk’ (below 10%) category. The sample was too small to allow meaningful gender comparisons across any of the measured cardiovascular risk factors.
Trajectory of cardiovascular risk over first year
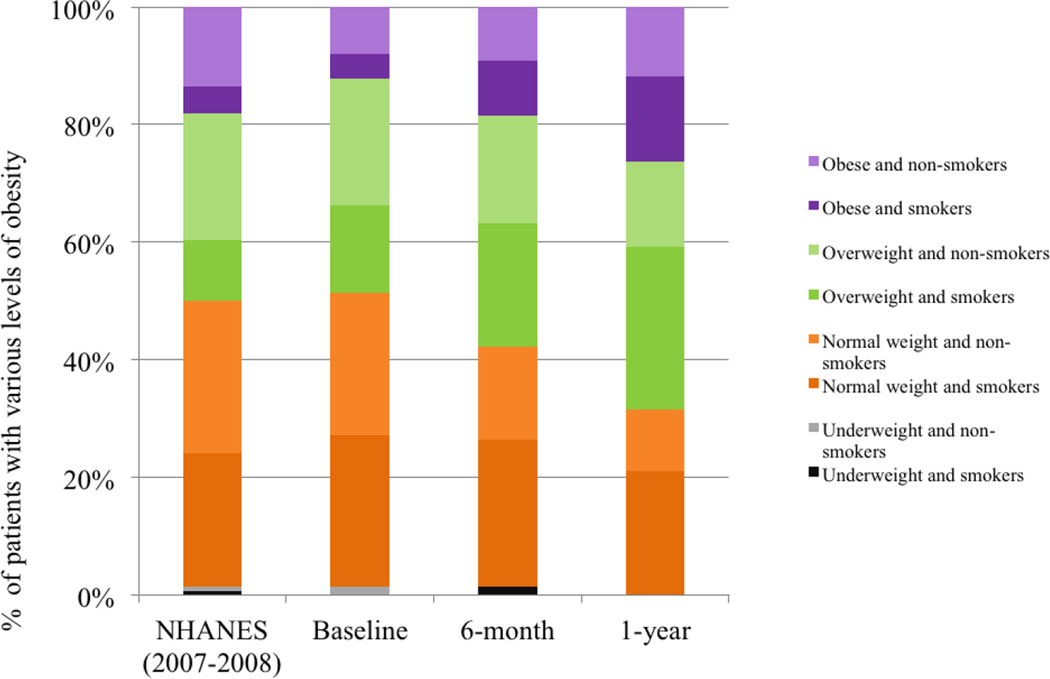
There was evidence of a consistent trajectory of worsening cardiovascular risk across all domains. During their first year in treatment, 16 of 44 initial non-smokers had become nicotine dependent with none of the 32 initial smokers having successfully quit. Similarly for weight, in the background of generally increasing BMI, 17 patients transitioned to the overweight (BMI 25–29.9, n=3) or obese (BMI>30, n=14) categories, while only 24 of the 38 (63%) initially normal weight individuals were able to retain this status by one year.
The prevalence of impaired fasting glucose by single measurement increased over the year, but none of the participants met ADA criteria for diabetes. Trends in blood pressure were analogous, with a statistically significant worsening in mean systolic and diastolic measures over time, but none of the participants met standard criteria for hypertension when single elevations were followed-up with repeat blood pressure measurement. Lipid measurements also showed adverse trends with small increases in LDL and Triglyceride levels and declines in HDL cholesterol.
These trends in individual risk factors were reflected in the prevalence of metabolic syndrome, which increased from 1.31% to 5.26% over a year. Overall, estimates of 10-year cardiovascular risk showed a small but significant trend upwards although the sample average remained in the low risk (<10%) range.
Discussion
The major finding of this analysis is that, in patients with schizophrenia spectrum disorders, there is a significant worsening in the prevalence of smoking and obesity relatively soon after the onset of psychotic symptoms. Additionally, these two risk factors accumulate in a significant subgroup of patients in their first year of treatment. This occurs in a background of worsening trends in all other established cardiovascular risk factors. We also replicated our previous finding (17) with a larger and partially overlapping sample i.e. upon entry into treatment, patients with psychosis are not significantly different from their peers in terms of overall cardiovascular risk, despite having an alarmingly high prevalence of smoking (42%), overweight or obesity (BMI≥25, 46%), and impaired fasting glucose (9%).
This study adds two principal elements to the growing understanding of elevated cardiovascular mortality in schizophrenia. First, it traces the early development of cardiovascular risk. The emergence of 2 specific risk factors – obesity and smoking – that escalate early in the illness course provide a rational focus for prevention in this population. Second, the use of a risk calculator models one way to personalize preventive efforts: while the increase in mean 10-year risk for the group was small in magnitude, the wide range of individual risk estimates with a peak of 10% suggests that some individuals will transition out of the lower risk category very early after the onset of a psychotic illness. Given multiple goals that compete for attention in the care of the seriously mentally ill, consistent clinical use of such risk estimating tools can help direct resources towards patients at highest risk for poor cardiovascular outcomes.
A limitation of this analysis was the use of a convenience or non - probability sample i.e. patients who were referred to an early intervention service, that may not represent the community burden of cardiovascular risk in early schizophrenia. While our sample was indistinguishable from an epidemiologic sample of peers in terms of baseline cardiovascular risk, we cannot rule out the possibility that our primarily urban, male, and minority population suffers a more adverse trajectory than the modal young patient with schizophrenia. On the other hand, 72% of this sample received treatment in a center with demonstrated success monitoring and lifestyle interventions for cardiovascular risk (Srihari et al. 2007;Ratliff et al. 2012). The alarming increase in rates of smoking and obesity might thus underestimate the emergence of cardiovascular risk in average community settings.
While our sample was appropriately young and ethnically diverse, there was an over-representation of males (89.5%). Repeating the analysis after excluding the females did not change alter any of the statistically significant trends in Table 2, with males having an overall higher risk. Notably smoking prevalence amongst males increased from 44% to 66% while the 10-year risk estimate increased from 0.95% to 2.2%. The small number of females precludes determination of potentially important gender-based differences in risk, but the overall findings are robust to the gender imbalance in this sample.
Some important predictors of cardiovascular risk were not measured because of feasibility issues (waist circumference and waist-to-hip ratio which are more reliable measures of abdominal obesity, insulin levels, and glucose tolerance tests) or comparisons could not be conducted because such measures were not collected in the NHANES comparison group (family history of coronary heart disease, true diagnoses of diabetes). Nevertheless, all risk factors used in established prediction tools were reliably determined for our patients.
Other possible threats to our overall inference of worsening cardiovascular trajectories in early psychosis are worth noting. We were able to report 6 month and 1-year outcomes on 76 of 123 (62%) eligible patients, and although patients lost to follow up assessment were not distinguishable at baseline, we cannot exclude attrition bias. Specifically, patients who could not be engaged in paid follow-up assessments might be expected to have worse cardiovascular parameters as a result of poorer monitoring of risk. On the other hand, they may be more likely to be non-adherent to medication treatment, and spared of the weight enhancing effects of antipsychotic medication, might enjoy a better cardiovascular risk trajectory over the first year. It is important to acknowledge that, to date, no cardiovascular risk engine has been validated in a population with serious mental illness. Given that patients with schizophrenia have a higher prevalence of risk factors that are not included in these risk calculators, such as sedentary lifestyles, poverty and poor access to adequate preventative healthcare, the tool used in this study likely underestimates their true risk.
Also, no annual follow-up data were available from NHANES or equivalent national epidemiologic samples to use as a control for our longitudinal outcomes in this early p sychosis sample. This gap in our public health database prohibits any inference that these patients accumulate more cardiovascular risk than their peers. However, the well-reported adverse cardiac profiles and outcomes for older schizophrenia populations makes this report of significant worsening in risk factors one year after psychosis onset salient. Future studies that can tie these worsening cardiac profiles to specific lifestyle (e.g. exercise or fitness) or treatment factors will allow for more targeted prevention.
The role of various antipsychotic medications in worsening cardiovascular risk has gathered justified attention with evidence of their adverse effects on weight, glucose regulation and lipid profiles. In addition, these medications are prescribed to individuals with a higher prevalence of sedentary lifestyles, poor diet and limited access to general medical care(Goldberg et al. 2007). Antipsychotic medications thus likely constitute one of several multi-component ‘sufficient causes’ of increased cardiovascular mortality(Rothman et al. 1988). There are measurable differences amongst medications that can guide meaningful choices within a framework of minimizing cardiovascular risk and is discussed elsewhere (Freudenreich 2012). The pragmatic design of the study from which this sample was drawn precludes inferences about the relative impact of the different antipsychotics used in this population (Phutane et al. 2011).
In the context of worsening cardiac profiles in the general U.S. adult population, increased attention is being paid to preventive approaches that enlarge the focus beyond traditional high-risk patients to shifting risk profiles in wider populations of concern(Lloyd-Jones 2012). The American Heart Association (AHA) introduced a new focus, in 2010, on ‘cardiovascular health ’ which includes 7 metrics to stratify population risk that include: smoking status, body mass index, dietary content, participation in physical activity, and levels of blood pressure, blood glucose, and total cholesterol and these have been shown to strongly correlate with cardiovascular mortality(Yang et al. 2012). Reassuringly, there is evidence that those who can maintain good metrics as young adults are more likely to retain such profiles in middle age(Liu et al. 2012). This increases the stakes for vigorous approaches to primary but also primordial prevention(Strasser T 1978) (i.e. preventing the onset of risk factors such as smoking and weight gain) for younger individuals.
Over the past 15 years ‘Early Intervention’ clinics for psychotic disorders have been established around the world (Edwards and McGorry 2002). These services are endeavoring to transform long term psychosocial outcomes in schizophrenia by delivering phase specific and comprehensive care in the early stages of illness. By focusing on a putative critical period(Birchwood et al. 1998) of 2–5 years after illness onset, such approaches have demonstrably improved the course of what was once considered a uniformly disabling set of illnesses. This study suggests that the first year of treatment constitutes an analogous critical period in the emergence of cardiovascular risk. Early Intervention services that specialize in the challenging task of engaging young patients with psychotic disorders thus face an opportunity to reduce premature cardiovascular mortality. Specifically, refining and applying measures to prevent or reduce obesity and smoking in younger populations with serious mental illnesses should become areas of focus for such services and a priority for all who care for these patients.
Figure 1.
Trajectories of weight gain in early psychosis
Acknowledgements
Role of Funding Source:
The Specialized Treatment Early in Psychosis (STEP) Program is supported by The Donaghue Foundation Grant number DF07-014 (Srihari, PI) and by the National Institute of Health (NIH), Grant number 1RC1MH088971-01 (Srihari, PI). Both The Donaghue Foundation and the NIH had no further role in the study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Dr. Tek's work on this project is supported by the National Institute of Health (NIH), Grant number 5R01 MH80048-2 / AS0002.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Srihari and Tek designed the study and wrote the protocol. Phutane, Ratliff and Ozkan extracted matching data from the NHANES database and with Srihari managed the literature searches and analyses. Srihari, Phutane, Tek, Chwastiak and Woods undertook the analyses, and Srihari wrote the first draft of the manuscript. All other authors contributed to and have approved the final manuscript.
Conflict of Interest:
All authors declare that they have no conflicts of interest.
References
- Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2004;27(90001):5S–10S. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012;35(Supplement 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br.J.Psychiatry Suppl. 1998;172(33):53–59. [PubMed] [Google Scholar]
- Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br.J.Psychiatry. 2010;196(2):116–121. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2011 http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. 2012 (December/20th)
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003a;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003b;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Chwastiak LA, Tek C. The unchanging mortality gap for people with schizophrenia. The Lancet. 2009;374(9690):590–592. doi: 10.1016/S0140-6736(09)61072-2. [DOI] [PubMed] [Google Scholar]
- Drake RE, Rosenberg SD, Mueser KT. Assessing substance use disorder in persons with severe mental illness. New Dir.Ment.Health Serv. 1996;70(70):3–17. doi: 10.1002/yd.23319960203. [DOI] [PubMed] [Google Scholar]
- Druss BG, Marcus SC, Campbell J, Cuffel B, Harnett J, Ingoglia C, Mauer B. Medical Services for Clients in Community Mental Health Centers: Results From a National Survey. Psychiatr.Serv. 2008;59(8):917–920. doi: 10.1176/ps.2008.59.8.917. [DOI] [PubMed] [Google Scholar]
- Edwards J, McGorry PD. Multi-component early intervention-models of good practice. In: Edwards J, McGorry PD, editors. Implementing Early Intervention in Psychosis. London: Martin Dunitz; 2002. p. 63. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL, (SCID-I/P Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch.Gen.Psychiatry. 2011;68(6):609–616. doi: 10.1001/archgenpsychiatry.2011.2. [DOI] [PubMed] [Google Scholar]
- Freudenreich O, McEvoy JP. Optimizing outcome with antipsychotic treatment in first-episode schizophrenia: balancing efficacy and side effects. Clin.Schizophr.Relat.Psychoses. 2012;6(3):115–121. doi: 10.3371/CSRP.6.3.3. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D'Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr.Res. 2005;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Goldberg RW, Kreyenbuhl JA, Medoff DR, Dickerson FB, Wohlheiter K, Fang LJ, Brown CH, Dixon LB. Quality of diabetes care among adults with serious mental illness. Psychiatr.Serv. 2007;58(4):536–543. doi: 10.1176/ps.2007.58.4.536. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J.Am.Coll.Cardiol. 2004;44(3):720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Liu K, Daviglus ML, Loria CM, Colangelo LA, Spring B, Moller AC, Lloyd-Jones DM. Healthy Lifestyle Through Young Adulthood and the Presence of Low Cardiovascular Disease Risk Profile in Middle Age / Clinical Perspective. Circulation. 2012;125(8):996–1004. doi: 10.1161/CIRCULATIONAHA.111.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones DM. Improving the Cardiovascular Health of the US Population. JAMA: The Journal of the American Medical Association. 2012;307(12):1314–1316. doi: 10.1001/jama.2012.361. [DOI] [PubMed] [Google Scholar]
- Phutane VH, Tek C, Chwastiak L, Ratliff JC, Ozyuksel B, Woods SW, Srihari VH. Cardiovascular risk in a first-episode psychosis sample: a 'critical period' for prevention? Schizophr.Res. 2011;127(1–3):257–261. doi: 10.1016/j.schres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff JC, Palmese LB, Reutenauer EL, Liskov E, Grilo CM, Tek C. The effect of dietary and physical activity pattern on metabolic profile in individuals with schizophrenia: a cross-sectional study. Compr.Psychiatry. 2012 doi: 10.1016/j.comppsych.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Lanes SF. Society for Epidemiologic Research . Meeting, 1988. Causal inference,. Epidemiology Resources. Chestnut Hill, MA: [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch.Gen.Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Srihari VH, Breitborde NJK, Pollard J. Public-Academic Partnerships: Early Intervention for Psychotic Disorders in a Community Mental Health Center. Psych Serv. 2009 doi: 10.1176/appi.ps.60.11.1426. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihari VH, Tek C, Chwastiak LA, Woods SW, Steiner JL. Best practices: surveillance and management of diabetes in a CMHC population. Psychiatr.Serv. 2007;58(9):1151–1153. doi: 10.1176/ps.2007.58.9.1151. [DOI] [PubMed] [Google Scholar]
- Strasser T. Reflections on cardiovascular diseases. Interdiscip Sci Rev. 1978;3(3):225. [Google Scholar]
- Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in Cardiovascular Health Metrics and Associations With All-Cause and CVD Mortality Among US Adults. JAMA: The Journal of the American Medical Association. 2012;307(12):1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]