Abstract
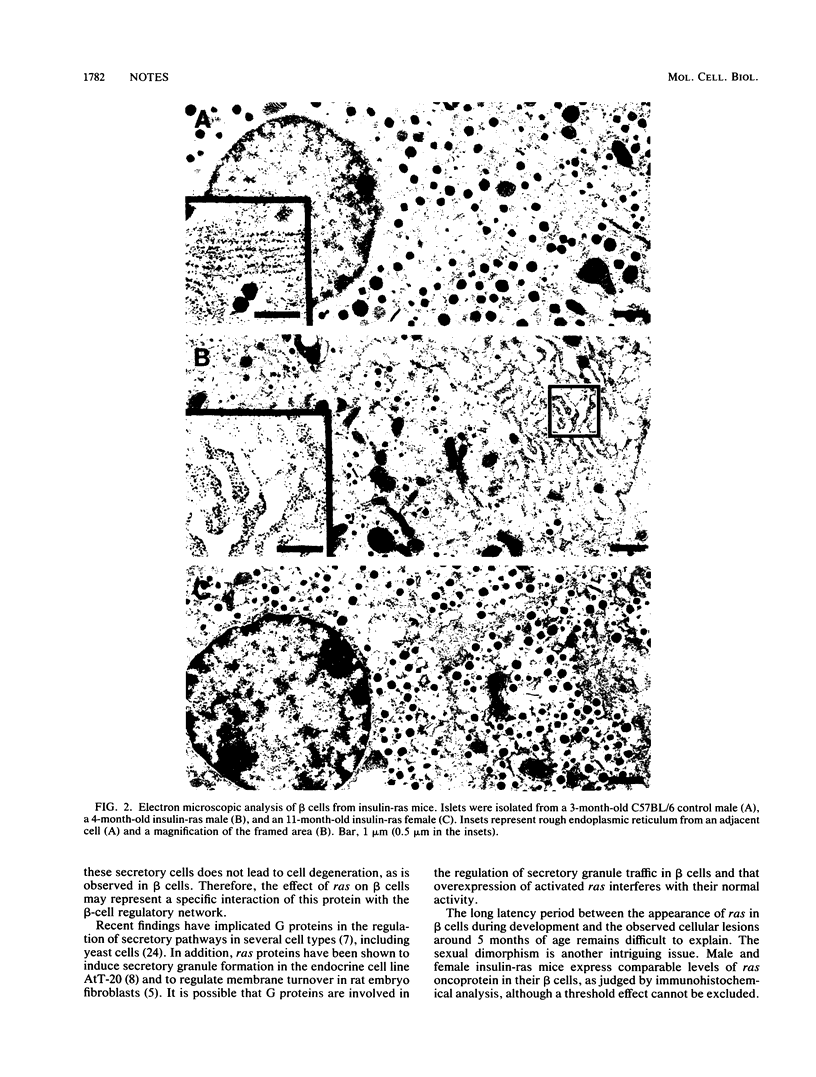
Transgenic mice expressing an insulin-promoted H-ras hybrid gene in pancreatic beta cells developed beta-cell degeneration and diabetes. The disease was manifested in male mice by hyperglycemia, glycosuria, and reduced plasma insulin levels, which appeared around 5 months of age and led to premature death. Histological analyses revealed large holes within the islets of Langerhans and a reduced number of beta cells. The destruction of the islets was not associated with an obvious inflammatory activity. Ultrastructural analysis showed extensive engorgement in the endoplasmic reticulum of the residual beta cells from diabetic males. The females carrying the insulin-promoted ras gene did not manifest any of the physiological abnormalities observed in males and showed only minor histological and ultrastructural changes, even at much greater ages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Campbell I. L., Morahan G., Mandel T. E., Harrison L. C., Miller J. F. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 1988 Jun 9;333(6173):529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Andres A. C., van der Valk M. A., Schönenberger C. A., Flückiger F., LeMeur M., Gerlinger P., Groner B. Ha-ras and c-myc oncogene expression interferes with morphological and functional differentiation of mammary epithelial cells in single and double transgenic mice. Genes Dev. 1988 Nov;2(11):1486–1495. doi: 10.1101/gad.2.11.1486. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Fernandez A., Feramisco J. R. Regulation of membrane turnover by ras proteins. Biosci Rep. 1987 May;7(5):427–434. doi: 10.1007/BF01362505. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Levinson A. D. A point mutation in the last intron responsible for increased expression and transforming activity of the c-Ha-ras oncogene. Nature. 1988 Jul 14;334(6178):119–124. doi: 10.1038/334119a0. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Rutter W. J., Hanahan D. Directed expression of NGF to pancreatic beta cells in transgenic mice leads to selective hyperinnervation of the islets. Cell. 1989 Jul 14;58(1):161–170. doi: 10.1016/0092-8674(89)90412-1. [DOI] [PubMed] [Google Scholar]
- Efrat S., Hanahan D. Bidirectional activity of the rat insulin II 5'-flanking region in transgenic mice. Mol Cell Biol. 1987 Jan;7(1):192–198. doi: 10.1128/mcb.7.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Linde S., Kofod H., Spector D., Delannoy M., Grant S., Hanahan D., Baekkeskov S. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P. N., Overbeek P. A., Means A. R. Calmodulin-induced early-onset diabetes in transgenic mice. Cell. 1989 Sep 22;58(6):1067–1073. doi: 10.1016/0092-8674(89)90505-9. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Aldrich T. H., Cordon-Cardo C. Expression of ras proto-oncogene proteins in normal human tissues. Oncogene. 1987 Mar;1(1):47–58. [PubMed] [Google Scholar]
- Gotoh M., Maki T., Kiyoizumi T., Satomi S., Monaco A. P. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985 Oct;40(4):437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Dissecting multistep tumorigenesis in transgenic mice. Annu Rev Genet. 1988;22:479–519. doi: 10.1146/annurev.ge.22.120188.002403. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. Control of spontaneous glucose intolerance, hyperinsulinemia, and islet hyperplasia in nonobese C3H.SW male mice by Y-linked locus and adrenal gland. Metabolism. 1988 Jul;37(7):689–696. doi: 10.1016/0026-0495(88)90092-3. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Le P. H., Coleman D. L. Susceptibility to db gene and streptozotocin-induced diabetes in C57BL mice: control by gender-associated, MHC-unlinked traits. Immunogenetics. 1987;26(1-2):6–13. doi: 10.1007/BF00345448. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):630–634. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Widera G., Cowing C., Flavell R. A., Palmiter R. D., Brinster R. L. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988 Apr 8;53(1):159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- Paik S. G., Michelis M. A., Kim Y. T., Shin S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes. 1982 Aug;31(8 Pt 1):724–729. doi: 10.2337/diab.31.8.724. [DOI] [PubMed] [Google Scholar]
- Quaife C. J., Pinkert C. A., Ornitz D. M., Palmiter R. D., Brinster R. L. Pancreatic neoplasia induced by ras expression in acinar cells of transgenic mice. Cell. 1987 Mar 27;48(6):1023–1034. doi: 10.1016/0092-8674(87)90710-0. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N., Liggitt D., Pitts S. L., Hansen S. E., Stewart T. A. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell. 1988 Mar 11;52(5):773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelman G., Alpert S., Hanahan D. Proliferation, senescence, and neoplastic progression of beta cells in hyperplasic pancreatic islets. Cell. 1988 Jan 15;52(1):97–105. doi: 10.1016/0092-8674(88)90534-x. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]





