Abstract
Background/Aims
The aim of this study was to determine the relationship between serum CRP levels and the prognosis of hepatocellular carcinoma (HCC) patients.
Methods
HCC patients who underwent the first session of transcatheter arterial chemoembolization (TACE) between January 2005 and December 2009 (n=211) were analyzed retrospectively. The patients were divided into two groups: high C-reactive protein (CRP; ≥1 mg/dL, n=51) and low CRP (<1 mg/dL, n=160). They were followed for a mean of 22.44 months and their clinicoradiological variables and overall survival were compared.
Results
There were significant differences between the two groups in regard to tumor type, tumor-progression-free survival, 10-month mortality, white blood cell (WBC) count, tumor size, and TNM stage. Multivariate analysis revealed that a high serum CRP level was independently associated with tumor size and tumor type. Subgroup analysis of CRP groups according to tumor size demonstrated that a high serum level of CRP was significantly associated with poorly defined (diffuse) tumor type in the tumor size <5 cm group [hazard ratio (HR)=4.81, P=0.018]. A Lipiodol dose exceeding 7 mL (HR=5.55, P=0.046) and the 10-month mortality (HR=7.693, P=0.004) were significantly associated with high serum CRP level in the group of patients with a tumor size of ≥5 cm. In addition, subgroup analysis of matched CRP according to TNM stage revealed that elevated serum CRP was independently associated with tumor type, WBC count, and tumorprogression-free survival.
Conclusions
A high serum CRP level is associated with large tumors and a poorly defined tumor type, and is significantly associated with 10-month mortality in patients with large HCC (size ≥5 cm) who undergo TACE.
Keywords: C-reactive protein, Chemoembolization, Hepatocellular carcinoma, Prognosis, Progression-free survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is the seventh most common carcinoma worldwide, and the third most common cause of cancer-related mortality.1 Recent advances in treatment modalities have improved the survival rate for patients with HCC.2,3
There have been numerous reports about the relationship between chronic inflammation and cancer. The inflammatory cells and cytokine found in tumor highly likely to contribute to tumor growth, progression, and immune-suppression compared to cope with an effective host anti-tumor response. Examples of predispositions to particular cancer due to infection and chronic inflammation include susceptibility to colon cancer due to chronic inflammatory bowel disease, gastric cancer due to helicobacter pylori, hepatocellular carcinoma due to hepatitis B and C virus, and cervical cancer due to human papilloma virus. Some examples of chemical agents that may induce both chronic inflammation and cancer include tobacco smoke, which predisposes to lung cancer, and asbestos, which predispose to mesothelioma. Persistent infection of the host induces chronic inflammation and inflammatory cells induce DNA damage in proliferating cell, by generating reactive oxygen species.4-6
C-reactive protein (CRP) is an acute-phase reactant synthesized by hepatocytes. It is regulated by pro-inflammatory cytokines such as IL-6 in responding to acute inflammation, infection, tissue damage and cancer. Also cancer cells show autocrine production of IL-6 in multiple myeloma, as well as in prostate, renal, colorectal and esophageal cancer in vivo.7-10
Recently, some studies have showed that an elevation of serum CRP level is associated with poor prognosis in patients with various malignancies, such as esophageal cancer, gastric cancer, colorectal cancer, pancreatic cancer, breast cancer and ovarian cancer.11-16 In regard to HCC, a few studies directly addressed the relationship between CRP level and disease prognosis.17-19
In this study, we evaluate the relationship between serum CRP and HCC, and investigated serum CRP as a potential prognostic serum marker in patients with HCC.
MATERIALS AND METHODS
Patients and methods
From January 2005 to December 2009, four hundred forty three patients with untreated hepatocellular carcinoma were admitted to the division of gastroenterology, at Chonnam National University Hospital, Gwangju, Korea for TACE. 232 patients were whose entire set of laboratory data was not available at the end of follow-up period and who had suspicious infection were excluded. In total, the data of 211 patients with HCC who underwent 1st TACE were finally evaluated (Fig. 1).
Figure 1.
Flow chart of the study patients.
The diagnosis of HCC was confirmed histologically or based on consistent findings obtained at least two imaging techniques: ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and/or selective hepatic arterial angiography.20
Clinical staging was determined based on the TNM classification of malignant tumor of the American Joint Committee on Cancer (AJCC).21
Serum CRP levels were measured by a turbidimetric immunoassay (Mitsubishi Chemical Company, Ltd, Japan).
Clinicoradiological variables were compared between two groups. Host related variables were; age, sex, viral status, cause of HCC, Child-Pugh score, Eastern Cooperative Oncology Group (ECOG) performance status, white blood cell counts (WBC), aspartate aminotransferase (AST), alanine aminotransferase (ALT), α-fetoprotein (AFP), 10 months mortality, 20 months mortality. Tumor related variables; maximal tumor size, numbers of tumors, TNM stage, radiological finding (poorly defined or well defined), portal vein thrombosis, CT response after 1st TACE. Subsequently, we performed a subgroup analysis to exclude the possibility of tumor size and TNM stage effect on serum CRP. Patients were divided into two groups according to tumor size (≥5 cm vs. <5 cm) (Fig. 1), and patients with high CRP group (n=39) were matched to those in the low CRP group (n=38) according to TNM stage. Then overall mortality and clinical variables between the two groups were compared. Mean survival time of intermediate and advanced stage based on BCLC system were 20 months, 10 months. Therefore, we analyzed 20 months and 10 months mortality for evaluation of prognosis.
Our institutional review board did not require approval, because the procedures were performed for clinical reasons. Informed consent was obtained from all patients after the nature and purpose of the TACE procedure had been fully explained.
Treatment and follow-up of patients
All patients were admitted before 1st TACE, blood samples were obtained from all patients before treatment. Serum AFP, CRP, blood chemistry and ECOG score at admission were measured. After 1st TACE, patients were carefully followed up. Dynamic CT was performed after 4 weeks and then every 3-6 months.
Definitions
Similarly to the Hashimoto's report,17 we defined patients with the serum CRP level ≥1 mg/dL as the high-CRP group and the patients with the serum CRP level <1 mg/dL as the low-CRP group.
Poorly defined tumor type was defined as diffuse type HCC, well defined tumor type was defined as nodular type HCC. Poor blood glucose control was defined as mean blood glucose >200 mg/dL.
Tumor progression free survival was defined as interval of time during and after treatment in which a patient remained alive but the disease did not worsen. (interval from 1st TACE to 2nd TACE). Shorter progression free survival means early recurrence of HCC after TACE.
For response assessment of TACE, all patients underwent dynamic spiral CT after 1 month following TACE. The efficacy of TACE was evaluated by comparing the CT scans obtained before and after chemoembolization in terms of the pattern of iodized oil uptake in the tumor that could be considered necrotic and the tumor extent.20 If the oily contrast medium was clearly visible and evenly dispersed throughout all viable target tumors, it was considered as a compact uptake. Otherwise, it was considered noncompact.21 Tumor response to TACE was defined as a compact uptake of iodized oil or at least a 30% decrease in the sum of longest diameters of viable tumors despite noncompact iodized oil uptake.
Statistical analysis
The correlation between serum CRP level and other categorical clinical variables was compared by Pearson's chi-square test and Independent Student's t-test. Factors were significant in the univariate analysis were entered into a stepwise multivariate analysis to find the most significant risk factors. The hazard function data were estimated using Kaplan-Meier curve and compared using log rank test, Multivariate analysis were performed to find prognostic factors using Cox proportional hazards model. A p-value of less than 0.05 was considered to be statistically significant. We performed statistical analysis using SPSS 20.0 (SPSS Inc., Chicago, USA).
RESULTS
Serum CRP and patient characteristics
Mean follow-up duration was 22.44 months in average (0-81 months). The mean age of patients was 62.47 (range 36-86) years. There were 178 men (84.2%) and 33 women (15.8%). 115 patients were positive for hepatitis B surface antigen (HBsAg), 33 patients were positive for antibody for hepatitis C virus (anti-HCV Ab), 41 patients were chronic alcohol drinker. A pretreatment elevation of the serum CRP level (≥1 mg/dL) was found in 51 patients (24.1%), whereas no such elevation (<1 mg/dL) was noticed in 160 patients (75.9%).
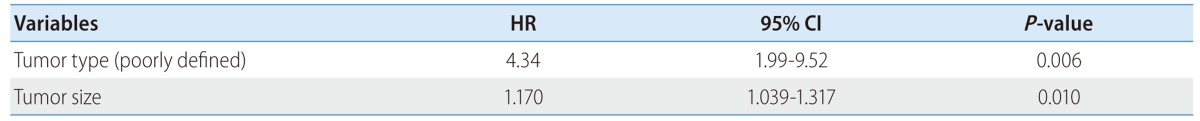
The clinical characteristics of the two groups (high CRP group vs. low CRP group) are shown in Table 1. There were no significant difference between the two groups in age, sex, cause of HCC, complication of TACE, AST, ALT, AFP, Child-Pugh score and total TACE sessions. A significant difference was found in tumor type, 10 months mortality, tumor progression free survival, WBC, tumor size and TNM stage. Patients with high CRP group had higher WBC, tumor size, TNM stage, poorly defined (diffuse) type on CT than patients with low CRP group. Among these variables, there was statistically significant correlation between the serum high CRP and poorly defined (diffuse) tumor type (HR: 4.34, P=0.006), large tumor size (HR: 1.17, P=0.01) in th multivariate analysis (Table 2).
Table 1.
Baseline clinical characteristics in each patient group
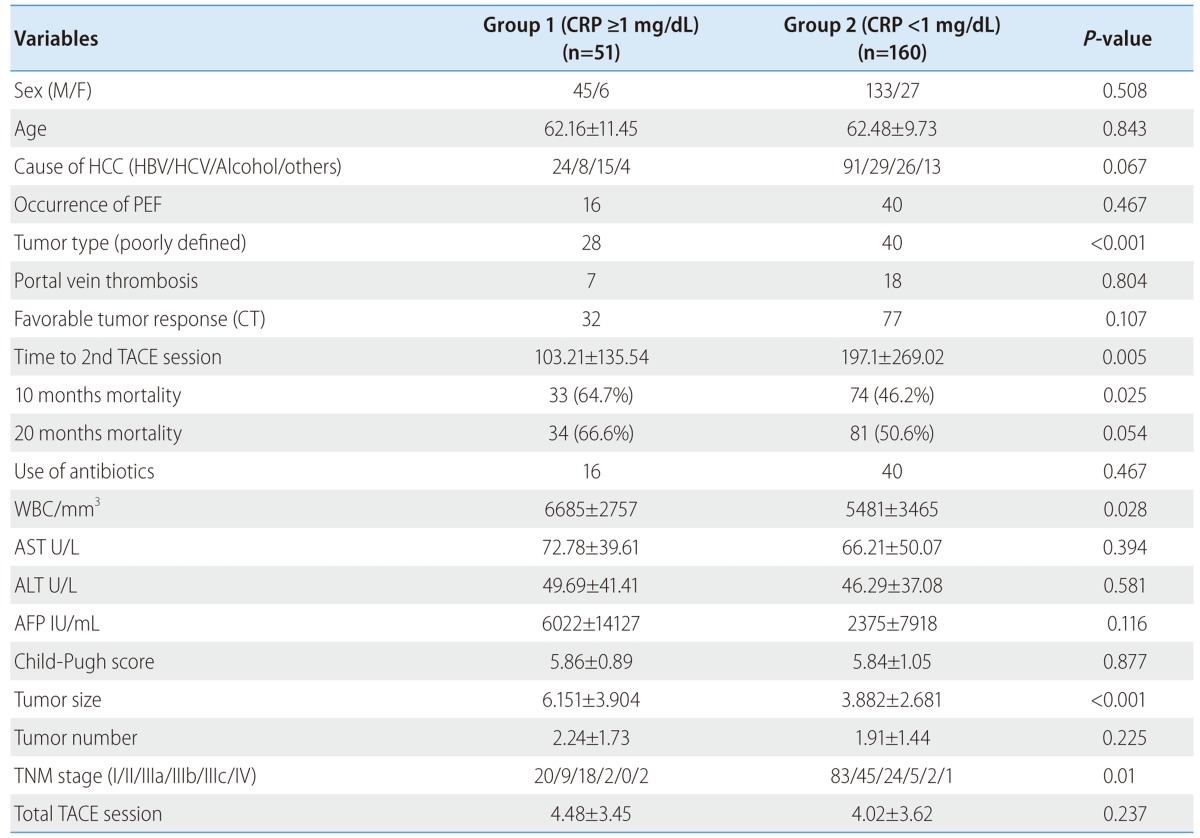
CT, computed tomography; PEF, post-embolization fever; TACE, transcatheter arterial chemoembolization.
Table 2.
Multivariate analysis of independent variables related to high CRP (≥1 mg/dL)
Serum CRP and matched patients based on tumor size
Tumor size in high serum CRP group was significantly larger than that in low CRP group. To exclude the possibility of tumor size effect on serum CRP level, so we performed subgroup analysis according to tumor size between over 5 cm (n=66) and under 5 cm (n=145) (Table 3, 4). Univariate analysis showed viral replication (HR: 4.4, P=0.031), poorly defined tumor type (HR: 2.76, P=0.036) are associated with high serum CRP in tumor size under 5 cm group. Multivariate analysis showed that high serum CRP is significantly associated with poorly defined (diffuse) tumor type in tumor size under 5 cm group (HR: 4.81, P=0.018) (Table 5), but high serum CRP was not significantly associated with 10 months, 20 months mortality, and progression free survival in tumor size under 5 cm group. Also, univariate analysis showed lipiodol dose over 7 mL (HR: 4.81, P=0.05), 10 months mortality (HR: 6.74, P=0.006), and 20 months mortality (HR: 5.43, P=0.015) are associated with high serum CRP in tumor size over 5 cm group. Multivariate analysis showed that lipiodol dose over 7 mL (HR: 5.55, P=0.046) and 10 months mortality (HR: 7.693, P=0.004) were significantly associated with high serum CRP level in tumor size over 5 cm group (Table 6).
Table 3.
Clinical characteristics in each group of the patients with a tumor size of ≥5 cm
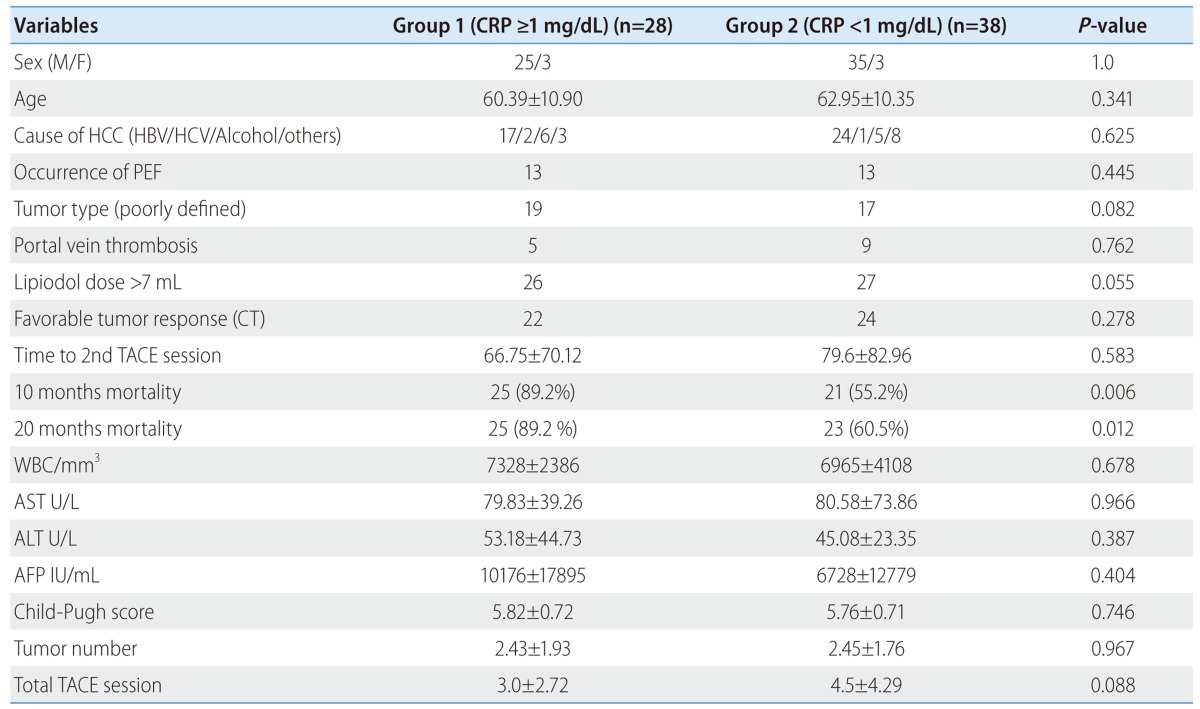
CT, computed tomography; PEF, post-embolization fever; TACE, transcatheter arterial chemoembolization.
Table 4.
Clinical characteristics in each group of the patients with a tumor size of <5 cm
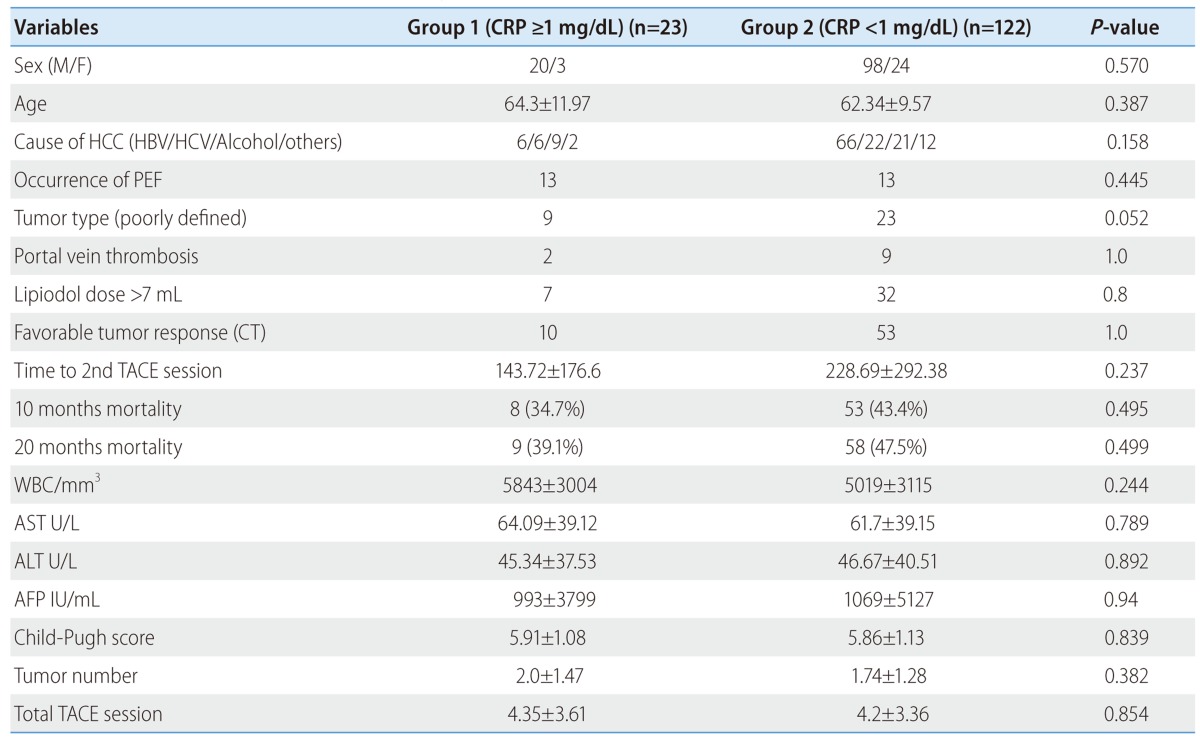
CT, computed tomography; PEF, post-embolization fever; TACE, transcatheter arterial chemoembolization.
Table 5.
Multivariate analysis of independent variables related to elevated serum CRP (≥1 mg/dL) among those with a tumor size of <5 cm

Table 6.
Multivariate analysis of independent variables related to elevated serum CRP (≥1 mg/dL) among those with a tumor size of ≥5 cm

Serum CRP and matched CRP group based on TNM stage
Subgroup analysis of matched CRP according to TNM stage, patients with high CRP group (n=39) were matched to those in the low CRP group (n=38) according to TNM stage. There were no significant differences between the two groups in age, sex, cause of HCC, complication of TACE, AST, ALT, AFP, Child-Pugh score and total TACE sessions. A significant difference was found in tumor type, 10 months and 20 months mortality, tumor progression free survival, WBC, tumor size.
On multivariate analysis, high CRP was independently associated with poorly defined tumor type (HR: 5.0, P=0.033), WBC (HR: 1.01, P=0.004) and tumor progression free survival (HR: 0.99, P=0.035).
Tumor progression free survival
The tumor progression free survival in the poorly defined tumor (diffuse) type was significantly shorter than well defined tumor type (nodular) group (P<0.001). Additionally, size (size ≥5 cm vs. size <5 cm, P<0.001), anti-viral treatment (yes vs. no, P=0.019), tumor response on CT (favorable vs. poor, P<0.001), Lipiodol dose (dose≥7 mL vs. dose <7 mL, P<0.001), use of antibiotics (yes vs. no, P=0.021) and CRP (CRP ≥1 mg/dL vs. CRP <1 mg/dL, P=0.003) were statistically significant factor in hazard function using Kaplan-Meier curve (Fig. 2). But high serum CRP was not significant associated with progression free survival on Cox proportional hazards model.
Figure 2.

HR of serum CRP level for progression-free survival (log-rank test, P=0.003)
Risk factors for 10 months and 20 months mortality
On univariate analysis for 10 months mortality, poor blood sugar control (HR: 2.41, P=0.002), high CRP (HR: 2.105, P=0.025), AFP (>400 IU/mL) (HR: 2.283, P=0.023), tumor size (≥5 cm) (HR: 3.125, P=0.001), portal vein thrombosis (HR: 8.656, P=0.03), TNM stage (≥II) (HR: 2.008, P=0.029), Lipiodol dose (≥7 mL) (HR: 1.972, P=0.017) and poor CT response after 1st TACE (HR: 2.77, P<0.001).
On multivariate analysis for 10 months mortality, viral replication (HR: 2.403, P=0.023) and poor CT response after 1st TACE (HR: 2.631, P=0.013) were independently associated with 10 months mortality.
On multivariate analysis for 20 months mortality, poor blood sugar control (HR: 2.601, P=0.021), portal vein thrombosis (HR: 8.130, P=0.013) and poor CT response after 1st TACE (HR: 3.058, P=0.006) were independently associated with 20 months mortality.
DISCUSSION
Serum CRP levels, measurement of which is relatively inexpensive and easy quantify in daily clinical practice, can be elevated in various acute and chronic benign inflammatory conditions and various malignancies. As state above, there have been many efforts to investigate the relationship between serum CRP and poor prognosis in several types of cancer.5,11-17
The mechanism by which an elevation of serum CRP level might influence survival in patients with cancer is not clear. One possible mechanism is coexisting infection or inflammation which could increase white blood cell counts (WBC), subsequently increasing pro-inflammatory cytokine CRP concentration. In the present study, we demonstrated the correlation between CRP and WBC in matched CRP group. The other possibility is that pro-inflammatory cytokine are produced by tumor necrosis or local tissue damage which is caused by tumor-host cell interaction. The presence of inflammatory factors may also promote tumor growth which, in turn, may further stimulate the systemic inflammatory response.22,23 In recently, some reports showed that high serum CRP level were also associated with several other tumor prognostic factors such as distant metastasis, tumor size, lymph node metastasis, vascular invasion and tumor recurrence.11,12,24,25
Chun et al24 reported preoperative high CRP was associated with the aggressiveness of early recurrent HCC. In consistent with Chun's report, subgroup analysis (matched CRP according to TNM stage) of our study shows that high CRP is on independent risk factor for shorter progression free survival. However, our study is distinct from Chun's report in patient selection (recurrence after surgical resection vs. recurrence after TACE).
Lin et al26 reported high serum CRP levels in patients with diffuse-type HCC and Hashimoto et al17 reported CRP as a prognostic factor and developed a scoring system including CRP for HCC patients treated by surgical resection. In consistent with other studies, subgroup analysis of matched CRP according to tumor size (≥5 cm vs. <5 cm), high CRP is independent variable related to 10 months mortality in tumor size over 5 cm group, and related to poorly defined tumor type in tumor size under 5 cm group. As far as we know, our study is the first study that high CRP is significant variable related to tumor type and 10 months mortality according to tumor size.
As for overall mortality, we found a positive trend between high serum CRP and 10 months mortality within the same stage, but this was not statistically significant. However, as above mentioned, high CRP is independent variable related to large tumor size (≥5 cm), diffuse tumor type, and it is well known that large tumor size (≥5 cm) and diffuse HCC are independent prognostic factors in patients with HCC undergoing TACE.21,27 In conclusion, these findings suggest that high CRP can be serve as an indicator of the aggressive potential of tumor and their prognosis in patients with large HCC undergoing TACE.
In summary, our study is distinct from other study in that high serum CRP is independent variable related to 10 months mortality and diffuse tumor type according to tumor size. Additionally high CRP has positive trend in early recurrence after TACE.
The limitation of our study is retrospective study, single center study and limited patients selection who underwent TACE in early and intermediate stage. Although its pivotal role in HCC tumoral proliferation has been discussed earlier, CRP is also elevated in several clinical syndromes including infectious diseases, chronic inflammatory disease. It is possible that the presence of 1 or multiple inflammatory conditions might have contaminated our data. However, patients with cancer generally have been shown to have higher CRP concentrations than healthy controls and benign disease.5 Some epidemiologic studies suggest that in patients with several types of solid cancer, elevated levels of CRP are associated with poor prognosis. Therefore, despite this potential contamination, CRP still yielded highly accurate prognostic marker of HCC.
CONCLUSION
Serum CRP is associated with 10 months mortality in large HCC over 5 cm, and associated with poorly defined tumor type in HCC under 5 cm. This suggests that high serum CRP level can be a poor prognosis marker of large HCC.
Abbreviations
- ALT
alanine aminotransferase
- AFP
α-fetoprotein
- AST
aspartate aminotransferase
- CRP
C-reactive protein
- CT
computed tomography
- ECOG
Eastern Cooperative Oncology Group
- HCC
Hepatocellular carcinoma
- MRI
magnetic resonance imaging
- PEIT
percutaneous ethanol injection therapy
- RFA
radiofrequency ablation
- TACE
transcatheter arterial chemoembolization
- US
ultrasonography
- WBC
White blood cell counts
Footnotes
The authors have no conflicts to disclose.
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61:824–833. doi: 10.1136/jech.2006.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- 8.Miki S, Iwano M, Miki Y, Yamamoto M, Tang B, Yokokawa K, et al. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989;250:607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- 9.Miki C, Konishi N, Ojima E, Hatada T, Inoue Y, Kusunoki M. C-reactive protein as a prognostic variable that reflects uncontrolled upregulation of the IL-1-IL-6 network system in colorectal carcinoma. Dig Dis Sci. 2004;49:970–976. doi: 10.1023/b:ddas.0000034556.48527.6e. [DOI] [PubMed] [Google Scholar]
- 10.Nozoe T, Korenaga D, Futatsugi M, Saeki H, Maehara Y, Sugimachi K. Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus - significance as a tumor marker. Cancer Lett. 2003;192:89–95. doi: 10.1016/s0304-3835(02)00630-4. [DOI] [PubMed] [Google Scholar]
- 11.Fujikawa K, Matsui Y, Oka H, Fukuzawa S, Takeuchi H. Serum Creactive protein level and the impact of cytoreductive surgery in patients with metastatic renal cell carcinoma. J Urol. 1999;162:1934–1937. doi: 10.1016/S0022-5347(05)68072-X. [DOI] [PubMed] [Google Scholar]
- 12.Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176:335–338. doi: 10.1016/s0002-9610(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 13.Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. doi: 10.1016/s0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 14.Rashid SA, O'Quigley J, Axon AT, Cooper EH. Plasma protein profiles and prognosis in gastric cancer. Br J Cancer. 1982;45:390–394. doi: 10.1038/bjc.1982.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno H, Okada S, Okusaka T, Ikeda M. Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Oncology. 2000;59:296–301. doi: 10.1159/000012186. [DOI] [PubMed] [Google Scholar]
- 16.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856–1864. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita A, Onoda H, Takano K, Imai N, Saeki C, Fushiya N, et al. Pretreatment serum C-reactive protein level predicts poor prognosis in patients with hepatocellular carcinoma. Med Oncol. 2012;29:2800–2808. doi: 10.1007/s12032-012-0220-1. [DOI] [PubMed] [Google Scholar]
- 19.Nagaoka S, Yoshida T, Akiyoshi J, Akiba J, Torimura T, Adachi H, et al. Serum C-reactive protein levels predict survival in hepatocellular carcinoma. Liver Int. 2007;27:1091–1097. doi: 10.1111/j.1478-3231.2007.01550.x. [DOI] [PubMed] [Google Scholar]
- 20.Takayasu K, Arii S, Matsuo N, Yoshikawa M, Ryu M, Takasaki K, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699–704. doi: 10.2214/ajr.175.3.1750699. [DOI] [PubMed] [Google Scholar]
- 21.Kanematsu T, Furuta T, Takenaka K, Matsumata T, Yoshida Y, Nishizaki T, et al. A 5-year experience of lipiodolization: selective regional chemotherapy for 200 patients with hepatocellular carcinoma. Hepatology. 1989;10:98–102. doi: 10.1002/hep.1840100119. [DOI] [PubMed] [Google Scholar]
- 22.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 23.Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264–267. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun JM, Kwon HJ, Sohn J, Kim SG, Park JY, Bae HI, et al. Prognostic factors after early recurrence in patients who underwent curative resection for hepatocellular carcinoma. J Surg Oncol. 2011;103:148–151. doi: 10.1002/jso.21786. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002;4:250–255. doi: 10.1007/s11912-002-0023-1. [DOI] [PubMed] [Google Scholar]
- 26.Lin ZY, Wang LY, Yu ML, Chen SC, Chuang WL, Hsieh MY, et al. Role of serum C-reactive protein as a marker of hepatocellular carcinoma in patients with cirrhosis. J Gastroenterol Hepatol. 2000;15:417–421. doi: 10.1046/j.1440-1746.2000.02149.x. [DOI] [PubMed] [Google Scholar]
- 27.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]



