Abstract
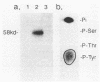
The c-fgr gene product was shown by immune complex protein kinase assay with specific antibodies to be a 58-kilodalton protein (p58c-fgr) with tyrosine-specific autophosphorylating activity. On examination of peripheral blood cells by immunoblotting with anti-c-fgr antibodies, p58c-fgr was found only in the fractions of monocytes, granulocytes, and natural killer cells. On the other hand, histochemical studies of hybridization demonstrated accumulation of c-fgr transcripts on most monocytes and large lymphocytes. In hematopoietic cell lines, p58c-fgr was detected in differentiated granulocytic cells as well as in differentiated monocytic cells of HL-60-cell origin. These data suggest a specific role for p58c-fgr in natural immunity effector cells.
Full text
PDF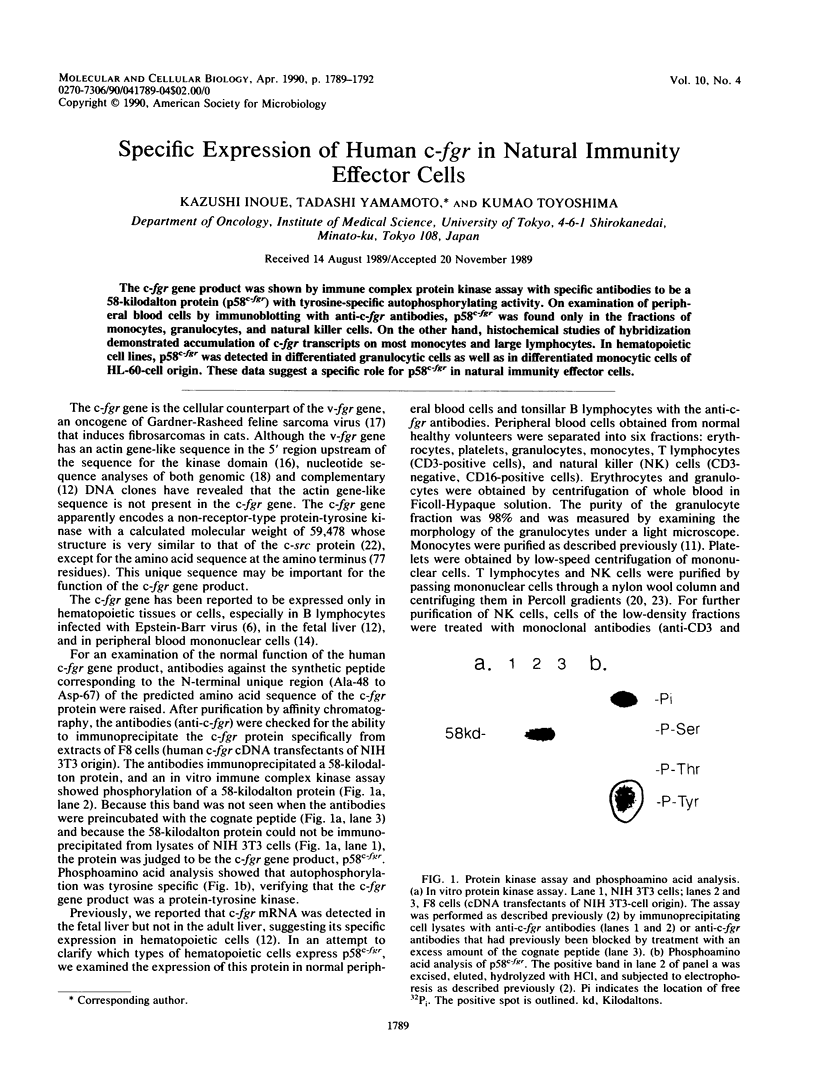


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Sudo C., Ogawara H., Toyoshima K., Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986 Jun 27;232(4758):1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- Amrein P. C., Stossel T. P. Prevention of degradation of human polymorphonuclear leukocyte proteins by diisopropylfluorophosphate. Blood. 1980 Sep;56(3):442–447. [PubMed] [Google Scholar]
- Birnboim H. C. Rapid extraction of high molecular weight RNA from cultured cells and granulocytes for Northern analysis. Nucleic Acids Res. 1988 Feb 25;16(4):1487–1497. doi: 10.1093/nar/16.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M. S., Ley T. J., Tronick S. R., Robbins K. C. fgr proto-oncogene mRNA induced in B lymphocytes by Epstein-Barr virus infection. Nature. 1986 Jan 16;319(6050):238–240. doi: 10.1038/319238a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Hercend T., Schmidt R. E. Characteristics and uses of natural killer cells. Immunol Today. 1988 Oct;9(10):291–293. doi: 10.1016/0167-5699(88)91317-5. [DOI] [PubMed] [Google Scholar]
- Inoue K., Ikawa S., Semba K., Sukegawa J., Yamamoto T., Toyoshima K. Isolation and sequencing of cDNA clones homologous to the v-fgr oncogene from a human B lymphocyte cell line, IM-9. Oncogene. 1987;1(3):301–304. [PubMed] [Google Scholar]
- Katamine S., Notario V., Rao C. D., Miki T., Cheah M. S., Tronick S. R., Robbins K. C. Primary structure of the human fgr proto-oncogene product p55c-fgr. Mol Cell Biol. 1988 Jan;8(1):259–266. doi: 10.1128/mcb.8.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley T. J., Connolly N. L., Katamine S., Cheah M. S., Senior R. M., Robbins K. C. Tissue-specific expression and developmental regulation of the human fgr proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):92–99. doi: 10.1128/mcb.9.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Naharro G., Tronick S. R., Rasheed S., Gardner M. B., Aaronson S. A., Robbins K. C. Molecular cloning of integrated Gardner-Rasheed feline sarcoma virus: genetic structure of its cell-derived sequence differs from that of other tyrosine kinase-coding onc genes. J Virol. 1983 Sep;47(3):611–619. doi: 10.1128/jvi.47.3.611-619.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Semba K., Yamamoto T., Toyoshima K. Human c-f gr gene does not contain coding sequence for actin-like protein. Jpn J Cancer Res. 1985 Mar;76(3):155–159. [PubMed] [Google Scholar]
- Notario V., Gutkind J. S., Imaizumi M., Katamine S., Robbins K. C. Expression of the fgr protooncogene product as a function of myelomonocytic cell maturation. J Cell Biol. 1989 Dec;109(6 Pt 1):3129–3136. doi: 10.1083/jcb.109.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo J. R. Regulation of natural killer activity. Cancer Metastasis Rev. 1987;6(4):637–651. doi: 10.1007/BF00047471. [DOI] [PubMed] [Google Scholar]
- Oshimi K., Oshimi Y., Satake M., Mizoguchi H. Natural killer-mediated lysis of normal and malignant target cells, and its regulation by monocytes. J Exp Med. 1985 Aug 1;162(2):472–486. doi: 10.1084/jem.162.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalde H. R. A simple method of obtaining monocytes in suspension. J Immunol Methods. 1984 Apr 13;69(1):71–77. doi: 10.1016/0022-1759(84)90278-3. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]