Abstract
Sudden Cardiac Death (SCD) is responsible for an estimated 300,000 US deaths per year. Despite sophisticated resuscitation techniques and first responder systems, survival rates are very low. This is especially true for the majority of cases where the onset is unexpected and without prior cardiac symptoms; and further underscores the importance of finding better ways of early identification of subjects at risk of SCD. Although important contributions have been added from cohort studies as well as community-based studies, more pieces of the puzzle need to be solved. The use of plasma biomarkers is a common instrument for assessing cardiovascular risk in different subsets. In this review, we weigh the evidence regarding a potential role for plasma biomarkers in predicting SCD in the general population and suggest future investigative approaches that could be of clinical utility.
Keywords: sudden cardiac arrest, sudden death, biomarker, ventricular arrhythmia, risk marker
Sudden cardiac death (SCD) accounts for the majority of cardiovascular deaths in the US, claiming an estimated 300,000 lives per year 1. Despite intense efforts to develop resuscitation techniques survival rates are still low at around 5 % 2. This has led to a renewed focus in this field directed towards optimizing risk prediction in order to identify the candidate that would qualify for prophylactic or preventive interventions 3. At present, the clinical risk factors are largely limited to the use of left ventricular ejection fraction (LVEF) for identifying candidates for primary prophylaxis, but specificity is modest 4. Moreover, the absolute numbers of these high-risk patients in the general population are low. Instead, the highest prevalence of SCD is likely observed among previously asymptomatic individuals, even though it is well known that coronary artery disease (CAD) is present in the majority of SCD cases 5. This underscores the need for the development of risk stratification models that can be applied to the general population where the largest health benefit is likely to be seen.
The underpinnings of SCD are complex and it is reasonable to believe that the risk stratification instrument of the future is likely to involve other components beyond clinical risk markers. Novel genomic variants show promise but are still in early phases of development as risk stratification targets. However, the ‘unbiased’ approaches made possible through genome-wide association studies have led to the recent identification of specific genetic variants that could predict SCD 6, 7. This has re-ignited the interest in plasma biomarkers as a means for risk stratification. This article will review the emerging evidence and role of plasma biomarkers in predicting SCD in the general population and suggest a blueprint for future studies of potential utility.
Risk markers for SCD
Uniform definition of SCD
Given the complexity and dynamic nature of the condition there has been some variability in defining SCD in the published literature 1. The need for a uniform definition of SCD is increasingly acknowledged and at a recent consensus conference 3 the condition was defined as follows: ‘A case of established SCD is an unexpected death without obvious extracardiac cause, occurring with a rapid witnessed collapse, or if unwitnessed, occurring within 1 hour after the onset of symptoms. A probable SCD is an unexpected death without obvious extracardiac cause that occurred within the previous 24 hours. In any situation, the death should not occur in the setting of a prior terminal condition, such as a malignancy that is not in remission or end-stage chronic obstructive lung disease’.
Current status of risk markers for SCD
Traditional cardiovascular risk factors such as diabetes, obesity, dyslipidemia, and hypertension have all been linked to SCD 8, 9. Furthermore, several electrocardiographic risk markers have emerged from cohorts and community-based studies more likely to reflect the general population. These include prolonged QT-interval 10 and the interval from the peak to the end of the T wave 11. At present, the LVEF is the most common clinically utilized risk predictor of SCD, with LVEF below 35% indicating the need for ICD implantation 4. However, it is now well recognized that this selection parameter is likely to have limited effectiveness. The ICD cohort studies indicate that less than a quarter of all patients implanted with an ICD based on the LVEF criterion will receive adequate therapies over an intermediate follow-up period of 3-5 years 4. Furthermore, the vast majority (>65%) of SCD patients do not have severely reduced LVEF 12, 13 and therefore cannot be risk-stratified based on this parameter. Moreover, a significant proportion of the individuals at risk will present with SCD as their first cardiac manifestation. Taken together, it is likely that previous studies of biomarkers performed in populations with ischemic heart disease or heart failure will be of limited generalizability to the general population. This is also applicable to the use of ICD-shocks as a surrogate marker for SCD as was recently pointed out in a consensus document 3. Consequently, more investigational efforts are warranted that utilize studies that reflect events in the general population. Effective risk stratification in such subjects will require the availability of tools that can be employed at an early stage in the natural history of the condition 14. Ideally, such tools should be inexpensive and cost efficient, easy to use and interpret, as well as widely available. Since plasma biomarkers could fulfill these criteria we would suggest that there is a critical need to effectively incorporate plasma biomarkers for SCD within the risk stratification paradigm.
In search of the ideal biomarker
A recent NIH consensus document defined a biomarker as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention’ 15. While this is a very broad definition, more commonly a cardiac biomarker is a substance that is measured in blood or plasma which is the definition adopted in this review.
In order to make it into clinical practice, a biomarker needs to fulfill certain criteria (Figure 1). The first requirement is evaluation and replication in prospective and/or community-wide studies 16. There is universal agreement that a biomarker should improve three different model characteristics: discrimination, classification, and calibration of risk. Discrimination would mean the ability to separate individuals with or without risk. Classification denotes the ability of a marker to move an individual to a different risk classification, thus changing the clinical course. Finally, calibration denotes how well the predicted risk of a model matches the actual observed risk.
Figure 1. Qualities of an Ideal Plasma Biomarker for SCD.
In the box the requirements for an ideal biomarker are listed. This includes clinical utility such as the ability to successfully shift individuals between different risk classes.
Given these somewhat stringent criteria in the setting of a complex and dynamic condition that can occur in the absence of warning signs mostly in the out-of-hospital setting, it is not surprising that only a limited number of biomarkers have been identified for SCD and thus far, none of these are in clinical use for risk stratification. In summary, SCD is a complex manifestation where there is a need for early identification of risk in individuals that will often be asymptomatic. Such a strategy will likely incorporate different risk markers where biomarkers are likely to contribute. Even though the task of exploring risk markers when the incidence is low as in the general population involves studies of large cohorts, an increasing number of reports are emerging from biomarkers for SCD in community-based and cohort studies (summarized in Table 1). What follows is a critical evaluation of each of these potential biomarkers as well as a discussion of the challenges and opportunities involved in moving this area of research and clinical development forward.
Table 1.
A Summary of Studies on Biomarkers and SCD.
| BIOMARKER | PUBLICATION | POPULATION | RISK | PERIOD |
|---|---|---|---|---|
| C-reactive protein | 2002 (34) | 97/192 ♂ | RR 2.65 (0.79-8.83)* | 17 |
| hsCRP | 2009 (42) | 99/294 ♀ | n.s.* | 16 |
| hsCRP | 2010 (44) | 50/100 ♂ | n.s‡ | 10 |
| NT-proBNP | 2009 (42) | 99/294 ♀ | RR 1.49 (1.09-2.05)* | 16 |
| NT-proBNP | 2011 (50) | 289/ ♂, ♀ | HR 2.5 (1.6-3.8)∥ | 16 |
| NEFA | 2001 (52) | 91/ ♂ | RR 1.70 (1.21-2.13)† | 22 |
| LCn3FA | 2002 (53) | 94/184 ♂ | RR 0.19 (0.05-0.71)* | 17 |
| TC | 1995 (33) | 106/ ♂ | RR 1.48§ | 8 |
| TC, TG, LDL, HDL | 2002 (34) | 97/192 ♂ | n.s.* | 17 |
| Homocystein | 2002 (34) | 97/192 ♂ | n.s.* | 17 |
| Magnesium | 2010 (58) | 264/ ♂, ♀ | HR 0.62 (0.42-0.93)* | 12 |
| Magnesium | 2010 (59) | 505/ ♀, | RR 0.23 (0.09-0.60)* | 26 |
| Glucose | 2005 (15) | 2040/3800 ♂, ♀ | OR 1.20 (1.12-1.28)† | 14 |
| Cystatin C | 2009 (61) | 91/ ♂, ♀ | HR 2.67 (1.33-5.35)‡ | 12 |
| Interleukin 6 | 2010 (44) | 50/100 ♂ | HR 3.06 (1.20-7.81)‡ | 10 |
| Fibrinogen | 2010 (44) | 50/100 ♂ | n.s‡ | 10 |
| Fibrinogen | 2009 (55) | 207/ ♂, ♀ | RR 2.56 (1.76-3.73)‡ | 12 |
| vWf | 2009 (55) | 207/ ♂, ♀ | RR 2.67 (1.80-3.96)‡ | 12 |
| Factor VIIIc | 2009 (55) | 207/ ♂, ♀ | RR 2.58 (1.77-3.78)‡ | 12 |
The table summarizes existing larger studies on biomarkers and risk of SCD that have reported risk variables including confidence intervals and P-values. Publication denotes year published and the number in the reference list. Population denotes number of cases/controls and gender.
Period indicates the follow-up period in years.
highest versus lowest quartile
per 1 SD increment
highest versus lowest tertile
per 1 mmol/L increase
highest versus lowest quintile
RR=relative risk, OR=odds ratio, HR=hazard ratio, n.s.=non significant, ♂=male, ♀=female, hsCRP=highly sensitive C-reactive protein, NT-proBNP= N-terminal pro B-type natriuretic peptide, NEFA= Nonesterified free fatty acids, LCn3FA= long-chain n–3 fatty acids, TC= total cholesterol, TG= triglyceride, LDL=LDL cholesterol, HDL= HDL cholesterol, vWf= von Willebrand factor
SCD biomarkers from cohort studies
Blood lipids
The link between blood lipids and CAD development is widely accepted but the association between lipids and the risk of SCD is less clear. A prospective study of nearly 8,000 British middle-aged men by Wannamethee et al 17 reported an association of cholesterol with increased risk of SCD in men both with and without preexisting ischemic heart disease. The relative risk (RR) of SCD was 3.5 for subjects without preexisting ischemic heart disease when comparing extreme quintiles (P<0.01) and 1.48 per 1 mmol/L increase of cholesterol when looking at subjects both with and without preexisting ischemic heart disease. However, the small number of SCD cases is a limitation of this study as well as the fact that the presence of preexisting ischemic heart disease was largely ascertained through self-reporting.
A prospective nested case-control study 18, analysing data from the Physicians’ Health Study, could not verify an association between plasma lipid levels (total cholesterol, triglyceride, LDL- and HDL cholesterol) and SCD. Another marker with lack of any association reported in this study was homocysteine. The study population comprised 22,000 presumably healthy middle-aged men with 97 cases of SCD occurring over the 17-year follow-up period. The authors speculated that the study was underpowered to detect small effects, emphasizing the difficulties associated with studying biomarkers in the general population through prospective cohort studies.
It might also be that plasma lipids are not adequate markers for the early stages of CAD. Instead remnant proteins could be more promising as has been suggested by Nakajima et al reporting post-mortem-data 19. The potential role of blood lipids in predicting SCD in the general population merits further investigation.
Inflammatory markers
A significant proportion of SCD cases are related to CAD with the possibility of plaque rupture playing a significant mechanistic role. The importance of inflammation in the development of the vulnerable plaque is well known 20 and consequently biomarkers reflecting increased inflammatory activity have been the focus of extensive research in CAD, but increasingly also for SCD (Figure 2). C-reactive protein (CRP), an acute phase reactant, is the inflammatory marker that has been studied the most. In particular, the analysis of highly sensitive CRP (hsCRP) has allowed for ascertainment of elevated CRP levels that are sub-clinical. Several studies have demonstrated that hsCRP has a predictive value in relation to CAD 21, 22 . However, whether or not this biomarker is useful in reclassifying an individual's risk level for CAD in a clinically significant manner, remains controversial 23.
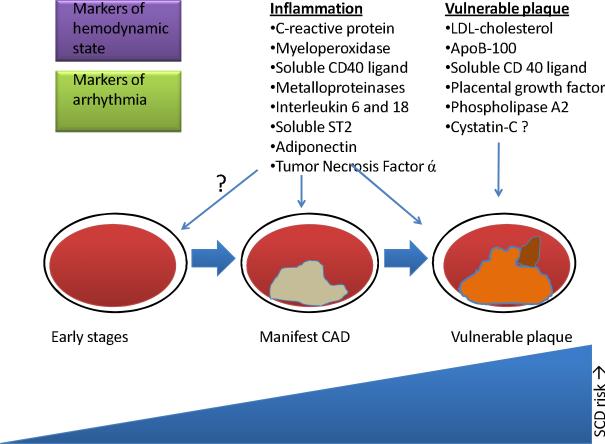
Figure 2. Promising Biomarkers Linked to CAD.
The schematic illustrates that different plasma biomarkers seem to be useful at different stages of CAD. Markers of other conditions such as hemodynamic compromise might also prove to be linked to CAD. The biomarkers show promise but require further evaluation. CAD=coronary artery disease, SCD=sudden cardiac death
In the context of SCD in the general population, analyses from the Physicians’ Health Study 18 showed that CRP levels were an independent risk factor for SCD in males after correcting for potential confounders (RR for highest versus lowest quartile 2.65, 95% confidence interval (CI) 0.79 to 8.83; P=0.03). This study was the first to evaluate CRP in association with SCD as a specific endpoint. In contrast, a prospective nested case-control study performed in a large cohort (more than 120,000 presumably, healthy women) did not show any significant correlation between SCD and hsCRP 24, (P=0.34). Again the number of SCD cases was low (n=99). As is often the case, the analysis was based on single assays conducted at baseline which is likely to constitute a limitation when studying an association with an end-point occurring several years or even decades later in time.
In 2007, Blangy et al reported an association between CRP levels and ventricular tachycardia in a group of ICD-recipients with ischemic heart disease 25. Again, one must bear in mind that this might be of limited relevance to SCD cases without established CAD and that recurrence of ventricular arrhythmia in an ICD population may not be an effective surrogate for SCA occurrence 3.
Interleukin (IL) 6 is another inflammatory marker that has been associated with CAD. To date, one large prospective investigation, the PRIME study, has investigated the association of SCD and IL 6 26. The investigators followed nearly 10,000 asymptomatic European middle-aged men over 10 years and reported that IL 6 was associated with an increased risk of SCD (adjusted hazard ratio (HR) for extreme tertiles 3.06 (95% CI 1.20-7.81, P for trend 0.02). Interestingly and in contrast, no significant associations were seen for hsCRP and SCD. This study was also limited by a low number of events.
Other biomarkers that participate in development of CAD and vulnerable plaques and also hold promise in SCD are shown in Figure 2 and include for example myeloperoxidase, metalloproteinases, and IL 18. All these require further evaluations particularly in the context of SCD and in large cohorts.
Hemodynamic markers
Natriuretic peptides are hormones secreted during cardiac hemodynamic stress. At present, this biomarker is mainly used to rule out cardiac hemodynamic compromise in a clinical setting 27. Several studies have reported the ability of B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) to predict risk of SCD as well as appropriate ICD-therapies 28, 29. Most have reported a strong association between elevated levels of these biomarkers and risk of SCD. However, the vast majority of the studies were performed in patients with established ischemic heart disease and/or heart failure. Indeed, there are only two large studies performed in presumably healthy subjects with SCD as a specific outcome 24. Korngold and co-workers reporting from the Nurses’ Health Study found that NT-proBNP is an independent risk marker for SCD in presumably healthy women (RR for 1 standard deviation (SD) increment 1.49; 95% CI, 1.09 to 2.05). This is consistent with the findings of Patton et al observed in an older study population (n=5,447) comprising men and women as well as Caucasians and African Americans 30. They reported an association between higher base-line levels of NT-proBNP and SCD over a 16-year follow-up period when comparing extreme quintiles (adjusted HR 2.5; 95% CI, 1.6 to 3.8, P <.001). The authors concluded that NT-proBNP provided information regarding the risk of SCD in a community-based population of older adults, beyond traditional risk factors but also pointed out that the specificity of elevated peptide levels for SCD compared to other causes of cardiovascular mortality is unclear.
Free Fatty Acids
Nonesterified free fatty acids (NEFA) are thought to be proarrhythmic based on their ability to modulate potassium- and calcium channels, and perhaps also due to direct toxic effects 31. Based on the association of NEFA with arrhythmias and SCD in ischemic patients, Jouven and co-workers set out to study NEFA as a risk factor in a non-ischemic population. More than 5,000 men (age 42-53) were followed within the framework of the Paris Prospective Study I for a mean of 22 years 32. NEFA were found to be an independent risk factor for SCD in this population (adjusted RR 1.70; 95% CI, 1.21 to 2.13).
In contrast to NEFA, long-chain n–3 fatty acids found in fish have been assigned cardio-protective properties. In a prospective nested case-control analysis within the Physicians’ Health Study 33, Albert et al reported that blood levels of long-chain n–3 fatty acids were inversely related to the risk of SCD in men without known cardiovascular disease. The finding remained significant after correcting for possible confounders (men with levels in the highest quartile had an 81% lower risk of sudden death compared to the lowest quartile). The mechanism of the possible antiarrhythmic properties of long-chain n–3 fatty acids are not yet fully understood but have sparked an interest for possible dietary interventions on a primary prophylactic level. Taken together, these findings are also in keeping with data from a case-control study of 95 cases of SCD nested in the Cardiovascular Health Study 34. This study from Lemaitre and co-workers performed in an elderly cohort found that elevated levels of trans-18:2 fatty acids were associated with higher risk for SCD (odds ratio 2.34; 95% CI, 1.27 to 4.31) and higher trans-18:1 with lower risk (0.18, 95% CI, 0.06 to 0.54). This is an interesting field of biomarker research that merits further validation in other cohorts.
Other biomarkers
A few studies have reported that markers of hemostasis are associated with SCD. Kucharska-Newton et al, analyzing data from the Atherosclerosis Risk in Communities cohort 35, reported that elevated levels of von Willebrand factor, factor VIIIc, and fibrinogen were all associated with SCD (adjusted RR for extreme tertiles 2.67 (1.80-3.96), 2.58 (1.77-3.78), and 2.56 (1.76-3.73) respectively). From the same study, a moderate inverse relationship between albumin and risk of SCD was also reported. In contrast, Empana et al did not find any statistically significant association between fibrinogen levels and SCD in the PRIME study 26.
Magnesium regulates membrane electrical stability of the cardiac myocyte and may have antiarrhythmic properties 36. Recently, two studies were published that showed an inverse relationship between increased magnesium levels and risk of SCD. Peacock and co-workers reported that in a middle-aged biracial population 37, individuals in the highest quartile of serum magnesium had a 40% reduced risk of SCD (HR 0.62, 95% CI 0.42-0.93) compared to the lowest quartile. A second study performed in healthy women reported that a rise of 1 SD in plasma magnesium was associated with a 41% (95% CI 15-58) lower risk of SCD 38.
Elevated plasma renin levels were recently reported to be independently and strongly associated with an increased risk for death due to heart failure, cardiovascular events, as well as SCD 39. This study was performed in older Caucasians referred for coronary angiography and has limited generalizability. Previous similar studies in smaller numbers of subjects have been inconsistent. However, the finding is interesting and merits further investigation in a larger prospective community-wide setting.
There is a growing body of evidence supporting the association between diabetes mellitus and increased risk of SCD 9, 40. This might not be surprising given the link between diabetes and CAD but large, prospective, community-wide studies are lacking. In a study by Jouven and co-workers 40, a 1 SD increase in glucose levels was associated with a slightly elevated risk for SCD (OR 1.20, 1.12-1.28, 95 % CI) after adjusting for possible confounders.
The association between renal disease and cardiovascular morbidity and mortality is well established. To date, there is one nested case-control analysis of the Cardiovascular Health Study that has identified Cystatin C (a marker of renal insufficiency) as a potential risk marker for SCD 41. In 91 cases of SCD, Deo et al found an associated risk for SCD with elevated levels of Cystatin C (HR 2.67, 1.33-5.35, 95% CI) when comparing extreme tertiles. However, the relatively high age of the study population again is a limitation. Interestingly, emerging data suggest that Cystatin C is not only a maker of renal insufficiency but also associated with inflammation and atherosclerosis 42 as well as an indicator of risk for development of CAD 43.
Challenges and future directions
There is significant potential for development of biomarkers that will facilitate early identification of asymptomatic individuals at risk of SCD in the general population. However, on an individual basis currently known candidate biomarkers will require significant additional evaluation to extend beyond the moderate efficacy mark. Most importantly, there is a need for the evaluation of large numbers of subjects especially to assess the distinct possibility that specific biomarkers may be more efficacious in prediction risk in specific subgroups of the SCD population. Another widely held hypothesis is that a multi-marker strategy (Figure 3) is more likely to be useful 3, 14. So far, these models seem to add only moderately to risk prediction 44. As ongoing evaluations of the genome and proteome yield additional results 6, 7 these are going to provide a growing number of additional candidate biomarkers. A systematic strategy will need to be employed to evaluate these potential SCD biomarkers.
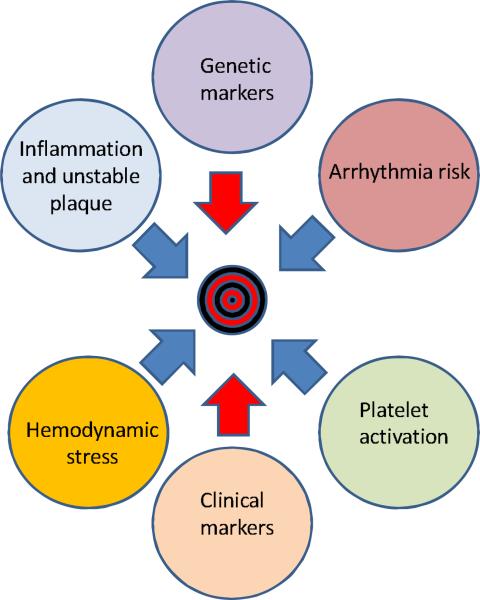
Figure 3. The Multi-marker Strategy.
A multi-marker strategy is likely to be needed in order to provide the cardiologist with a tool of adequate specificity and clinical utility. Markers to be included in a risk stratification model for SCD are likely to reflect different pathophysiological manifestations as illustrated above by the circles. Blue arrows indicate possible input from plasma biomarkers and red arrows other kinds of risk markers.
Thus far, the cohort studies that have evaluated biomarkers have significant similarities in study design with samples being drawn at baseline. However the relevance of an elevated biomarker for an event that occurred 10-16 years subsequently has been questioned 45. One could argue that these biomarkers indicate early stages of SCD-risk producing cardiac disease development. However, this may also represent a coincidental finding unrelated to disease mechanisms. Such results underscore the need for replicating findings in distinct and different populations.
Moreover, the pathophysiological processes also merit further investigation as the issue of causality is still unexplored in most cases of biomarkers. This includes evaluation of the possible temporal variations of risk characteristics. Future studies could plan to repeat measurements throughout the follow-up period in order to minimize the time between sample collection and event occurrence. In order to further assess potential competing risks as well as specificity for SCD, it would be prudent to make comparisons of candidate biomarkers in subjects that suffer non-sudden cardiac death as well as non-cardiac death.
An important challenge that remains to be overcome is that of sample size. Existing cohorts, even when combined may not be large enough to be adequately powered for biomarker evaluation. There is an existing model for prospective community-based investigation of SCD biomarkers that provides significant larger sample sizes and overcomes the difficulty of an uncommon end-point 10, 46. However this approach poses a separate set of research design challenges. Since the samples are drawn by first responders during the resuscitation process for SCA, identified biomarkers need to be evaluated for potential association with the SCA event itself, likely in animal models. Lastly, it is important to make a clear distinction between statistical significance for association a particular biomarker with SCD, and the clinical usefulness of that biomarker.
Designing the Ideal SCD Biomarker Study
What then, would be the ideal study design for identification of early risk markers for SCD? The first requirement is the availability of a sufficiently large, adequately powered sample size of a well-defined and carefully characterized SCD phenotype. Ideally, repeated clinical measures of the phenotype such as the EKG, echocardiogram etc would be made available in a large community-wide study. Repeated measurements of biomarkers would be performed in parallel with genomic evaluation, with subsequent validation of identified candidate markers in separate populations with comparisons conducted between the sexes as well as within and across multiple ethnicities.
Conclusions
There is a critical need for better methods to identify individuals in the general population at high risk of SCD. Future risk stratification models will rely on multiple predictors and one or more plasma biomarkers are likely to solve a piece of this puzzle. At present, some biomarkers have shown promise but none of these are ready for clinical utilization at the present time. Future investigative approaches should utilize a uniform definition of SCD and perform evaluations in large numbers of subjects representing the general population, with analyses performed within and across the sexes, multiple ethnicities as well as age groups.
Acknowledgments
Funding Sources: Funded in part by National Heart Lung and Blood Institute R01HL088416 and R01HL105170 to SSC. RH is supported by a grant from the Swedish Research Council. SSC is the Pauline and Harold Price Professor of Cardiac Electrophysiology at the Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA.
Footnotes
Disclosures: None
References
- 1.Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51(3):213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O'Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 122(22):2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 5.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 6.Arking DE, Junttila MJ, Goyette P, Huertas-Vazquez A, Eijgelsheim M, Blom MT, Newton-Cheh C, Reinier K, Teodorescu C, Uy-Evanado A, Carter-Monroe N, Kaikkonen KS, Kortelainen ML, Boucher G, Lagace C, Moes A, Zhao X, Kolodgie F, Rivadeneira F, Hofman A, Witteman JC, Uitterlinden AG, Marsman RF, Pazoki R, Bardai A, Koster RW, Dehghan A, Hwang SJ, Bhatnagar P, Post W, Hilton G, Prineas RJ, Li M, Kottgen A, Ehret G, Boerwinkle E, Coresh J, Kao WH, Psaty BM, Tomaselli GF, Sotoodehnia N, Siscovick DS, Burke GL, Marban E, Spooner PM, Cupples LA, Jui J, Gunson K, Kesaniemi YA, Wilde AA, Tardif JC, O'Donnell CJ, Bezzina CR, Virmani R, Stricker BH, Tan HL, Albert CM, Chakravarti A, Rioux JD, Huikuri HV, Chugh SS. Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals. PLoS Genet. 7(6):e1002158. doi: 10.1371/journal.pgen.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westaway SK, Reinier K, Huertas-Vazquez A, Evanado A, Teodorescu C, Navarro J, Sinner MF, Gunson K, Jui J, Spooner P, Kaab S, Chugh SS. Common Variants in CASQ2, GPD1L and NOS1AP Are Significantly Associated with Risk of Sudden Death in Patients with Coronary Artery Disease. Circ Cardiovasc Genet. 2011;4(4):397–402. doi: 10.1161/CIRCGENETICS.111.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkau B, Jouven X, Ducimetiere P, Eschwege E. Diabetes as a risk factor for sudden death. Lancet. 1999;354(9194):1968–1969. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 9.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99(15):1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 10.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119(5):663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged Tpeak to Tend Interval on the Resting Electrocardiogram Is Associated with Increased Risk of Sudden Cardiac Death. Circ Arrhythm Electrophysiol. 2011;4(4):441–7. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 13.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24(13):1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 14.Chugh SS. Early identification of risk factors for sudden cardiac death. Nat Rev Cardiol. 7(6):318–326. doi: 10.1038/nrcardio.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 16.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 17.Wannamethee G, Shaper AG, Macfarlane PW, Walker M. Risk factors for sudden cardiac death in middle-aged British men. Circulation. 1995;91(6):1749–1756. doi: 10.1161/01.cir.91.6.1749. [DOI] [PubMed] [Google Scholar]
- 18.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105(22):2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima K, Nakajima Y, Takeichi S, Fujita MQ. ApoB-100 carrying lipoprotein, but not apoB-48, is the major subset of proatherogenic remnant-like lipoprotein particles detected in plasma of sudden cardiac death cases. Atherosclerosis. 2007;194(2):473–482. doi: 10.1016/j.atherosclerosis.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 20.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 30(7):1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 21.Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, Pestaner J, Smialek J, Virmani R. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105(17):2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins JT, Lloyd-Jones DM. Biomarkers for coronary heart disease clinical risk prediction: a critical appraisal. Counterpoint. Prev Cardiol. 13(4):160–165. doi: 10.1111/j.1751-7141.2010.00081.x. [DOI] [PubMed] [Google Scholar]
- 24.Korngold EC, Januzzi JL, Jr., Gantzer ML, Moorthy MV, Cook NR, Albert CM. Amino-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein as predictors of sudden cardiac death among women. Circulation. 2009;119(22):2868–2876. doi: 10.1161/CIRCULATIONAHA.108.832576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blangy H, Sadoul N, Dousset B, Radauceanu A, Fay R, Aliot E, Zannad F. Serum BNP, hs-C-reactive protein, procollagen to assess the risk of ventricular tachycardia in ICD recipients after myocardial infarction. Europace. 2007;9(9):724–729. doi: 10.1093/europace/eum102. [DOI] [PubMed] [Google Scholar]
- 26.Empana JP, Jouven X, Canoui-Poitrine F, Luc G, Tafflet M, Haas B, Arveiler D, Ferrieres J, Ruidavets JB, Montaye M, Yarnell J, Morange P, Kee F, Evans A, Amouyel P, Ducimetiere P. C-reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: the PRIME study. Arterioscler Thromb Vasc Biol. 30(10):2047–2052. doi: 10.1161/ATVBAHA.110.208785. [DOI] [PubMed] [Google Scholar]
- 27.Vaes B, de Ruijter W, Gussekloo J, Degryse J. The accuracy of plasma natriuretic peptide levels for diagnosis of cardiac dysfunction and chronic heart failure in community-dwelling elderly: a systematic review. Age Ageing. 2009;38(6):655–662. doi: 10.1093/ageing/afp157. [DOI] [PubMed] [Google Scholar]
- 28.Tapanainen JM, Lindgren KS, Makikallio TH, Vuolteenaho O, Leppaluoto J, Huikuri HV. Natriuretic peptides as predictors of non-sudden and sudden cardiac death after acute myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2004;43(5):757–763. doi: 10.1016/j.jacc.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 29.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105(20):2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 30.Patton KK, Sotoodehnia N, DeFilippi C, Siscovick DS, Gottdiener JS, Kronmal RA. N-terminal pro-B-type natriuretic peptide is associated with sudden cardiac death risk: the Cardiovascular Health Study. Heart Rhythm. 8(2):228–233. doi: 10.1016/j.hrthm.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet. 1994;343(8890):155–158. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 32.Jouven X, Charles MA, Desnos M, Ducimetiere P. Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation. 2001;104(7):756–761. doi: 10.1161/hc3201.094151. [DOI] [PubMed] [Google Scholar]
- 33.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346(15):1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 34.Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH, Tracy RP, Siscovick DS. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: the cardiovascular health study. Circulation. 2006;114(3):209–215. doi: 10.1161/CIRCULATIONAHA.106.620336. [DOI] [PubMed] [Google Scholar]
- 35.Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, Chakravarti A, Folsom AR, Siscovick DS, Rosamond WD. Hemostasis, inflammation, and fatal and nonfatal coronary heart disease: long-term follow-up of the atherosclerosis risk in communities (ARIC) cohort. Arterioscler Thromb Vasc Biol. 2009;29(12):2182–2190. doi: 10.1161/ATVBAHA.109.192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verduyn SC, Vos MA, van der Zande J, van der Hulst FF, Wellens HJ. Role of interventricular dispersion of repolarization in acquired torsade-de-pointes arrhythmias: reversal by magnesium. Cardiovasc Res. 1997;34(3):453–463. doi: 10.1016/s0008-6363(97)00067-9. [DOI] [PubMed] [Google Scholar]
- 37.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 160(3):464–470. doi: 10.1016/j.ahj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiuve SE, Korngold EC, Januzzi JL, Jr., Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 93(2):253–260. doi: 10.3945/ajcn.110.002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomaschitz A, Pilz S, Ritz E, Morganti A, Grammer T, Amrein K, Boehm BO, Marz W. Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J. 2011 May 23; doi: 10.1093/eurheartj/ehr150. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26(20):2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 41.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, Kestenbaum B, Psaty BM, Siscovick DS, Shlipak MG. Cystatin C and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes. 3(2):159–164. doi: 10.1161/CIRCOUTCOMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem. 2009;55(11):1932–1943. doi: 10.1373/clinchem.2009.128397. [DOI] [PubMed] [Google Scholar]
- 43.Luc G, Bard JM, Lesueur C, Arveiler D, Evans A, Amouyel P, Ferrieres J, Juhan-Vague I, Fruchart JC, Ducimetiere P. Plasma cystatin-C and development of coronary heart disease: The PRIME Study. Atherosclerosis. 2006;185(2):375–380. doi: 10.1016/j.atherosclerosis.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Wang TJ. Multiple biomarkers for predicting cardiovascular events: lessons learned. J Am Coll Cardiol. 55(19):2092–2095. doi: 10.1016/j.jacc.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Chugh SS, Reinier K. Predicting sudden death in the general population: another step, N terminal B-type natriuretic factor levels. Circulation. 2009;119(22):2863–2864. doi: 10.1161/CIRCULATIONAHA.109.865436. [DOI] [PubMed] [Google Scholar]
- 46.Teodorescu C, Reinier K, Dervan C, Uy-Evanado A, Samara M, Mariani R, Gunson K, Jui J, Chugh SS. Factors associated with pulseless electric activity versus ventricular fibrillation: the Oregon sudden unexpected death study. Circulation. 122(21):2116–2122. doi: 10.1161/CIRCULATIONAHA.110.966333. [DOI] [PubMed] [Google Scholar]