Abstract
The treatment of maternal hypothyroidism presents clinicians with a unique challenge, because dosing regimens previously developed and validated for nonpregnant women cannot be easily extrapolated to dosing in pregnancy. Thyroid hormone requirement increases by 20% to 40% early during pregnancy, persisting throughout gestation. Accordingly, women with treated hypothyroidism need to increase their levothyroxine dose to prevent maternal hypothyroidism and the associated impaired cognitive development and increased fetal mortality. We investigated the pharmacokinetic properties of levothyroxine during pregnancy through the use of a novel, traceable form of levothyroxine. The objective was to conduct a longitudinal study to determine whether levothyroxine pharmacokinetics differ in the pregnant versus nonpregnant state. We used a unique 13C-levothyroxine-tracer method to distinguish between endogenous and exogenous levothyroxine and studied the pharmacokinetics of a single oral dose of levothyroxine using tandem mass spectrometry. Moreover, we were able to detect single dose amounts of the drug, in picogram/mL concentrations. The area under the curve was 23.0 ng*h/mL in pregnancy and 14.8 ng*h/mL in nonpregnant women (P < 0.03) with median serum half-lives of 32.1 hours and 24.1 hours, respectively (P < 0.04). Further research involves the measurement of free thyroxine on these samples using tandem mass spectrometry. Future work should focus on the mechanisms responsible for the gestational differences in pharmacokinetics and whether these should necessitate dose schedule changes in pregnancy.
Keywords: levothyroxine LT4, Thyroid, gestation, clinical pharmacology, thyroid disorder/drug therapy/physiopathology/hypothyroidism
INTRODUCTION
Primary hypothyroidism is a relatively common condition with a prevalence of 3% to 10% in women aged 18 years and older. Oral levothyroxine sodium (L-thyroxine [LT4]) is commonly administered lifelong with periodic monitoring with the aim of maintaining serum thyroxine and thyrotropin (thyroid-stimulating hormone [TSH]) concentrations within the normal range. The search for an effective and optimal LT4 replacement dose has been a challenge, because dosages vary according to the individual condition of the patient, pregnancy, lean body mass, age, diet, proximity to meals, and other medications and compliance.1–3
Approximately 2% of all pregnant women receive levothyroxine therapy for hypothyroidism. The management of thyroid disease during pregnancy requires special considerations because pregnancy induces major changes in thyroid function and because maternal thyroid disease can have adverse effects on the pregnancy and on fetal development. Gestational hypothyroidism has been associated with higher rates of spontaneous abortions, pre-eclampsia, stillbirth, intrauterine growth restriction, and prematurity.4 Because maternal thyroxine is critical for fetal brain development, low thyroxine can result in subtle neurodevelopmental and neuropsychologic deficits5 and in extreme cases, in mental retardation.6 Thus, hypothyroidism in pregnant women presents clinicians with a unique challenge, because dosing regimens previously developed and validated for nonpregnant women cannot be easily extrapolated to dosing in pregnancy.
After conception, there is a dynamic equilibrium and a very rapid rise in circulating thyroxine-binding globulin concentrations, the key thyroid hormone-binding protein leading to an elevation in serum total thyroxine.7,8 Concomitantly, as a consequence of the weak thyroid-stimulating activity of chorionic gonadotropin, TSH concentrations decrease.7 In healthy, euthyroid women, thyroxine production increases by approximately 20% to 40%,1 evident as early as the fifth week of gestation, a likely important time for maternal provision of T4 for fetal neurodevelopment and for placental development and function.9 T4 requirements remain high throughout pregnancy. In women with primary hypothyroidism, there is a similar need to increase levothyroxine replacement dose very early in pregnancy10 and maintain hormone levels within trimester-specific reference intervals reflecting normal physiology.11–13
Our objective in this study was to determine the pharmacokinetics of LT4 in pregnant women using, for the first time, carbon 13-labeled levothyroxine (13C-LT4) as a tracer molecule. Thyroid hormone analysis was conducted using isotope dilution tandem mass spectrometry—a methodology independent of interference by binding proteins—providing more accurate data than the traditional immunoassays.13,14
METHODS
The research protocol was approved by the Georgetown University Institutional Review Board and written informed consent was obtained from all study participants. All women were recruited from and studies were conducted at Georgetown University Medical Center. Hypothyroid women rendered euthyroid by LT4 replacement were recruited during pregnancy to participate in the study. Target therapeutic TSH levels were maintained at approximately 1 mIU/L. In each pharmacokinetic study session, on the first day of the study, the patients were admitted to the General Clinical Research Center; they returned to conduct the postpartum part of the study within 3 to 12 months after delivery, when maternal metabolism returns to normal. In both study periods (during and after pregnancy), the patient’s own daily LT4 maintenance dose was replaced by one 13C-LT4 dose. Only the dose on Day 1 of the study was LT4 replaced by 13C-LT4; on each of the next days of the pharmacokinetic study (120 hours), the patient continued taking the daily dose of their own LT4 (not 13C-LT4).
Subjects were included in the study when meeting the following criteria: 18 years of age or older at the time of consent, euthyroid (LT4-treated hypothyroid) women with no other serious illness, able to give written informed consent, prescribed LT4 by their physicians for therapeutic reasons during a pregnancy, and anticipating continuing LT4 medication postpartum as prescribed by their physician. Subjects were excluded from the study for any of the following reasons: baseline hematocrit was lower than 28.0%, TSH greater than 4.5 mIU/L, kidney dysfunction, women taking other drugs that affect thyroidal axis interactions such as drugs that may alter TSH and thyroid hormone secretion, transport, or metabolism.
Carbon-13 (13C) is a stable isotope of carbon. It is nonradioactive (thus “stable”) with a half-life, if any, greater than 0.5 billion years and possesses no harmful or radiation-related effects. Tests have shown stable isotopes to be safe in newborn infants, and they have been used for over 20 years in studies with infants, children, and adults. It is also safe for use in pregnant women. The 13C-LT4 derivative of LT4 is highly stable and is not converted to the 12C-LT4 analog (the prescribed levothyroxine).
Participants were required to fast (other than water) for at least 5 hours before and 2 hours after ingesting the morning 13C-LT4 dose during each study visit. Patients did not take any other medication within 2 hours of ingesting 13C-LT4 to prevent any interference with 13C-LT4’s rate of absorption and ate a simple breakfast based on eggs, toast, fruit, yogurt, and tea. The women were requested to discontinue taking any iron-containing multivitamins or pills at least 1 week before and throughout the study. All pregnant and nonpregnant women were given their prescribed dose on the first day of the pharmacokinetic study to maintain thyroxine steady state. For each woman, the dose during pregnancy was at most times a higher dose than postpartum. Patients kept a record of time and LT4 dose taken daily during the pharmacokinetic study.
The methods for the measurement of thyroid hormone and 13C-LT4 were developed in the Georgetown Bioanalytical Core Laboratory.14–17 The pharmacokinetics of 13C-LT4 and T4 were conducted after 13C-LT4 administration. Blood samples were drawn at predose (0 hour) and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, 96, and 120 hours postdose. All blood samples were drawn into 10-mL tubes without anticoagulant. After a 10-minute centrifugation at 2500 rpm, serum aliquots were stored at −80°C before assay.
13C6-LT4 derivative was synthesized for the purpose of this study and purchased from IsoSciences, King of Prussia, PA. The 13C-LT4 was compounded in 70-mg and 100-mg capsules by a special compounding pharmacy. The thyroxine content of the compounded capsules was verified by high-performance liquid chromatography. 13C-LT4 capsules were kept at room temperature (temperature-controlled and monitored hourly) in the dark in a firmly closed dessicator until use. Quality control potency and stability testing were conducted biannually by Eagles, Dynalabs, St. Louis, MO, a specifically designated and independently licensed quality control laboratory. Any replacement that was not a multiple of 70 or 100 μg LT4 was supplemented by a complimentary dose of non-13C-LT4 levothyroxine by the Georgetown research pharmacists. However, we note in our results outlined in Table 1 only the 13C-LT4 dosages. For example, if a woman was prescribed by her physician a daily LT4 dose of 225 μg, she received 210 μg 13C-LT4 and an additional 12.5 μg 12C LT4 (half of a 25-μg tablet) reflected in Table 2 as receiving 210 μg for this study.
TABLE 1.
Individual Hormone and Laboratory Data
| TSH |
FT4 |
TBG |
UI |
TPOAb |
TgAb |
Weight |
13CLT4 |
|
|---|---|---|---|---|---|---|---|---|
| Patient No. | (mIU/mL) | (ng/dL) | (μg/mL) | (μg/dL) (>15) | (IU/mL) (>10) | (IU/mL) (<20) | (kg) | Dose (μg) |
| 1 | 1.39 | 1.07 | 30.2 | NA | 18 | 246 | 105.1 | 70 |
| 1NP nursing | 1.32 | 0.71 | 12.1 | 3.3 | 116.5 | 1072 | 82.0 | 70 |
| 2 | 2.84 | 1.43 | 48.3 | NA | <10 | 88.2 | 81.1 | 210 |
| 2NP | 1.69 | 0.79 | 28.1 | 21.4 | <8.3 | 140 | 69.0 | 210 |
| 3 | 2.36 | 1.07 | 30.8 | 19.2 | 48.6 | <20 | 65.2 | 70 |
| 3NP | 1.01 | 0.69 | 26.3 | 10.2 | 127.5 | <20 | 60.4 | 70 |
| 4 | 0.13 | 1.39 | 37.6 | 19.9 | 28.3 | <20 | 78.1 | 210 |
| 4NP nursing | 1.45 | 0.89 | 17.5 | 12.2 | 20.8 | <20 | 65.7 | 210 |
| 5 | 2.18 | 1.15 | 42.3 | 7.5 | <10 | <20 | 64.2 | 70 |
| 5NP | 1.51 | 0.70 | 13.3 | 4.4 | <1.9 | <20 | 58.0 | 70 |
| 6 | 1.04 | 1.35 | 40.7 | NA | 129 | 27.9 | 66.6 | 70 |
| 6NP | 2.74 | 1.15 | 34.2 | 20.4 | 192 | 40.2 | 63.1 | 70 |
| 7 | 0.58 | 1.08 | 39.6 | 7.6 | <10 | <20 | 90.8 | 70 |
| 8 | 0.80 | 1.14 | 42.7 | 20.1 | <10 | <20 | 78.9 | 70 |
| 9 | 1.32 | 1.17 | 13.7 | 12.3 | 19.2 | 42 | 59.3 | 70 |
| 10 NP | 0.61 | 1.39 | 25.8 | 15.6 | 15 | <20 | 87.7 | 140 |
TSH, thyroid-stimulating hormone; NP, not pregnant; NA, not available; UI, urinary iodide; TPOAb, antithyroid peroxidase antibodies; TgAb, antithyroglobulin antibodies.
TABLE 2.
Characteristics of Study Participants
| Patient No. | Patient Age (years) | 13CLT4 Dosage (μg) | Trimester Studied | Maternal Weight (kg) | GW | Newborn Weight (lbs) | Race |
|---|---|---|---|---|---|---|---|
| 1 | 31 | 70 | Third | 105 | 36 | 7.11 | Asian |
| 2 | 41 | 210 | Second | 81 | 22 | 15.5 | White |
| 3 | 34 | 70 | Third | 65 | 30 | 7.14 | White |
| 4 | 34 | 210 | First | 78 | 13 | 8.3 | AA |
| 5 | 30 | 70 | Second | 64 | 16 | 6.11 | White |
| 6 | 35 | 70 | Second | 67 | 25 | 7.9 | White |
| 7 | 34 | 70 | Third | 91 | 36 | 7.8 | White |
| 8 | 35 | 70 | Third | 79 | 30 | 7.11 | White |
| 9 | 42 | 70 | Second | 59 | 26 | NA | Asian |
| 10 | 38 | 140 | Nonpregnant | 88 | NA | NA | AA |
GW, gestation week; AA, African American; NA, not available.
Serum concentrations of 13C-LT4 and T4 were measured using a validated, sensitive, and specific isotope dilution tandem mass spectrometry.17 The method used precipitation of proteins from 100 μL serum with 150 μL methanol containing the deuterated internal standards. After vortexing and centrifugation, 100 μL of supernatant was diluted with 100 microliter water and 100 microliter were injected into the high-performance liquid chromatography. Samples were passed through a Supelco LC-18-DB (Bellefonte, PA) (3.3 cm × 3.0 mm, 3 μm internal diameter) analytical column and eluted with a methanol gradient. Detection was performed by tandem mass spectrometry in the negative-ion mode, in which the ions of 13C-LT4 (m/z 782-127), d-T4 (m/z 778-127), and T4 (m/z 776-127) were monitored (API-4000 tandem mass spectrometer; Sciex, Toronto, Canada). The standard curve had a linear dynamic range of 0.01 to 2.0 ng/mL for 13C-LT4 and 5 to 150 ng/mL for T4. The limit of quantification is 0.002 ng/mL for 13C-LT4 and 5 ng/mL for T4. Interday and intraday coefficients of variation for the assay were less than 5%.
Pharmacokinetic assessments were conducted by a standard two-stage approach using noncompartmental techniques in WinNonLin (Version 5.1.1; Pharsight Corporation, Mountain View, CA). Calculated pharmacokinetic variables included peak concentration (Cmax), time to peak concentration (Tmax), area under the concentration–time curve (AUC0–∞), clearance rate (defined as the ratio of dose administered and AUC), the apparent plasma terminal rate constant (λ), and the half-life of the terminal disposition phase estimated by ln(2)/λ. A combination of linear trapezoidal during ascending phase and log linear method during the descending phase were used for estimating AUC. The primary outcome involved within-subjects comparison of apparent oral drug clearance (CL/F) for study days during pregnancy and postpartum. Levothyroxine pharmacokinetic parameters in pregnant versus nonpregnant women were compared using Wilcoxon signed-rank test using significance level of α = 0.05.
Urinary iodide was measured by inductively coupled plasma mass spectrometry (Mayo Clinic, Rochester, MN). TSH and all other analytes were measured using Immulite 2000 (Bohemia, NY) and standard immunoassay techniques.
RESULTS
Pharmacokinetic data was obtained from seven nonpregnant women and nine pregnant women, six of whom were studied longitudinally, namely during pregnancy and then postpartum. The characteristics of study participants are listed in Table 1. Thyroid status data are shown in Table 2, including TSH, free thyroxine, thyroxine-binding globulin, urinary iodide, antithyroid peroxidase antibodies and antithyroglobulin antibodies as well as individual pharmacokinetic data. All values were within the normal intervals. Urine iodide levels of some of the women indicated possible low iodine status (population sufficient levels are 15 μg/dL) that could have accounted for some differences in pharmacokinetics (see “Discussion”). The normal ranges for thyroxine in nonpregnant women using similar liquid chromatography–tandem mass spectroscopy methodology are the following: for nonpregnant women 4.8 to 11.7 μg/dL (60.9–150.4 nmol/L); first trimester 5.8 to 14.2 μg/dL (74.6–181.9 nmol/L); second trimester 6.3 to 14.4 μg/dL (80.3–184.7 nmol/L); and third trimester 5.9 to 14.2 μg/dL (75.1–181.5 nmol/L).13 The interassay coefficient of variation for T4 was 3.5% to 8% and intra-assay coefficient of variation 3.7% to 4.6%. The normal range for TSH was 0.3 to 3.0 mIU/L (slightly lower in the first trimester) with an intra-assay coefficient of variation of 6% to 8% and interassay coefficient of variation less than 4%.
Pharmacokinetic parameters of 13C-LT4 after oral administration are shown in Table 3. For each pharmacokinetic study, to maintain thyroxine steady state, on Day 1 of the study, each of the women studied was administered one single dose of 13C-LT4, each at her normal prescribed dose of 12C-LT4. Because 13C-LT4 and 12C-LT4 are the same identical drug, we make the assumption that pharmacokinetic parameters for the drug are interchangeable. The results from the postpartum and nonpregnant women were calculated together.
TABLE 3.
13C-Levothyroxine Pharmacokinetic Measures After Oral Administration
|
Tmax (hours) |
Cmax (ng/mL) |
AUC (ng h/mL) |
CL (L/h) |
||
|---|---|---|---|---|---|
| Pregnant women (n = 9) |
Median | 8.0 | 0.9 | 14.8 | 4.5 |
| Minimum | 6.0 | 0.6 | 12.0 | 1.7 | |
| Maximum | 12.0 | 3.1 | 47.6 | 12.5 | |
| Nonpregnant women (n = 7) |
Median | 8.0 | 0.7 | 10.5 | 7.0 |
| Minimum | 6.0 | 0.5 | 5.7 | 5.3 | |
| Maximum | 8.0 | 0.9 | 12.0 | 16.3 |
CL, apparent oral clearance rate; AUC, area under the curve.
Pharmacokinetic data obtained were best described by a two-compartment model with zero order absorption.18 13C-LT4 was slowly absorbed with a median Tmax of 8 hours in pregnant and nonpregnant women (± 2.0) consistent with a transporter-mediated process. There were no significant differences in Tmax and Cmax between pregnant and nonpregnant women (P > 0.05).
The median apparent oral CL/F based on AUC was 4.5 L/h in pregnant women and 7.0 L/h in nonpregnant women (Table 3). Figure 1 depicts percent change in apparent oral clearance. 13C-LT4 apparent oral clearance rate was significantly slower in pregnant women when compared with nonpregnant women (P < 0.04). The median percent decrease in clearance rate was 52%. Mean oral CL/F was 6.7 ± 6.4L/h during pregnancy and 11.8 ± 5.5L/h postpartum (P < 0.03).
FIGURE 1.
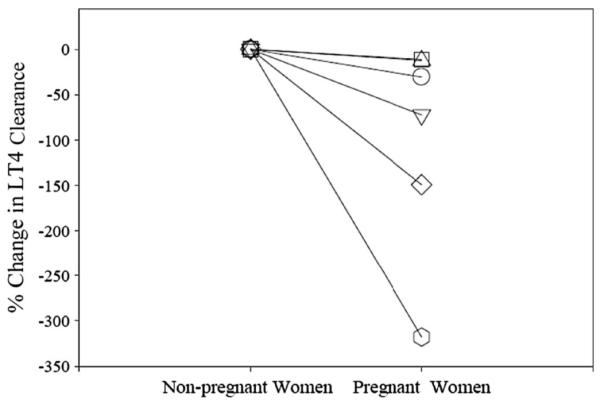
Percent change in 13C-LT4 clearance in pregnant women compared with nonpregnant women.
Systemic drug exposure measured by AUC was 14.8 ng*h/mL in pregnant women and 10.5 ng*h/mL in nonpregnant women (P < 0.03). AUC∞ was 23.08 (± 13.5) ng*h/mL (range, 13.4–47.7 ng*h/mL) during pregnancy and 14.8 (±9.6) ng*h/mL (range, 6.5–35.9 ng*h/mL) in nonpregnant women (P < 0.03). AUC0–72 was significantly higher (P < 0.03) in pregnant women with median percent increase of 37%. Figure 2 shows percent changes in AUC for the drug in pregnant and the nonpregnant women.
FIGURE 2.
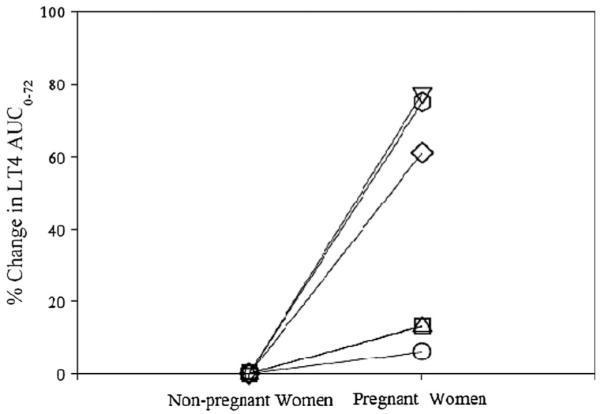
Percent change in 13C-LT4 AUC0–72 in pregnant women compared with nonpregnant women.
DISCUSSION
A comprehensive understanding of drug pharmacokinetics is important for optimal dosing in women, men, and especially in pregnant women. Levothyroxine has a narrow therapeutic index; therefore, precise and accurate dosing is critical. Ordinarily, any estimates of levothyroxine concentrations are affected by baseline concentrations of the hormone. We address the issue of adequate levothyroxine replacement during pregnancy, a clinically significant problem as a result of the narrow therapeutic index of thyroxine, high incidence of hypothyroidism in women (2–10%), the negative effects of hypothyroidism on pregnancy outcome, and the lack of data on thyroxine pharmacokinetics.
This study uses a unique 13C-LT4 tracer method to conduct single-dose pharmacokinetics of levothyroxine in pregnant women. By using this biomarker, we were able to differentiate between the endogenous thyroxine and exogenous synthetic drug replacement LT4. 13C-LT4 and 12C-LT4 are pharmacologically identical and we therefore make the assumption that 13C-LT4 pharmacokinetic and 12C-LT4 pharmacokinetic parameters are interchangeable. In addition, we use a specific and accurate method to estimate the pharmacokinetic parameters after a single oral ingestion of LT4, whereas in other published studies, LT4 was measured indirectly and using immunoassays known to be the less accurate method for measuring thyroxine, especially during pregnancy.13,19,20 Tandem mass spectrometry afforded the detection of 13C-LT4 concentrations as low as 1 pg/mL (lower limit of detection = 0.001 ng/mL), whereas the lower limit of quantification was 2 pcg/mL (lower limit of quantification = 0.002 ng/mL). It is important to explain that our study was conducted while all of the women were at steady state for thyroxine (but using a tracer dose of 13C-levothyroxine). Although high-performance liquid chromatography is incapable of differentiating between 13C-levothyroxine and 12C-levothyroxine, tandem mass spectrometry technology is capable of differentiating between these two molecules. Serum TSH was measured to assure patients are within the normal TSH range and consistently euthyroid during testing and for valid comparisons between patient groups and each of the patients maintained their daily levothyroxine dose on the days of pharmacokinetic analysis. The patients were studied longitudinally so that each woman served as her own control.
Like with any other drug, there is a need for the direct measurement and knowledge of levothyroxine pharmacokinetics, although TSH measurements are considered a valid biomarker of both replacement and suppressive therapy. Hypothyroxinemia, for example, cannot be prevented when relying on TSH results because, by definition, TSH is the within normal range. Also, in pregnancy, TSH concentrations are normally lower in the first trimester and therefore TSH reference ranges are not as reliable. Most importantly, there are very fast changes in thyroid hormone concentrations requiring specific increases in levothyroxine doses very early in gestation.
Evidence that levothyroxine requirements are markedly enhanced during pregnancy in hypothyroid-treated women strongly suggests that not only thyroxine degradation is decreased in early pregnancy, but also that an increased thyroxine production must occur throughout gestation for maintaining the homeostasis of free thyroxine concentrations.21 Our data suggest considerably slower elimination rates of levothyroxine in nonpregnant women than previously reported in the literature, casting doubt on the values reported previously. Moreover, our data illustrate that LT4 elimination is even slower in pregnant women, underscoring the need to apply novel experimental approaches to various other pharmacokinetic studies of drugs in pregnancy. As a comparison, caffeine is a different example of a slower elimination in pregnancy; a significant decrease in the rate that caffeine is eliminated is the result of increases in sex hormone levels in pregnancy or even oral contraceptive use.22 It has also been suggested that caffeine clearance during the follicular and luteal phases suggests that systemic clearance of caffeine is slower in the luteal phase likely resulting from higher progesterone levels.23
In general, the AUC0–∞ during pregnancy was significantly higher than in the same women approximately 6 months postpartum. Furthermore, as pregnancy progressed, there was a trend toward higher systemic exposure in comparison to the postpartum state (Table 4). The increase in LT4 exposure in pregnant women represented by the elevated AUC could be attributed to a decrease in LT4 clearance. However, the data indicate large variability of pharmacokinetic measurements in pregnant women and a relatively narrow range of variability in nonpregnant women. In pregnancy, the minimum value relative to maximum value was a difference of two- to sevenfold with the corresponding variation in nonpregnant women of 1.3- to threefold. The source for this variability between subjects in pharmacokinetic measurements in pregnant women compared with nonpregnant women is not known at this time and requires further investigation. As described, the data are based on a span of gestational ages between 13 and 36 weeks providing a potential effect based on the different gestational ages and the increasing levels of progesterone.
TABLE 4.
Area Under the Curve (AUC) Relative to Week of Gestation
| Gestation Week | AUC Ratio Pregnant/Nonpregnant |
|---|---|
| 13 | 1.03 |
| 16 | 1.11 |
| 22 | 1.33 |
| 25 | 1.11 |
| 30 | 2.50 |
| 36 | 4.15 |
More than 99.96% of circulating T4 and T3 are bound to plasma-binding proteins. During pregnancy, estrogen-driven increase (100% or greater) in thyroxine-binding globulin concentrations is accompanied by an increased number of binding sites for T4 and T4 concentrations. A higher thyroxine-binding globulin and transthyretin (prealbumin) affinity for T4 may also explain, in part, the higher serum T4 concentrations and slower metabolism.24,25 Theoretically, the decrease in LT4 clearance may also be the result of changes in deiodination and glucuronidation; either or both may decrease in activity during pregnancy.
The pharmacokinetic data obtained in our studies were best described by a two-compartment model with zero-order absorption. 13C-LT4 was slowly absorbed with a median Tmax of 8 hours in pregnant and nonpregnant women (± 2.0) consistent with a transporter-mediated process rather than diffusion. Several transporter families have been identified, but only the organic anion-transporting polypeptide 1c1 and the monocarboxylate transporter 8 and monocarboxylate transporter 10 have demonstrated a high specificity to thyroid hormones.26–28
13C-LT4 appeared to decline in a biexponential way with a median apparent oral CL/F of 4.5 L/h in pregnant women and 7.0 L/h in nonpregnant women. 13C-LT4 clearance was significantly slower in pregnant women when compared with nonpregnant women (P < 0.04). This is intriguing because drug clearance increases during pregnancy for nearly all drugs along with increased cardiac output. The analysis of the ratio between the drug exposure expressed as AUC in pregnant women relative to AUC in nonpregnant women illustrates that the ratio increases consistently from 1.3 in gestation Week 13 to 2.5 during gestation Week 30 and 4.15 in gestation Week 36 (Table 4). Hence, bioavailability increases during gestation and does not remain constant.
Other available oral levothyroxine pharmacokinetic data suffer from the unavoidable shortcomings of inability to separate endogenous from administered levothyroxine. Moreover, the studies are usually conducted in euthyroid, nonpregnant adults who received supratherapeutic “megadoses” of LT4 (usually 600–1000 μg).29 Earlier studies used radioactive 131I or 125I-labeled thyroxine injected intravenously, and not the oral preparation. Patients had to be given potassium iodine to prevent thyroidal uptake of inorganic 131I or 125I generated by peripheral deiodination of T4 or T3. In some of these studies, serum T4 concentrations appeared unexplainably elevated.30 Others have documented that serum thyroxine levels were significantly elevated from trough levels at 2 to 9 hours from the time of dose ingestion,31–34 producing increases in measured total and free thyroxine. It was argued that transient elevations in free and total T4 levels that occur over 9 hours are artifacts of hormone equilibration in the serum compartment and do not reflect steady-state values. Others argue that for accurate T4 assessment at least 10 hours are required from the last administered LT4 dose realizing the time to reach Cmax is longer than 2 to 3 hours as previously believed.
In summary, we used stable isotope-labeled levothyroxine to conduct LT4 pharmacokinetic studies in pregnant women and in the same women postpregnancy. There was a large intersubject variability in the primary outcome suggesting further studies are needed to evaluate LT4 pharmacokinetics throughout the first, second, and third trimesters. Future work should focus on the mechanisms responsible for gestational differences in pharmacokinetics and whether these should necessitate dose schedule changes in pregnancy.
Despite the importance of maternal dosing, the key issue for LT4 replacement adequacy in early pregnancy is not just maternal response, but more importantly the adequacy of placentally and transplacentally delivered thyroid hormone for placental development and function and for fetal development.35 It is likely that fetal levels adequate for neurodevelopment will not exactly parallel those required to support TSH secretion in the mother on which we currently base our determination of maternal euthyroidism.
Finally, the combination of serum TSH with the measurement of circulating thyroid hormones greatly improves sensitivity and specificity of thyroid disease diagnosis. Although the estimation of serum free T4 accommodates variations in the concentration of thyroxine-binding globulin, current techniques are unreliable and especially during pregnancy. In addition, FT4 levels can be underestimated as a result of sample dilution and overestimated as a result of nonesterified fatty acids generated during sample storage and incubation. The current measurement of total thyroid hormones using tandem mass spectrometry overcomes some of these issues and can provide a more reliable estimation of T4 in pregnancy.
ACKNOWLEDGMENT
We thank the members of the Obstetric–Fetal Pharmacology Research Unit (OPRU) Network for their support and for constructive discussions on the study and its design.
O.P.S. is Chair of the American Thyroid Association Laboratory Services Committee, a member of the American Thyroid Association Clinical Guidelines Task Force on Thyroid and Pregnancy, and a member of the American Thyroid Association Committee on Thyroid in Pregnancy. O.P.S. is supported by a National Institutes of Health/National Institute of Child Health and Human Development (NIH/NICHD) supplement to the Obstetric–Fetal Pharmacology Research Unit Network Grant 5U10HD0478925, funds from the Office of Research on Women’s Health, and by the GCRC at Georgetown University Medical Center. A.A.V.’s contribution was supported in part by NIH/NICHD Pediatric Pharmacology Research Unit grant 5U10HD037249-10 (AAV).
This project was conducted through the General Clinical Research Center at Georgetown University and supported by the National Institutes of Health National Center for Research Resources, Grant M01RR-023942.
Footnotes
The authors declared no conflict of Interest. O.P.S. reports having served as a consultant for Abbott Laboratories.
Presented in part at the annual meeting of the American Thyroid Association, Palm Beach, FL, September 23, 2009.
REFERENCES
- 1.Mandel SJ, Brent GA, Larsen PR. Levothyroxine therapy in patients with thyroid disease. Ann Intern Med. 1993;119:492–502. doi: 10.7326/0003-4819-119-6-199309150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Lazarus JH, Soldin OP, Evans C. Assessing thyroid function in pregnancy. In: Brent GA, editor. Thyroid Function Testing. Springer; New York: 2010. pp. 209–233. [Google Scholar]
- 3.Santini F, Pinchera A, Marsili A, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab. 2005;90:124–127. doi: 10.1210/jc.2004-1306. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus JH, Kokandi A. Thyroid disease in relation to pregnancy: a decade of change. Clin Endocrinol (Oxf) 2000;53:265–278. doi: 10.1046/j.1365-2265.2000.01087.x. [DOI] [PubMed] [Google Scholar]
- 5.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 6.Glinoer D. Feto-maternal repercussions of iodine deficiency during pregnancy. An update. Ann Endocrinol (Paris) 2003;64:37–44. [PubMed] [Google Scholar]
- 7.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 8.Kahric-Janicic N, Soldin SJ, Soldin OP, et al. Tandem mass spectrometry improves the accuracy of free thyroxine measurements during pregnancy. Thyroid. 2007;17:303–311. doi: 10.1089/thy.2006.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151(Suppl 3):U25–U37. doi: 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- 10.Mandel SJ, Larsen PR, Seely EW, et al. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96. doi: 10.1056/NEJM199007123230204. [DOI] [PubMed] [Google Scholar]
- 11.Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2007;92:S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 12.Soldin OP. Thyroid function testing in pregnancy and thyroid disease: trimester-specific reference intervals. Ther Drug Monit. 2006;28:8–11. doi: 10.1097/01.ftd.0000194498.32398.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soldin OP, Hilakivi-Clarke L, Weiderpass E, et al. Trimester-specific reference intervals for thyroxine and triiodothyronine in pregnancy in iodine-sufficient women using isotope dilution tandem mass spectrometry and immunoassays. Clin Chim Acta. 2004;349:181–189. doi: 10.1016/j.cccn.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soukhova N, Soldin OP, Soldin SJ. Isotope dilution tandem mass spectrometric method for T4/T3. Clin Chim Acta. 2004;343:185–190. doi: 10.1016/j.cccn.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu J, Soldin OP, Soldin SJ. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin Biochem. 2007;40:1386–1391. doi: 10.1016/j.clinbiochem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Soldin O, de Ridder S, Vinks A, et al. Levothyroxine pharmacokinetics in pregnancy. 79th Annual Meeting of the American Thyroid Association; Chicago, IL. October 1–5, 2008. [Google Scholar]
- 17.Soldin OP, Mendu DM, Soldin SJ. Development of a method for the simultaneous measurement of stable isotope C13- and C12-thyroxine in human serum or plasma. Thyroid. 2008;18:S84–S85. [Google Scholar]
- 18.Soldin OP, Soldin SJ, Mattison DR, et al. Changes in thyroxine pharmacokinetics during pregnancy: a stable isotope study. 80th Annual Meeting of the American Thyroid Association Meeting; Palm Beach FL. September 2009; Thyroid 2009. [Google Scholar]
- 19.Soldin OP. Therapeutic drug monitoring during pregnancy and lactation: thyroid function assessment in pregnancy—challenges and solutions. Ther Drug Monit. 2010;32:265–268. doi: 10.1097/FTD.0b013e3181ddf729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soldin OP, Soldin SJ. Thyroid hormone testing by tandem mass spectrometry. Clin Biochem. 2010 Aug 4; doi: 10.1016/j.clinbiochem.2010.07.020. [Epub ahead print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 22.Lane JD, Steege JF, Rupp SL, et al. Menstrual cycle effects on caffeine elimination in the human female. Eur J Clin Pharmacol. 1992;43:543–546. doi: 10.1007/BF02285099. [DOI] [PubMed] [Google Scholar]
- 23.Zaigler M, Rietbrock S, Szymanski J, et al. Variation of cyp1a2-dependent caffeine metabolism during menstrual cycle in healthy women. Int J Clin Pharmacol Ther. 2000;38:235–244. doi: 10.5414/cpp38235. [DOI] [PubMed] [Google Scholar]
- 24.Glinoer D, Abalovich M. Unresolved questions in managing hypothyroidism during pregnancy. BMJ. 2007;335:300–302. doi: 10.1136/bmj.39189.513935.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schussler GC. The thyroxine-binding proteins. Thyroid. 2000;10:141–149. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Abe T. Thyroid hormone transporters in the brain. Cerebellum. 2008;7:75–83. doi: 10.1007/s12311-008-0029-9. [DOI] [PubMed] [Google Scholar]
- 27.Ceballos A, Belinchon MM, Sanchez-Mendoza E, et al. Importance of monocarboxylate transporter 8 for the blood–brain barrier-dependent availability of 3,5,3′-triiodo-l-thyronine. Endocrinology. 2009;150:2491–2496. doi: 10.1210/en.2008-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts LM, Woodford K, Zhou M, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (slc16a2) and organic ion transporter-14 (slco1c1) at the blood–brain barrier. Endocrinology. 2008;149:6251–6261. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- 29.Abbott Laboratories . Physician Prescribing Information Package Insert. Abbott Laboratories, Inc; North Chicago, IL: Synthroid (levothyroxine sodium tablets, USP) 03-A119-R3; revised March 2008. [Google Scholar]
- 30.Braverman LE, Vagenakis A, Downs P, et al. Effects of replacement doses of sodium l-thyroxine on the peripheral metabolism of thyroxine and triiodothyronine in man. J Clin Invest. 1973;52:1010–1017. doi: 10.1172/JCI107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ain KB, Pucino F, Shiver TM, et al. Thyroid hormone levels affected by time of blood sampling in thyroxine-treated patients. Thyroid. 1993;3:81–85. doi: 10.1089/thy.1993.3.81. [DOI] [PubMed] [Google Scholar]
- 32.Maxon H, Volle C, Hertzberg V, et al. Variations in serum thyroxine concentrations with time after an oral replacement dose. Clin Nucl Med. 1987;12:389–390. doi: 10.1097/00003072-198705000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Sturgess I, Thomas SH, Pennell DJ, et al. Diurnal variation in TSH and free thyroid hormones in patients on thyroxine replacement. Acta Endocrinol (Copenh) 1989;121:674–676. doi: 10.1530/acta.0.1210674. [DOI] [PubMed] [Google Scholar]
- 34.Soppi E, Irjala K, Viikari J. Thyroid hormone concentrations after exogenous thyroxine. Br Med J (Clin Res Ed) 1984;288:1157. doi: 10.1136/bmj.288.6424.1157-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glinoer D. Maternal thyroid function in pregnancy. J Endocrinol Invest. 1993;16:374–378. doi: 10.1007/BF03348861. [DOI] [PubMed] [Google Scholar]


