Abstract
Objective
To examine the relationship between estimated maximal cardiorespiratory fitness (CRF) and metabolic syndrome (MetS).
Patients
Aerobics Center Longitudinal Study (N=38,659) participants examined between 1979–2006.
Methods
We performed a cross-sectional analysis of participants to examine CRF levels defined as low (lower 20%), moderate (middle 40%) and high (upper 40%) of age/gender specific distributions versus NCEP derived MetSyn expressed as a summed z-score continuous variable. We used a general linear model for continuous variables, chi-square for distribution of categorical variables, and multiple linear regression for single and cumulative MetS scores adjusted for BMI, smoking status, alcohol intake and family history of cardiovascular disease.
Results
We observed significant inverse trends for MetS vs. CRF in both genders (p-for-trend, < 0.001). CRF associations vs. individual components were: Waist circumference (men, β= −0.14, r2 = 0.78; women, β= −0.04, r2 = 0.71), triglycerides (men, β= −0.29, r2 = 0.18; women, β= −0.17, r2 = 0.18), HDL-cholesterol (men, β= 0.25, r2 = 0.17; women, β= 0.08, r2 = 0.19), fasting glucose (men, β= −0.09, r2 = 0.09; women, β= 0.09, r2 = 0.01), systolic blood pressure (men, β= −0.09, r2 = 0.09; women, β= −0.01, r2 = 0.21), and diastolic blood pressure (men, β= −0.07, r2 = 0.12; women, β= −0.05, r2 = 0.14). All associations except for systolic blood pressure (both genders) and glucose (women) are significant (p<0.001).
Conclusion
CRF demonstrated a strong inverse relationship with MetS in both genders with the strongest single associative component being waist circumference.
Keywords: Metabolic syndrome, exercise, fitness, syndrome X
INTRODUCTION
Metabolic syndrome (MetSyn) represents a clustering of physiologic disorders within the same individual.1 Though a number of earlier works laid the groundwork for its conception, MetSyn, Reaven (1988) presented the original hypothesis for this clustering of characteristics, referring to them Syndrome X. In his original treatise, Reaven proposed that insulin resistance was the mediating component and root cause of dyslipidemia and hypertension.2 Over time his original hypothesis evolved to include obesity and, in particular, abdominal adiposity.3 It is now widely accepted that the MetSyn plays a prominent role in the development of cardiovascular disease and type 2 diabetes.1,4 Accordingly, it has been estimated that those individuals with the MetSyn have a higher relative risk of type 2 diabetes, coronary heart disease, and cardiovascular disease.5 As with many diseases and syndromes, positive lifestyle behaviors such as physical activity and nutrition play an important role in the development and progression of each component of the MetSyn.6–8
Epidemiology studies have demonstrated a significant, inverse, independent association between physical activity, maximal cardiorespiratory fitness (i.e. fitness), and both the composite MetSyn score and each component taken in singularity.9–12 Further evidence also demonstrates that the protection afforded by physical activity and fitness occurs in a dose dependent fashion.11–13 While these results may be intuitive, we propose that the categorical nature of the MetSyn assessment may not fully elucidate the cumulative benefits afforded by fitness and the consequent improvements within each component category. Supporting this premise is the concept that MetSyn represents a constellation of risk factors influenced by multiple physiologic systems. Likewise, exercise also affects multiple physiologic systems that may not be fully explained during the analysis of an intervention aimed at improving the syndrome as a whole. Further, the categorical nature of the MetSyn does not allow for intra-individual response differences within each component. Thus, the global effects of exercise may be blunted if one views each component in solidarity where, despite improvements in a particular MetSyn feature, those who do not show enough improvement to move out of a qualifying category are not “given credit” for their improvement. For example, NCEP ATP III MetSyn guidelines categorize men as qualifying for higher risk if their waist circumference is ≥ 102 cm. Consequently, if an individual presents with a waist circumference of 120 cm and reduces said circumference to 103 cm through behavioral change, they are still scored as positive for that particular risk component.
One way to avoid this complication is to examine the MetSyn score as a summed Z score as recently proposed by Brage et al. (2004). In brief, individual components of MetSyn statistically normalized and expressed as Z scores. A MetSyn score is then computed as the mean of these Z scores.14 The use of such a score may have particular utility when examining the effectiveness of clinical interventions for those participants who are at a higher risk for type 2 diabetes and cardiovascular disease. Herein we examine the role of cardiorespiratory fitness as it relates to the MetSyn in the Aerobics Center Longitudinal Study (ACLS).
METHODS
Study Population
We performed a cross-sectional analysis of participants from the Aerobic Center Longitudinal Study (ACLS) by examining the association between MetS and estimated maximal cardiorespiratory fitness (CRF). Study participants came to the Cooper Clinic (Dallas, TX) for periodic preventive health examinations. We initially considered 47,398 participants with complete data and having medical examinations from 1979–2006. We excluded participants with histories of CVD (heart attack or stroke; n=740), cancer (n=2,294), underweight (body mass index, BMI <18.5kg/m2; n=528), abnormal ECG (n=4,199) or failed to achieve at least 85% of age-predicted maximum heart rate during exercise testing (n=978). This analysis includes 38,659 individuals (20–90 y; 22% women) who were predominantly white, well-educated, and within middle-to-upper socioeconomic strata. All participants gave written, informed consent to participate in the study, which was approved annually by The Cooper Institute Institutional Review Board.
Clinical examination
Details of the ACLS clinical examination are detailed elsewhere 3. Briefly, examinations were completed after an overnight fast and included an extensive physical examination inclusive of BMI (kg/m2), waist circumference measured at the umbilicus, and resting blood pressure measured in the seated position using standard auscultation methods after 5 min of sitting quietly and the averaging of two or more readings separated by 2 min.4 Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or by physician diagnosis. Triglycerides, total cholesterol, HDL-cholesterol and fasting plasma glucose were measured using automated techniques in accordance with the standards of the Centers for Disease Control and Prevention lipid standardization program. Hypercholesterolemia and diabetes were defined as a total cholesterol concentration of ≥ 240 mg/dL, fasting glucose ≥126 mg/dL, respectively, or by previous physician diagnosis.
Participants completed a standardized questionnaire on medical history including a personal history of myocardial infarction, stroke, hypertension, diabetes, and cancer; parental history of premature CVD”, defined as Heart attacks, coronary bypass, angioplasty, or angina under age 50; smoking status; alcohol intake and physical activity. Physical inactivity was defined as reporting no physical activity during leisure time 3 months before the examination.
Cardiorespiratory Fitness
We assessed fitness using a modified Balke maximal treadmill exercise test 5. Test endpoints included volitional exhaustion or termination for medical reasons. We have shown that total exercise test time correlates highly (r ≥ 0.92) with measured maximal oxygen uptake in men and women.6,7 To standardize interpretation of exercise test performance, maximal metabolic equivalents (METs, 1 MET = 3.5 ml oxygen uptake/kg/min) were estimated based on the final treadmill speed and grade 8. Maintaining consistency with previous ACLS reports, fitness was classified as low, moderate and high fitness corresponding to the lower 20%, the middle 40% and the upper 40%, respectively, of the age-/gender-specific distributions for treadmill exercise duration.5
Metabolic Syndrome
We calculated MetS according to the NCEP ATP III criteria by creating a continuous score based on the average individual component z-scores comprising MetS 2,9
Statistical Analyses
We checked all variables for distribution normality before the analysis, subsequently transforming several variables for skewness. Natural logarithms were applied to BMI, triglycerides, glucose and systolic blood pressure. Square roots were applied to total-/HDL-cholesterol. However, untransformed data summarizing participant characteristics are presented for ease of interpretation. The mean levels of continuous variables were compared using analysis of the variance (ANOVA), while chi-square tests compared the distribution of categorical variable values. Multiple linear regression models were used to examine the association between fitness, single, and clustered MetS scores using three models:
Model 1: Adjusted for age, and examination year
Model 2: Adjusted for age, examination year, BMI, smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not), and family history of CVD.
Model 3: A limitation to the ACLS data set is a lack of data on medications and diet information., which may bias our findings. In Model 3, we adjusted age, examination year, BMI, physical activity (active or inactive), smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not), hypercholesterolemia, hypertension and diabetes (present or not for each), and family history of CVD (present or not).
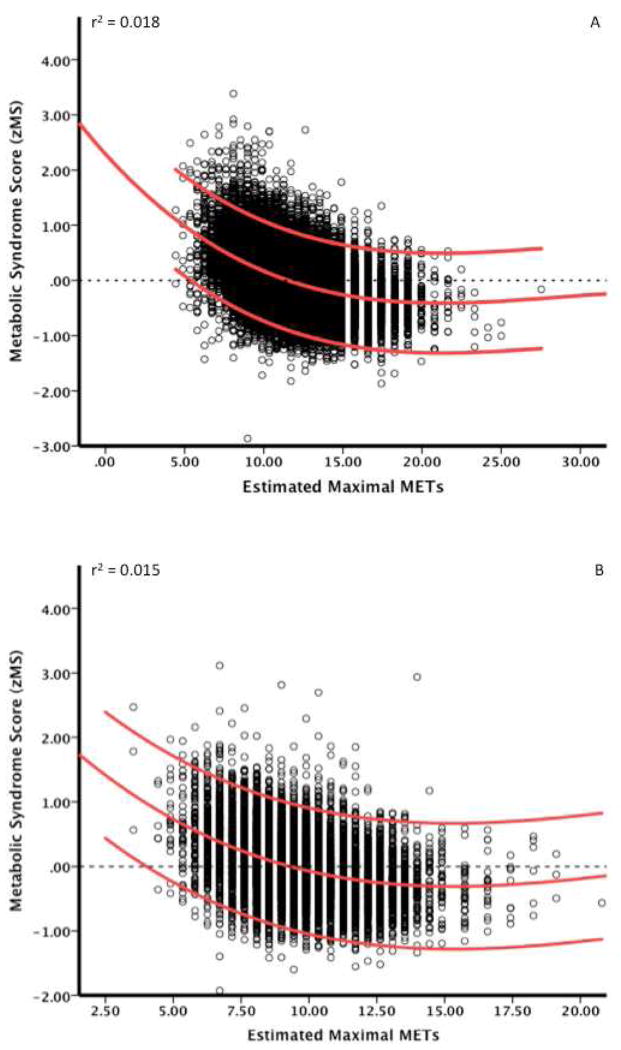
Analysis of covariance (ANCOVA) was used to assess differences in single and clustered MetS components across levels of fitness (low, moderate and high) after adjusting for confounders. ANOVA post hoc analyses were conducted for each between group comparison (low vs. moderate; moderate vs. high; low vs. high) using Bonferroni adjustments. Quadratic regression analyses were performed to examine the reduction in zMS vs. estimated CRF (i.e., METs and presented in Figure 2. Data analyses were performed using PWSA statistical package version 18.0 (SPSS Inc., Chicago, IL, USA) and all p values are 2-sided with an alpha level of 0.05.
Figure 2.
Data present the quadratic regression of individual zMS scores and accompanying 95% confidence intervals vs. estimated CRF (i.e., METs) from graded exercise testing for men (panel A) and women (panel B).
RESULTS
Demographic characteristics (Table 1), independent MetS composite scores for men (Table 2) and women (Table 3) and composite MetS scores (Figure 1) are presented below.
Table 1.
Characteristics of the study population. Aerobics Center Longitudinal Study, 1979–2006
| Low | Moderate | High | ||||
|---|---|---|---|---|---|---|
| All | (N = 38,659) | (n = 4,016) | (n = 13,480) | (n = 21,163) | ||
| Men | (n = 30,167) | (n = 3,254) | (n = 10,861) | (n = 16,052) | ||
| Women | (n = 8,492) | (n = 762) | (n = 2,619) | (n = 5,111) | ||
| Age (years) | All | 45.6 ± 10.1 | 43.6 ± 9.2 | 45.1 ± 9.8 | 46.3 ± 10.3 | * |
| Men | 45.8 ± 9.9 | 43.6 ± 9.0 | 45.3 ± 9.6 | 46.5 ± 10.3 | * | |
| Women | 45.0 ± 10.5 | 43.4 ± 10.1 | 44.6 ± 10.5 | 45.4 ± 10.5 | * | |
| Body mass index (kg/m2) b | All | 26.0 ± 4.1 | 30.3 ± 5.6 | 26.9 ± 3.8 | 24.6 ± 3.0 | * |
| Men | 26.7 ± 3.8 | 30.8 ± 5.3 | 27.5 ± 3.5 | 25.3 ± 2.7 | * | |
| Women | 24.0 ± 4.1 | 28.2 ± 6.4 | 24.5 ± 4.2 | 22.4 ± 2.8 | * | |
| Maximal MET capacity | All | 11.5 ± 2.5 | 8.2 ± 1.3 | 10.2 ± 1.4 | 12.9 ± 2.2 | * |
| Men | 11.9 ± 2.4 | 8.6 ± 1.1 | 10.7 ± 1.1 | 13.5 ± 1.9 | * | |
| Women | 9.8 ± 2.1 | 6.7 ± 0.9 | 8.4 ± 1.0 | 10.9 ± 1.7 | * | |
| Total cholesterol (mg/dL) c | All | 203.5 ± 39.4 | 213.9 ± 43.0 | 208.5 ± 40.4 | 198.4 ± 37.3 | * |
| Men | 204.9 ± 39.9 | 216.2 ± 43.6 | 210.2 ± 40.5 | 199.1 ± 37.6 | * | |
| Women | 198.5 ± 37.4 | 204.3 ± 39.0 | 201.8 ± 38.9 | 195.9 ± 36.1 | * | |
| No (%) of physically inactive | All | 8,852 (22.9) | 2,093 (52.1) | 4,323 (32.1) | 2,436 (11.5) | * |
| Men | 7,064 (23.4) | 1,721 (52.9) | 3,501 (32.2) | 1,842 (11.5) | * | |
| Women | 1,788 (21.1) | 372 (48.8) | 822 (31.4) | 594 (11.6) | * | |
| No (%) of current smokers | All | 5,417 (14.0) | 1,023 (25.5) | 2,499 (18.5) | 1,985 (9.0) | * |
| Men | 4,707 (15.6) | 918 (28.2) | 2,191 (20.2) | 1,598 (10.0) | * | |
| Women | 710 (8.4) | 105 (13.8) | 308 (11.8) | 297 (5.8) | * | |
| No (%) of heavy drinkers | All | 3,514 (9.1) | 275 (6.8) | 1,135 (8.4) | 2,104 (9.9) | * |
| Men | 2,538 (8.4) | 243 (7.5) | 906 (8.3) | 1,389 (8.7) | § | |
| Women | 976 (11.5) | 32 (4.2) | 229 (8.7) | 715 (14.0) | * | |
| No (%) with medical condition | ||||||
| Hypertension | All | 4,791 (12,4) | 894 (22.3) | 1,958 (14.5) | 1,939 (9.2) | * |
| Men | 3,991 (13.2) | 756 (23.2) | 1,671 (15.4) | 1,564 (9.7) | * | |
| Women | 800 (9.4) | 138 (18.1) | 287 (11.0) | 375 (7.3) | * | |
| Hypercholesterolemia | All | 6,929 (17.9) | 843 (21.0) | 2,684 (19.9) | 3,402 (16.1) | * |
| Men | 5,639 (18.7) | 719 (22.1) | 2,263 (20.8) | 2,657 (16.6) | * | |
| Women | 1,290 (15.2) | 124 (16.3) | 421 (16.1) | 745 (14.6) | ||
| Diabetes mellitus | All | 973 (2.5) | 161 (4.0) | 386 (2.9) | 426 (2.0) | * |
| Men | 699 (2.3) | 131 (4.0) | 292 (2.7) | 276 (1.7) | * | |
| Women | 274 (3.2) | 30 (3.9) | 94 (3.6) | 150 (2.9) |
Values expressed as means ± SD or numbers (percentage).
Differences between genders and fitness categories were examined by analysis of the variance (ANOVA) and chi-square tests for continuous and categorical variables, respectively.
Values were natural log-transformed before analysis, but non-transformed values are presented.
Values were square root transformed before analysis, but non-transformed values are presented.
SI Conversions. To convert total cholesterol to mmol/L multiply by 0.0259.
Levels of significance are
P<0.001,
P = 0.08
Table 2.
Single and clustered metabolic syndrome components by cardiorespiratory fitness level in men (n = 30,167)
| Cardiorespiratory Fitness | p | Post-hoc comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Low | Moderate | High | trend | Low vs. Moderate | Low vs. High | Moderate vs. High | ||
| Waist circumference (cm) | Model 1 | 105.9 ± 0.2 | 96.9 ± 0.1 | 89.6 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 95.8 ± 0.1 | 94.9 ± 0.1 | 93.0 ± 0.0 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Model 3 | 95.6 ± 0.1 | 94.9 ± 0.0 | 93.1 ± 0.0 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Triglycerides (mg/dL) a | Model 1 | 196.4 ± 1.8 | 155.1 ± 1.0 | 110.0 ± 0.8 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 173.0 ± 2.0 | 150.3 ± 1.0 | 118.0 ± 0.8 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Model 3 | 168.1 ± 2.0 | 148.2 ± 1.0 | 120.4 ± 0.8 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| HDL-cholesterol (mg/dL) b | Model 1 | 41.0 ± 0.2 | 44.0 ± 0.1 | 50.0 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 44.2 ± 0.2 | 44.6 ± 0.1 | 48.8 ± 0.1 | < 0.001 | 0.018 | < 0.001 | < 0.001 | |
| Model 3 | 44.6 ± 0.2 | 44.7 ± 0.1 | 48.6 ± 0.1 | < 0.001 | 0.20 | < 0.001 | < 0.001 | |
| Fasting blood glucose (mg/dL) a | Model 1 | 106.6 ± 0.3 | 100.6 ± 0.2 | 97.8 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 104.0 ± 0.3 | 100.1 ± 0.2 | 98.7 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Model 3 | 102.9 ± 0.3 | 99.8 ± 0.2 | 99.1 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Systolic blood pressure (mmHg) a | Model 1 | 124.9 ± 0.2 | 121.5 ± 0.1 | 120.2 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 121.9 ± 0.2 | 121.0 ± 0.1 | 121.2 ± 0.1 | 0.015 | 0.003 | 0.045 | 0.71 | |
| Model 3 | 121.5 ± 0.2 | 120.9 ± 0.1 | 121.3 ± 0.1 | 0.85 | 0.14 | 0.016 | 1.00 | |
| Diastolic blood pressure (mmHg) | Model 1 | 85.1 ± 0.2 | 82.4 ± 0.1 | 80.0 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 82.6 ± 0.2 | 82.0 ± 0.1 | 80.8 ± 0.1 | < 0.001 | 0.002 | < 0.001 | < 0.001 | |
| Model 3 | 88.2 ± 0.2 | 81.9 ± 0.1 | 81.0 ± 0.9 | < 0.001 | 0.19 | < 0.001 | < 0.001 | |
Results expressed as adjusted mean (SEM).
Values were natural log-transformed before analysis, but non-transformed values are presented.
Values were square root transformed before analysis, but non-transformed values are presented.
SI Conversions.
To convert HDL-C to mmol/L multiply by 0.0259.
To convert triglycerides to mmol/L multiply by 0.0113.
To convert glucose to mmol/L multiply by 0.0555.
Model 1: Adjustment for age, examination year;
Model 2: Adjustment for age, examination year, BMI, smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not) and family history of cardiovascular disease;
Model 3: Adjustment for age, examination year, BMI, physical activity (active or inactive), smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not), hypercholesterolemia, hypertension and diabetes (present or not for each), and family history of cardiovascular disease.
Table 3.
Single and clustered metabolic syndrome components by cardiorespiratory fitness level in women (n = 8,492)
| Cardiorespiratory Fitness | p | Post-hoc comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Low | Moderate | High | trend | Low vs. Moderate | Low vs. High | Moderate vs. High | ||
| Waist circumference (cm) | Model 1 | 85.2 ± 0.3 | 76.9 ± 0.2 | 71.1 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 75.0 ± 0.2 | 74.6 ± 0.1 | 73.8 ± 0.1 | < 0.001 | 1.00 | < 0.001 | < 0.001 | |
| Model 3 | 74.7 ± 0.2 | 74.5 ± 0.1 | 73.8 ± 0.1 | 0.001 | 1.00 | 0.004 | < 0.001 | |
| Triglycerides (mg/dL) a | Model 1 | 122.7 ± 2.2 | 105.2 ± 1.2 | 83.7 ± 0.8 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 103.2 ± 2.3 | 100.7 ± 1.2 | 88.9 ± 0.9 | < 0.001 | 0.05 | < 0.001 | < 0.001 | |
| Model 3 | 101.8 ± 2.3 | 99.8 ± 1.2 | 89.5 ± 0.9 | < 0.001 | 0.07 | < 0.001 | < 0.001 | |
| HDL-cholesterol (mg/dL) b | Model 1 | 55.7 ± 0.5 | 60.7 ± 0.3 | 66.0 ± 0.2 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 61.6 ± 0.6 | 62.1 ± 0.3 | 64.4 ± 0.2 | < 0.001 | 0.53 | < 0.001 | < 0.001 | |
| Model 3 | 61.8 ± 0.6 | 62.2 ± 0.3 | 64.3 ± 0.2 | < 0.001 | 0.65 | < 0.001 | < 0.001 | |
| Fasting blood glucose (mg/dL) a | Model 1 | 97.1 ± 0.5 | 93.8 ± 0.3 | 92.6 ± 0.2 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 94.3 ± 0.5 | 93.2 ± 0.3 | 93.3 ± 0.2 | 0.30 | 0.34 | 0.91 | 1.00 | |
| Model 3 | 93.9 ± 0.5 | 93.0 ± 0.3 | 93.5 ± 0.2 | 0.88 | 0.81 | 1.00 | 0.12 | |
| Systolic blood pressure (mmHg) a | Model 1 | 118.3 ± 0.5 | 113.8 ± 0.3 | 111.9 ± 0.2 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 114.2 ± 0.5 | 112.9 ± 0.3 | 112.9 ± 0.2 | 0.039 | 0.90 | 0.12 | 1.00 | |
| Model 3 | 113.7 ± 0.5 | 112.8 ± 0.2 | 113.1 ± 0.2 | 0.38 | 0.50 | 1.00 | 1.00 | |
| Diastolic blood pressure (mmHg) | Model 1 | 79.8 ± 0.3 | 77.2 ± 0.2 | 75.4 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Model 2 | 77.2 ± 0.3 | 76.7 ± 0.2 | 76.1 ± 0.1 | 0.004 | 0.54 | 0.011 | 0.018 | |
| Model 3 | 76.9 ± 0.3 | 76.7 ± 0.2 | 76.2 ± 0.1 | 0.052 | 1.00 | 0.16 | 0.60 | |
Results expressed as adjusted mean (SEM).
Values were natural log-transformed before analysis, but non-transformed values are presented.
Values were square root transformed before analysis, but non-transformed values are presented.
SI Conversions.
To convert HDL-C to mmol/L multiply by 0.0259.
To convert triglycerides to mmol/L multiply by 0.0113.
To convert glucose to mmol/L multiply by 0.0555.
Model 1: Adjustment for age, examination year;
Model 2: Adjustment for age, examination year, BMI, smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not) and family history of cardiovascular disease;
Model 3: Adjustment for age, examination year, BMI, physical activity (active or inactive), smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not), hypercholesterolemia, hypertension and diabetes (present or not for each), and family history of cardiovascular disease.
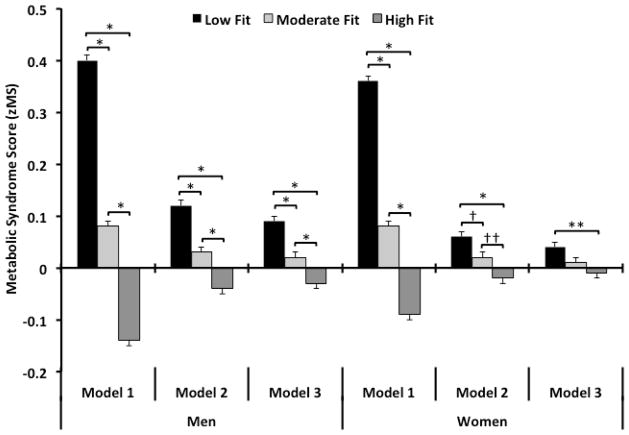
Figure 1.
Data represent the adjusted mean (SEM) for men (left panel) and women (right panel) for averaged zMS scores based on three statistical models. Model 1: Adjustment for age, examination year; Model 2: Adjustment for age, examination year, BMI, smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not) and family history of cardiovascular disease; Model 3: Adjustment for age, examination year, BMI, physical activity (active or inactive), smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not), hypercholesterolemia, hypertension and diabetes (present or not for each), and family history of cardiovascular disease. Levels of significance are: * p < 0.001; ** p = 0.02); † p = 0.04; †† p = 0.008.
Metabolic Syndrome
Men
We observed a significant trend showing a lower MetS score across fitness groups regardless of analytical model (Fig. 1, all models, p for trend < 0.001). Between group post-hoc assessments demonstrated significant differences for: low vs. moderate, low vs. high, and moderate vs. high fitness levels (all models, p < 0.001).
Women
We observed a significant trend showing a lower MetS score across fitness groups. Models 1 and 2 demonstrated the same level of significance (p for trend < 0.001), while Model 3 was slightly lower (p for trend < 0.007). Between group post-hoc assessments showed significant differences for: Low vs. moderate, low vs. high, and moderate vs. high fitness levels (Model 1, p < 0.001). Model 2 was also significant for each comparison for: Low vs. moderate (p = 0.036), low vs. high (p < 0.001) and moderate vs. high fitness levels (p = 0.008). Model 3 only showed a significant difference between the low and high fit participants (p = 0.02).
Waist Circumference
Men
We observed significant trends showing a smaller waist circumference across fitness groups (all models, p for trend < 0.001) with significant post-hoc differences for the low vs. moderate, low vs. high, and moderate vs. high fitness levels (all models and comparisons, p < 0.001)
Women
We observed significant trends showing smaller waist circumferences across fitness groups (all models, p for trend < 0.001). Contrasting with men, we observed several post-hoc differences. In Model 1, we observed significant differences for: low vs. moderate, low vs. high, and moderate vs. high fitness levels (all, p < 0.001). For Models 2 and 3 we found no significant differences between the low and moderate fitness groups. In Model 2 the comparisons between the low vs. high and moderate vs. high groups remained consistent (p < 0.001); however, Model 3 showed the low vs high fitness group to be slightly different (p = 0.004).
Triglycerides
Men
We observed a significant trend showing lower triglyceride concentration across fitness groups (all models, p for trend < 0.001). Our post-hoc assessments further showed significant differences for: low vs. moderate fitness levels, low vs. high, and moderate vs. high fitness levels (all models and comparisons, p < 0.001).
Women
We observed a significant trend showing a lower triglyceride concentration across fitness groups (all models, p for trend < 0.001). In contrast to men, we observed several post-hoc analysis differences. In Model 1, we observed significant differences for: low vs. moderate, low vs. high, and moderate vs. high fitness levels (all, p < 0.001). When using Models 2 and 3 we found no significant differences between the low and moderate fitness groups, while the low vs. high and moderate vs. high group comparisons showed the same level of significance (p < 0.001).
HDL-Cholesterol
Men
We observed a significant trend showing a higher HDL-C concentration across fitness groups (all models, p for trend < 0.001). Between group post-hoc assessments were: low vs. moderate, low vs. high, and moderate vs. high fitness levels for Model 1 (all, p < 0.001) with a similar pattern noted for Model 2: low vs. moderate group difference (p < 0.02). No significant difference was noted for the low vs. moderate groups in Model 3.
Women
We observed a significant trend showing a higher HDL-C concentration across fitness groups (all models, p for trend < 0.001). Contrasting men, we observed several differences for our post-hoc analyses. In Model 1, we observed significant differences for: low vs. moderate, low vs. high, and moderate vs. high fitness levels (all, p < 0.001). However, when using Models 2 and 3 we found no significant differences between the low and moderate fitness groups, while the low vs. high and moderate vs. high comparisons remained significant (p < 0.001).
Fasting Blood Glucose
Men
We observed a significant trend showing a lower glucose concentration across fitness groups (all models, p for trend < 0.001). Between group post-hoc analyses further showed significant differences for: low vs. moderate, low vs. high, and moderate vs. high fitness levels (all models and comparisons, p < 0.001).
Women
We only observed a significant trend for Model 1 (p for trend < 0.001). Accordingly our post-hoc assessment between groups further showed significant differences for: low vs. moderate, low vs. high, and moderate vs. high fitness levels (Model 1, all comparisons, p < 0.001).
Systolic Blood Pressure
Men
We observed a significant trend showing lower systolic blood across fitness groups for Model 1 (p for trend < 0.001) and Model 2 (p for trend < 0.02). Post-hoc between group assessments in Model 1 are: Low vs. moderate, low vs. high, and moderate vs. high fitness levels (p < 0.001). For Model 2, significant between group differences were noted for: Low vs. moderate (p = 0.003) and low vs. high fitness levels (p < 0.05). In Model 3, only the low vs. high fitness group comparison was significant (p < 0.02).
Women
Women also showed a significant trend for Model 1 (p for trend < 0.001), Model 2 (p for trend <0.04), but not for Model 3. For our post-hoc comparison, all between group comparisons were significant for Model 1 (all, p < 0.001). No significant between group comparisons were noted for Model 2 or Model 3.
Diastolic Blood Pressure
Men
We observed a significant trend showing for lower diastolic blood pressure across fitness groups (all models, p for trend < 0.001). Our post-hoc assessment between groups further showed significant differences for Model 1: Low vs. moderate, low vs. high, and moderate vs. High fitness levels (all models, p < 0.001) For Model 2, all between group comparisons were also found to be significant: Low vs. moderate (p = 0.002), Low vs. high and moderate vs. high (p < 0.001). For Model 3, no significant differences were observed for the low vs. moderate group comparison. However, the low vs. high and moderate vs. high were both significant (p < 0.001).
Women
Women also showed a significant trend for Model 1 (p for trend < 0.001), Model 2 (p for trend = 0.004), and Model 3 (p for trend = 0.05). Within Model 1, significant between group differences were noted for: Low vs. moderate, low vs. high, and moderate vs. high fitness levels (all models, p < 0.001). Within Model 2, significant differences were only noted between the low and high fitness groups (p = 0.01) and the moderate and high fitness groups (p = 0.02).
DISCUSSION
The primary finding from our current study is the observation of a significant inverse relationship between fitness and MetS for both men and women regardless of the analytical model we used (Fig. 1). For men, each model demonstrated significant group differences between each fitness category. In women, models 1 and 2 demonstrated the same pattern as men; however, model 3 did not show a significant difference between the moderate and high fitness groups. This latter finding may be due in part to the degree of adjustment in model 3, which is covaried for hypercholesterolemia, hypertension and diabetes, the smaller sample of women in this study, and subtle differences for factors influencing insulin resistance between genders due to the use of oral contraceptives.10 When considering model 3, readers should note that the medications we adjusted for are important to the calculation of MetS and should be interpreted accordingly. For the purposes of our discussion we will focus on model 2, yet have presented information from all three models in our tables and figure so that the reader can make draw their own interpretations of our findings.
Numerous cross sectional and prospective studies have examined the relationship between physical activity, fitness and MetS and have thoroughly been reviewed elsewhere.11 Moreover, two reports from the ACLS have previously been published examining the influence of fitness on MetS in a much smaller cohort (N=9,007).12 Herein, we are able to extend previous findings based on a more than four-fold increase the amount of participants included in our current study. An important distinction in discussing these reports is the separation of terms used to evaluate various findings. In our current analysis we report on the effects of fitness while covarying our analysis (model 3) for physical activity. However, it can be argued that fitness is largely genetic, though trainable with exercise, and physical activity is a practiced behavior that does not always carry with it higher levels of fitness. In an effort to separate these two distinctions, Wareham et al. (1999) examined a small mixed gender cohort for the prevalence of MetS, where physical activity level and fitness, predicted by a sub-maximal exercise test, were measured.13 While the authors reported that fitness did indeed show a stronger relationship with MetS, they also demonstrated that physical activity, adjusted for fitness, also plays a significant role as well. Nonetheless, some caution should be exercised interpreting these findings given the sub-maximal nature of the “VO2max” assessment.
In a larger study using more robust measures of CRF via measured maximal oxygen uptake (i.e., VO2max, ml−1•kg−1•min−1) Lakka et al. (2003) showed that men who engaged in moderate-intensity (≥ 4.5 METS) leisure time physical activity < 1.0 h/wk were 60% more likely to have MetS study compared to counterparts engaging in > 3.0 h/wk after adjusting for covariates, including VO2max.14 Further analysis of this same cohort also showed that men with a VO2max < 29.1 ml−1•kg−1•min−1 were almost seven times more likely to have the MetS than those with ≥ 35.5 ml−1•kg−1•min−1. Therefore, physical activity and fitness appear to play a role in the prevalence of MetS. While others have reported similar findings, the association between physical activity and MetS is much steeper in those individuals who are less fit.15,16 An examination of model 3 from our current analysis supports this given its adjustment for physical activity. Based on these results, several clinical considerations should be considered.
The foremost consideration is the long held fact that fitness can be improved and the subsequent health effects are quite notable. For example, Blair et al (1995) have shown that low fit individuals substantially improve their risk for all-cause and CVD mortality improving their fitness and moving into a higher fitness category.17 In their paper, Blair showed that changing fitness categories was synonymous with a 2–4 minute (1–2 MET) improvement in time to exhaustion on a standardized treadmill test. This is clinically important because each 1 MET improvement in fitness carries with it a 13% and 15% reduction in all-cause and CVD risk mortality, respectively.18 An advantage to using estimated METs and not directly measuring VO2max is body mass. In essence, those with higher METs and excess adiposity will have a reduced per kg oxygen consumption value during VO2max testing. Estimated METs potentially avoids this controversy, Further, the minimal requirement for changing fitness category should be emphasized as such an improvement is easily achievable my most individual participating in exercise training at a low-to-moderate fitness intensity.19,20 As it pertains to MetS, Lee et al. (2012) recently examined changes in fitness versus development of CVD risk factors, inclusive of MetS, in a cross-sectional study and found that participants maintaining or improving fitness showed lower risk of developing each outcome even after adjusting for possible confounders inclusive of fatness and fitness for each.21 Our premise is clinically supported by trials examining the prevalence of MetS in those undertaking exercise training.
In the Diabetes Prevention Program, individuals with type 2 diabetes undergoing treatment with increased physical activity and dietary modification showed a 41% reduction in developing the MetS accompanying weight loss.22 Similar results have been reported from the Oslo Diet and Exercise Study.23 In studies using exercise training only, Katzmarzyk et al. (2003) reported that 30% of participants classified as having MetS in the Heritage Family Study and undertaking 20 weeks of aerobic exercise, no longer qualified as having MetS following exercise training.24 These findings are supported by others.25,26
Johnson (2007) et al. have extended the body of research on exercise training and MetS by examining the exercise dose in men and women assigned to a 6-month sedentary control group or 3 exercises doses: (1) low amount/moderate intensity (equivalent to walking ~19 km/wk), (2) low amount/vigorous intensity (equivalent to jogging ~19 km/wk), or (3) high amount/vigorous intensity (equivalent to jogging ~32 km/wk).27 The authors of this study reported that while low-amount/moderate-intensity exercise and high amount/vigorous intensity exercise improved MetS relative to inactive controls, the low amount/vigorous intensity group did showed no such relationship. This latter study presents an interesting, though counterintuitive, finding possibly suggesting that short duration, higher intensity exercise, may not be effective in reducing the MetS. However, two exercise trials have shown that interval training, which is short duration and high intensity exercise by nature, to have a greater effect in reducing MetS in men.28,29
While we have attempted to focus our discussion on the effect of fitness and MetS as a singular component, it would be remiss not to briefly address of our findings regarding individual MetS components scores. Our most noteworthy finding is the strong, inverse association between fitness and waist circumference in both men (models 2 & 3; r2=0.78) and women (models 2 & 3; r2=0.71; Table 4). This is an important observation as Reaven’s hypothesis suggests that insulin resistance is the mediating cause of obesity; while in contrast, the NCEP definition of MetS implies that central adiposity has the central role for driving insulin resistance as overweight and obese individuals show a greater prevalence of MetS than their non-obese counterparts.30 Though our findings preclude a thorough discussion surrounding the mechanisms of action involved with visceral adiposity and MetS, the topic has been thoroughly reviewed elsewhere.31 Briefly, however, it has been suggest by some that MetS alone may not adequately predict CVD and that abdominal obesity, hence, “dysfunctional adipose tissue,” may be more essential for clinical purposes.31 Morever, regardless of the clinical significance of MetS vs. abdominal obesity, all of our statistical models show a strong relationship between fitness and waist circumference. In addition, weight loss is not essential for reducing waist circumference via exercise participation and therefore, should be encouraged in participants in need of reducing MetS risk, regardless of weight loss.32
Table 4.
Standardized regression coefficients examining the association between cardiorespiratory fitness and single and clustered metabolic syndrome components in men (n = 30,167) and women (n = 8,492)
| Cardiorespiratory Fitness (METs) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||||||
| Men | R2 | β | R2 Change | P value | R2 | β | R2 Change | P value | R2 | β | R2 Change | P value |
| Waist circumference (cm) | 0.32 | −0.57 | 0.296 | < 0.001 | 0.78 | −0.14 | 0.012 | < 0.001 | 0.78 | −0.13 | 0.010 | < 0.001 |
| Triglycerides (mg/dL) a | 0.15 | −0.41 | 0.150 | < 0.001 | 0.18 | −0.29 | 0.053 | < 0.001 | 0.23 | −0.26 | 0.040 | < 0.001 |
| HDL-cholesterol (mg/dL) b | 0.13 | 0.34 | 0.106 | < 0.001 | 0.17 | 0.25 | 0.038 | < 0.001 | 0.18 | 0.23 | 0.031 | < 0.001 |
| Fasting blood glucose (mg/dL) a | 0.06 | −0.18 | 0.029 | < 0.001 | 0.09 | −0.09 | 0.005 | < 0.001 | 0.14 | −0.05 | 0.002 | < 0.001 |
| Systolic blood pressure (mmH g) a | 0.06 | −0.11 | 0.011 | < 0.001 | 0.11 | 0.00 | 0.000 | 0.782 | 0.16 | 0.02 | 0.000 | 0.007 |
| Diastolic blood pressure (mmHg) | 0.07 | −0.20 | 0.035 | < 0.001 | 0.12 | −0.07 | 0.003 | < 0.001 | 0.17 | −0.05 | 0.002 | < 0.001 |
| zMS | 0.20 | −0.37 | 0.121 | < 0.001 | 0.38 | −0.11 | 0.008 | < 0.001 | 0.42 | −0.08 | 0.004 | < 0.001 |
| Women | ||||||||||||
| Waist circumference (cm) | 0.22 | −0.45 | 0.172 | < 0.001 | 0.71 | −0.04 | 0.001 | < 0.001 | 0.71 | −0.03 | 0.001 | < 0.001 |
| Triglycerides (mg/dL) a | 0.14 | −0.30 | 0.073 | < 0.001 | 0.18 | −0.17 | 0.017 | < 0.001 | 0.21 | −0.15 | 0.014 | < 0.001 |
| HDL-cholesterol (mg/dL) b | 0.11 | 0.24 | 0.049 | < 0.001 | 0.19 | 0.08 | 0.004 | < 0.001 | 0.20 | 0.07 | 0.003 | < 0.001 |
| Fasting blood glucose (mg/dL) a | 0.06 | −0.10 | 0.008 | < 0.001 | 0.09 | 0.01 | 0.000 | 0.336 | 0.12 | 0.04 | 0.001 | 0.006 |
| Systolic blood pressure (mmHg) a | 0.16 | −0.13 | 0.014 | < 0.001 | 0.21 | −0.01 | 0.000 | 0.604 | 0.26 | 0.01 | 0.000 | 0.456 |
| Diastolic blood pressure (mmHg) | 0.10 | −0.16 | 0.021 | < 0.001 | 0.14 | −0.05 | 0.001 | < 0.001 | 0.20 | −0.04 | 0.001 | 0.006 |
| zMS | 0.27 | −0.27 | 0.063 | < 0.001 | 0.44 | −0.05 | 0.002 | < 0.001 | 0.48 | −0.03 | 0.001 | 0.002 |
Values were natural log-transformed before analysis.
Values were square root transformed before analysis.
Model 1: Adjustment for age, examination year;
Model 2: Adjustment for age, examination year, BMI, smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not) and family history of cardiovascular disease;
Model 3: Adjustment for age, examination year, BMI, physical activity (active or inactive), smoking (current smoker or not), alcohol intake (>14 drinks/week for men and >7 for women or not), hypercholesterolemia, hypertension and diabetes (present or not for each), and family history of cardiovascular disease.
Limitations of the current study include a focus on participants who were primarily white well-educated, and with middle to upper socioeconomic status. The results may not apply to other racial groups. However, the homogeneity of our sample strengthens the internal validity of our findings by reducing potential confounding by unmeasured factors related to socioeconomic status, such as income, education, or prestige. Overall, our study shows that fitness is strongly and inversely related to MetS. The strength of these associations persists to waist circumference, which, regardless of hypotheses focusing on insulin resistance or central adiposity, demonstrate the effectiveness of fitness on the overall MetS score and the one individual component most highly associated with the development of MetS, type 2 diabetes, and CVD mortality risk.
Supplementary Material
Acknowledgments
Funding/Support: National Institutes of Health grants (AG06945, HL62508, R21DK088195) and the Spanish Ministry of Education (EX-2010-1008).
We thank Cooper Clinic physicians, technicians and staff at the Cooper Institute.
Footnotes
Trial Registration: N/A
No other Disclosures are reported.
Disclosures
Dr. Blair receives book royalties (<$5,000/year) from Human Kinetics; honoraria for service on the Scientific/Medical Advisory Boards for Alere, Technogym, Santech, Clarity, and Jenny Craig; and honoraria for lectures and consultations from scientific, corporate, educational, and lay groups. He has received research grants from the National Institutes of Health, The Coca-Cola Company, Department of Defense, and Body Media
Dr. Church receives honoraria for lectures from scientific, educational, and lay groups. Dr. Church has a book entitled “Move Yourself: The Cooper Clinic Medical Director’s Guide to All the Healing Benefits of Exercise.” Dr. Church has received research funding from the American Heart Association and the National Institutes of Health as well as unrestricted research funding from Coca-Cola. Dr. Church has overseen study sites for large pharmaceutical trials funded by Sanofi Aventis, Orexigen, Arena and Amylin. Dr. Church is a member of the Jenny Craig Medical Advisory Board and has served as a consultant to Technogym, Trestle Tree, Vivus, Lockton-Dunning and Neuliven Health. In addition, he serves as the Senior Medical Advisor for Catapult Health
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Brage S, Wedderkopp N, Ekelund U, et al. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS) Diabetes Care. 2004 Sep;27(9):2141–2148. doi: 10.2337/diacare.27.9.2141. [DOI] [PubMed] [Google Scholar]
- 3.Lee DC, Sui X, Ortega FB, et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011 May;45(6):504–510. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- 4.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005 Feb 8;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 5.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989 Nov 3;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 6.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976 Jul;92(1):39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 7.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982 Mar;103(3):363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 8.American College of Sports Medicine. ACSM’s Guidelines For Exercise Testing And Prescription. 7. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 9.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–3421. [PubMed] [Google Scholar]
- 10.Clausen JO, Borch-Johnsen K, Ibsen H, et al. Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians. Analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest. 1996 Sep 1;98(5):1195–1209. doi: 10.1172/JCI118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med. 2004;34(6):371–418. doi: 10.2165/00007256-200434060-00004. [DOI] [PubMed] [Google Scholar]
- 12.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005 Jul 26;112(4):505–512. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 13.Wareham NJ, Hennings SJ, Byrne CD, Hales CN, Prentice AM, Day NE. A quantitative analysis of the relationship between habitual energy expenditure, fitness and the metabolic cardiovascular syndrome. Br J Nutr. 1998 Sep;80(3):235–241. doi: 10.1017/s0007114598001287. [DOI] [PubMed] [Google Scholar]
- 14.Lakka TA, Laaksonen DE, Lakka HM, et al. Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc. 2003 Aug;35(8):1279–1286. doi: 10.1249/01.MSS.0000079076.74931.9A. [DOI] [PubMed] [Google Scholar]
- 15.Franks PW, Ekelund U, Brage S, Wong MY, Wareham NJ. Does the association of habitual physical activity with the metabolic syndrome differ by level of cardiorespiratory fitness? Diabetes Care. 2004 May;27(5):1187–1193. doi: 10.2337/diacare.27.5.1187. [DOI] [PubMed] [Google Scholar]
- 16.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002 Sep;25(9):1612–1618. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- 17.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995 Apr 12;273(14):1093–1098. [PubMed] [Google Scholar]
- 18.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009 May 20;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 19.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007 May 16;297(19):2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 20.Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005 Oct;99(4):1613–1618. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lee DC, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. Journal of the American College of Cardiology. 2012 Feb 14;59(7):665–672. doi: 10.1016/j.jacc.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBride PE, Einerson JA, Grant H, et al. Putting the Diabetes Prevention Program into practice: a program for weight loss and cardiovascular risk reduction for patients with metabolic syndrome or type 2 diabetes mellitus. The journal of nutrition, health & aging. 2008 Dec;12(10):745S–749S. doi: 10.1007/BF03028624. [DOI] [PubMed] [Google Scholar]
- 23.Torjesen PA, Birkeland KI, Anderssen SA, Hjermann I, Holme I, Urdal P. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care. 1997 Jan;20(1):26–31. doi: 10.2337/diacare.20.1.26. [DOI] [PubMed] [Google Scholar]
- 24.Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003 Oct;35(10):1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 25.Stewart KJ, Bacher AC, Turner K, et al. Exercise and risk factors associated with metabolic syndrome in older adults. American journal of preventive medicine. 2005 Jan;28(1):9–18. doi: 10.1016/j.amepre.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Watkins LL, Sherwood A, Feinglos M, et al. Effects of exercise and weight loss on cardiac risk factors associated with syndrome X. Archives of internal medicine. 2003 Sep 8;163(16):1889–1895. doi: 10.1001/archinte.163.16.1889. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JL, Slentz CA, Houmard JA, et al. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise) The American journal of cardiology. 2007 Dec 15;100(12):1759–1766. doi: 10.1016/j.amjcard.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Earnest CP, Lupo M, Thibodeaux J, et al. Interval training in men at risk for insulin resistance. Int J Sports Med. doi: 10.1055/s-0032-1311594. In press. [DOI] [PubMed] [Google Scholar]
- 29.Tjonna AE, Lee SJ, Rognmo O, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008 Jul 22;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Archives of internal medicine. 2003 Feb 24;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 Dec 14;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 32.Ross R, Freeman JA, Janssen I. Exercise alone is an effective strategy for reducing obesity and related comorbidities. Exercise and sport sciences reviews. 2000 Oct;28(4):165–170. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.