ABSTRACT
Levels of the anaphylatoxin C3a are increased in patients with asthma compared with those in nonasthmatics and increase further still during asthma exacerbations. However, the role of C3a during sensitization to allergen is poorly understood. Sensitization to fungal allergens, such as Aspergillus fumigatus, is a strong risk factor for the development of asthma. Exposure to chitin, a structural polysaccharide of the fungal cell wall, induces innate allergic inflammation and may promote sensitization to fungal allergens. Here, we found that coincubation of chitin with serum or intratracheal administration of chitin in mice resulted in the generation of C3a. We established a model of chitin-dependent sensitization to soluble Aspergillus antigens to test the contribution of complement to these events. C3−/− and C3aR−/− mice were protected from chitin-dependent sensitization to Aspergillus and had reduced lung eosinophilia and type 2 cytokines and serum IgE. In contrast, complement-deficient mice were not protected against chitin-induced innate allergic inflammation. In sensitized mice, plasmacytoid dendritic cells from complement-deficient animals acquired a tolerogenic profile associated with enhanced regulatory T cell responses and suppressed Th2 and Th17 responses specific for Aspergillus. Thus, chitin induces the generation of C3a in the lung, and chitin-dependent allergic sensitization to Aspergillus requires C3aR signaling, which suppresses regulatory dendritic cells and T cells and induces allergy-promoting T cells.
IMPORTANCE
Asthma is one of the fastest growing chronic illnesses worldwide. Chitin, a ubiquitous polymer in our environment and a key component in the cell wall of fungal spores and the exoskeletons of insects, parasites, and crustaceans, triggers innate allergic inflammation. However, there is little understanding of how chitin is initially recognized by mammals and how early recognition of chitin affects sensitization to environmental allergens and development of allergic asthma. The complement system is evolutionarily one of the oldest facets of the early or innate warning systems in mammals. We studied whether and how complement components influence the recognition of chitin and shape the downstream sensitization toward fungal allergens. We show here that complement recognition of chitin plays a critical role in shaping the behavior of dendritic cells, which in turn regulate the function of T cells that mediate allergic responses to fungi.
Introduction
Environmental exposure to molds, such as Aspergillus fumigatus, is correlated with an increased risk of asthma. Moreover, in patients with severe acute onset asthma, more than half are hypersensitive to Aspergillus (1). In addition, detection of Aspergillus in sputum culture and IgE sensitization to Aspergillus are predictive for poor lung function in patients with asthma (2). Following the inhalation of conidia or hyphae, sensitization to Aspergillus is mediated by fungal proteases that cleave and activate protease-activated receptors (PAR) and by fungal wall components that activate pattern recognition receptors (PRR) on structural and hematopoietic cells in the lung (3, 4). These diverse signals generated upon exposure to Aspergillus drive the Th2 adaptive response in individuals with asthma.
Dendritic cells (DC) integrate the signals generated in the lung following exposure to Aspergillus. Immature DC line the airways and parenchyma of the lung and consist of plasmacytoid DC (pDC), CD11b+ DC, and CD103+ DC (5, 6). In the absence of robust stimulation via PRR or cytokines, inhaled antigen taken up by DC induces tolerance by promoting the development of regulatory T cells (Treg) (7). On the other hand, exposure to an antigen in the context of another inflammatory stimulus, such as lipopolysaccharide (LPS), enhances the ability of DC to migrate from the lung to the lymph node (8) and upregulates costimulatory molecules (9) leading to the priming of effector T cells. The presence of inflammation also recruits Ly6Chi monocytes to the lung, which differentiate into inflammatory or monocyte-derived DC (10) and can also prime effector T cells. DC subsets vary in their ability to instruct T cell polarization, and the ultimate outcome of the DC-T cell interaction is dependent on the presented antigen, the tissue microenvironment, including cytokine and chemokine levels, and the interaction of costimulatory molecules on the DC and T cells. During sensitization to allergens in the lung, DC can be activated directly via PRR (11) or indirectly via PRR activation on lung stromal cells (12). This activation results in the maturation of DC, characterized by increased expression of CD80, CD86, CD273 (B7-DC or PD-L2), and CD274 (B7-H1 or PD-L1) (13). Thus, DC are able to modulate and direct inflammation in the lung following antigen exposure by influencing the balance between tolerance and inflammation.
In coordination with DC, the complement system also critically maintains the balance between tolerance and inflammation in the lung. The complement system is an evolutionarily ancient protein cascade of the animal immune system that augments innate immune function by opsonizing targeted surfaces, modifying pathogenic or damaged cell surfaces to promote lysis, and recruiting innate effector cells to the site of inflammation (14). Complement factor 3C3 is the lynchpin of the complement cascade, and the initial cleavage of (C3) produces the anaphylatoxin C3a and a larger fragment, C3b. C3a mediates its effects via its receptor, C3aR, which is expressed on lymphoid and myeloid cells (15) and on pulmonary epithelial and smooth muscle cells (16). On DC, engagement of C3aR leads to cAMP-dependent increased antigen uptake and augments the ability of DC to promote T cell polarization (17). Thus, C3a is well suited to act on lung parenchymal cells as well as lung-resident and recruited immune cells to direct the immune response to encountered antigen.
Chitin is a ubiquitous polysaccharide common to the cell walls of fungi, including Aspergillus (18). Upon exposure, chitin triggers responses from organisms across the kingdoms of life, identifying it as an evolutionarily conserved and stimulatory molecular pattern (19–22). In the lung, chitin exposure promotes innate allergic inflammation that is characterized by alternatively activated macrophages, eosinophilia, and neutrophilia (21, 22). Following intraperitoneal injection of chitin particles with the model antigen ovalbumin, chitin can also serve as an adjuvant that drives polarization of CD4+ T cells into Th1, Th2, and Th17 cells (23). However, whether chitin can serve as an adjuvant to promote allergic inflammation and sensitization in the lung is unknown. Since many inhaled allergens of insect or fungal origin contain chitin (24, 25), understanding the mechanism of chitin-induced allergic inflammation in the lung is relevant to understanding allergic sensitization in asthma.
In the present study, we sought to determine whether chitin-induced allergic inflammation is dependent on C3 and C3aR signaling in the lung and to evaluate the impact of chitin administration on the maturation of lung DC during sensitization to Aspergillus antigens. We demonstrate that chitin activates complement and that C3 and C3aR promote adaptive allergic inflammatory responses by modulating lung DC function in a model of chitin-induced allergy to Aspergillus.
RESULTS
Chitin activates complement in vitro and in vivo.
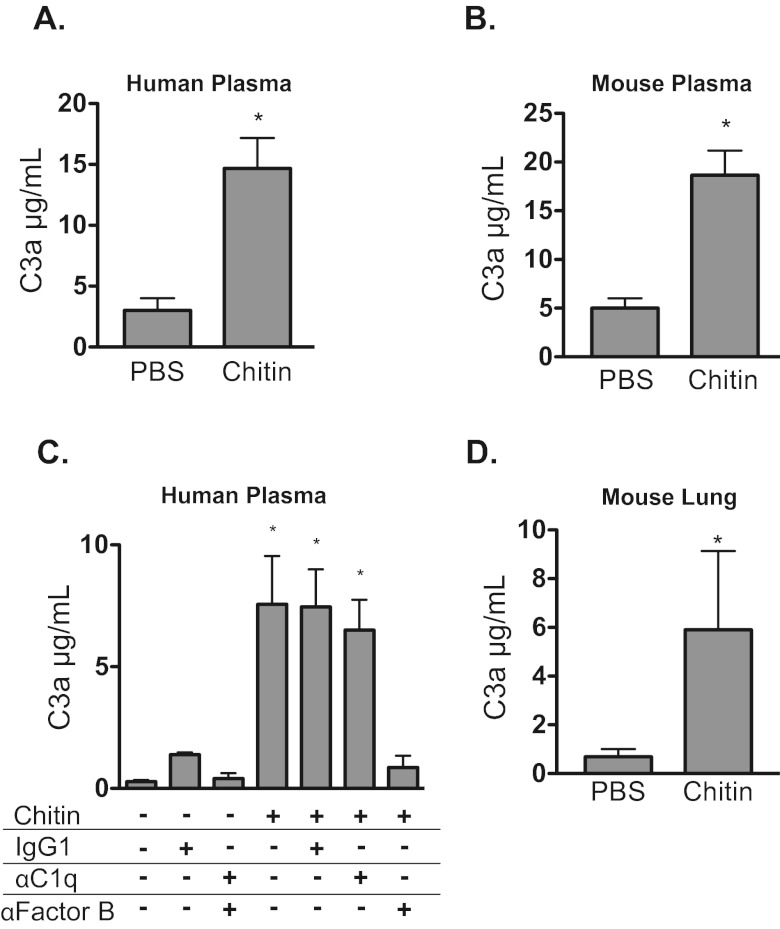
To investigate whether chitin particles are able to activate complement directly, we measured the production of the anaphylatoxin C3a in chitin-exposed human serum. Chitin particles induced the production of C3a in human serum (Fig. 1A) and mouse serum (Fig. 1B). Because chitin is a surface component of various pathogens such as fungi, parasite cysts, and helminth eggs, activation of complement could proceed via the antibody-mediated classical pathway or the antibody-independent alternative pathway. In addition to antibodies potentially generated by exposure to environmental sources of chitin, natural IgM antibodies to chitin have been described in animals across the evolutionary spectrum (26). To delineate the mechanism of chitin-induced complement activation, we added chitin to serum in the presence of neutralizing antibody against C1q to block the classical activation pathway or against factor B to block the alternative complement activation pathway. Neutralizing antibodies to factor B but not to C1q blocked the activation of complement by chitin particles in serum, suggesting that the major route of complement activation by chitin is via the alternative pathway (Fig. 1C).
FIG 1 .
Chitin particles generate C3a in vivo and via the alternative pathway in vitro. Chitin particles or Aspergillus antigens were coincubated with plasma and neutralizing antibodies. C3a levels were determined by ELISA. Chitin induced C3a generation in human plasma (n = 3) (A) and in mouse plasma (n = 3) (B). (C) Chitin induces C3a generation in human plasma via the alternative pathway. IgG1, isotype control antibody (Ab); αC1q, C1q neutralizing Ab; αFactorB, factor B neutralizing Ab (n = 3). (D) Chitin particles or PBS was administered intratracheally (i.t.). Within 24 h, perfused lung homogenate was obtained and C3a levels were determined by ELISA (n = 5). Values are presented as means ± standard errors of the means (SEM); *, P < 0.05 versus PBS.
To determine if chitin could activate complement in the lung, we assayed lung homogenates from naive and chitin-exposed mice for the presence of C3a by enzyme-linked immunosorbent assay (ELISA). Lung homogenate from chitin-exposed mice had significantly more C3a than did lung homogenates from naive mice (Fig. 1D). Thus, chitin promotes C3a generation in vitro and in vivo in the lung.
A model of chitin-dependent sensitization to Aspergillus antigens.
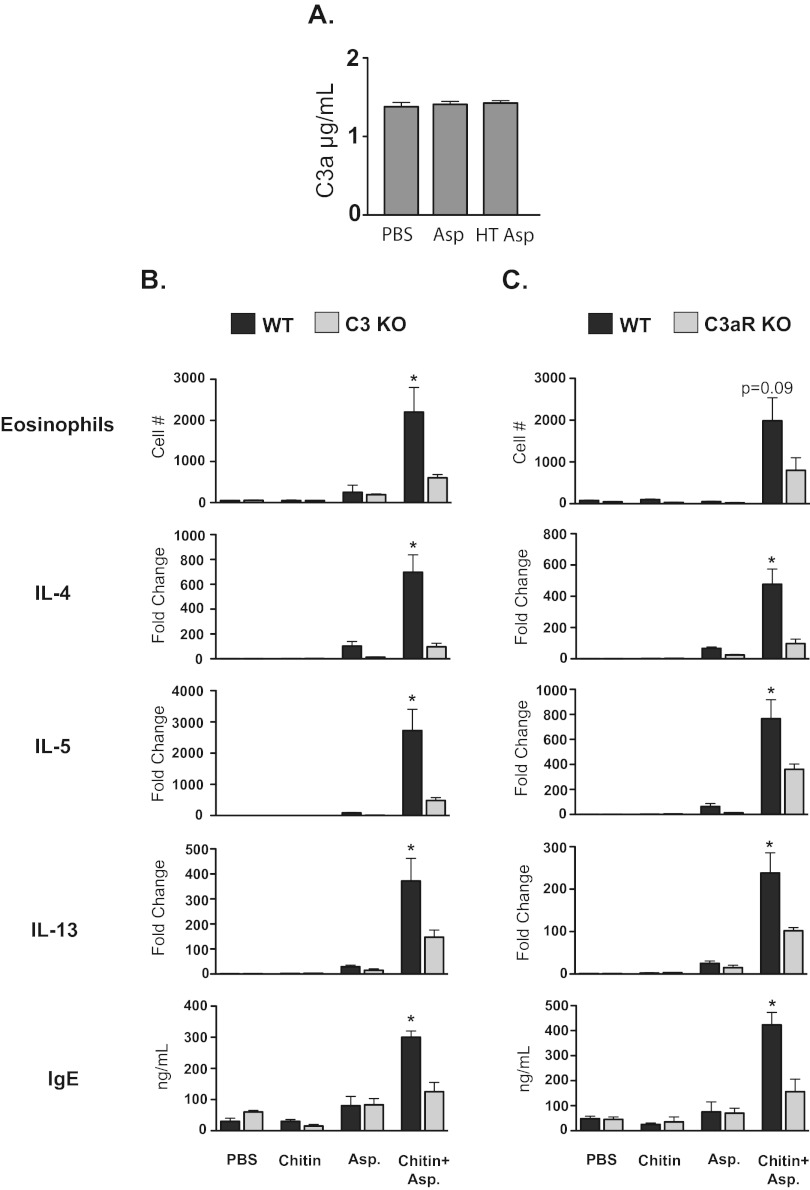
We established a model of chitin-dependent sensitization to allergen (below and manuscript in preparation) by utilizing soluble Aspergillus proteins, which can be important in the development of allergic asthma (27). Protease allergens from Aspergillus possess the ability to cleave C3 directly (28, 29) and could conceivably drive complement activation. We therefore tested whether the soluble Aspergillus protein antigens themselves could activate complement directly. Incubation of serum with Aspergillus antigens before or after heat treatment (to neutralize potential protease activity) failed to result in the production of C3a (Fig. 2A). Thus, soluble Aspergillus antigens alone lack the ability to generate C3a in vitro.
FIG 2 .
Chitin promotes C3- and C3aR-dependent allergic inflammation to Aspergillus antigens. (A) Aspergillus antigens fail to generate C3a in human plasma. HT Asp, heat-treated Aspergillus antigens (n = 3). (B) C3KO mice versus wild type (n = 5). (C) C3aRKO mice versus wild type (n = 5). In panels B and C, wild-type, C3KO, and C3aRKO mice were sensitized to Aspergillus as described in Materials and Methods. Eosinophils were enumerated from lung homogenates by flow cytometry. IL-4, IL-5, and IL-13 expression levels were determined by real-time PCR from mRNA isolated from lung homogenates. IgE was measured in serum by ELISA. Values are presented as means ± SEM. *, P < 0.05, for comparison of wild-type versus respective KO mice.
Chitin-induced allergic sensitization is C3-C3a dependent.
Aspergillus antigens alone fail to induce markers of allergic inflammation in the lung, whereas mice exposed to chitin particles during sensitization to Aspergillus demonstrate increased pulmonary eosinophilia (Fig. 2B). Furthermore, mice sensitized to Aspergillus in the presence of chitin showed increased serum IgE levels and increased expression of the allergy-associated cytokines IL-4, IL-5, and IL-13 in lung homogenates, compared with mice exposed to either chitin or Aspergillus antigen alone (Fig. 2B). Thus, chitin acts as an adjuvant during Aspergillus-induced allergic sensitization.
Because chitin can activate complement, we tested whether complement is required for chitin-dependent allergic inflammation. C3KO mice were protected against allergic sensitization in this model and exhibited lower pulmonary eosinophilia; reduced expression of IL-4, IL-5, and IL-13; and lower levels of serum IgE (Fig. 2B). To test whether the anaphylotoxin C3a is required for chitin-induced allergic inflammation to Aspergillus antigens, we sensitized wild-type and C3aRKO mice to Aspergillus in the presence or absence of chitin. Mirroring the results from C3KO mice, C3aRKO mice had reduced pulmonary eosinophilia; lower IL-4, IL-5, and IL-13 expression in the lung; and lower serum IgE levels than did wild-type mice (Fig. 2C). Thus, the ability of chitin particles to promote sensitization to Aspergillus and adaptive allergic inflammation is dependent on C3 and signaling via C3aR.
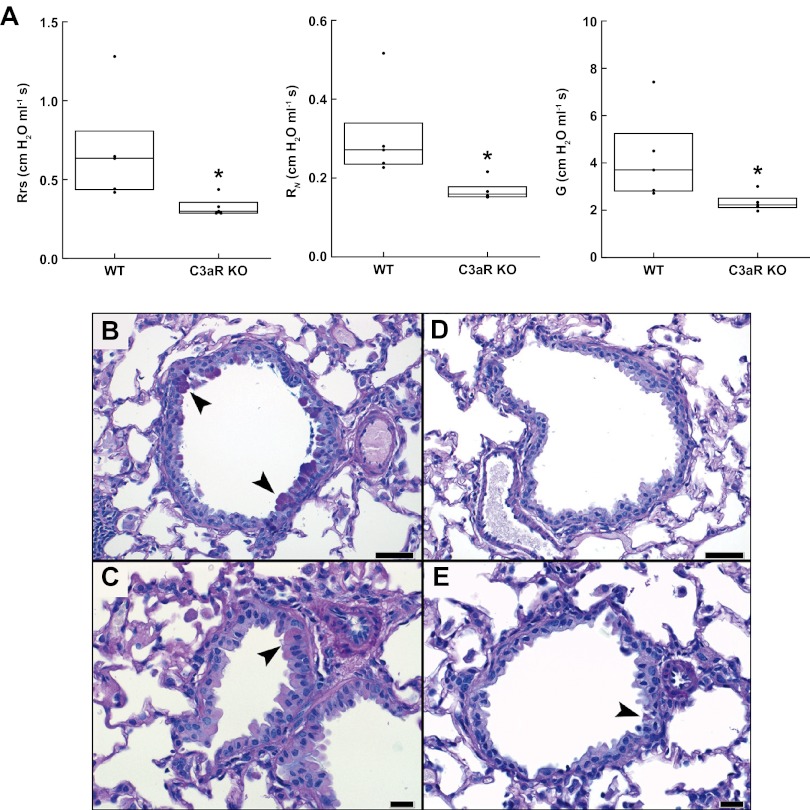
In addition to analyzing cellular influx in wild-type and complement knockout mice, we analyzed physiological correlates of asthma by looking at airflow resistance and lung tissue histology. Measures of airway function showed that respiratory system resistance (Rrs), Newtonian resistance (RN), and tissue damping (G), which were elevated in wild-type (WT) mice, were each significantly attenuated in the C3aRKO mice (Fig. 3A). Histologically, the airways of WT mice showed significantly more goblet cell hyperplasia than that observed in C3aRKO mice (Fig. 3B to E). Using a scoring system of 0 to 5 for the extent of epithelium composed of goblet cells (1 = <10%; 5 = >75%), the values were 3.1 ± 1.5 versus 0.9 ± 0.9 for WT and C3aRKO mice, respectively (P = 0.005). All fields of WT mice showed goblet cell hyperplasia (scores, 1 to 5), whereas half of the fields from KO mice showed no hyperplasia. Thus, physiological and histological studies buttressed other cellular features of allergic lung inflammation.
FIG 3 .
Physiological and histological analysis of airway inflammation in wild-type and complement-deficient mice. (A) Comparison of respiratory system resistance (Rrs), Newtonian resistance (RN), and tissue damping (G) in WT and C3aRKO mice after sensitization with Aspergillus antigens and chitin. Box plots indicate medians and interquartile ranges for each group. *, P = 0.016 (Rrs), 0.009 (RN), and 0.028 (G) compared with the WT group. (B) Photomicrographs of alcian blue PAS-stained, formalin-fixed, paraffin-embedded lung sections from WT (B, C) and C3aR KO (D, E) mice. Medium-caliber airways (above), bar = 50 µm; and smaller-caliber nonrespiratory airways (below), bar = 20 µm. The arrowheads depict epithelial cells with apical intracytoplasmic granules exhibiting positive PAS staining (goblet cells). Images are representative of an average of seven sized-matched large airways and eight size-matched small airways.
Chitin-induced innate allergic inflammation is not dependent on C3 or C3aR.
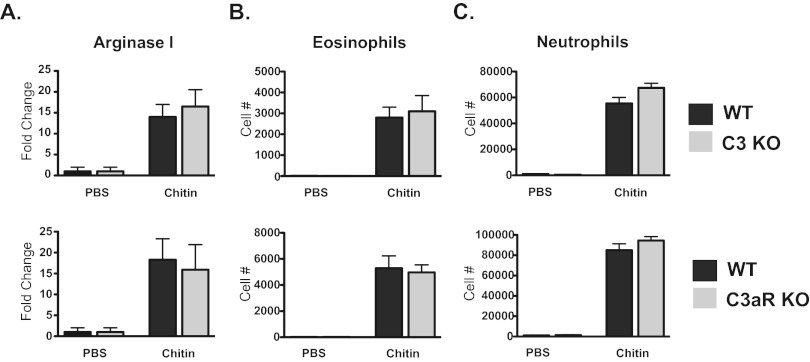
Because chitin alone can induce innate allergic inflammation characterized by alternatively activated macrophages and recruitment of eosinophils (21, 22), we tested whether C3 or signaling via C3aR was required for chitin-induced innate allergic inflammation. Forty-eight hours after chitin exposure, we compared expression of the macrophage alternative activation marker arginase I in lung homogenates from wild-type, C3KO, and C3aRKO mice. Neither C3KO mice nor C3aRKO mice demonstrated a defect in chitin-induced alternative activation of macrophages as measured by arginase I expression (Fig. 4A). We also quantified the recruitment of SiglecF+/CD11c− eosinophils to the lung following chitin exposure. Eosinophil recruitment in C3KO or C3aRKO mice was similar to that in wild-type mice following chitin exposure (Fig. 4B). Severe asthma and allergic bronchopulmonary aspergillosis (ABPA) are associated with airway neutrophilia (30–32), as is exposure to chitin (21, 22). Following exposure to chitin, wild-type, C3KO, and C3aRKO mice did not demonstrate a difference in recruited neutrophils in the lung (Fig. 4C). Therefore, C3 and signaling via C3aR are not required for chitin-induced innate pulmonary eosinophilia, neutrophilia, or alternative activation of macrophages.
FIG 4 .
Chitin-induced innate allergic inflammation is not C3 or C3aR dependent. Forty-eight hours following chitin exposure, lung homogenates were obtained. (A) Arginase I expression was determined by real-time PCR (n = 5). Values are presented as mean fold changes in arginase expression relative to PBS ± SEM. Eosinophils (B) and neutrophils (C) were enumerated from lung homogenates by flow cytometry (n = 5). Values are presented as means ± SEM.
C3/C3aR signaling blocks lung DC from acquiring a tolerogenic phenotype following chitin exposure.
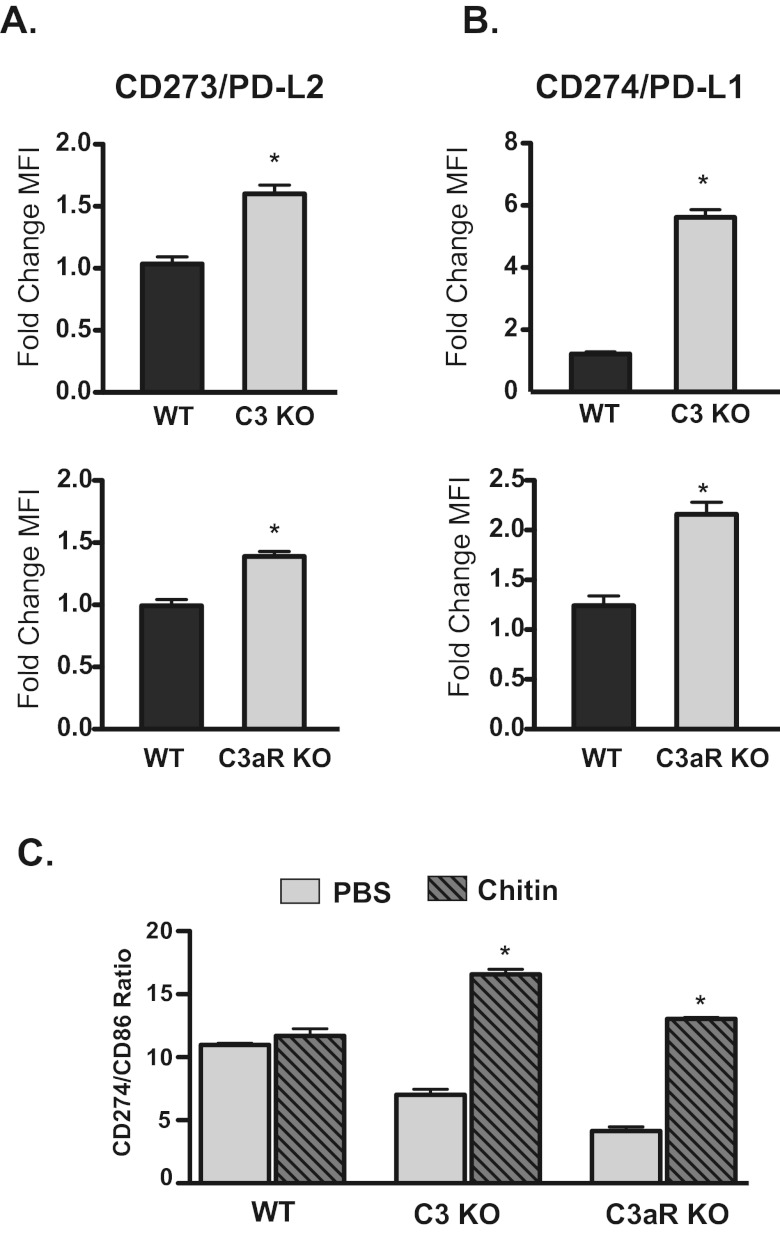
DCs play a critical role in shaping the adaptive immune response to antigen. Since C3KO and C3aRKO mice fail to mount a robust allergic response to Aspergillus antigens in the presence of chitin, we tested whether chitin exposure modulated DC maturation in a C3- and C3aR-dependent fashion. The failure of C3KO and C3arKO mice to mount an allergic response to Aspergillus in the presence of chitin could be the result of either a reduction in the ability of DC to stimulate T cells or an increase in the ability of DC to suppress T cell activation. In the lung, pDC promote tolerance to inhaled antigen by suppressing effector T cells and promoting regulatory T cells (33), a process that depends on the expression of CD273 and CD274 (34). Upon exposure to chitin, pDC in the lungs of wild-type mice failed to upregulate CD273 or CD274 (Fig. 5A and B). However, pDC from chitin-exposed C3KO mice and C3aRKO mice significantly increased expression of CD273 and CD274 (Fig. 5A and B). Tolerance to antigen is correlated with an elevated ratio of CD274 to CD86 expression on pDC (35). After exposure to chitin, pDC from C3KO and C3aRKO mice had a significantly increased CD274/CD86 expression ratio, whereas pDC from wild-type mice did not (Fig. 5C). Thus, pDC from C3KO and C3aRKO mice acquire a tolerogenic phenotype after exposure to chitin, whereas pDC from wild-type mice do not. C3 and C3aR signaling may thus retard the development of a tolerogenic phenotype in response to chitin.
FIG 5 .
Chitin induced a tolerogenic phenotype on lung pDC in the absence of C3 and C3aR. Forty-eight hours following chitin exposure, lung homogenates were obtained and the expression of CD273, CD274, and CD86 on pDC was determined by flow cytometry. pDC were defined as CD11b− CD11cdim CD62L+ Siglec H+. (A) Expression of CD273 on lung pDC (n = 5). Values are mean fold change ± SEM of the mean fluorescent intensity (MFI) of chitin-exposed mice versus PBS-exposed mice. *, P < 0.05 KO versus wild type. (B) Expression of CD274 on lung pDC (n = 5). Values are mean fold change ± SEM of the MFI of chitin-exposed mice versus PBS-exposed mice. *, P < 0.05 for comparison of KO versus wild type. (C) The tolerogenic phenotype of pDC in the absence of C3 and C3aR. Values are the ratios ± SEM between the MFI of CD274 and the MFI of CD86 on lung pDC. *, P < 0.05 for comparison of chitin versus PBS.
Chitin promotes robust CD80/CD86 activation on CD11b+ and inflammatory DC subsets in the lung.
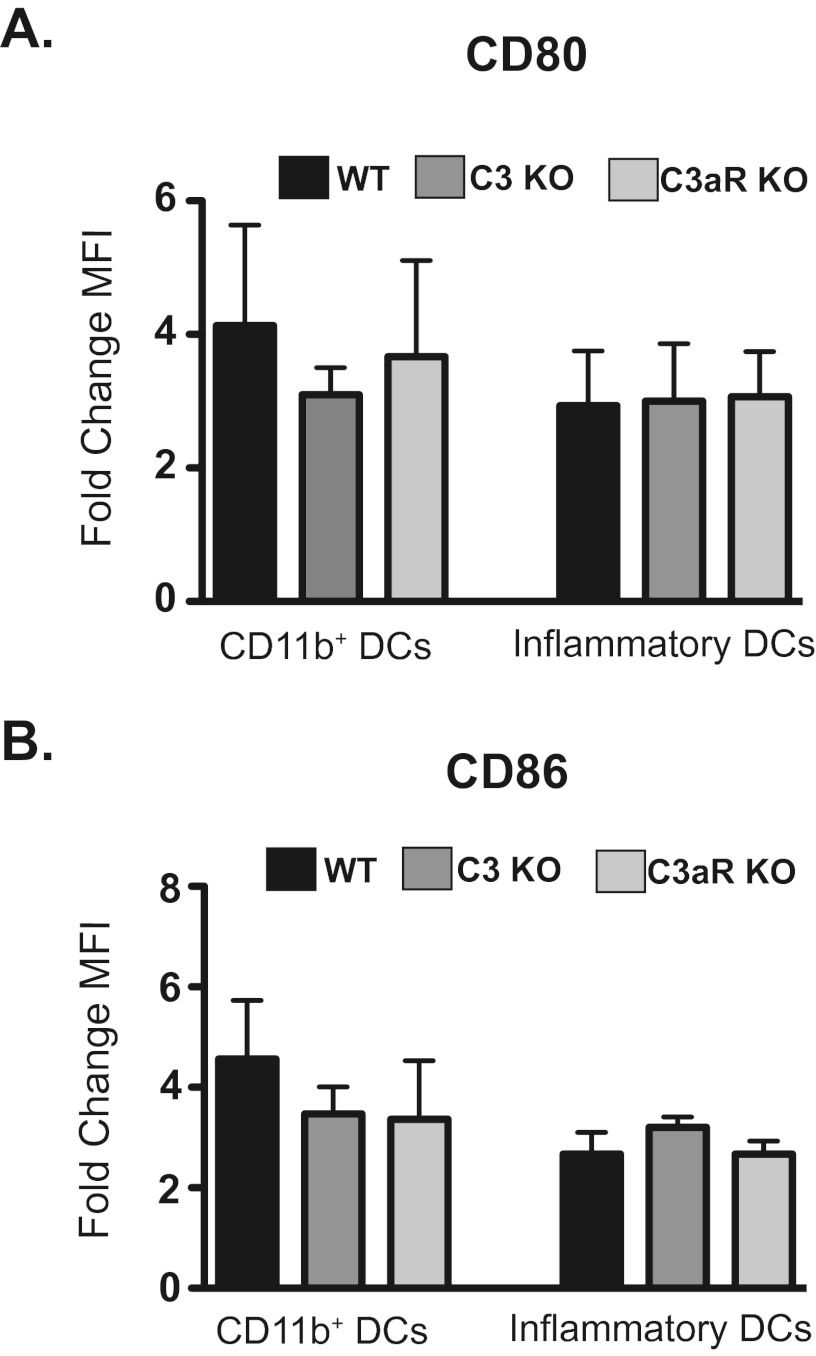
We also investigated how C3 or C3aR signaling influenced the activation of myeloid DC subsets in the lung 48 h after chitin exposure. We measured the surface expression of the costimulatory molecules CD80 and CD86 on CD11b+, CD103+, and inflammatory DC. Chitin exposure failed to increase CD80 or CD86 expression on CD103+ DC from wild-type, C3KO, or C3aRKO mice (data not shown). Resident CD11b+ DC upregulated CD80 and CD86 expression following chitin exposure, as did monocyte-derived inflammatory DC (Fig. 6A and B). The upregulation of CD80 and CD86 on CD11b+ DC and inflammatory DC was equivalent in wild-type, C3KO, and C3aRKO mice (Fig. 6A and B). Thus, chitin exposure induces the maturation of myeloid DC subsets in the lung in a manner that is independent of C3 and C3aR signaling.
FIG 6 .
Chitin-induced CD80 and CD86 expression on pulmonary myeloid DC is not C3 or C3aR dependent. Forty-eight hours following chitin exposure, lung homogenates were obtained and the expression of CD80 and CD86 on myeloid DC subsets was determined by flow cytometry. CD11b+ DC were defined as CD11b+ CD11c+ Ly6C− CD103−. Inflammatory DC were defined as CD11b+ CD11c+ Ly6C+. (A) Expression of CD80 on lung CD11b+ and inflammatory DC (n = 5). Values are mean fold change ± SEM of the mean fluorescent intensity (MFI) of chitin-exposed mice versus PBS-exposed mice. (B) Expression of CD86 on lung CD11b+ and inflammatory DC (n = 5). Values are mean fold changes ± SEM of the mean fluorescent intensity (MFI) of chitin-exposed mice versus PBS-exposed mice.
Chitin promotes Th2 and Th17 differentiation and inhibits Treg differentiation in a C3-dependent manner.
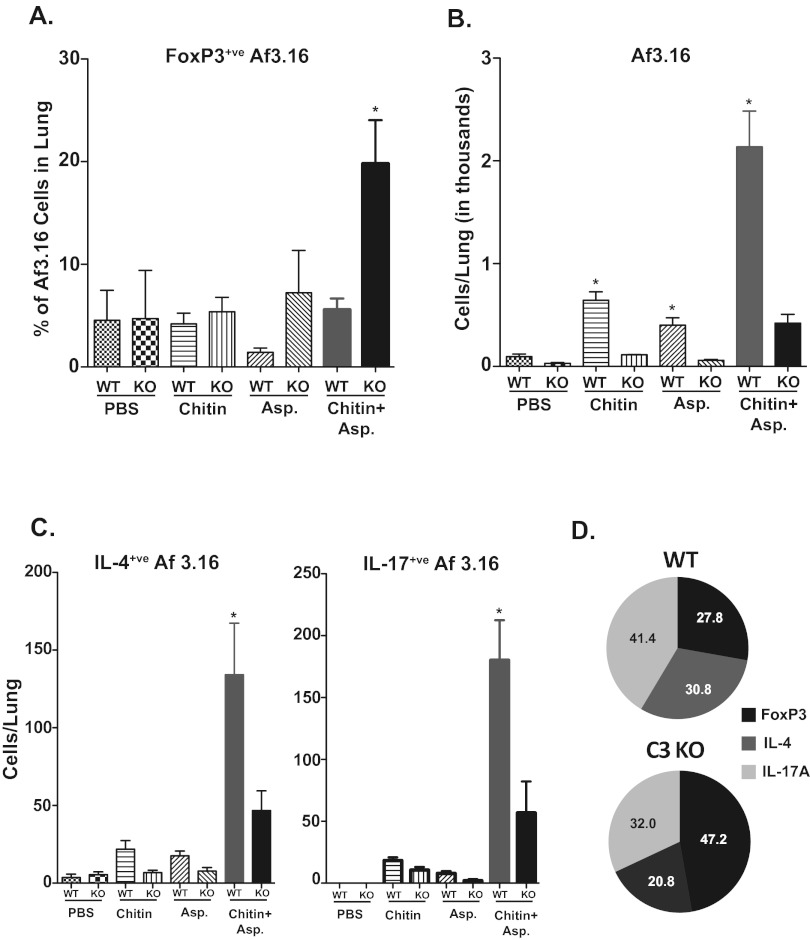
To ascertain the functional consequence of C3a inhibition of tolerogenic DCs, we evaluated CD4+ T cell responses after pulmonary exposure to chitin and Aspergillus antigen. We adoptively transferred Aspergillus-specific Af3.16-transgenic CD4+ T cells one day before exposing the mice to chitin and Aspergillus antigen. Using this technique allowed us to track the fate of antigen-specific cells, adjust precursor frequency, and preclude any developmental abnormalities found in C3KO mice. On day 7 (the peak of the T cell response), lungs were harvested to analyze the CD4+ T cells. The frequency of Af3.16 FoxP3+ T regulatory cells after exposure to chitin and Aspergillus was significantly higher in C3KO mice than in WT mice, even though the numbers of Af3.16 cells were reduced in the former group (Fig. 7A and B). Conversely, the numbers and percentages of IL-4- and IL-17-producing Af3.16 cells were curtailed significantly in chitin- and Aspergillus-sensitized C3KO mice compared to those for WT mice (Fig. 7C and D). Similar results were found with endogenous CD4 T cells (data not shown). Thus, in C3KO mice, we observed reciprocal changes in the distribution of Af3.16 T regulatory cells (elevated) versus IL-4- and IL-17-producing Af3.16 cells (reduced). Thus, C3 is required to induce Th2 and Th17 responses and inhibit Tregs. Collectively, our data show that chitin-mediated C3 signaling inhibits tolerogenic DC and Tregs while promoting the differentiation of Aspergillus-specific Th2 and Th17 responses.
FIG 7 .
Chitin-induced differentiation of Af3.16 CD4 T cells is regulated by C3. A total of 1 × 106 magnetic bead-enriched Af3.16 CD4 T cells were adoptively transferred into WT and C3KO mice by the intravenous route. One day later, chitin and Aspergillus antigens were administered, and this was repeated on days 3 and 6. On day 7, lungs were perfused with ~5 ml of PBS and cells were harvested and stimulated with anti-CD3 and anti-CD28 antibodies in the presence of Golgi Stop for 5 h. After incubation, cells were surface stained before staining for intracellular cytokine and FoxP3 using specific antibodies. Cells were analyzed by flow cytometry. (A) Percentage of FoxP3+ cells among Thy1.1+ Af3.16 cells; (B) total numbers of Af3.16 cells in the lung; (C) total numbers of IL-4+ and IL-17+ Af3.16 cells in the lung; (D) pie chart showing relative distribution of FoxP3+, IL-4+, and IL-17+ cells among Af3.16 cells in WT and C3KO mice that received chitin and Asp. In panel A, the mean total numbers of lung cells for the respective groups were as follows: PBS, WT, 0.67 × 106 versus KO, 0.74 × 106; chitin, WT, 0.99 × 106 versus KO, 0.69 × 106; Asp, WT, 0.89 × 106 versus KO, 0.95 × 106; and chitin and Asp, WT, 2.92 × 106 versus KO, 2.67 × 106. Values are means ± SEM from 5 mice/group.
DISCUSSION
The fungal cell wall contains a variety of carbohydrate polymers that are recognized by the mammalian immune system and that shape the ultimate immune response. Exposure to chitin particles triggers innate allergic inflammation; however, the role of chitin in promoting allergic sensitization in the lung is unknown. We developed our model to test whether chitin particles could promote allergic sensitization to Aspergillus antigens and to assess the role of C3 in chitin-dependent allergic sensitization. Our study further establishes that exposure to chitin particles promotes allergic inflammation in the lung. Furthermore, we demonstrate that chitin exposure impacts the maturation of DC in the lung and promotes the development of allergic sensitization to Aspergillus antigens. Finally, we establish a role for C3, the central component of the complement cascade, and the anaphylatoxin receptor C3aR in mediating chitin-dependent allergic sensitization in the lung.
The ability of chitin to generate C3a in the lung and to promote C3aR-dependent allergic inflammation has relevance for human disease, particularly asthma. Polymorphisms in C3 and C3aR are linked to an increased risk of asthma (36), and in individuals with asthma exacerbations, plasma concentrations of C3a rise, and higher C3a levels are associated with increased hospitalizations (37). Other environmental exposures linked to asthma, such as ozone, diesel exhaust, and cigarette smoke, also generate C3a and drive airway pathology (38–40). Our study demonstrates that environmental exposure to chitin particles also triggers the production of C3a via the alternative pathway, potentially linking chitin exposure to the development of allergic airway pathology. While chitin particles can generate C3a directly in plasma, the elevated C3a levels we observed in the lung following chitin exposure could proceed by one of two pathways, both of which are physiologically relevant. First, chitin particles could activate C3 already present in the lung via the alternative pathway (as with plasma) or trigger additional C3 production from lung resident cells. Second, because chitin particles trigger robust innate inflammation, plasma proteins from the blood could leak across into the inflamed airways. C3 present in the plasma could then be activated by chitin particles via the alternative pathway or via the activation of thrombin in the setting of inflammation (41). In each case, exposure to chitin particles results in an increase in C3a in the lung, which can drive allergic sensitization and inflammation (42).
Consistent with a role for C3a in the pathophysiology of allergic inflammation, we found that C3KO and C3aRKO mice were protected against chitin-dependent sensitization to Aspergillus antigens. Our results agree with previous studies using C3KO or C3aRKO mice, which found protection against pulmonary eosinophilia, elevated Th2 cytokines, and elevated IgE in response to ambient particulate matter, house dust mite, or the model antigen ovalbumin (38, 43, 44). While blockade of C3a or C3aR protects the airways from allergic inflammation following challenge with an allergen, less is known about the role of C3a in the sensitization to allergen. Upon engagement of C3aR by C3a, DC demonstrate an increase in the ability to phagocytose antigen and to activate T cells (17). Furthermore, C3aR signaling leads to reduced expression of C5aR on the surface of DC (34), shifting DC from a C5aR-dependent tolerogenic phenotype toward an activated phenotype capable of promoting Th2 cytokine production from T cells. Our results demonstrate that in the absence of C3 or C3aR, chitin exposure promotes the expression of the regulatory costimulatory molecules CD273 and CD274 on pDC. This suggests that production of C3a following chitin exposure helps to suppress the expression of the regulatory molecules CD273 and CD274, thereby promoting the sensitization to Aspergillus antigen.
While clearly impacting adaptive immune responses, the absence of C3 or C3aR had no impact on innate allergic inflammation or the expression of the costimulatory markers on myeloid DC following chitin administration. C3a mediates the adhesion of eosinophils to the walls of postcapillary venules but is unable to affect transmigration in vivo, whereas C5a can mediate adhesion and transmigration (45). In addition, chitin-induced eosinophilia has been shown to be dependent on CCR2 (22) and LTB4 (21), which likely represent complement-independent eosinophil recruitment pathways. Furthermore, chitin particles trigger cytokine production in vitro in experiments using complement-inactivated serum, which argues against a role for complement in innate immune responses to chitin (22, 46). While myeloid DC from chitin-exposed lungs had increased expression of CD80 and CD86 even in the absence of C3 and C3aR, pDC also showed enhanced expression of CD273 and CD274 in C3KO and C3aRKO mice compared to that in wild-type mice. Since CD273- and CD274-expressing pDC suppress the ability of myeloid DC to activate T cells (34, 42), these results may explain why C3 and C3aR mice are protected from chitin-induced sensitization to Aspergillus antigens.
We also studied how elimination of C3 influenced T cell polarization in chitin-induced allergic inflammation. In line with the tolerogenic phenotype that emerged in pDC from C3KO and C3aRKO mice, adoptively transferred Af3.16 Aspergillus-specific T cells polarized toward a Treg phenotype in C3KO mice and away from allergy-prone Th2 and Th17 cells. Conversely, in wild-type mice, where a tolerogenic phenotype in pDC did not emerge, Af3.16 T cells polarized toward the allergy-associated Th2 and Th17 phenotypes. In line with our study, Park et al. (47) have reported that peptidoglycan recognition protein 1 (Pglyrp1)-deficient mice are protected from asthma in a house dust mite model and that this protection reflects increased recruitment of pDC to the lung and Treg differentiation.
The role of anaphylatoxins as a bridge between innate and adaptive responses in pulmonary allergic models has garnered special interest (48). Unlike in epicutaneous allergic (49) and contact dermatitis (50) models, C3a promotes Th2 responses in an airway allergic model (44), indicating the contrasting roles of C3a in different organs. Our study suggests that chitin-induced C3a and C3aR signaling promotes allergic inflammation in the lung by blocking the differentiation of Tregs, enhancing Th2 and Th17 responses, or both. Our findings are consistent with the report of Lajoie et al. (51), who showed that mice deficient in C3aR had fewer IL-17-producing helper T cells and less airway hyperresponsiveness after allergen challenge with house dust mites, although the contribution of chitin in the model was unexplored.
Preparations of chitin vary widely between studies of allergic inflammation and may impact the results and conclusions of studies. Recent work with fungal glycans, including the β1→4 glycans chitin and chitosan, showed that they minimally activated C3 (51). We utilized commercial preparations of chitin that were acid extracted to remove impurities (22). They were tested to ensure limited residual protein and endotoxin contamination. Still, impurities beyond N-acetyl glucosamine could have influenced our results, explaining differences with the work of Agarwal et al. (52) regarding the extent of complement activation. However, our results may more closely model the mix of material in chitin from environmental sources. For example, chitin inhaled along with environmental allergens is not likely to represent purified N-acetyl glucosamine. Thus, while we cannot exclude a role for other constituents in complement activation, we feel our results reasonably model the role of complement in Aspergillus sensitization that occurs when allergens are inhaled with chitin as the adjuvant.
In conclusion, our study demonstrates that chitin exposure can promote allergic sensitization to Aspergillus antigens. Furthermore, we add chitin particles to the list of environmental insults that can generate C3a in the lung, and we link C3a generation to chitin’s ability to promote allergic inflammation via induction of Th2 and Th17 T cells and inhibition of Tregs. Finally, we suggest that chitin exposure modulates costimulatory molecule expression on lung DC subsets in order to potentiate allergic responses. These results uncover the requisite role of complement in mediating chitin-dependent allergic sensitization.
MATERIALS AND METHODS
Mice.
C3aR knockout (C.129S4-C3ar1tm1Cge/J, BALB/c background, generation N15 + N1F12, stock number 005712), C3 knockout (B6.129S4-C3tm1Crr/J, C57/BL6 background, generation N7F10; stock number 003641) mice, 5 to 8 weeks old, were obtained from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 wild-type mice (strain code 01C55) and BALB/c wild-type mice (strain code 01B05), 5 to 8 weeks old, were obtained from Jackson Laboratory (Frederick, MD). Congenic Aspergillus-specific transgenic Af3.16 mice (53) were provided by Tobias Hohl (Seattle, WA) with the permission of Amariliz Rivera (Newark, NJ) and Eric Pamer (New York, NY). For all experiments, five mice were used per group. Mice were housed and cared for according to guidelines from the University of Wisconsin Animal Care and Use Committee, who approved this work.
Reagents.
Chitin purified from crab shells was purchased from Sigma (C9752) and purified as described (22). Briefly, ground chitin particles were dissolved in 12.5 M HCl and incubated for 30 min at 40°C with agitation. The solution was transferred to a cooled beaker and neutralized with NaOH. The insoluble fraction was collected and washed in H2O and then ethanol. Purified chitin particles were dried in a SpeedVac and stored at −20°C. Before use, chitin particles were resuspended in endotoxin-free phosphate-buffered saline (PBS), sonicated, and filtered through a 10-µm-pore-size nylon filter. Protein was <2% by mass as determined by bicinchoninic acid assay. Endotoxin levels measured by Pyrogent Plus assay (Lonza) were <0.03 EU/ml. Aspergillus fumigatus antigens were obtained from Greer (M3 skin test vial) through the University of Wisconsin Hospital Pharmacy. Prior to use, Aspergillus antigens were dried by SpeedVac and reconstituted at 10 mg/ml in sterile PBS.
Measurement of C3a.
Whole blood was obtained from healthy human volunteers or from C57BL/6 mice and mixed with heparin to prevent clotting. Following centrifugation, plasma was incubated for 1 h with chitin particles or Aspergillus antigens, either with or without blocking antibodies to C1q or factor B.
Following exposure to chitin, C3a levels in plasma or in lung homogenate were determined by ELISA. Prior to lung homogenization, the pulmonary vasculature was perfused with 5 ml PBS via the right ventricle to minimize blood in the homogenate. The human C3a ELISA was obtained from Hycult Biotech, and the mouse C3a ELISA was obtained from BD BioScience.
Administration of chitin/Aspergillus antigens.
Mice were anesthetized via inhalation of isoflurane. The anesthetized mouse was then suspended from their front incisors and intubated using a BioLite intubation system (54). Chitin particles were suspended at 10 mg/ml in PBS. A total of 20 µl of PBS, chitin particles, Aspergillus antigens, or chitin particles plus Aspergillus antigens were administered via the intubation tube into the airway. For sensitization, Aspergillus antigens in the presence or absence of chitin were administered every four days for five exposures, and mice were euthanized two days following the final exposure. For measuring CD4+ T cell responses, mice were treated with antigen and chitin on days 0, 3, and 6, and the lungs were harvested on day 7.
Physiological studies.
Five mice each from sensitized WT and C3aRKO groups were used for physiological studies. Mice were anesthetized with pentobarbital, instrumented with a tracheal cannula, paralyzed with pancuronium, and ventilated with room air at 10 ml/kg tidal volume, 200 breaths/min, and 2.5 cmH2O positive end-expiratory pressure (PEEP). A FlexiVent apparatus (SCIREQ, Montreal, Quebec, Canada) was employed for mechanical ventilation and measurements of airflow resistance using a single frequency, one-compartment model for respiratory system resistance (Rrs; a global measure of airflow resistance) and a multifrequency constant phase model of impedance that included estimates of Newtonian resistance (RN; a measure primarily of central conducting airways) and tissue damping (G; a measure of peripheral airways and tissue resistance). All measures were conducted under conditions of stable ventilation, without administration of airway provocation agents.
Histological examinations.
Formalin-fixed lungs were from pooled from 3 mice in each group, and transverse sections were collected from the middle 1/3 of the right cranial, right caudal, and left lung lobes. The tissues were processed for routine paraffin embedding, and serial sections of each block were stained with hematoxylin and eosin (HE) and alcian blue-Periodic acid-Schiff (PAS) (the latter stains goblet cells). Histologic examination was performed on 12 to 16 transverse lung sections from each group.
Flow cytometry.
Lung cell suspensions were prepared by mincing lungs through a 70-µm filter using a 3-ml syringe plunger. The resulting homogenate was digested with Liberase/DNAse I, and the red blood cells were lysed with ammonium chloride-potassium bicarbonate buffer. All samples were blocked with anti-mouse CD16/32 antibody prior to staining with fluorochrome-conjugated antibodies. Events were gated on front-scatter (FSC)/side-scatter (SSC) parameters to exclude debris and on live cells based on Violet Fixable Live-Dead stain (Molecular Probes). Eosinophils were defined as Siglec Fhi CD11b+ SSChi CD11c− Ly6G− Thy-1−. Neutrophils were defined as CD11b+ SSChi CD11c− Ly6G+ Thy-1−. Plasmacytoid DC were defined as CD11b− CD11cdim CD62L+ SiglecH+. CD11b+ DC were defined as CD11b+ CD11c+ Ly6C−. CD103+ DC were defined as CD103+ CD11b− CD11c+ langerin+. Inflammatory DC were defined as CD11b+ CD11c+ Ly6C+. Antibodies were obtained from BD Biosciences, eBioscience, and BioLegend; reagents for staining FoxP3 were obtained from eBioscience. Cells were collected for analysis on a BD Biosciences LSRII cytometer and data analyzed with FloJo software (TreeStar).
Real-time PCR.
Total RNA was isolated from lung homogenates using a Qiagen RNeasy minikit, and cDNA was prepared using the Bio-Rad iScript cDNA synthesis kit. cDNA was amplified using Bio-Rad SsoFast EvaGreen supermix in a Bio-Rad MyIQ real-time PCR detection system. Primers were designed using Primer-BLAST (55). Relative transcript quantity was calculated using the comparative cycle threshold (CT) method, with β-actin transcript as the control transcript (56).
Statistics.
Samples were compared to the control using an unpaired t test with a two-tailed P value of <0.05 considered statistically significant. All statistical analysis was performed using Prism software (Graph Pad). In all figures, error bars represent the standard error of the mean of the data. Physiological data are shown as box plots (median and interquartile range) for each group, and statistical comparisons were performed with the Mann-Whitney test.
ACKNOWLEDGMENTS
We thank Marlene Klaila and Titilayo Omobesi for excellent animal care and Jenny Gumperz and Subramanya Hegde for assistance in obtaining human blood samples.
This work was supported by funds from the American Asthma Foundation and the National Institutes of Health, including grant AI35681 and AI40996 (B.S.K.), T32ES007015 and F30ES019048 (R.M.R.), and 9T32OD010423 and 5T32RR023916 (D.G.).
Footnotes
Citation Roy RM, Paes HC, Nanjappa SG, Sorkness R, Gasper D, Sterkel A, Wüthrich M, Klein BS. 2013. Complement component 3C3 and C3a receptor are required in chitin-dependent allergic sensitization to Aspergillus fumigatus but dispensable in chitin-induced innate allergic inflammation. mBio 4(2):e00162-13. doi:10.1128/mBio.00162-13.
REFERENCES
- 1. Agarwal R, Nath A, Aggarwal AN, Gupta D, Chakrabarti A. 2010. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with acute severe asthma in a respiratory intensive care unit in North India. Mycoses 53:138–143 [DOI] [PubMed] [Google Scholar]
- 2. Fairs A, Agbetile J, Hargadon B, Bourne M, Monteiro WR, Brightling CE, Bradding P, Green RH, Mutalithas K, Desai D, Pavord ID, Wardlaw AJ, Pashley CH. 2010. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am. J. Respir. Crit. Care Med. 182:1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. 2002. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169:5904–5911 [DOI] [PubMed] [Google Scholar]
- 4. Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, Yao Z, Martin R, Hamzeh N, Adelagun R, Amar S, Kheradmand F, Corry DB. 2007. A new mechanism regulating the initiation of allergic airway inflammation. J. Allergy Clin. Immunol. 120:334–342 [DOI] [PubMed] [Google Scholar]
- 5. Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. 1991. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J. Exp. Med. 173:1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung SS, Fu SM, Rose CE, Gaskin F, Ju ST, Beaty SR. 2006. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing langerin and tight junction proteins. J. Immunol. 176:2161–2172 [DOI] [PubMed] [Google Scholar]
- 7. Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. 2002. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8:1024–1032 [DOI] [PubMed] [Google Scholar]
- 8. Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hochweller K, Anderton SM. 2005. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur. J. Immunol. 35:1086–1096 [DOI] [PubMed] [Google Scholar]
- 10. Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. 2009. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J. Immunol. 183:8044–8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. 2009. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457:585–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. 2009. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15:410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. 2005. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201:981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carroll MC. 1998. The role of complement and complement receptors in induction and regulation of immunity. Annu. Rev. Immunol. 16:545–568 [DOI] [PubMed] [Google Scholar]
- 15. Zwirner J, Götze O, Begemann G, Kapp A, Kirchhoff K, Werfel T. 1999. Evaluation of C3a receptor expression on human leucocytes by the use of novel monoclonal antibodies. Immunology 97:166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drouin SM, Kildsgaard J, Haviland J, Zabner J, Jia HP, McCray PB, Tack BF, Wetsel RA. 2001. Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J. Immunol. 166:2025–2032 [DOI] [PubMed] [Google Scholar]
- 17. Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, Wang N, Sacks SH, Zhou W. 2008. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood 112:5084–5094 [DOI] [PubMed] [Google Scholar]
- 18. Maubon D, Park S, Tanguy M, Huerre M, Schmitt C, Prévost MC, Perlin DS, Latgé JP, Beauvais A. 2006. AGS3, an alpha, (1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet. Biol. 43: 366–375 [DOI] [PubMed] [Google Scholar]
- 19. Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, Minami E, Shibuya N. 2006. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. U. S. A. 103:11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuesta A, Esteban MA, Meseguer J.In vitro. 2003. effect of chitin particles on the innate cellular immune system of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 15:1–11. DOI: 10.1016/S1050-4648(02)00134-1. PubMed.
- 21. Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. 2007. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447:92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roy RM, Wuthrich M, Klein BS. 2012. Chitin elicits CCL2 from airway epithelial cells and induces CCR2-dependent innate allergic inflammation in the lung. J. Immunol. 189:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Da Silva CA, Pochard P, Lee CG, Elias JA. 2010. Chitin particles are multifaceted immune adjuvants. Am. J. Respir. Crit. Care Med. 182:1482–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. 2011. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J. Immunol. 187:2261–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dickey BF. 2007. Exoskeletons and exhalation. N. Engl. J. Med. 357:2082–2084 [DOI] [PubMed] [Google Scholar]
- 26. Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengtén E, Kolls JK. 2010. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J. Exp. Med. 207:2907–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koth LL, Rodriguez MW, Bernstein XL, Chan S, Huang X, Charo IF, Rollins BJ, Erle DJ. 2004. Aspergillus antigen induces robust Th2 cytokine production, inflammation, airway hyperreactivity and fibrosis in the absence of MCP-1 or CCR2. Respir. Res. 5:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behnsen J, Lessing F, Schindler S, Wartenberg D, Jacobsen ID, Thoen M, Zipfel PF, Brakhage AA. 2010. Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect. Immun. 78:3585–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagata S, Glovsky MM. 1987. Activation of human serum complement with allergens. I. Generation of C3a, C4a, and C5a and induction of human neutrophil aggregation. J. Allergy Clin. Immunol. 80: 24–32 [DOI] [PubMed] [Google Scholar]
- 30. Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, Saijo S, Iwakura Y, Fallert Junecko BA, Reinhart TA, Foreman O, Ray P, Kolls J, Ray A. 2011. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. U. S. A. 108:5360–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER, National Heart, Lung, and Blood Institute Severe Asthma Research Program; . 2010. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J. Allergy Clin. Immunol. 125:1028–1036 PubMed; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wark PA, Saltos N, Simpson J, Slater S, Hensley MJ, Gibson PG. 2000. Induced sputum eosinophils and neutrophils and bronchiectasis severity in allergic bronchopulmonary aspergillosis. Eur. Respir. J. 16:1095–1101 [DOI] [PubMed] [Google Scholar]
- 33. de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang X, Lewkowich IP, Köhl G, Clark JR, Wills-Karp M, Köhl J. 2009. A protective role for C5a in the development of allergic asthma associated with altered levels of B7-H1 and B7-DC on plasmacytoid dendritic cells. J. Immunol. 182:5123–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abe M, Wang Z, de Creus A, Thomson AW. 2005. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am. J. Transplant. 5:1808–1819 [DOI] [PubMed] [Google Scholar]
- 36. Hasegawa K, Tamari M, Shao C, Shimizu M, Takahashi N, Mao XQ, Yamasaki A, Kamada F, Doi S, Fujiwara H, Miyatake A, Fujita K, Tamura G, Matsubara Y, Shirakawa T, Suzuki Y. 2004. Variations in the C3, C3a receptor, and C5 genes affect susceptibility to bronchial asthma. Hum. Genet. 115:295–301 [DOI] [PubMed] [Google Scholar]
- 37. Nakano Y, Morita S, Kawamoto A, Suda T, Chida K, Nakamura H. 2003. Elevated complement C3a in plasma from patients with severe acute asthma. J. Allergy Clin. Immunol. 112:525–530 [DOI] [PubMed] [Google Scholar]
- 38. Walters DM, Breysse PN, Schofield B, Wills-Karp M. 2002. Complement factor 3 mediates particulate matter-induced airway hyperresponsiveness. Am. J. Respir. Cell. Mol. Biol. 27:413–418 [DOI] [PubMed] [Google Scholar]
- 39. Kanemitsu H, Nagasawa S, Sagai M, Mori Y. 1998. Complement activation by diesel exhaust particles (DEP). Biol. Pharm. Bull. 21:129–132 [DOI] [PubMed] [Google Scholar]
- 40. Park JW, Taube C, Joetham A, Takeda K, Kodama T, Dakhama A, McConville G, Allen CB, Sfyroera G, Shultz LD, Lambris JD, Giclas PC, Holers VM, Gelfand EW. 2004. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am. J. Respir. Crit. Care Med. 169:726–732 [DOI] [PubMed] [Google Scholar]
- 41. Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Brückner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. 2010. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 185:5628–5636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X, Köhl J. 2010. A complex role for complement in allergic asthma. Expert Rev. Clin. Immunol. 6:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drouin SM, Corry DB, Kildsgaard J, Wetsel RA. 2001. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. J. Immunol. 167:4141–4145 [DOI] [PubMed] [Google Scholar]
- 44. Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. 2002. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J. Immunol. 169:5926–5933 [DOI] [PubMed] [Google Scholar]
- 45. DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao P. 1999. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J. Immunol. 162:1127–1136 [PubMed] [Google Scholar]
- 46. Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. 2008. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 181:4279–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park SY, Jing X, Gupta D, Dziarski R. 18 February 2013, posting date Peptidoglycan recognition protein 1 enhances experimental asthma by promoting Th2 and Th17 and limiting regulatory T cell and plasmacytoid dendritic cell responses. J. Immunol. [Epub ahead of print]. http://dx.doi.org/10.4049/jimmunol.1202675 [DOI] [PMC free article] [PubMed]
- 48. Hawlisch H, Wills-Karp M, Karp CL, Köhl J. 2004. The anaphylatoxins bridge innate and adaptive immunity responses in allergic asthma. Mol. Immunol. 41:123–131 [DOI] [PubMed] [Google Scholar]
- 49. Kawamoto S, Yalcindag A, Laouini D, Brodeur S, Bryce P, Lu B, Humbles AA, Oettgen H, Gerard C, Geha RS. 2004. The anaphylatoxin C3a downregulates the Th2 response to epicutaneously introduced antigen. J. Clin. Invest. 114:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Niebuhr M, Bäumer W, Kietzmann M, Wichmann K, Heratizadeh A, Werfel T. 2012. Participation of complement 3a receptor (C3aR) in the sensitization phase of Th2 mediated allergic contact dermatitis. Exp. Dermatol. 21:52–56 [DOI] [PubMed] [Google Scholar]
- 51. Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. 2010. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat. Immunol. 11:928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Agarwal S, Specht CA, Haibin H, Ostroff GR, Ram S, Rice PA, Levitz SM. 2011. Linkage specificity and role of properdin in activation of the alternative complement pathway by fungal glycans. mBio 2(5):e00178-11 http://dx.doi.org/10.1128/mBio.00178-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant’Angelo DB, Pamer EG. 2006. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity 25:665–675 [DOI] [PubMed] [Google Scholar]
- 54. Rayamajhi M, Redente EF, Condon TV, Gonzalez-Juarrero M, Riches DW, Lenz LL. 2011. Non-surgical intratracheal instillation of mice with analysis of lungs and lung draining lymph nodes by flow cytometry. J. Vis. Exp. 51:e2702 http://dx.doi.org/10.3791/2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rozen S, Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365–386 [DOI] [PubMed] [Google Scholar]
- 56. Schmittgen TD, Livak KJ. 2008. Clyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108 [DOI] [PubMed] [Google Scholar]