Abstract
The role of the β2 Adrenergic Receptor (β2AR) in the regulation of chronic neurodegenerative inflammation within the CNS is poorly understood. The purpose of this study was to determine neuroprotective effects of long-acting β2AR agonists such as salmeterol in rodent models of Parkinson’s disease. Results showed salmeterol exerted potent neuroprotection against both LPS and MPTP/MPP+-induced dopaminergic neurotoxicity both in primary neuron-glia cultures (at sub-nanomolar concentrations) and in mice (1–10 μg/kg/day doses). Further studies demonstrated that salmeterol-mediated neuroprotection is not a direct effect on neurons; instead, it is mediated through the inhibition of LPS-induced microglial activation. Salmeterol significantly inhibited LPS-induced production of microglial pro-inflammatory neurotoxic mediators, such as TNFα, superoxide and nitric oxide, as well as the inhibition of TAK1-mediated phosphorylation of MAPK and p65 NF-κB. The anti-inflammatory effects of salmeterol required β2AR expression in microglia, but were not mediated through the conventional GPCR/cAMP pathway. Rather, salmeterol failed to induce microglial cAMP production, could not be reversed by either PKA inhibitors or an EPAC agonist, and was dependent on beta-arrestin2 expression. Together, our results demonstrate that administration of extremely low doses of salmeterol exhibit potent neuroprotective effects by inhibiting microglial cell activation through a β2AR/β-arrestin2-dependent but cAMP/PKA independent pathway.
Keywords: Monocytes/Macrophages, Neuroimmunology, Inflammation
Introduction
The β2 adrenergic receptor (β2AR)3 is a G-protein coupled receptor (GPCR) which is known to regulate a variety of biological functions, including the regulation of smooth muscle activity in the airway and vasculature. β2AR expression has been identified on immune cells such as macrophages, microglia, T cells, and B cells, and signaling through this receptor can influence the inflammatory response of these cells (1–7). The functional role of β2AR in biological responses has been further elucidated by the development of short-acting and long-acting β2AR agonists for pharmacological studies and clinical usage. Numerous studies using these β2AR agonists have shown that they possess the ability to regulate inflammatory responses by immune cells, and several of these agonists such as salmeterol (Advair®) and formoterol (Symbicort®) are already being used as anti-inflammatory therapeutics to treat asthma and chronic obstructive pulmonary disease (COPD) (8–10). However, potential use of β2AR agonists in neurodegenerative diseases in the CNS has not been well studied.
It is now well accepted that inflammation plays a major role in the progression of a number of neurodegenerative disorders, including Parkinson’s disease (PD), Alzheimer’s disease, Multiple sclerosis, and AIDS-related dementia (11). PD is characterized by the progressive loss of dopaminergic (DA) neurons within the substantia nigra (SN), which results in movement disorders as well as psychological/psychiatric changes in diseased individuals. While the disease mechanisms that ultimately cause PD are still unclear, it is believed that the progressive nature of PD is characterized by chronic inflammation-induced DA neurodegeneration within the SN (12–14). The premise of microglial involvement and the elevation of pro-inflammatory mediators in PD have been supported by the analysis of post mortem brains from PD patients that provides clear evidence of microglial activation in the SN (15–17). Using various PD animal models, we and others have shown that microglia, the major immune cell of the CNS containing high levels of β2AR (18), play a critical role in mediating toxicity of DA neurons in the SN area. Recent studies have shown that activation of nigral microglia and the subsequent release of neurotoxic factors, including the pro-inflammatory cytokine TNFα, as well as NO and ROS, are considered key mediators of DA neurodegeneration in PD (11, 19, 20). Since most long-acting β2AR agonists are highly lipophilic and should readily gain access to the brain, it is likely that these compounds could have an immunomodulatory effect on the progression of inflammation in PD patients by inhibiting the activation of microglia. Therefore, we hypothesize that β2AR agonists might well serve as a new class of compounds that could be used as an anti-inflammatory treatment for PD.
In this study, we report that long-acting β2AR agonists have anti-inflammatory and DA-neuroprotective properties even at extremely low doses, and that administration of the long-acting β2AR agonist salmeterol significantly protects DA neurons against LPS and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (MPTP)-induced toxicity both in vitro and in vivo. Mechanistic studies using primary mesencephalic neuron-glia cultures demonstrated that salmeterol, as well as several other long-acting β2AR agonists, exert potent neuroprotection through their inhibition of microglial inflammatory mediator production. Further, we showed that the anti-inflammatory effects of salmeterol seen at these extremely low doses requires the presence of β2AR, are mediated through the inhibition of both MAPK and NF-κB signaling pathways in activated microglia, and function independently of the canonical GPCR/cAMP/PKA signaling pathway. These results may suggest a new mode of action for β2AR agonists as a therapeutic in the treatment of chronic inflammatory conditions of the CNS.
Materials and Methods
Animals
C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). Timed-pregnant Fisher F344 rats and C57/BL6 mice were obtained from Charles River Laboratories (Raleigh, NC). Congenic BALB/c β2AR-deficient mice were derived from FVB β2AR-deficient mice (Taconic, Hudson, NY). Housingand breeding of the animals were performed in strict accordancewith the National Institutes of Healthguidelines.
Reagents
Salmeterol were obtained from Tocris Cookson, Ballwin, MO. Rp-cAMPS and cAMP Enzyme Immunoassay Kit was purchased from Biomol (Plymouth Meeting, PA). Long-acting compounds including formoterol fumarate dehydrate, bambuterol hydrochloride and clenbuterol hydrochloride, as well as MPTP and 1-methyl-4-phenylpyridinium (MPP+) were purchased from Sigma-Aldrich. LPS used for in vitro studies (E.coli strain O111:B4) was purchased from Calbiochem (San Diego, CA), LPS used for in vivo studies was purchased from Sigma-Aldrich. Cell culture reagents were obtained from Invitrogen (Carlsbad, CA). [3H]-DA (30 Ci/mmol) was obtained from Perkin-Elmer Life Sciences (Boston, MA). The polyclonal anti-tyrosine hydroxylase antibody was a generous gift from Dr. John Reinhard (GlaxoSmithKline, Research Triangle Park, NC). The Vectastain ABC kit and biotinylated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA). The fluorescence probe Dichlorodihydro-fluorescein Diacetate (DCFH-DA) was obtained from Calbiochem (La Jolla, CA). Anti-phospho-ERK1/2 Ab, anti-ERK1/2 Ab, anti-phospho-p38 Ab, anti-p38 Ab, anti-phospho-JNK Ab or anti-JNK Ab, anti-phospho-p65 or anti-p65 Ab and anti-TAK1 Ab were purchased from Cell Signaling Technology (Danvers, MA). Negative control siRNA and β-arrestin2 siRNA are from invitrogen (Carlsbad, CA).
Primary mesencephalic neuron-glia cultures
Neuron-glia cultures were prepared from the ventral mesencephalic tissues of embryonic day 14–15 rats or day 13–14 mice, as described previously (21, 22). Briefly, dissociated cells were seeded at 1 × 105/well and 5 × 105/well in poly-D-lysine-coated 96-well and 24-well plates, respectively. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air, in MEM containing 10% FBS, 10% horse serum, 1 g/L glucose, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 50 U/ml penicillin and 50 μg/ml streptomycin. Seven-day-old cultures were used for drug treatments. At the time of treatment, immunocytochemical analysis indicated that the rat neuron-glia cultures were made up of 11% microglia, 48% astrocytes, 41% neurons, and 1% tyrosine hydroxylase immunoreacitve (TH-IR) neurons. The composition of the neuron-glia cultures of mice is very similar to that of rat, which consist of 12% microglia, 48% astrocytes, 40% neurons, and 1% TH-IR neurons.
Primary mesencephalic neuron-enriched cultures
Midbrain neuron-enriched cultures were established as described previously (23). Briefly, 24 h after seeding, cytosine β-D-arabinocide was added to a final concentration of 10 μM to suppress glial proliferation. Three days later, the media was removed and replaced with maintenance medium. Cells were used for drug treatments 7 days after initial seeding. Routinely, the 7-day-old neuron-enriched cultures, which normally contain less than 0.1% microglia, and less than 3–5% astrocytes, were used for treatment. Among the neuronal population (Neu-N immunoreactive neurons), 2.7–3.9% were dopaminergic neurons (TH-IR positive neurons).
Primary midbrain neuron-astroglia cocultures
Rat primary neuron-astroglia cocultures were obtained by suppressing microglial proliferation with 1.5 mM LME 24 h after seeding the cells, as described previously (23). Three days later cultures were changed back to maintenance medium and used for treatment 7 days after initial seeding. The cultures stained with Iba1 or F4/80 antibody showed less than 0.1% microglia.
Primary microglia-enriched cultures
Mice microglia-enriched cultures with a purity of > 98%, were prepared from whole brains of 1-day-old mice pups as described previously (24). For superoxide assays, 105 cells were grown overnight in 96-well culture plates before use.
[3H]-DA uptake assay
[3H]-DA uptake assays were performed as described (24). Briefly, cells were incubated for 20 min at 37°C with 1 μM [3H]-DAin Krebs-Ringer buffer (16 mMsodium phosphate, 119 mM NaCl, 4.7 mM KCl, 1.8 mM CaCl2, 1.2mM MgSO4, 1.3 mM EDTA, and 5.6 mM glucose; pH 7.4). Cells were washed with ice-cold Krebs-Ringer buffer three times, after which the cells were collected in 1N NaOH. Radioactivity was determined by liquid scintillation counting. Nonspecific DA uptake observed in the presence of mazindol (10μM) was subtracted.
Immunocytochemistry
Immunostaining was performed as previously described (22). Briefly, formaldehyde (3.7%)-fixed cultures were treated with 1% hydrogen peroxide followed by sequential incubation with blocking solution, after which the cells were incubated overnight at 4°C with antibodies against TH (1:20,000). Cells were incubated with biotinylated secondary antibody for 2 h followed by incubation with ABC reagents for 40 min. Color was developed with 3,3′-diaminobenzidine. For morphological analysis, the images were recorded with an inverted microscope (Nikon, Tokyo, Japan) connected to a charge-coupled device camera (DAGE-MTI, Michigan City, IN) operated with the MetaMorph software (Universal Imaging Corporation, Downingtown, PA). For visual counting of TH-IR neurons, nine representative areas per well of the 24-well plate were counted under the microscope at 100 × magnification by three individuals. The average of these scores was reported.
Superoxide assay
The production of superoxide was determined by measuring the superoxide dismutase (SOD)-inhibitable reduction of the tetrazolium salt WST-1 (25, 26). Neuron-glia or microglia-enriched cultures in 96-well culture plates were washed twice with HBSS without phenol red. Cultures were then incubated at 37°C for 30 min with vehicle control (DMSO) or salmeterol in DMSO (50 μl/well). Then, 50 μl of HBSS with and without SOD (50 U/ml, final concentration) was added to each well along with 50 μl of WST-1 (1 mM) in HBSS, and 50 μl of vehicle or LPS (10 ng/ml). To measure superoxide production induced by MPP+, seven day-old mesencephalic neuron-glia cultures grown in 96-well plates were treated with salmeterol in the presence and absence of MPP+, or vehicle alone in 150 μl of phenol red-free treatment medium. Four days after treatment, 50 μl of HBSS with and without SOD (50 U/ml, final concentration) were added to each well along with 50 μl of WST-1 (1 mM) in HBSS. Fifteen minutes later, absorbance at 450 nm was read with a SpectraMax Plus microplate spectrophotometer (Molecular Devices Corp, Sunnyvale, CA). The difference in absorbance observed in the presence and absence of SOD was considered to be the amount of superoxide produced, and results were expressed as the percentage of vehicle-treated control cultures.
Intracellular ROS assay
Intracellular ROS were determined by using a 2′,7′-dichlorodi-hydrofluorescein (DCFH-DA) assay as described previously with minor modifications (27). DCFH-DA enters cells passively and is deacetylated by esterase to nonfluorescent DCFH. DCFH reacts with ROS to form DCF; the fluorescent product DCFH-DA was dissolved in methanol at 10 mM and was diluted 500-fold in HBSS to give DCFH-DA at 20 μM. The cells were exposed to DCFH-DA for 1 h and then treated with HBSS containing the corresponding concentrations of LPS for 2 h. The fluorescence was read immediately at wavelengths of 485 nm for excitation and 530 nm for emission using a SpectraMax Gemini XS fluorescence microplate reader (Molecular Devices). The experimental value minus the value of the control group was interpreted as the increase in intracellular ROS.
Nitrite and TNFα assays
The production of NO was determined by measuring the accumulated levels of nitrite in the supernatant with Griess reagent and the release of TNFα was measured with a mouse TNFα– enzyme-linked immunosorbent assay kit from R and D System (Minneapolis, MN, USA), as described (23).
Western blot analysis
Enriched microglia were treated with pre-treated with vehicle or salmeterol in the presence or absence of LPS, 15 min later, cells were lysed and collect total protein for assay. Equal amounts of protein (20 μg per lane) were separated by 4~12% Bis-Tris Nu-PAGE gel and transferred to polyvinylidene difluoride membranes (Novex, San Diego, CA). For detection the phosphorylation of MAPK, membranes were blocked with 5% BSA PBS buffer, washed three times followed by incubation with either anti-phospho-ERK1/2 Ab, anti-ERK1/2 Ab, anti-phospho-p38 Ab, anti-p38 Ab, anti-phospho-JNK Ab or anti-JNK Ab, anti-phospho-TAK1 Ab or anti-TAK1 Ab at 1:1000 dilution (Cell Signaling Technology, Danvers, MA) overnight at 4°C. Anti-rabbit horse radish peroxidase-linked secondary antibody (1:2000 dilution) was incubated for 1h at 25°C. The same detection system as above was used.
Cyclic AMP assay
Primary microglia cells were treated with 0, 10−8M~10−11M salmeterol for 5, 10 and 30 min. Cells were lysed in 0.1 M HCl for 10 min, the supernatant were used for cAMP assays following the manufacture’s protocol of cAMP Enzyme Immunoassay Kit from Biomol (Plymouth Meeting, PA). The absorbance was measured at 405 nm using a colorimetric 96-well plate reader. Data were expressed as pmol cAMP per 1 million cells.
DNA binding assay
DNA binding assay was performed by using NF-κB (p65) Transcription Factor Assay Kit from Cayman Chemical following the instruction.
RNA interference
SiRNA duplexes with sequences specifically targetingβ– arrestin2 RNA was 5′ UGGUGUCCUACAGGGUCAAGGUGAA3′ and negative control siRNA (Invitrogen) were transfected into primary mouse microglia cells using an Macrophage Nucleofector (Lonza, Allendale, NJ, USA) and the manufacturer’s buffer kit, and protocol optimized for mouse macrophage. Knockdown of expression of the target was determined by Western blotting.
Systemic LPS injection model
Three months after a single systemic LPS injection (5 mg/kg; ip) (28, 29), continuous infusion of extremely low doses (1~10 μg/kg/day, delivered subcutaneously via an Alzet osmotic pump) of salmeterol for two weeks. Rotarod activity was evaluated at 7 and 10 months after LPS injection. Ten months after LPS injection, mice were euthanized, brains were removed and postfixed in 4% paraformaldehyde overnight at 4°C. Brains were then placed into 30% sucrose/PBS solution at 4°C until the brains sank to the bottom of the container. Coronal sections including SN pars compacta (SNpc) were cut on a horizontal sliding microtome into 35-μm transverse free-floating sections.
MPTP model
For 6 consecutive days, 8-wk-old C57BL/6 male mice received daily MPTP injections (15 mg/kg of MPTP·HCl (14.52 mg/kg as a base), s.c.). Salmeterol (1 or 10 μg/kg/day) was given by continuous infusion (via an Alzet mini-pump) for 2 weeks starting at two days before the first MPTP injection. Mice used as controls received an equal volume of 0.9% saline. The mice were euthanized 21 days after the last MPTP/saline treatment with an i.p. injection of 120 mg/kg pentobarbital and then perfused through the left ventricle with saline. Mice were divided into two groups: 1) The striatum region was dissected and immediately frozen in dry ice for the analysis of levels of DA and its metabolite (DOPAC and HVA) by HPLC. 2) Saline perfusion was followed by 4% paraformaldehyde for immunocytochemical analyses. Brains were removed and postfixed in 4% paraformaldehyde overnight at 4°C. Brains were then placed into 30% sucrose/PBS solution at 4°C until the brains sank to the bottom of the container. Coronal sections including SNpc were cut on a horizontal sliding microtome into 35-μm transverse free-floating sections.
Analysis of striatal catecholamine content
The levels of DA and its metabolites (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)) were determined byHPLC and coupled with electrochemical detection as described (34). In brief, striatal tissues were sonicated in 0.2 M perchloric acid (20% w/v) containing the internal standard 3,4-dihydroxybenzylamine(100 ng/ml). After centrifugation, 150 μl of the supernatant was passed through a 0.2-μm Nylon-66 filter, and 25 μl of the filtrate representing 2.5 mg of striatal tissue was used. The concentrations of DA, DOPAC, and HVA were calculated using standard curves that were generated by determining, in triplicate, the ratios between three known amounts of the internal standard.
Rotarod test
The rotorod apparatus (Rotamex, Columbus Instruments, Columbus, OH) was used to evaluate the ability of staying on the rotating rod. Mice were transported (within their home cage) to acclimate to the testing room for 1 h prior to testing started. The parameters of rotor-rod system include start speed, acceleration and highest speed (2 rpm, accelerate 2 rpm/10 s, 20 rpm). Each mouse was placed on the confined section of the rod, turn on the rotarod that begins to rotate with a smooth increase in speed from 2 rpm. Mice received three consecutive trails. The rest period between each trial was 2 min. The mean latency for the three trails was used for the analysis. The latency to fall was measured in seconds. In all trials, if the mouse did not fall from the rod, it was removed from the rod after 2 min.
Analysis of Neurotoxicity
The loss of dopaminergic neurons was assessed by counting the number of TH-immunoreactive (TH-IR) neurons following immunostaining of brain sections. Twenty-four consecutive brain slices (35 μm thickness), which encompassed the entire SNpc, were collected. A normal distribution of the number of TH-IR neurons in the SNpc was constructed based on the counts of 24 slices from C57BL/6J mice. Eight evenly spaced brain slices from saline, LPS-injected or salmeterol-treated animals were immunostained with an antibody against TH and counted. The distribution of the cell numbers from each animal was matched with the normal distribution curve to correct for errors resulting from the cutting. Samples were counted in a double-bind manner with three individuals. Conclusions were drawn only when the difference was within 5%.
Microglia counting
Microglia were counted as previously described (30). Briefly, three vertical and horizontal lines were drawn in equal intervals in each well of the 24-well plate. For quantification of microglia, nine representative areas (0.9 mm × 0.7 mm/field) per well at the line intersections were focused, and 81 fields from three independent experiment in triplicate were selected at 100× magnification by using Nikon Diaphot equipped with Dage-MTI (DC 330) camera with MetaMorph software. The number of Iba-1 positive microglia was counted. Counting was performed in a double-blind manner by three individuals, and conclusions were drawn only when the difference was within 5%.
Statistical Analysis
The data were presented as the means ± SE. For multiple comparisons of groups, two-way or three-way ANOVA was used. Statistical significance between groups was assessed by paired Student’s t test, followed by Bonferroni correction using the JMP program (SAS Institute, Cary, NC, USA). A value of P < 0.05 was considered statistically significant.
Results
Low doses of β2AR agonists are neuroprotective against LPS-induced DAb neurotoxicity
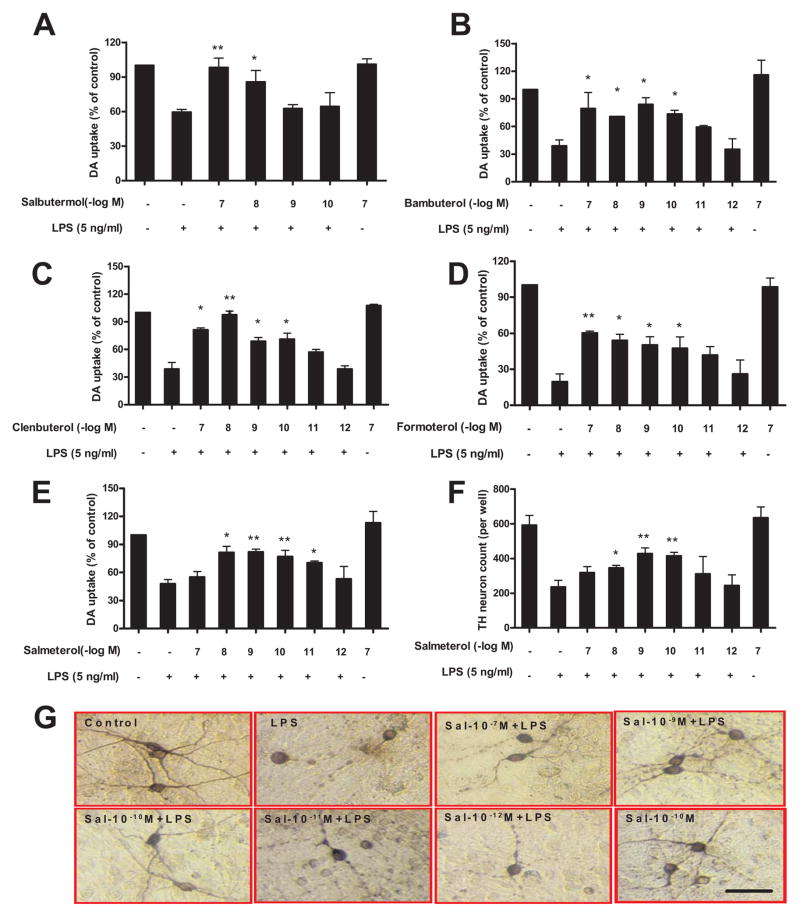
Since β2AR agonists have been shown to have potent anti-inflammatory effects in a number of different chronic inflammatory conditions, we explored whether β2AR agonists are neuroprotective against inflammation-induced toxicity of DA neurons (Fig. 1). Based on the blood half-life, β2AR agonists were classified into short-acting (serum half-life 3–4 hours) and long-acting (serum half-life ~12 hours), (31, 32), and both types were tested in this study. Mesencephalic neuron–glia cultures were pretreated with either short-acting β2AR agonist salbutermol (A), or long-acting β2AR agonists bambuterol (B), clenbuterol (C), formoterol (D), and salmeterol (E–G), at a wide range of concentrations (10−7 to 10−12 M) for 30 min prior to the addition of LPS. Seven days later, LPS-induced DA neurotoxicity was quantified by measuring DA neuronal function using the [3H]-DA uptake assay (A–E), by counting the number of TH-IR neurons (F), or by morphological analysis of TH-IR neurons (G). The [3H]-DA uptake assay showed that salbutermol (the short-acting β2AR agonist) can protect DA neurons only at concentrations of 10−7 and 10−8 M (Fig. 1A), while long-acting β2AR agonists clenbuterol, bambuterol, formoterol, and salmeterol can protect DA neurons from LPS-induced toxicity at concentrations ranging from 10−7 down to 10−10 M (Fig. 1B–E). These results demonstrate that the neuroprotective effects of short-acting β2AR are about 100–1000 fold less potent than long-acting compounds. Due to the slightly higher potency of salmeterol against LPS-induced DA neurotoxicity when compared to the other long-acting agonists (Fig. 1E), and its wide usage as a clinical treatment for asthma and COPD, we used salmeterol as our prototype long-acting β2AR agonist for further studies in this paper. Similar protective effects on DA neurons were observed with salmeterol when we quantified the number of TH-IR neurons remaining in culture after 7 days with immunostaining (Fig. 1F). Morphological analysis revealed that LPS treatment not only decreased the number of TH-IR neurons, but also caused a loss of neuronal processes, and these characteristics were also reversed by low doses of salmeterol pretreatment in a concentration-dependent manner (Fig. 1G).
FIGURE 1.
Low doses of β2AR agonists protect DA neurons against LPS-induced toxicity. Rat primary mesencephalic neuron-glia cultures were seeded in a 24-well culture plate at 5×105, then pretreated with vehicle or indicated concentrations of β2AR agonists, including short-acting β2AR agonist salbutermol (A), long-acting β2AR agonists bambuterol (B), clenbuterol (C), formoterol (D) and salmeterol (E–G) for 30 min before the addition of 5 ng/ml LPS. Seven days later, the LPS-induced DA neurotoxicity was quantified by the [3H]-DA uptake assay (A–E); the immunocytochemical analysis of salmeterol and LPS treated cells, including TH-IR neuron counts (F) and the representative pictures of TH+ neuron immunostaining (G). Results in A–E were expressed as a percentage of the vehicle-treated control cultures and were the means ± SE. from three independent experiments in triplicate. *P<0.05, **P<0.01 compared with the LPS-treated cultures. Scale bar: 50 μm.
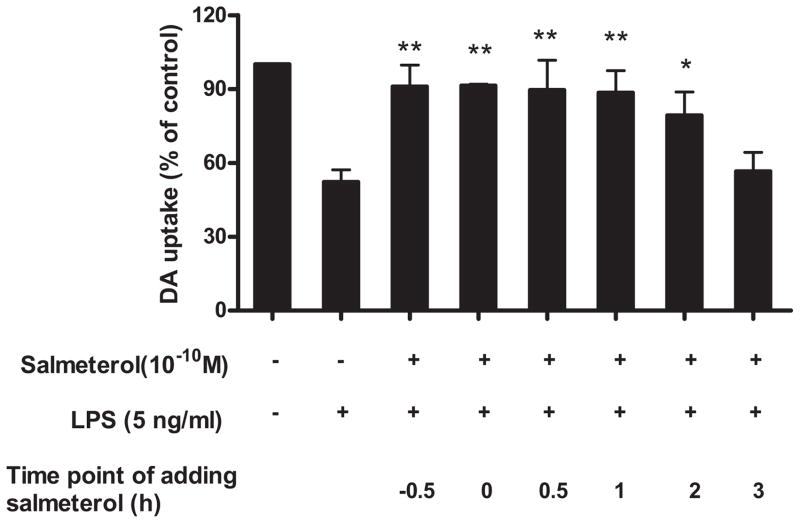
In addition to the pre-treatment studies, a post-treatment of salmeterol was performed. Salmeterol at 10−10 M was added to neuron-glia cultures 30 min prior, simultaneously with, or 30 min, 1, 2, and 3 hrs following the addition of LPS, and DA-neurotoxicity was measured 7 days later. We chose the 10−10 M concentration of salmeterol for these experiments because this was the lowest consistently effective concentration of salmeterol and other long-acting agonists that provided optimal neuroprotection in our pre-treatment studies. The results in Fig. 2 showed that when salmeterol was added up to 2 hrs after LPS treatment, significant neuroprotection of TH-IR neurons was observed. We also observed that the production of inflammatory mediators was inhibited by the addition of salmeterol up to 2 hours post LPS treatment (data not shown). However, when salmeterol was added 3 hrs post LPS treatment, no significant neuroprotection or inhibition of inflammatory mediator production was observed. These results demonstrate that the addition of salmeterol within the first few hours following exposure to LPS is critical in determining the survival of DA neurons.
FIGURE 2.
Salmeterol mediates neuroprotection when administered prior to or after exposure to LPS. Rat primary mesencephalic neuron-glia cultures were seeded in a 24-well culture plate at 5×105, vehicle or salmeterol at 10−10 M was added to neuron-glia cultures 30 min prior to, simultaneously with, 30 min, 1, 2, or 3 hrs following the addition of LPS. DA neurotoxicity was measured 7 days later. Results were expressed as a percentage of the vehicle-treated control cultures and were the means ± SE from 3 independent experiments in triplicate. *P<0.05, **P<0.01 compared with the LPS-treated cultures.
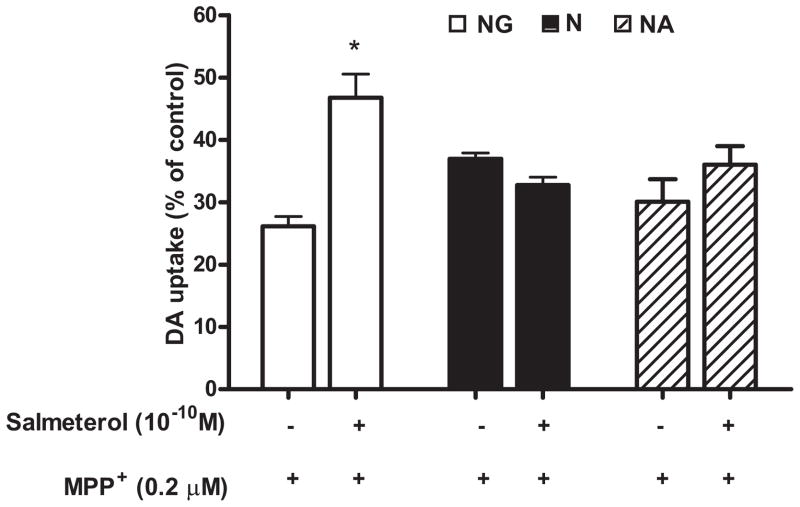
The neuroprotective effect of salmeterol was further studied using MPP+, the active metabolite of MPTP, as the initiator of DA neurotoxicity in neuron-glia cultures (33). MPP+ is known to kill DA neurons directly, and our previous work has shown that neuronal death induced by MPP+ is the result of both direct cytotoxic effects on DA neurons by MPP+, as well as by reactive microgliosis induced by toxic factors released by dying neurons (11, 33). Treatment with 0.2 μM MPP+ for 7 days resulted in a 74% decrease in DA neuron function in neuron-glia cultures (NG), and pretreatment with 10−10 M salmeterol restored significant but not complete DA neuronal function in these cultures (Fig. 3). To further determine how salmeterol protects DA neurons from the cytotoxic effects of MPP+, we treated cultures of rat midbrain lacking all glial cells (N, neuron enriched cultures) or cultures containing only neurons and astrocytes but not microglia (NA, neuron-astrocyte cultures), and measured DA neurons function 7 days after treatment. Our results show that salmeterol was not capable of protecting DA neurons from MPP+-induced toxicity in either the neuron enriched cultures or neuron-astrocyte cultures (Fig. 3). These results demonstrate that microglia, not neurons or astroglia, serve as the target of salmeterol-mediated effect against MPP+-induced toxicity, and might function by inhibiting the reactive microgliosis or by mediating direct neuroprotection elicited from MPP+-induced DA neuronal toxicity.
FIGURE 3.
Salmeterol mediated neuroprotection is microglia dependent. Mesencephalic midbrain neuron-glia cultures (open bars, NG, which contain ~10% microglia), neuron-enriched cultures (solid bars, N, which contain <0.1% microglia) and neuron-astrocyte cultures (cross-hatched bars, NA, which contain <0.1% microglia) were pretreated with either vehicle or 10−10 M salmeterol for 30 min prior to the addition of 0.2 μM MPP+. The [3H]-DA uptake measurements were performed 7 days following MPP+ treatment. Results were expressed as a percentage of the vehicle-treated control cultures and were the means ± SE from 3 independent experiments in triplicate. * P<0.05 compared with the MPP+-treated cultures.
Salmeterol significant attenuates the loss of DA neurons in SNpc induced by systemic injection MPTP or LPS in vivo
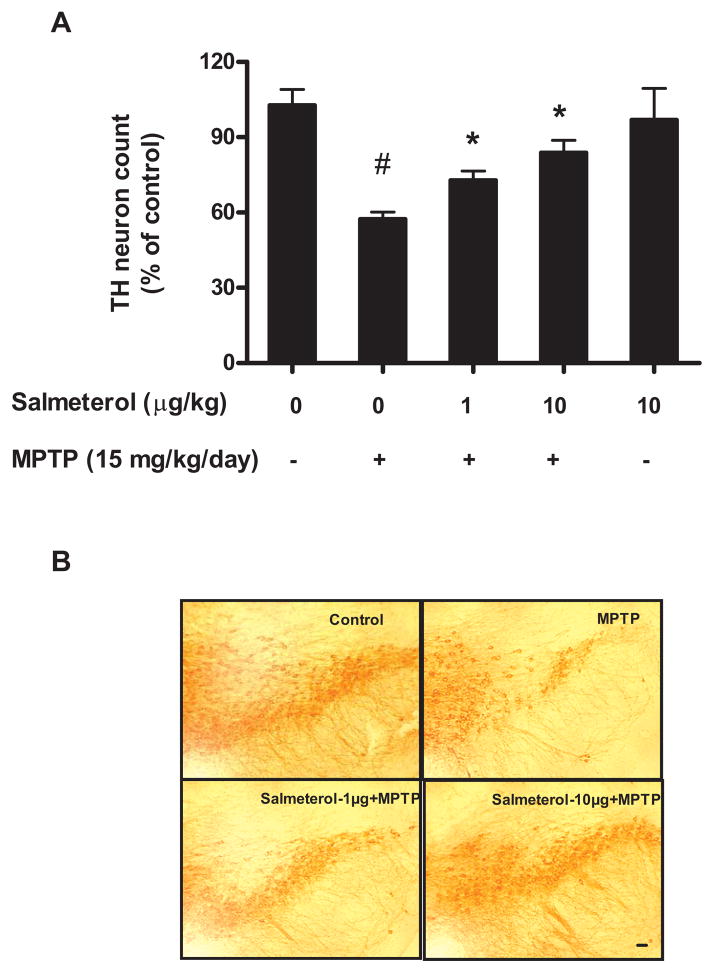
Since low doses of salmeterol shows potent DA-neuroprotective effects in vitro, two different animal PD models were used for the substantiation of in vivo efficacy of salmeterol in preventing DA neurodegeneration. The first one is the MPTP model, initially used in this study to validate the therapeutic efficacy of salmeterol (34). Mice received daily injections of MPTP (15 mg/kg; s.c.) for 6 consecutive days. Experimental mice were also given either saline or two different doses of salmeterol (1 or 10 μg/kg/day) by continuous infusion (via an Alzet mini-pump) for 2 weeks starting two days before the first MPTP injection. Three weeks following the last MPTP injection, mice were euthanized for morphologic analysis. MPTP-injected mice displayed a 50% loss of TH-positive neurons (DA neurons) in SNpc compared with saline-injected controls and this decrease was significantly prevented by treatment with both doses of salmeterol (Fig. 4A). In addition, the loss of DA neurites in the pars reticulata region of SN by injection of MPTP was also fully protected by salmeterol treatment (Fig. 4A and 4B). In addition to the analysis of DA neurons in the substantia nigra, levels of DA and its metabolites, DOPAC and HVA were measured in the striatum, the region containing DA nerve terminals projected from the substantia nigra. Six consecutive daily MPTP injections reduced the DA level by about 65% compared with saline injected group (Table 1). Although salmeterol treatment did not prevent the loss of DA levels caused by MPTP, interesting results were observed with the turn-over rate of DA (expressed by the ratio of DA and its metabolites), which reflect the functional status of the DA release. Both doses of salmeterol treatment (1μg/kg/day and 10μg/kg/day) showed much higher turn-over rates than those of either the saline- or MPTP-treated groups. These results suggest that salemeterol, due to its protective effect on cell bodies of DA neurons in the substantia nigra, was able to preserve and maintain higher activity of DA neurons. Therefore, similar to our previous report (34), it is highly likely that as long as the nigral DA neuronal bodies are preserved by the neuroprotective agents, the DA levels eventually will return to normal values.
FIGURE 4.
Salmeterol was also capable of inhibiting progressive DA neurodegeneration induced by injection of the neurotoxin MPTP. To induce DA neurodegeneration, MPTP (15 mg/kg) was injected s.c. into C57BL/6J mice for 6 consecutive days. Experimental mice (8 mice/group) were also given either saline or doses of salmeterol at 1μg/kg/day or at 10μg/kg/day by continuous infusion (via an Alzet mini-pump) for 2 weeks starting two days before the first MPTP injection. We then assessed the survival of DA neurons in the SNpc 21 days following the last MPTP injection through quantification of TH-positive neurons (A). Representative pictures of TH-IR neurons staining for different treatment groups are shown (B). *P<0.05, compared with the LPS-treated mice. #P<0.05, compared with saline-treated mice. Scale bar: 50 μm.
Table 1.
Salemeterol treatment increased the turn-over rate of dopamine in the mouse striatum in MPTP Parkinson’s disease model
| DA (Mean ± SE) | DOPAC (Mean ± SE) | % (DOPAC/DA) | HVA (Mean ± SE) | % (HVA/DA) | |
|---|---|---|---|---|---|
| Saline control | 748.5 ± 76.7 | 123.0 ± 14.3 | 16.4 | 107.1 ± 4.26 | 14.3 |
| MPTP | 266.0 ± 22.18 | 81.33 ± 10.2 | 30.5 | 73.11 ± 5.66 | 27.4 |
| Salmeterol (10 ug/kg/day) | 668.6 ± 67.05 | 193.3 ± 28.0 | 28.8 | 101.9 ± 7.81 | 16.5 |
| MPTP + Salmeterol (1ug/kg/day) | 214.1± 16.89 | 148.2 ± 9.7 | 69.2 | 85.68 ± 2.68 | 39.7 |
| MPTP + Salmeterol (10ug/kg/day) | 205.6 ± 16.23 | 165.9 ± 8.8 | 80.5 | 97.18 ± 5.77 | 47.3 |
For 6 consecutive days, 8-wk-old C57BL/6 male mice received daily MPTP injections (15 mg/kg of MPTP·HCl (14.52 mg/kg as a base), s.c.). Salmeterol (1or 10 μg/kg/day) was given by continuous infusion (via an Alzet mini-pump) for 2 weeks starting at two days before the first MPTP injection. Mice used as controls received an equal volume of 0.9% saline. The mice were euthanized 21 days after the last MPTP/saline treatment with an i.p. injectionof 120 mg/kg pentobarbital and then perfused through the left ventricle with saline. Striatum region was dissected for the analysis of levels of DA and its metabolites, DOPAC and HVA. The trun-over rates were expressed by the ratio of DOPAC/DA and HVA/DA. The values of the DA, DOPAC and HVA are expressed as ng/100 mg of wet weight. Numbers of animal are 5–8 per group.
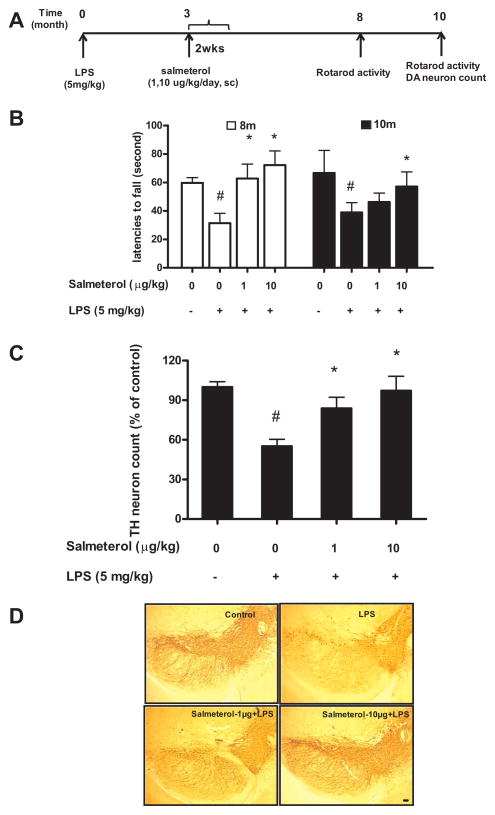
A second PD model is the chronic inflammatory-based LPS model. Our previous observations have shown that a single systemic injection of LPS elicits a time-dependent inflammatory-mediated progressive loss of DA neurons in the SN and a concomitant loss of motor activity (28, 29). Three months after a single injection of LPS (5 mg/kg, i.p), the mid-time point between this LPS injection and any observable DA-neuronal degeneration, C57BL/6J mice were infused with either saline alone or salmeterol given at two different doses (1μg/kg/day or 10 μg/kg/day) for a period of two weeks via an Alzet mini-pump. Eight months after the LPS injection, rotarod behavioral tests were performed, and again at 10 months, when mice were killed for histological analysis (Fig. 5A). Treatment with salmeterol at 10 μg/kg/day significantly protected the LPS-treated mice from motor deficits measured by rotarod activity at both 8 and 10 months, while with the 1μg/kg/day dose showed significant protection at 8 months but not 10 months (Fig. 5B). We further observed that salmeterol treatment significantly prevented LPS-induced loss of cell bodies and processes of DA neurons within the SNpc at 10 months (Fig. 5C and 5D). Together, our data showed that a salmeterol treatment significantly attenuates the loss of DA neurons in the SNpc induced by the injection of either MPTP or LPS in vivo.
FIGURE 5.
Salmeterol significantly attenuates the loss of rotarod activity and DA neurons in SNpc induced by systemic LPS injection. C57BL/6J mice received single LPS injection (5mg/kg, i.p), and 3 months later, mice were infused with either vehicle alone or salmeterol (1 or 10 μg/kg/day) for 2 weeks (via an Alzet mini-pump). Eight months and 10 months after LPS injection, rotarod tests were performed (A–B), and then at 10 months mice were sacrificed, brains were harvested and sectioned, and 24 sections (rostral to caudal: 4.52–5.36 mm posterior to bregma) were collected as described in methods. Eight evenly spaced brain sections from vehicle, LPS-injected, or LPS-injected salmeterol-treated animals were immunostained with anti-TH antibody, and the number of TH-IR neurons in the SN was counted. Data were expressed as percent loss compared to saline-injected controls (C). Representative pictures of TH-IR neurons staining for different treatment groups are shown (D). *P<0.05,compared with the LPS-treated mice. #P<0.05, compared with saline-treated mice. Scale bar: 50 μm.
Salmeterol inhibits the LPS-induced increase in the production of microglial pro-inflammatory factors
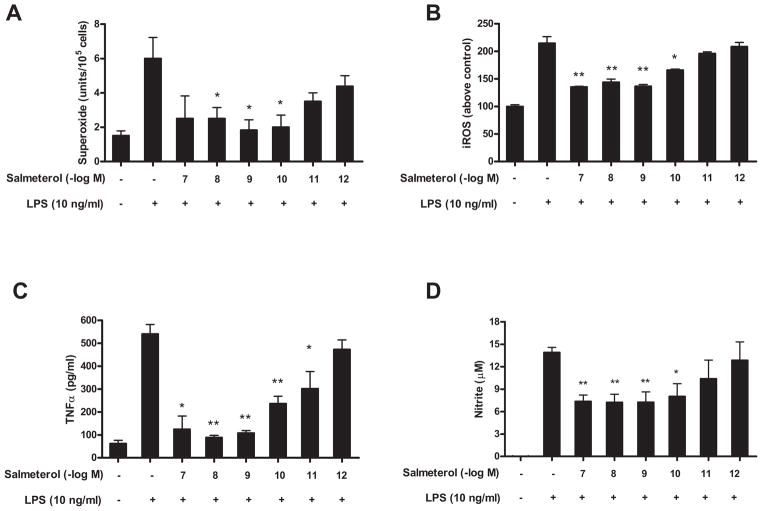
Activation of microglia either through the direct effects of LPS on microglia, or by reactive microgliosis via the death of neurons, produce an array of pro-inflammatory mediators, including ROS, TNFα, and NO, which are the pivotal products that mediate inflammation-mediated neurotoxicity (11, 35). To determine whether salmeterol affects the generation of inflammatory mediators, primary microglia were pretreated with salmeterol before the addition of LPS. At concentrations from 10−7 to 10−10 M but not 10−11 and 10−12 M, salmeterol significantly reduced LPS-mediated superoxide production (Fig. 6A), intracellular ROS production (Fig. 6B), TNFα (Fig. 6C), and NO (Fig. 6D), respectively. The inhibition of LPS-induced production of proinflammatory factors by salmeterol was not due to lower number of microglia, since microglial cell counts based on Iba-1 immunostaining showed that salemterol had no effect on the absolute number of microglia over the 7 day culture period (data not shown). These results show that low dose salmeterol can specifically inhibit LPS-induced activation and pro-inflammatory mediator production by microglia, but had no effect on microglia viability. Interestingly, other long-acting β2AR agonists including bambuterol, fomoterol and clenbuterol also show the similar pattern in the inhibition of the production of the pro-inflammatory mediators TNFα and NO from microglia, while short-acting agonist salbutermol failed to inhibit TNFα and NO production at 10−9 M (supplemental data Fig. 1).
FIGURE 6.
Effect of salmeterol on LPS-induced production of pro-inflammatory factors from microglia. Microglia-enriched cultures were seeded at a density of 1×105/well. Cells were pretreated with vehicle or various concentrations of salmeterol for 30 min followed by the addition of LPS. The production of LPS-induced extracellular superoxide production (A) was measured as SOD-inhabitable reduction of WST-1. LPS-induced intracellular ROS was determined by probe DCFH-DA (B). Salmererol’s effect on LPS-induced production of TNFα and nitrite were shown in Fig. 6C and Fig. 6D. Results were expressed as mean ± SE from three to five independent experiments in triplicate. *P<0.05, **P<0.01 compared with the LPS-treated cultures.
The anti-inflammatory effects of low dose salmeterol are β2AR- and β-arrestin2-dependent, but cAMP-independent
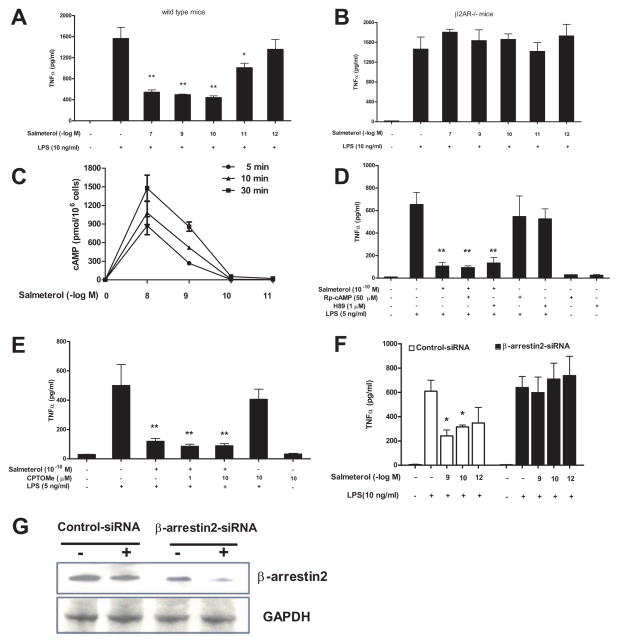
Previous reports have shown that anti-inflammatory responses of higher concentrations of β2AR agonists (between 10 nM to 10 μM) are mediated through the activation of the β2AR and its induction of cAMP (2, 4, 5, 36). Since much lower concentrations of β2AR agonists were examined in this study, a series of experiments were conducted aimed at elucidating the signaling pathway underlying the anti-inflammatory effect of salmeterol on microglial cells. For this purpose, TNFα production was used as the prototype of the inflammatory response induced by LPS. We first measured TNFα production from microglial cultures generated from β2AR-deficient or wild type mice to determine if low dose salmeterol inhibition was β2AR-dependent. Results show that while salmeterol displayed a concentration-dependent inhibition of TNFα production in wild-type mice (Fig. 7A), this β2AR agonist completely lost its inhibitory effect on TNFα production from the β2AR deficient microglia (Fig. 7B). β2AR activation normally leads to intracellular production of cAMP, resulting in the activation of the catalytic subunit of PKA (37). Surprisingly, enriched primary microglia from wide type mice did not produce detectable cAMP after treatment with salmeterol at concentrations 10−10 M or lower, while 10−8 M and 10−9 M salmeterol dose-dependent production of cAMP (Fig. 7C). Further, we found that the PKA inhibitors Rp-cAMP and H89 failed to abolish the inhibitory effects of low dose salmeterol (10−10 M) on the LPS-induced production of TNFα (Fig. 7D) or nitrite (supplemental Fig. 2A) in microglia. In addition to the stimulation of PKA, other effectors can also be activated by cAMP signaling including a small family of guanine nucleotide exchange factors (GEFs) also known as an exchange protein directly activated by cAMP (EPAC) (38). Furthermore, pretreatment with an EPAC agonist, 8CPT-2′-O-Me-cAMP (CPTOMe), did not inhibit the LPS-induced production of TNFα (Fig. 7E) or nitrite (supplemental Fig. 2B), nor did it significantly alter the salmeterol-mediated inhibition of microglial activation, indicating EPAC activation does not play a role in the salmeterol-mediated anti-inflammatory response. Since low dose salmeterol did not appear to activate the canonical β2AR/cAMP signaling pathway, we then investigated whether salmeterol’s effects were mediated through β-arrestin2, a known negative regulator of inflammatory responses in monocytes and macrophages (39, 40). To test this possibility, primary microglia were transfected with control or β-arrestin2 specific siRNA, treated with the indicated concentrations of salmeterol, then exposed to LPS. We found that the anti-inflammatory effects of salmeterol were abolished by the siRNA-mediated knockdown of β-arrestin 2, but not in the control siRNA treated cells (Fig. 7F). Western blot analysis demonstrated the efficiency of siRNA knockdown (Fig. 7G). Taken together, these data strongly suggest low dose salmeterol-mediated anti-inflammatory effects are dependent on β2AR/β-arrestin2, but independent of the cAMP/PKA and cAMP/EPAC pathways.
FIGURE 7.
Low dose salmeterol-mediated anti-inflammatory effects are β2AR/β-arrestin2 dependent, but cAMP/PKA and cAMP/EPAC-independent. Microglia culture prepared from C57/BL6 (A) or β2AR deficient mice (B) were pretreated with vehicle or indicated concentrations of salmeterol for 30 min prior to the addition of LPS. Supernatant were collected at 3h after LPS addition for TNFα analysis (A–B). Figure C: Enriched primary microglia were incubated with vehicle or indicated concentrations of salmeterol at 37°C for 5, 10, or 30 min. After incubation, the cells were lysed and cAMP levels were determined using a cAMP assay kit. Data were expressed as pmol cAMP per 1 million cells (C). Figures D and E: Enriched primary microglia cells were pretreated with vehicle or PKA inhibitors, including H89 (1 μM for 45 min), Rp-cAMP (50 μM for 45 min) (D), or EPAC agonist 8CPT-2′-O-Me-cAMP (CPTOMe) (10 μM for 45 min) (E) prior to stimulation with salmeterol (10−10M) and LPS (5 ng/ml). Supernatants were collected 3h after LPS addition to measure TNFα levels. Figure F and G: Primary microglia cells were transfected with 100 pmol specific β-arrestin2 siRNA or control siRNA, and 48 hrs after transfection cells were treated with indicated concentrations of salmeterol for 30 min prior to addition of LPS. Supernatants were collected for TNFα assay (F). Knockdown of expression of the β-arrestin2 was determined by western blot analysis (G). Results in A–F were expressed as mean ± SE from three to four independent experiments in triplicate. *P<0.05, **P<0.01 compared with the LPS-treated cultures.
Salmeterol significantly suppresses LPS-induced MAPK and NF-κB activation through inhibition of TAK-1 phosphorylation
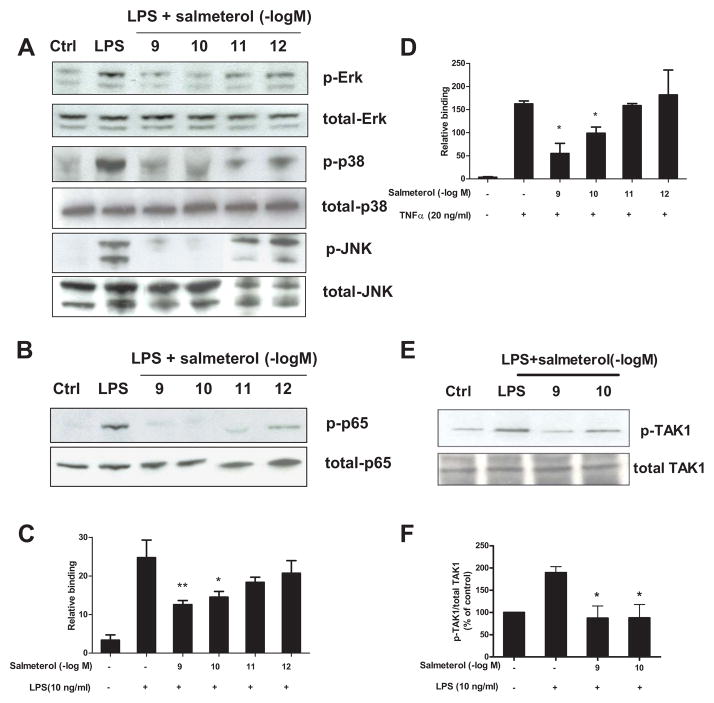
It has been previously shown that β2AR-dependent responses mediated by agonists at micromolar concentrations exert their anti-inflammatory effect primarily through the inhibition of NF-κB. However, the target(s) of the anti-inflammatory activity we observed at doses of 10−10 M salmeterol are not known. Thus, we investigated whether this low dose of salmeterol showed any inhibitory effect on the major LPS-induced pro-inflammatory pathways, namely MAPK and NF-κB (Fig. 8). Primary microglia cells were pretreated with salmeterol at concentrations ranging from 10−9 to 10−12 M, then followed by stimulation with LPS for 15 min. ERK1/2, p38, and JNK were all phosphorylated in microglia following LPS stimulation (Fig. 8A), and the phosphorylation of ERK1/2, p38, and JNK were all significantly reduced by pretreatment with salmeterol at concentrations of 10−9–10−10M but not 10−12M. Salmeterol at 10−10M also reduced the phosphorylation of p65-NF-κB (Fig. 8B) and the LPS-induced NF-κB nuclear translocation and binding to the κB site (Fig. 8C). Identical results were seen when TNFα rather than LPS was used to activate microglial cells, i.e. NF-κB nuclear translocation and κB-binding were inhibited by low dose salmeterol (Fig. 8D), suggesting this inhibition was not specific for the TLR4-mediated pathway. Since TAK-1 is the converging target for LPS- and TNFα– elicited activation of both the MAPK and NF-κB pathways, we investigated whether TAK-1 phosphorylation induced by LPS stimulation of microglial cells was inhibited by salmeterol. The results show that salmeterol at concentrations of 10−9 and 10−10 M inhibited the LPS-induced phosphorylation of TAK-1 (Fig. 8E–F), suggesting that low doses salmeterol-mediated potent anti-inflammatory effects are through the inhibition of TAK1-mediated MAPK and NF-κB signaling pathways.
FIGURE 8.
Salmeterol significantly suppresses LPS-induced MAPK and NF-κB activation through inhibition of TAK-1 phosphorylation. Enriched microglia were pretreated with vehicle or salmeterol (10−9–10−12 M) for 30 min followed by treatment with LPS (10 ng/ml) for 15 min. Cells were then harvested, and the amounts of phosphorylated and total MAPK (ERK1/2, p38, and JNK1/2) (A) and phosphorylated and total NF-κB p65 (B) were determined by western blot analysis, respectively. Representative western blots for ERK1/2, p38, JNK and p65 phosphorylation are shown from 3 independent experiments. Figures C and D: primary microglia were pretreated with salmeterol (10−9–10−12 M) for 30 min, then treated with LPS (C) or TNFα (D) for 30 min. Nuclear extracts were prepared from these cells, and NF-κB (p65) DNA binding activity was detected using the NF-κB (p65) Transcription Factor Assay Kit. Figure E and F: Enriched microglia were pretreated with vehicle or salmeterol (10−9–10−10 M) for 30 min followed by the treatment with LPS (10 ng/ml) for 15 min, cells were then harvested, and the amounts of phosphorylated TAK1 and total TAK1 are show in western blot analysis using specific antibodies (E). ImageJ software was used to quantitate the intensity of the phosphorylated TAK1 and total TAK1 bands in western blot, and the results given in figure F represents the percentage difference of the ratio of phosphorylated TAK1 compared with total TAK1 normalized to the vehicle-treated control (F).
Discussion
One of the most salient features in this report is the finding that long-acting β2AR agonists exhibit potent neuroprotective and anti-inflammatory effects at concentrations 100 fold less than have been previously found to have specific biological activity. Using well-established LPS and MPP+-mediated in vitro and in vivo PD models, we demonstrated for the first time thatβ2AR agonists are protective against inflammation-induced degeneration of DA containing neurons, and that this protection is mediated through the β2AR-mediated inhibition of microglial activation and its pro-inflammatory factors production. Mechanistic studies demonstrate that this low dose salmeterol-mediated potent anti-inflammatory effect is mediated through the inhibition of the phosphorylation of MAPK and p65 NF-κB in activated microglia, and function independent of the conventional GPCR/cAMP/PKA or GPCR/cAMP/EPAC signaling pathways; rather this inhibition is dependent on the expression of β-arrestin 2, which suggests a novel mode of action for the long-acting β2AR agonists in regulating CNS inflammatory conditions.
Long-acting β2AR agonists including salmeterol are now currently in use for the treatment of chronic pulmonary diseases such as asthma and COPD (8, 9). Our data proposes a new indication for this class of compounds in the treatment of chronic neurodegenerative diseases such as PD. This possibility is based on the efficacy of salmeterol shown in two different in vivo PD models, a subacute neurotoxin MPTP model and a chronic inflammatory LPS model. Interestingly, our results showed that salmeterol displays protective effects in both of these PD models, even though the mode of action of these two toxins is different. In the LPS model, we have previously reported that a single systemic injection produces chronic neuroinflammation which results in delayed, progressive motor deficits and selective loss of nigral DA neurons over a period of 7–10 months (28, 29). Our in vitro studies have shown that the most likely mechanism of this chronic LPS-mediated neurodegeneration is through the activation of microglia, which results in the acute production of inflammatory mediators. This initial acute inflammatory response results in the generation of dying or damaged neurons, which in turn release toxic substances to further activate microglia through a process called “reactive microgliosis”, which causes additional neurodegeneration (41). Thus, between microglia activation and neuronal death, a vicious, self-propelling cycle is formed and drives progressive neurodegeneration (33). It is interesting to note that 3 months after LPS injection, a two-week treatment with extremely low dose of salmeterol (1–10 μg/kg/day) is sufficient to achieve neuroprotection and improvement of motor deficits. This finding lends further credence to the notion that breaking the vicious cycle of inflammation-mediated neurodegeneration in the diseased brain is a new and efficient therapy for the treatment of PD, and perhaps other long-term chronic neurodegenerative diseases (33).
Equally potent efficacy of salmeterol’s action in protecting against DA-neurodegeneration was also observed in MPTP model. It is interesting to note that MPTP initially exerts a specific neurotoxic effect on DA neurons, and our studies show that salmeterol has no direct protective effect on DA neurons against MPP+-mediated toxicity (Fig. 3). This finding, and our previous work (42), suggests that a significant amount of neurodegeneration seen in the MPTP model is mediated by microglia-mediated inflammatory killing, and one possible mechanism underlying salmeterol-elicited protection is through inhibition of reactive microgliosis. This observation is consistent with previous results both from our laboratory and from others using anti-inflammatory therapies such as NF-κB inhibitors (43), Treg cells (44, 45) or anti-inflammatory cytokines (46) to prevent MPTP-mediated neurodegeneration. In summary, both our in vivo and in vitro results clearly demonstrate that, despite the difference in the mode of action, salmeterol displays potent neuroprotection in both LPS and MPTP-induced PD models. Based on these findings, we propose that the anti-inflammatory effect of low dose long-acting β2AR agonists is capable of inhibiting both MPTP-induced reactive microgliosis and LPS-induced acute microglial activation, and ultimately suppress neuroinflammation that mediates chronic neurodegeneration in PD.
Increasing evidence from both in vivo and in vitro studies suggest that β2AR agonists possess important immunomodulatory potential. Our studies provide a novel pathway which is different from the conventional cAMP/PKA/NF-κB-dependent pathway mediated byβ2AR activation. These findings also provide opportunities for novel therapeutic approaches in inflammation-mediated CNS disorders such as PD. While bronchodilation occurs via the classical β2AR/cAMP/PKA pathway (47), it is clear that low dose salmeterol-mediated anti-inflammatory and neuroprotective effects are not PKA or EPAC dependent since PKA inhibitors could not block the anti-inflammatory activity of salmeterol, and EPAC agonists could not mimic their anti-inflammatory effect (Fig. 7D–E). For this reason, we looked for an alternative pathway mediating salmeterol-related protective effects. Arrestins, originally discovered as terminators of GPCR signaling by facilitating desensitization and internalization of β2AR, have recently been recognized as multifunctional adaptor/scaffold proteins in regulating cellular processes such as chemotaxis, apoptosis, metastasis and inflammation (40, 48, 49). β-arrestin2 has been found to be a negative regulator of inflammatory responses in monocytes and macrophages (39, 40), but its role in microglia remains unknown. Our studies demonstrate that low doses salmeterol-mediated anti-inflammatory effects are dependent on the expression of both β2AR and β-arrestin2, which suggests a novel GPCR-coupled signaling pathway may be involved, and suggests β-arrestin2 may represent a new target for anti-inflammatory therapy.
Previous results have shown that β2 AR agonists are known to activate MAPKs via both Gs-dependent and Gs-independent mechanisms, and we have previously found that a Gs-independent increase in phosphorylation of ERK occurred following high doses of salmeterol treatment (10−5–10−6M) in RAW264 macrophage cells and primary microglia cells (50), which mediated a pro-inflammatory and neurotoxic effect (51) Conversely, we found that much lower doses salmeterol (10−10–10−11M) have no pro-inflammatory effect, but rather shows dramatic inhibition of MAPK molecules ERK, JNK, and p38 in primary microglia when these cells are activated by LPS. While both effects appear to work independently of PKA activation, the pro-inflammatory effect of high dose salmeterol is through the activation of the cAMP/EPAC pathway, and the inhibitory effect of low dose salmeterol is independent of cAMP induction as well as PKA and EPAC activity. In addition, low doses of salmeterol had a significant inhibitory effect on the LPS-mediated activation of NF-κB and the production of inflammatory mediators normally under NF-κB regulation, such as TNFα and NO. However, it appears that the anti-inflammatory action of salmeterol may be selective only for certain pro-inflammatory pathways in microglial cells, as low dose salmeterol was able to inhibit the activation of superoxide production by LPS but not by PMA (data not shown). Therefore, it appears that low dose salmeterol can be potently but selectively anti-inflammatory in microglial cells by targeting the MAPK and NF-κB signaling pathways following LPS activation.
The neuroprotective effects of β2AR activation by higher doses of β2AR agonists have been reported in other conditions such as ALS (52), cerebral ischemic (53), and spinal cord injury-induced locomotor dysfunction (54). While it is not yet clear how the β2AR agonists exhibit all these neuroprotective properties, several studies suggested thatβ2AR agonists function to stimulate glutathione-dependent antioxidant processes from nerve cells (54), and others reported that neurotrophic factors from activated astocytes induced by β2AR agonists contribute to neuroprotection, (55, 56). We propose that the major neuroprotective activity of β2AR agonists in this study was due to their anti-inflammatory properties. Our results clearly show that the effectiveness of the β2AR agonist salmeterol and the other long-acting β2AR agonists at the low concentrations tested here is due to their anti-inflammatory effect on microglia, not to a direct protective effect on DA-neurons or through an astrocyte-dependent effect (Fig. 3). Given the effectiveness of these compounds at such low concentrations in inhibiting inflammatory responses, they appear to have significant potential in regulating CNS inflammation and the treatment of chronic inflammatory disorders of CNS.
Supplementary Material
Acknowledgments
We thank Drs. Robert Langenbach and Robert Oakley at NIEHS for their helpful suggestions for this paper.
This work was supported by NIH grant DE-13079 from the National Institute for Dental and Craniofacial Research, a grant from the Michael J. Fox Foundation, and was also supported in part by the Intramural Research Program of the NIH/NIEHS.
Abbreviations used in this paper
- GPCR
G-protein coupled receptor
- β2AR
β2 adrenergic receptor
- DA
dopaminergic
- PD
Parkinson’s disease
- TH-IR
tyrosine hydroxylase-immunoreactive
- SOD
superoxide dismutase
- DCFH-DA
dichlorodihydrofluorescein Diacetate
- iROS
intracellular ROS
- SN
substantia nigra
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- COPD
chronic obstructive pulmonary disease
- SNpc
SN pars compacta
- GEFs
guanine nucleotide exchange factors
- EPAC
exchange protein directly activated by cAMP
- DOPAC
3,4-dihydroxyphenylacetic acid
- and HVA
homovanillic acid
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Koff WC, Fann AV, Dunegan MA, Lachman LB. Catecholamine-induced suppression of interleukin-1 production. Lymphokine Res. 1986;5:239–247. [PubMed] [Google Scholar]
- 2.van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62:2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekut L, Champion BR, Page K, Menius JA, Jr, Connolly KM. Anti-inflammatory activity of salmeterol: down-regulation of cytokine production. Clin Exp Immunol. 1995;99:461–466. doi: 10.1111/j.1365-2249.1995.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992;148:3441–3445. [PubMed] [Google Scholar]
- 5.Farmer P, Pugin J. beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:L675–682. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- 6.Kin NW, V, Sanders M. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 7.Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tashkin DP, Cooper CB. The role of long-acting bronchodilators in the management of stable COPD. Chest. 2004;125:249–259. doi: 10.1378/chest.125.1.249. [DOI] [PubMed] [Google Scholar]
- 9.McKeage K, Keam SJ. Salmeterol/fluticasone propionate: a review of its use in asthma. Drugs. 2009;69:1799–1828. doi: 10.2165/11202210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Koto H, Mak JC, Haddad EB, Xu WB, Salmon M, Barnes PJ, Chung KF. Mechanisms of impaired beta-adrenoceptor-induced airway relaxation by interleukin-1beta in vivo in the rat. J Clin Invest. 1996;98:1780–1787. doi: 10.1172/JCI118977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov Disord. 2007;22:1852–1856. doi: 10.1002/mds.21552. [DOI] [PubMed] [Google Scholar]
- 14.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 15.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 16.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 17.Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka KF, Kashima H, Suzuki H, Ono K, Sawada M. Existence of functional beta1- and beta2-adrenergic receptors on microglia. J Neurosci Res. 2002;70:232–237. doi: 10.1002/jnr.10399. [DOI] [PubMed] [Google Scholar]
- 19.Qian L, Flood PM. Microglial cells and Parkinson’s disease. Immunol Res. 2008 doi: 10.1007/s12026-008-8018-0. [DOI] [PubMed] [Google Scholar]
- 20.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293:607–617. [PubMed] [Google Scholar]
- 22.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 23.Qian L, Block ML, Wei SJ, Lin CF, Reece J, Pang H, Wilson B, Hong JS, Flood PM. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther. 2006;319:44–52. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Du L, Kong LY, Hudson PM, Wilson BC, Chang RC, Abel HH, Hong JS. Reduction by naloxone of lipopolysaccharide-induced neurotoxicity in mouse cortical neuron-glia co-cultures. Neuroscience. 2000;97:749–756. doi: 10.1016/s0306-4522(00)00057-9. [DOI] [PubMed] [Google Scholar]
- 25.Peskin AV, Winterbourn CC. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1) Clin Chim Acta. 2000;293:157–166. doi: 10.1016/s0009-8981(99)00246-6. [DOI] [PubMed] [Google Scholar]
- 26.Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Shen HM, Ong CN. Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in Salvia miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells. Life Sci. 2001;69:1833–1850. doi: 10.1016/s0024-3205(01)01267-x. [DOI] [PubMed] [Google Scholar]
- 28.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Qin L, Wilson B, Wu X, Qian L, Granholm AC, Crews FT, Hong JS. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008;29:864–870. doi: 10.1016/j.neuro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu HM, Tzeng NS, Qian L, Wei SJ, Hu X, Chen SH, Rawls SM, Flood P, Hong JS, Lu RB. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology. 2009;34:2344–2357. doi: 10.1038/npp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanania NA, Moore RH. Anti-inflammatory activities of beta2-agonists. Curr Drug Targets Inflamm Allergy. 2004;3:271–277. doi: 10.2174/1568010043343598. [DOI] [PubMed] [Google Scholar]
- 32.Johnson M. Effects of beta2-agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol. 2002;110:S282–290. doi: 10.1067/mai.2002.129430. [DOI] [PubMed] [Google Scholar]
- 33.Qian L, Tan KS, Wei SJ, Wu HM, Xu Z, Wilson B, Lu RB, Hong JS, Flood PM. Microglia-Mediated Neurotoxicity Is Inhibited by Morphine through an Opioid Receptor-Independent Reduction of NADPH Oxidase Activity. J Immunol. 2007;179:1198–1209. doi: 10.4049/jimmunol.179.2.1198. [DOI] [PubMed] [Google Scholar]
- 34.Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, Liu Y, Qian L, Wilson B, Di Monte DA, Ali SF, Zhang J, Block ML, Hong JS. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J Immunol. 2008;181:7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. Faseb J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- 36.Hung CH, Chu YT, Hua YM, Hsu SH, Lin CS, Chang HC, Lee MS, Jong YJ. Effects of formoterol and salmeterol on the production of Th1- and Th2-related chemokines by monocytes and bronchial epithelial cells. Eur Respir J. 2008;31:1313–1321. doi: 10.1183/09031936.00121406. [DOI] [PubMed] [Google Scholar]
- 37.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 38.Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 39.Loniewski K, Shi Y, Pestka J, Parameswaran N. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol Immunol. 2008;45:2312–2322. doi: 10.1016/j.molimm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Xu M, Zhang YY, He B. Fenoterol, a beta(2)-adrenoceptor agonist, inhibits LPS-induced membrane-bound CD14, TLR4/CD14 complex, and inflammatory cytokines production through beta-arrestin-2 in THP-1 cell line. Acta Pharmacol Sin. 2009;30:1522–1528. doi: 10.1038/aps.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 42.Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML. Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain. 133:808–821. doi: 10.1093/brain/awp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol. 2007b;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, El-Benna J, Hong JS, Flood PM. Potent Anti-Inflammatory and Neuroprotective Effects of TGF-{beta}1 Are Mediated through the Inhibition of ERK and p47phox-Ser345 Phosphorylation and Translocation in Microglia. J Immunol. 2008;181:660–668. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussein A, Al-Wadei N, Takahashi T, Schuller HM. Theophylline stimulates cAMP-mediated signaling associated with growth regulation in human cells from pulmonary adenocarcinoma and small airway epithelia. Int J Oncol. 2005;27:155–160. [PubMed] [Google Scholar]
- 48.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Qian L, Hu X, Zhang D, Snyder A, Wu HM, Li Y, Wilson B, Lu RB, Hong JS, Flood PM. beta2 Adrenergic receptor activation induces microglial NADPH oxidase activation and dopaminergic neurotoxicity through an ERK-dependent/protein kinase A-independent pathway. Glia. 2009;57:1600–1609. doi: 10.1002/glia.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teng YD, Choi H, Huang W, Onario RC, Frontera WR, Snyder EY, Sabharwal S. Therapeutic effects of clenbuterol in a murine model of amyotrophic lateral sclerosis. Neurosci Lett. 2006;397:155–158. doi: 10.1016/j.neulet.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Culmsee C, Junker V, Kremers W, Thal S, Plesnila N, Krieglstein J. Combination therapy in ischemic stroke: synergistic neuroprotective effects of memantine and clenbuterol. Stroke. 2004;35:1197–1202. doi: 10.1161/01.STR.0000125855.17686.6d. [DOI] [PubMed] [Google Scholar]
- 54.Zeman RJ, Peng H, Feng Y, Song H, Liu X, Etlinger JD. Beta2-adrenoreceptor agonist-enhanced recovery of locomotor function after spinal cord injury is glutathione dependent. J Neurotrauma. 2006;23:170–180. doi: 10.1089/neu.2006.23.170. [DOI] [PubMed] [Google Scholar]
- 55.Junker V, Becker A, Huhne R, Zembatov M, Ravati A, Culmsee C, Krieglstein J. Stimulation of beta-adrenoceptors activates astrocytes and provides neuroprotection. Eur J Pharmacol. 2002;446:25–36. doi: 10.1016/s0014-2999(02)01814-9. [DOI] [PubMed] [Google Scholar]
- 56.Semkova I, Krieglstein J. Neuroprotection mediated via neurotrophic factors and induction of neurotrophic factors. Brain Res Brain Res Rev. 1999;30:176–188. doi: 10.1016/s0165-0173(99)00013-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.