Abstract
The drug release and degradation behavior of two double-walled microsphere formulations consisting of a doxorubicin loaded poly(D,L-lactic-co-glycolic acid) (PLGA) core (~46 kDa) surrounded by a poly(D,L-lactic acid) (PDLLA) shell layer (~55 and 116 kDa) were examined. It was postulated that different molecular weights of the shell layer could modulate the erosion of the outer coating and limit the occurrence of water penetration into the inner drug-loaded core on various time scales, and therefore control the drug release from the microspheres. For both microsphere formulations, the drug release profiles were observed to be similar. The degradation of the microspheres was monitored for a period of about nine weeks and analyzed using scanning electron microscopy, laser scanning confocal microscopy, and gel permeation chromatography. Interestingly, both microsphere formulations exhibited occurrence of bulk erosion of PDLLA on a similar time scale despite different PDLLA molecular weights forming the shell layer. The shell layer of the double-walled microspheres served as an effective diffusion barrier during the initial lag phase period and controlled the release rate of the hydrophilic drug independent of the molecular weight of the shell layer.
Keywords: Double-walled microspheres, PLGA, PDLLA, doxorubicin, degradation, erosion
1. Introduction
With the advent of biocompatible and biodegradable polymers, much research has focused on the development of suitable polymeric drug delivery systems and their design for sustained drug release applications. Polymeric drug delivery systems have the potential to protect drugs from degradation, and at the same time, provide their release at the targeted site in a predesigned manner to achieve more effective therapies while eliminating the potential for both under- and over-dosing. Polymeric drug delivery systems such as biodegradable polymer microspheres are simple to fabricate. Moreover, they offer facile administration via routes including oral, pulmonary and parenteral injection, and they do not need surgical removal upon complete drug release. However, the use of conventional single-polymer microspheres is severely undermined by several limitations, including the initial burst release caused by rapid release of drug found on or near the external surface, difficulty in achieving zero-order drug release, and a lack of timedelayed or pulsatile release of drugs [1, 2].
Since an important goal of drug delivery systems is to attain well-controlled drug release rates, double-walled microspheres with a drug-encapsulating particle core surrounded by a drug-free shell layer are introduced [3–12]. These double-walled microspheres often exhibit a reduction in the initial burst release as compared to single-polymer microspheres [3, 4, 8, 9], and provide a sustained drug release that is tunable by adjusting the shell material or thickness [6, 7]. In addition, these microspheres enable the encapsulation of multiple drugs in the core and shell phases, and allow their release in various stages, thus achieving synergistic therapeutic effects [10–12]. For example, the parallel or sequential release of multiple drugs would be useful for expediting a variety of growth factor driven tissue regenerative processes in tissue engineering [10] or formulating a successful tumor inhibition strategy in cancer therapy [11, 12]. Recently, the development of triple-walled microspheres have also gained significant interest, and such multi-layered drug delivery systems could provide a versatile approach to deliver several drugs and control their respective drug release profiles [13–18].
Drug release from biodegradable polymeric delivery systems is intricately linked to the degradation of the polymer matrix, and is dependent on the degree of crystallinity of the polymer [19] and the release condition [20]. Polymer degradation is often preceded by a sequence of processes including water absorption, polymer hydrolysis and matrix erosion that occur simultaneously [21]. For common materials such as polyesters, the process of polymer degradation involves hydrolytic chain scission, during which polymer chains are cleaved into oligomers and monomers. This leads to the mass loss of the polymer matrix that is characteristic for erosion [22]. Degradable polymers are typically classified into bulk- and surface-eroding materials [23]. For bulk-eroding polymers such as polyesters [24, 25], the rate of water penetration into the polymer matrix is higher than the rate of hydrolysis. In this case, water diffuses into the polymer inducing swelling and degradation throughout the matrix simultaneously. For surface-eroding polymers such as polyanhydrides [26, 27] and poly(ortho esters) [28], the rate of hydrolysis is higher than the rate of water penetration. In this case, hydrolysis is confined to the outer polymer surface, and the interior of the matrix remains relatively unchanged.
Many groups have produced double-walled microspheres from a variety of materials including bulk- and surface-eroding polymers, and investigated their degradation behavior [29–34]. In one study, the degradation of double-walled microspheres with a core of poly(1,3-bis-(p-carboxyphenoxypropane)- co-(sebacic anhydride)) 20:80 (P(CPP:SA)20:80) and an external coat of poly(L-lactic acid) (PLLA) was monitored in vitro and in vivo for 6 months [30, 31]. The inner core of the more hydrolytically labile P(CPP:SA)20:80 degraded first while the shell layer remained relatively intact. In another study, the degradation of double-walled microspheres consisting of a poly(ortho ester) (POE) core surrounded by a poly(D,L-lactic-co-glycolic acid) 50:50 (PLGA) shell layer was examined [32]. Similar to the previous study, preferential degradation of the POE core was observed, and formation of hollow microspheres became pronounced after the first week of incubation. In an attempt to limit water penetration into the inner core phase, a surface-eroding polymer, poly(1,6-bis-(p-carboxyphenoxyhexane)) (PCPH), was used to encapsulate a PLGA core [34]. However, the slow eroding PCPH shell layer could not prevent water penetration, and the PLGA core was completely eroded by 6 weeks of incubation.
Overall, these studies showed that the preferential degradation of the inner core is highly dependent on the occurrence of water penetration through the shell layer. It would be interesting if different molecular weights of the shell layer could modulate the erosion of the outer coating and limit the occurrence of water penetration into the inner drug-loaded core on various time scales, and therefore control the drug release from the microspheres. Thus, the main focus of this study is to investigate the effect of molecular weight of polymer shell on the drug release and degradation behavior of double-walled microspheres.
Here, the drug release and degradation behavior of double-walled microspheres consisting of a doxorubicin-loaded PLGA core surrounded by a poly(D,L-lactic acid) (PDLLA) shell layer were reported. Doxorubicin was employed as a hydrophilic model drug loaded selectively in the core phase of the microspheres. For the purpose of this study, two different PDLLA molecular weights were used to form the shell layer of the double-walled microspheres since they exhibited different trends of time-dependent molecular weight change [24]. The in vitro release profile of doxorubicin was determined, while the degradation behavior of the microspheres was monitored using scanning electron microscopy, laser scanning confocal microscopy and gel permeation chromatography. The compilation of the results from the three analytical tools would allow elucidation of the dominant mechanism controlling drug release at different stages of the degradation process and account for the drug release profiles obtained experimentally.
2. Materials and methods
2.1. Materials
Poly(D,L-lactic-co-glycolic acid) (PLGA) copolymer (50:50 lactic acid:glycolic acid; inherent viscosity (i.v.) = 0.61 dL/g in hexafluoroisopropanol (HFIP)), and poly(lactic acid) (PLA) polymers including poly(D,L-lactic acid) (PDLLA) (i.v. = 0.37 and 0.70 dL/g in chloroform) and poly(L-lactic acid) (PLLA) (i.v. = 1.05 dL/g in chloroform) were purchased from Lactel Absorbable Polymers (Pelham, AL). Poly(vinyl alcohol) (PVA) (Mw = 25,000 Da), 88 mol% hydrolyzed, was purchased from Polysciences, Inc. (Warrington, PA). Doxorubicin, in the form of hydrochloride salt with more than 99% purity, was purchased from LC Laboratories (Woburn, MA). Dichloromethane (DCM) and HFIP were acquired from Sigma-Aldrich Corp. (St. Louis, MO) while HPLC-grade tetrahydrofuran (THF) was acquired from Tedia (Fairfield, OH). Phosphate-buffered saline (PBS) with a pH of 7.4 was acquired from Mediatech, Inc. (Manassas, VA).
2.2. Fabrication of double-walled PLA(PLGA) microspheres
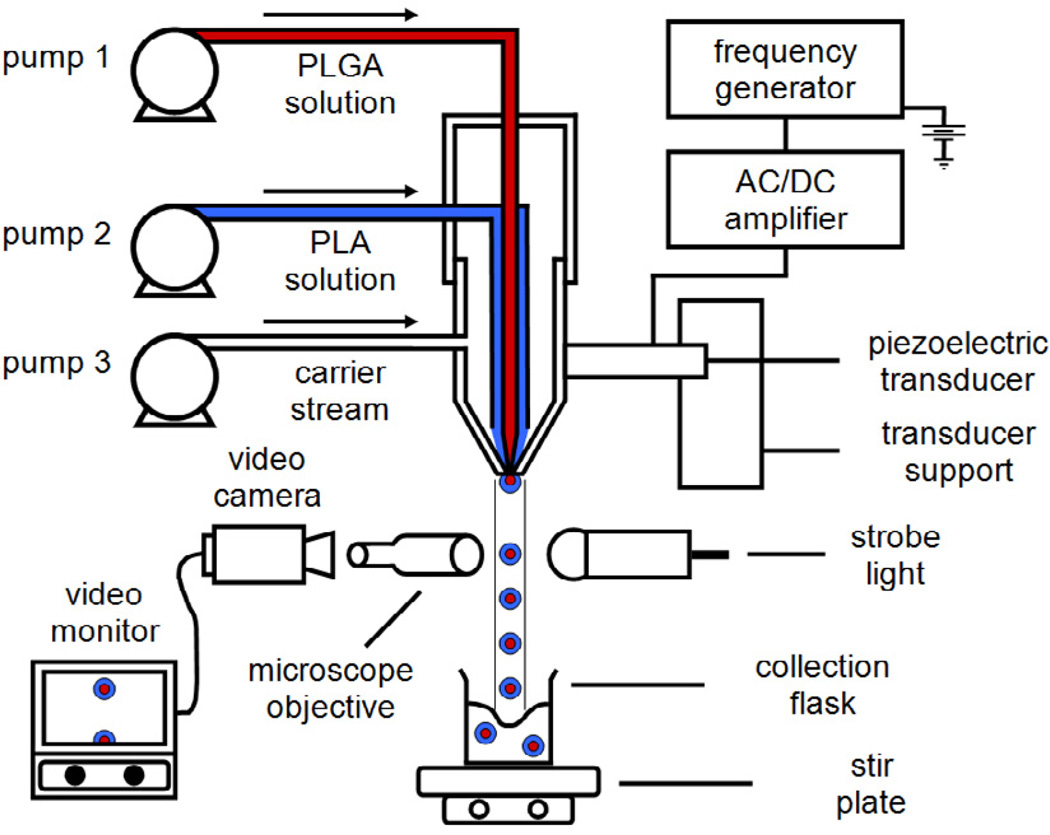
Double-walled PLA(PLGA) microspheres consisting of a PLGA core surrounded by a PLA shell were produced by using the established precision particle fabrication (PPF) technique (Fig. 1). Solutions containing 20 to 40% (w/v) PLGA and 5% (w/v) PLA in DCM were individually prepared. In this technique, a coaxial nozzle was used to produce a jet of core PLGA surrounded by an annular stream containing PLA. The core-shell polymer jet, protected by a non-solvent 0.5% (w/v) PVA carrier stream, was disrupted into uniform nascent double-walled droplets by an ultrasonic transducer controlled by a frequency generator. In order to control monodispersity of the double-walled microspheres, the fabrication process was monitored to ensure there was a steady disruption of the core-shell polymer jet by adjusting ultrasonic frequency and flow rate of the carrier stream. The droplets were collected in a beaker containing 0.5% (w/v) PVA solution, before they were stirred continuously for ~2 h, filtered and rinsed with an equal volume of distilled water to remove residual PVA from the microspheres. Finally, the microspheres were freeze-dried for 3 days and stored at −20°C under desiccant. To prepare microspheres loaded with doxorubicin in the PLGA core phase, a stock solution of doxorubicin was first prepared in water (50 mg/ml), before an appropriate amount of drug solution was further diluted in water and added to 10 ml of PLGA/DCM solution to obtain the desired drug to polymer loading. The resultant mixture was sonicated using a Model 500 Sonic Dismembrator (Thermo Fisher Scientific, Inc., Pittsburgh, PA) at 30% amplitude in an ice bath for 90 s to form a stable emulsion.
Figure 1.
Schematic diagram of precision particle fabrication apparatus for the production of uniform double-walled microspheres of controlled shell thickness.
2.3. Particle size distribution
The size distributions of the hardened double-walled microspheres were determined using a Multisizer 3 (Beckman Coulter, Inc., Fullerton, CA) with a 120 µm aperture. The microspheres were suspended in Isoton II Diluent (Beckman Coulter, Inc., Fullerton, CA) before measurement. At least 10,000 microspheres were measured for every sample.
2.4. Optical microscopy
The hardened double-walled microspheres were examined using an Invertoskop inverted microscope (Carl Zeiss Microscopy LLC, Thornwood, NY). A few droplets of the aqueous microsphere suspension were placed directly onto a microscope slide. Images were captured using Digital Microscope Suite software.
2.5. Drug loading
The drug loading was determined by dissolving approximately 50 mg of microspheres (2% (w/w) theoretical loading of doxorubicin with respect to PLGA) in 1 ml of HFIP. The samples were allowed to stand until complete dissolution of the polymers. The samples were then centrifuged at 10,000 rpm for 10 min, and the supernatants were carefully extracted. In order to measure doxorubicin concentration, the supernatant was added in triplicate in a 96-well plate, and the absorbance was analyzed using a SpectraMax 340PC spectrophotometer (Molecular Devices LLC, Sunnyvale, CA) at a wavelength of 480 nm.
2.6. In vitro drug release
The release profile was determined by suspending approximately 150 mg of microspheres (2% (w/w) theoretical loading of doxorubicin with respect to PLGA) in 5 ml of PBS in centrifuge tubes. The tubes were placed in an incubator maintained at 37°C and shaken at 240 rpm. At selected time points, the tubes were centrifuged at 10,000 rpm for 10 min before 1 ml of supernatant was collected and 1 ml of fresh PBS was replaced. This is done to ensure sink conditions. In order to measure doxorubicin concentration, the supernatant was sufficiently diluted in PBS before adding in triplicate in a 96-well plate, and the fluorescence was analyzed using the fluorescence spectrophotometer at excitation and emission wavelengths of 480 and 590 nm, respectively.
2.7. In vitro degradation
The in vitro degradation study was performed by suspending approximately 20 mg of microspheres (0.06% (w/w) loading of doxorubicin with respect to PLGA) in 660 µl of PBS in centrifuge tubes. Similar to the in vitro drug release study, the tubes were placed in an incubator maintained at 37°C and shaken at 240 rpm. Based on the in vitro drug release time points, the tubes were centrifuged at 10,000 rpm for 10 min before 132 µl of supernatant was removed, and 132 µl of fresh PBS was replaced. This is done to ensure sink conditions. At pre-determined time points, the tubes were centrifuged, and PBS was removed. The microspheres were then rinsed twice with distilled water, and imaged using scanning electron microscopy (SEM) and laser scanning confocal microscopy. Samples were also collected for gel permeation chromatography (GPC) analysis. In this case, the samples were further freeze-dried for 3 days before dissolving them in HPLC-grade THF.
2.8. Scanning electron microscopy
The surface morphology of the degrading double-walled microspheres was examined using a JEOL JSM-5600LV scanning electron microscope (SEM) (JEOL Ltd., Tokyo, Japan). A few droplets of the aqueous microsphere suspension were placed directly onto a SEM sample holder coated with conductive carbon tape and air-dried overnight. The samples were sputter-coated with gold palladium prior to imaging at 10 kV.
2.9. Laser scanning confocal microscopy
The distribution of doxorubicin in the degrading double-walled microspheres was examined using a Fluoview FV1000 laser scanning confocal microscope (Olympus Corp., Tokyo, Japan) equipped with argon ion laser tuned to 488 nm. A few droplets of the aqueous microsphere suspension were placed directly onto a microscope slide and sealed with a cover glass. The samples were then visualized using an oil immersion objective lens under ×60 magnification and ×1 zoom with the following calibrations: 4.0 µs/pixel sampling speed, laser at 720 V and transmissivity of 10%. The fluorescence emission was collected using a 505 nm long pass interference filter. These settings were used for all the samples to ensure consistency. Optical cross-sections were taken at various depths for each sample in order to determine drug distribution at the centerline of the microspheres. Images were captured using Olympus Fluoview software. The fluorescence intensity profiles were obtained using ImageJ software.
2.10. Molecular weight analysis
The molecular weights of pure polymers and double-walled microspheres were determined using a gel permeation chromatography (GPC) system consisting of a Waters 1515 Isocratic HPLC Pump, Waters 717plus Autosampler and Waters 2414 Refractive Index Detector (Waters Corp., Milford, MA). The samples were eluted in HPLC-grade THF through 5 µm Jordi Gel DVB 1,000, 10,000 and 100,000 Ǻ columns (Jordi Labs LLC, Bellingham, MA) connected in series at a flow rate of 1 ml/min and a temperature of 35°C. The samples were filtered before injecting into the column to remove insoluble particulates when present. The calibration curve was generated using polystyrene standards (Polymer Laboratories Ltd., Church Stretton, Shropshire, UK) prepared at concentrations of 1 mg/ml. The semi-logarithmic calibration curve of molecular weight versus elution time is linear (R2 = 0.998). The weight-averaged and peak molecular weights of the samples were obtained using Waters Breeze software. The molecular weights were reported based on the average of two measurements.
3. Results and discussion
3.1. Effect of polymer concentration and flow rate on the formation of double-walled PLLA(PLGA) microspheres
The first experiment was conducted to examine the effect of polymer concentration and flow rate on the formation of double-walled PLLA(PLGA) microspheres. As shown in Table 1, the PLLA shell concentration was fixed at 5% (w/v) while the PLGA core concentration was 20, 30 or 40% (w/v) in DCM. In addition, the PLGA core flow rate was maintained at 4 ml/h while the PLLA shell flow rate was 12, 24 or 36 ml/h, leading to various PLLA:PLGA mass ratios.
Table 1.
Summary of polymer concentrations and flow rates used to produce double-walled PLLA(PLGA) microspheres. The calculation of PLLA:PLGA mass ratio is based on the assumption that the volumes of polymer and solvent in the solution are additive. The following constant density values are used: ρPLLA = 1.34 g/cm3, ρPLGA = 1.24 g/cm3 and ρDCM = 1.33 g/cm3.
| Sample | PLLA shell concentration (% (w/v)) |
PLGA core concentration (% (w/v)) |
PLLA shell flow rate (ml/h) |
PLGA core flow rate (ml/h) |
PLLA:PLGA polymer mass ratio |
Mean diameter (µm) |
Full PLGA encapsulation (Yes/No) |
|---|---|---|---|---|---|---|---|
| A1 | 5 | 20 | 12 | 4 | 0.46:0.54 | 50.1 ± 3.6 | No |
| A2 | 5 | 20 | 24 | 4 | 0.63:0.37 | 57.2 ± 2.0 | No |
| A3 | 5 | 20 | 36 | 4 | 0.72:0.28 | 74.9 ± 4.2 | Yes |
| B1 | 5 | 30 | 12 | 4 | 0.37:0.63 | 49.2 ± 2.3 | No |
| B2 | 5 | 30 | 24 | 4 | 0.54:0.46 | 59.6 ± 3.6 | No |
| B3 | 5 | 30 | 36 | 4 | 0.64:0.36 | 66.1 ± 2.5 | Yes |
| C1 | 5 | 40 | 12 | 4 | 0.32:0.68 | 58.7 ± 1.6 | No |
| C2 | 5 | 40 | 24 | 4 | 0.49:0.51 | 65.6 ± 1.3 | No |
| C3 | 5 | 40 | 36 | 4 | 0.59:0.41 | 74.0 ± 2.7 | No |
Based on the above conditions, microspheres with a mean diameter ranging from 50 to 75 µm could be produced using the PPF apparatus, with each sample showing a narrow size distribution (Table 1). Overall, the increase in the PLLA flow rate led to an increase in the size of the microspheres since the ultrasonic frequency was fixed at 5 kHz, thus leading to a constant rate of droplet formation. Most importantly, the efficient production of fully formed double-walled microspheres depended on the PLLA:PLGA mass ratio, and it occurred only above a critical polymer mass ratio. When using a PLGA core concentration of 20% (w/v) in DCM, the critical PLLA mass fraction was found to lie between 0.63 and 0.72. Similarly, when using a PLGA core concentration of 30% (w/v) in DCM, the critical PLLA mass fraction was found to lie between 0.55 and 0.64. However, it appeared that a critical PLLA mass fraction higher than 0.59 was required when a PLGA core concentration of 40% (w/v) in DCM was used. Increasing the PDLLA flow rate may aid the spreading of the shell phase onto the more concentrated core phase to form core-shell structured microspheres [5]. The PLGA core concentration, on the other hand, did not seem to have a significant effect on the critical polymer mass ratio needed for forming double-walled microspheres.
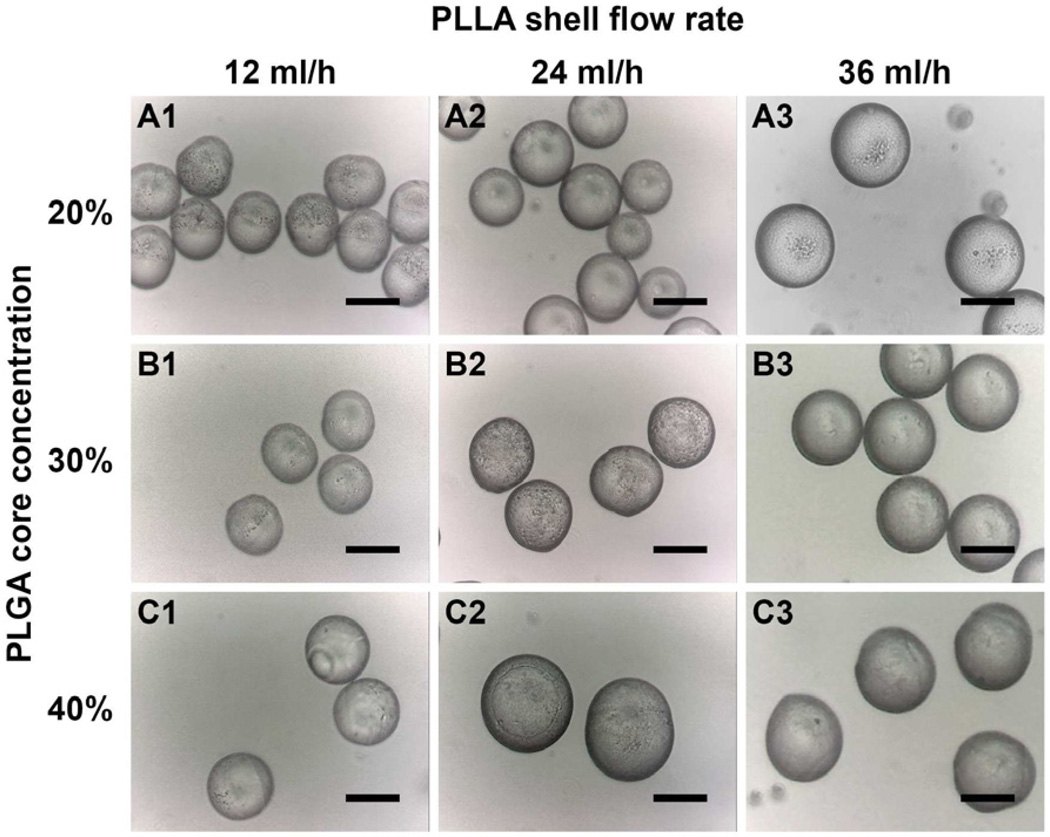
Fig. 2 shows the optical images for various microsphere samples listed in Table 1. Partial encapsulation of PLGA core by PLLA polymer was found in microspheres when a PLLA shell flow rate below the critical polymer mass ratio was selected (Samples A1, A2, B1, B2 and C1- C3). The polymer interface between PLLA and PLGA could be observed on the microsphere surface. Above the critical polymer mass ratio, fully formed double-walled microspheres were observed with no sign of partial encapsulation configuration (Samples A3 and B3).
Figure 2.
Optical images depicting the surface morphology of double-walled PLLA(PLGA) microspheres for various microsphere samples listed in Table 1. Partial encapsulation was observed for samples A1, A2, B1, B2, and C1 to C3. Fully formed double-walled microspheres were observed in samples A3 and B3. Scale bar = 50 µm.
3.2. Encapsulation efficiency and in vitro drug release study of double-walled PDLLA(PLGA) microspheres
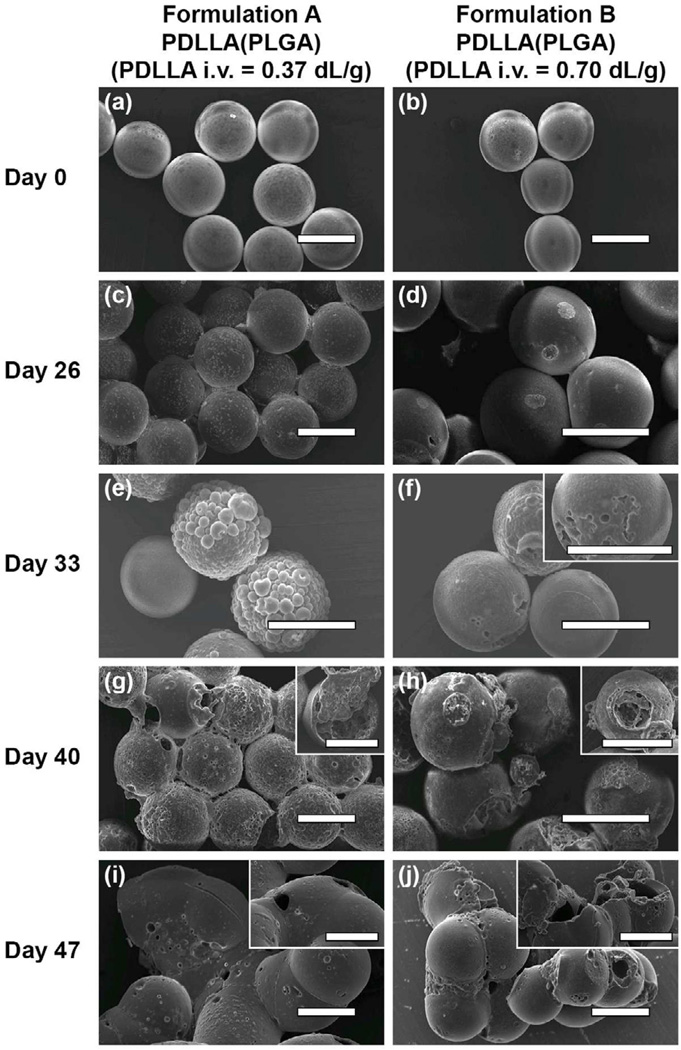
The formation of double-walled PDLLA(PLGA) microspheres could also be achieved by using different molecular weights of PDLLA as the shell layer. In addition, doxorubicin could be encapsulated within the PLGA core successfully. Here, two microsphere formulations consisting of a doxorubicin loaded PLGA core surrounded by a low PDLLA molecular weight shell layer (i.v. = 0.37 dL/g; formulation A) and a high PDLLA molecular weight shell layer (i.v. = 0.70 dL/g; formulation B) were fabricated based on the same polymer concentrations and flow rates used to produce sample B3 microspheres. Based on the measured overall particle diameter (about 65 µm) and known mass flow rates of the polymer solutions, and using an assumption of complete polymer phase separation, the shell thickness of both formulations is approximately 8 µm. From the SEM images, the as-prepared microspheres for both formulations were fairly smooth with minimal surface pores (Fig. 3a and 3b). From the confocal images, doxorubicin was localized within the PLGA core, although microspheres having a high PDLLA molecular weight shell layer exhibited a better core-shell structure than those having a low PDLLA molecular weight shell layer (Fig. 4a(i) and 4a(ii)).
Figure 3.
SEM images depicting the surface morphology of double-walled PDLLA(PLGA) microspheres with a low PDLLA molecular weight shell layer (formulation A) and a high PDLLA molecular weight shell layer (formulation B) at different stages of the degradation process. (a) and (b) are images of initial microspheres before degradation, (c) and (d) 26 days, (e) and (f) 33 days, (g) and (h) 40 days, and (i) and (j) 47 days after degradation. The inserts show microspheres with pore or cavity formation. Scale bar = 50 µm.
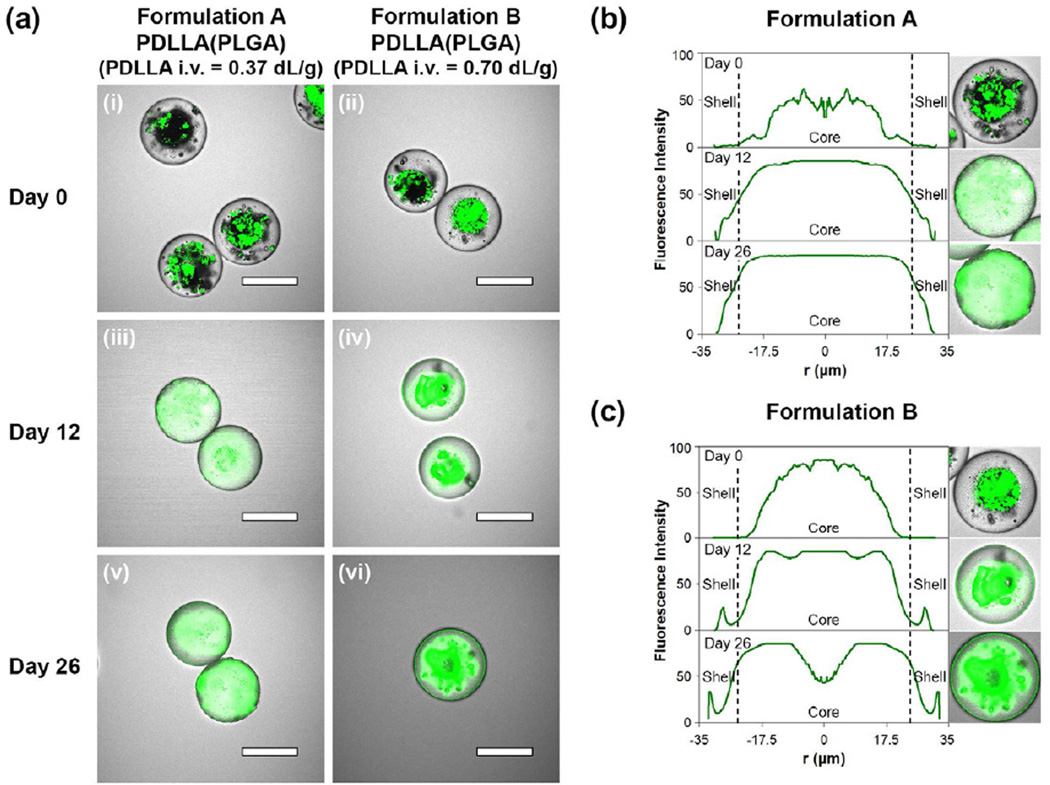
Figure 4.
Laser scanning confocal images and fluorescence intensity profiles depicting the distribution of doxorubicin in the double-walled PDLLA(PLGA) microspheres during the initial stage of the degradation process (0 to 26 days). (a(i)) and (a(ii)) are images of initial microspheres before degradation, (a(iii)) and (a(iv)) 12 days, and (a(v)) and (a(vi)) 26 days after degradation. Scale bar = 50 µm. (b) and (c) are the fluorescence intensity profiles of doxorubicin in representative microspheres of formulations A and B respectively. The inserts are the confocal images captured at the centerline of the microspheres, and the profile is based on the radial average fluorescence intensity from the center of the microsphere.
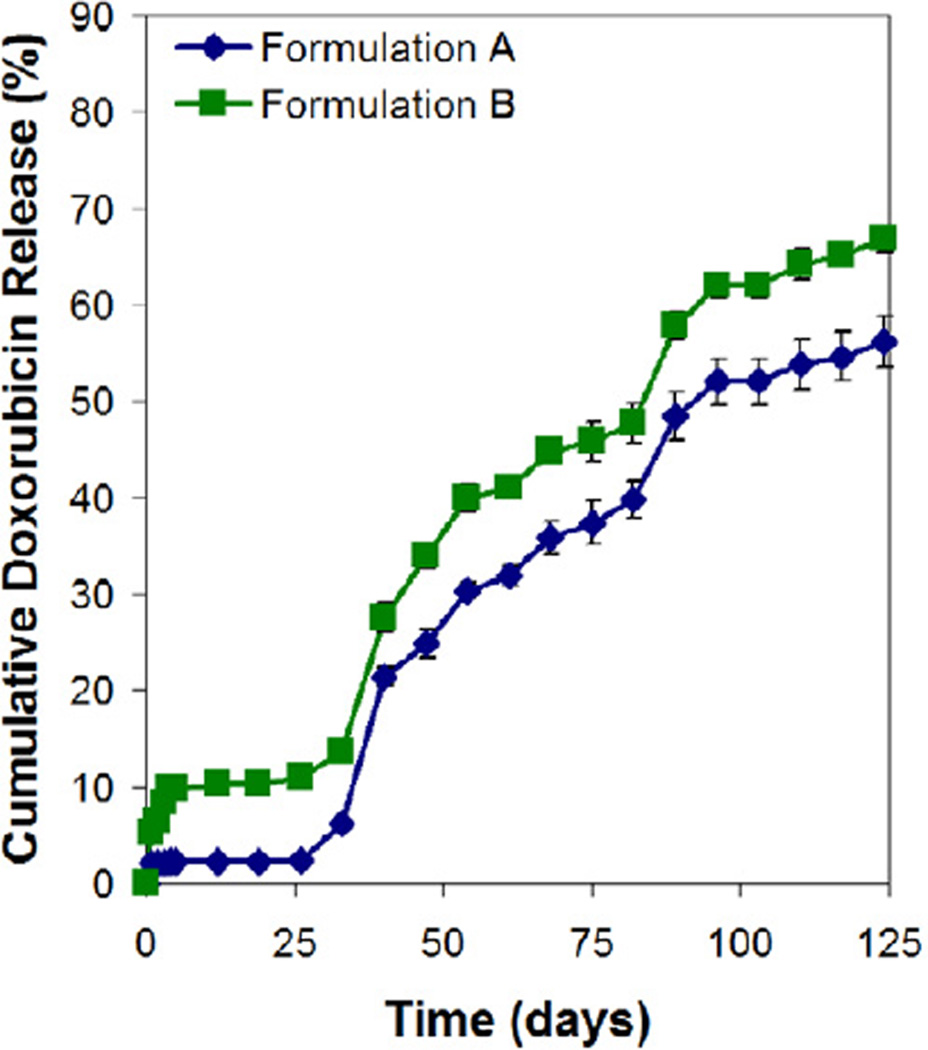
Both microsphere formulations were further examined for their respective encapsulation efficiency and in vitro drug release profile. The encapsulation efficiency of doxorubicin in formulation A and B microspheres was 79.3 ± 1.5% and 80.0 ± 2.6%, respectively. The release profiles of doxorubicin for both formulations were similar (Fig. 5). Typically, the profiles showed a small initial burst (~2% for formulation A and ~10% for formulation B), before entering a lag phase of about 25 days. The drug subsequently released slowly (~55%) at nearly constant rate over a period of 100 days. It is interesting to note that the molecular weight of the PDLLA shell layer did not have a significant effect on the lag phase period and the subsequent drug release rate. These observations are not expected since slower polymer erosion rate and hence slower water penetration rate are associated with the use of higher PDLLA molecular weight as the shell layer [24]. Consequently, it is predicted that formulation B microspheres would exhibit a longer lag phase period and a slower drug release rate than formulation A microspheres. In fact, the similar drug release behavior for both formulations is closely related to the degradation mechanism of these core-shell structured microspheres, and this phenomenon will be investigated in the next section.
Figure 5.
In vitro release of doxorubicin from double-walled PDLLA(PLGA) microspheres.
3.3. In vitro degradation study of double-walled PDLLA(PLGA) microspheres
In an attempt to further examine the influence of the molecular weight of PDLLA shell layer on the drug release behavior, the degradation study of the microspheres was performed. From the drug release profiles, 26 days was determined to be the critical time point at which the drug release began. Thus, the degradation of the microspheres was monitored for a period of about nine weeks and analyzed using SEM, laser scanning confocal microscopy and GPC. The findings will be used to elucidate the underlying degradation mechanism and explain the similar drug release behavior for these microsphere formulations.
3.3.1. Changes in surface morphology
During degradation, surface defects such as pores and cavities are formed on the microspheres, and these features are important in evaluating the extent of water penetration. Fig. 3 presents visual evidence that the initial stage of the degradation process is characterized by water penetration into the microspheres and pore formation on the PDLLA shell layer. After 26 days of incubation, formulation A microspheres showed increase in surface roughness and presence of numerous pores on the shell layer (Fig. 3c). Formulation B microspheres showed localized pore formation most likely due to slower PDLLA shell degradation (Fig. 3d). These surface defects formed by water penetration expand and extend into the PLGA core, leading to the formation of large cavities by 40 days.
The next stage of the degradation process is characterized by fast erosion of the PLGA core and slow erosion of the PDLLA shell layer. By 40 days, formulation A microspheres showed continuous erosion of the PDLLA polymer, resulting in a high level of porosity that extends throughout the shell layer (Fig. 3g). Some large cavities were formed, resulting in the exposure of the PLGA core to the aqueous medium. Formulation B microspheres that showed localized pore formation continued to extend to a large cavity, resulting in the exposure of the PLGA core (Fig. 3h). The shell layer showed a slow erosion rate based on the level of porosity exhibited. By 47 days, formulation A microspheres showed a thin PDLLA shell layer that lacked the PLGA core (Fig. 3i). Formulation B microspheres showed bulk erosion of the PDLLA shell layer, leading to the formation of larger cavities (Fig. 3j). The PLGA core may be degraded partially or completely. Here, degradation proceeds faster internally than externally for the microspheres, likely due to internal build-up of acidic degradation products that catalyze the PLGA degradation, resulting in the formation of hollow microspheres.
3.3.2. Changes in drug distribution
Fig. 4 shows the confocal images and the fluorescence intensity profiles of doxorubicin for both microsphere formulations during the initial stage of the degradation process from 0 to 26 days. As mentioned, doxorubicin was initially localized within the PLGA core for both formulations (Fig. 4a(i) and 4a(ii)). Minimal or no fluorescence signal was detected for ~10 µm from both ends of the microspheres (Day 0 profile in Fig. 4b and 4c), thus indicating a core-shell configuration. During 26 days of incubation, the fluorescence intensity profiles showed a gradual spread of fluorescence signal along the radial direction of the microspheres with time, from the core regions (Day 0 profile in Fig. 4b and 4c), into the shell layers, and then towards the external surfaces of the particles (Day 26 profile in Fig. 4b and 4c). The spread of fluorescence signal within the microspheres is consistent with the mass transfer of drug by diffusion via aqueousfilled pores. Despite the occurrence of outward drug diffusion from core regions as early as 12 days of incubation, the release of the drug was not detected in the release profiles (Fig. 5). Thus, during the lag phase period of 26 days, the PDLLA shell layer acts as an effective barrier preventing the premature release of the drug. After 26 days of incubation, fluorescence signal detected along the circumferential regions of the microspheres supports the commencement of drug release into the aqueous medium.
3.3.3. Erosion extent of PDLLA and PLGA polymers
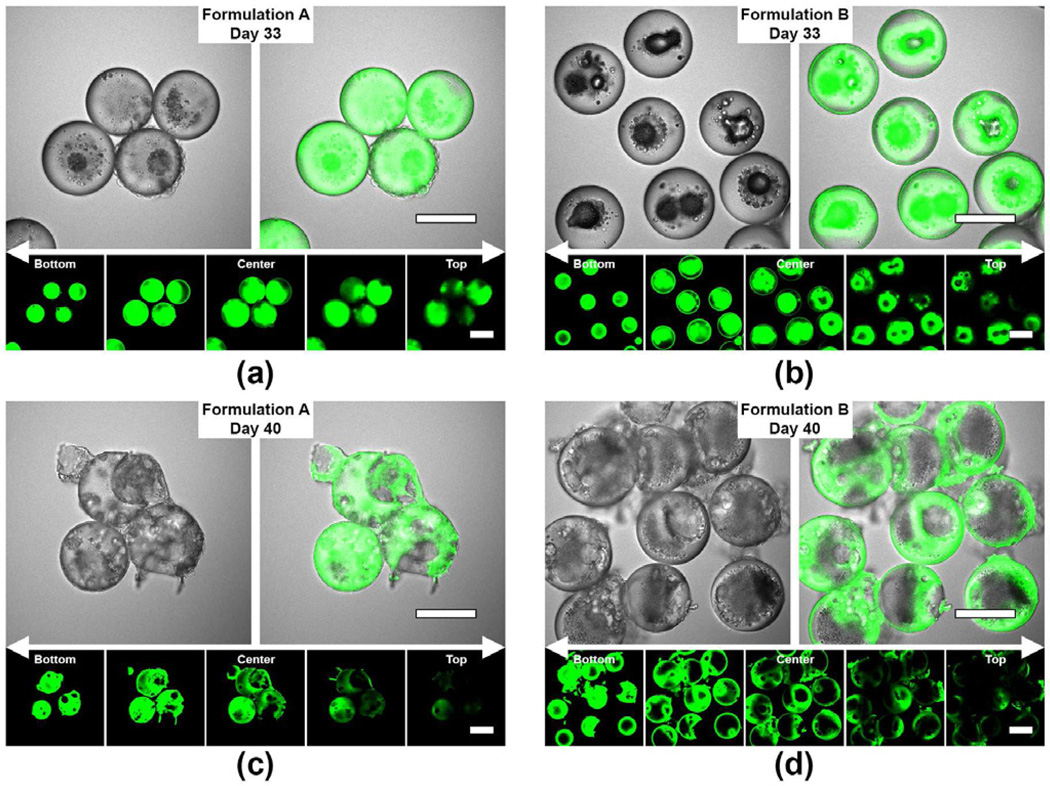
Fig. 6 shows the transmitted light and confocal images for both microsphere formulations during the later stage of the degradation process from 33 to 40 days. In addition, a composite z-stack consisting of five confocal sections of the same microspheres was captured to visualize the internal drug distribution three dimensionally, identify the locations of pores and/or cavities, and verify the erosion extent of PDLLA and PLGA polymers in the particles. After 33 days of incubation, optical images (Fig. 6a and 6b) reveal the presence of fluorescence-free cavities within the microspheres and suggest that the PLGA core phase underwent partial degradation after significant water penetration. Here, formulation B showed more extensive cavity formation as compared to formulation A microspheres. In particular, the z-stack fluorescence images of formulation B microspheres after 33 days of incubation showed the presence of a cavity at the center plane (Fig. 6b, z-stack, center image) that extended continuously to the particle surface (Fig. 6b, z-stack, top image). After 40 days of incubation, both formulations showed multiple pore formation on the particle surface and significant degradation of the PLGA core. Intense fluorescence signal detected around the polymer region of the cavities is corroborative evidence of the eroded PLGA core (Fig. 6c and 6d).
Figure 6.
Laser scanning confocal images depicting the development of multiple pores and/or cavities in the double-walled PDLLA(PLGA) microspheres during the later stage of the degradation process (33 to 40 days). A composite z-stack consisting of 5 confocal sections of the same microspheres was captured based on a z-interval of 12.5 µm between images measured above and below the center plane of the microspheres. (a) and (c) are the confocal images of formulation A microspheres after 33 and 40 days of degradation respectively. (b) and (d) are the confocal images of formulation B microspheres after 33 and 40 days of degradation respectively. Scale bar = 50 µm.
3.3.4. Changes in polymer molecular weight
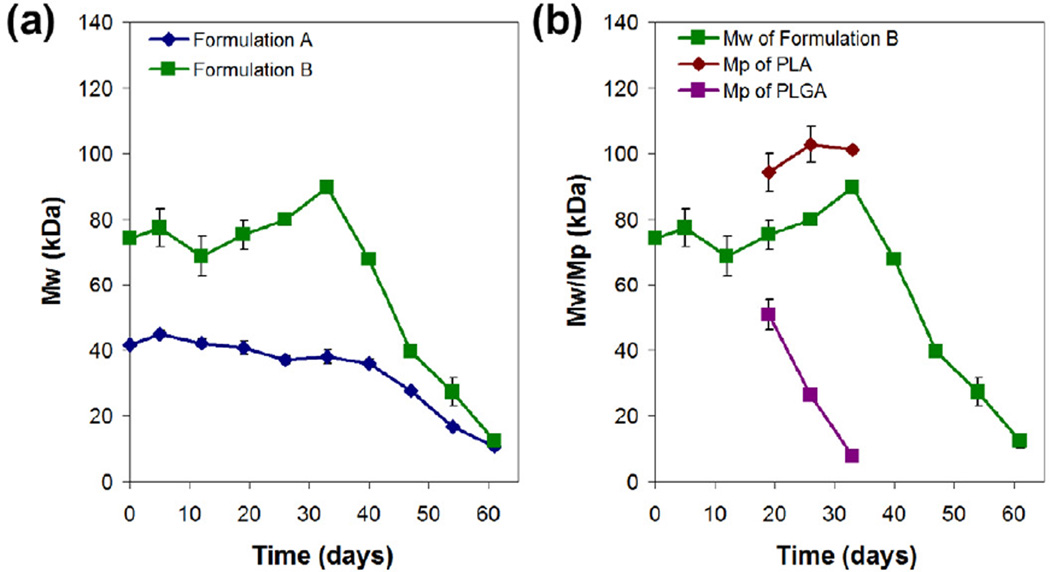
The degradation process was monitored by GPC, and the molecular weight profiles as a function of incubation time for both microsphere formulations are shown in Fig. 7. The initial weight-averaged molecular weight of pure PLGA polymer (i.v. = 0.61 dL/g in HFIP) was measured to be about 46 kDa, while that of pure PDLLA polymer with i.v. 0.37 and 0.70 dL/g in chloroform was about 55 and 116 kDa, respectively. Thus, the initial double-walled microspheres before degradation (0.64:0.36 mass ratio of PDLLA:PLGA) would be expected to have a weightaveraged molecular weight of 52 and 75 kDa for formulation A and B, respectively. As shown in Fig. 7a, the measured values of 42 and 74 kDa before degradation for formulation A and B, respectively, were in close agreement with the expected values.
Figure 7.
Molecular weight profiles as a function of incubation time for double-walled PDLLA(PLGA) microspheres during degradation. (a) Weight-averaged molecular weight (Mw) profiles for formulations A and B microspheres. (b) Weight-averaged molecular weight (Mw) profile for formulation B microspheres together with the corresponding peak molecular weight (Mp) profiles of PDLLA and PLGA polymers from 19 to 33 days of degradation.
Formulation A microspheres did not show appreciable change in weight-averaged molecular weight for the first 19 days, followed by a gradual decrease over the next 6 weeks (Fig. 7a). After 19 days of incubation, the average molecular weight decreased from 42 to 28 kDa (~67% of the initial value) by 47 days, to 17 kDa (~40%) by 54 days, and to only 11 kDa (~26%) by 61 days. Increase in surface roughness and formation of numerous pores on the shell layer after 26 days of incubation, as shown by SEM image in Fig. 3c, further supports the erosion of PDLLA polymer, and consequently a decrease in molecular weight in the following weeks of degradation. During degradation, distinct PDLLA and PLGA elution peaks were not observed as the molecular weights of PDLLA and PLGA polymers are comparable, and this makes tracking of individual polymer degradation difficult.
Formulation B microspheres showed a similar trend in which the weight-averaged molecular weight remained relatively constant at 74 kDa for the first 19 days (Fig. 7a). The molecular weight then increased and peaked at 90 kDa after 33 days of incubation. After that, the molecular weight began to decrease to 40 kDa (~54% of the initial value) by 47 days, to 27 kDa (~36%) by 54 days, and to only 12 kDa (~16%) by 61 days. Unlike formulation A microspheres, formulation B microspheres exhibited distinguishable PDLLA and PLGA elution peaks during 19, 26 and 33 days of incubation (Fig. 7b). Here, the PLGA core showed rapid degradation between 19 and 33 days of incubation as estimated from the decrease in peak molecular weight from 51 to 8 kDa. On the other hand, the PDLLA shell only appeared to start degrading from 33 days onwards, after PLGA has been degraded significantly. The peak at day 33 was due to the decrease in the amount of PLGA which led to an increase in the weight-averaged molecular weight of the microspheres. Despite having a higher PDLLA molecular weight as the shell layer, a delayed decrease in molecular weight was not observed. Instead, the molecular weight decreased rapidly between 33 and 61 days of incubation. Significant bulk erosion of the PDLLA shell layer after 47 days of incubation as shown by SEM image in Fig. 3j further supports the rapid decrease in molecular weight.
For both formulations, the microspheres reached a weight-averaged molecular weight of ~10 kDa after 61 days of incubation (Fig 7a). Here, the degradation of the higher molecular weight PDLLA shell layer proceeded rapidly, and this could be attributed to the internal build-up of acidic degradation products of PLGA core that catalyze the degradation of the outer layer. However, for the lower molecular weight PDLLA shell layer, the acidic degradation products of PLGA core could be released into the aqueous medium more readily due to the porous surface, resulting in less accumulation of acidic degradation products.
3.4. Drug release and degradation mechanism of double-walled PDLLA(PLGA) microspheres
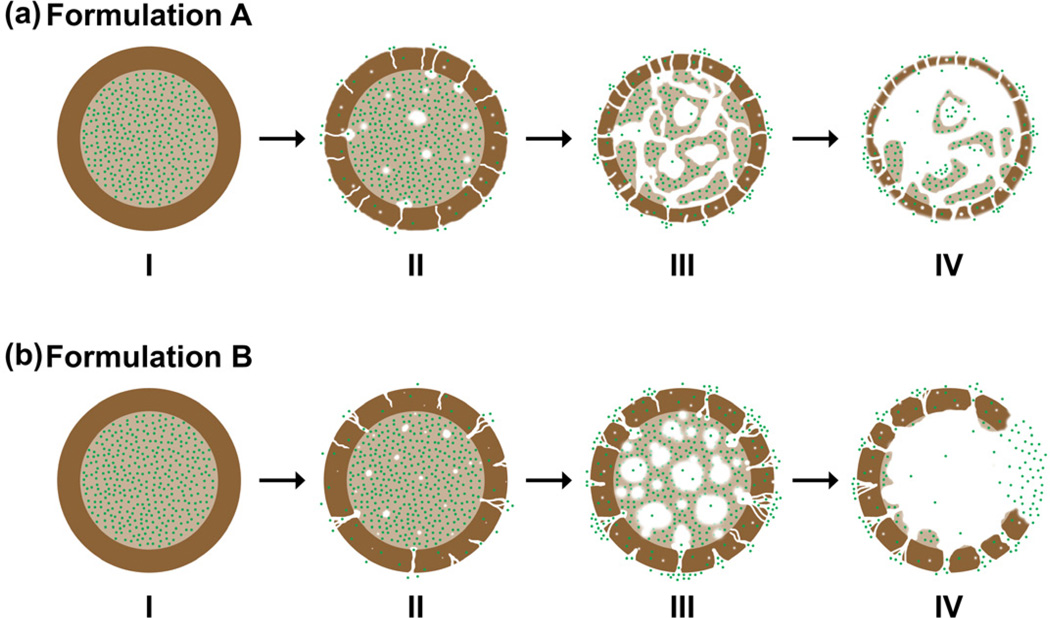
Given that most of the doxorubicin molecules were loaded in the PLGA core surrounded by a PDLLA shell layer for both microsphere formulations, a possible degradation mechanism for each formulation is proposed (Fig. 8). During the initial period of incubation from 0 to 26 days, the PDLLA shell layer was an effective barrier preventing the premature release of doxorubicin into the aqueous medium. This is despite the fact that drug diffusion was already occurring within the microspheres as early as 12 days of incubation, and no detectable drug was released during the lag phase period of 26 days. By 26 days of incubation, formulation A microspheres were presented with numerous pore formation (Fig. 8a, Stage II), while formulation B microspheres were presented with localized pore formation on the shell layer (Fig. 8b, Stage II). Significant degradation of the PLGA cores accounted for the drug release after the lag phase period. By 40 days of incubation, formulation A microspheres exhibited high porosity and thinning of the shell layer (Fig. 8a, Stages III and IV), while formulation B microspheres exhibited large cavities and bulk erosion of the shell layer (Fig. 8b, Stages III and IV). During the later stage of the degradation process, the rapid degradation of the PDLLA shell layer no longer acts as an effective barrier for drug diffusion. The similar erosion time scale of PDLLA shell layers for both formulations is consistent with the similar drug release profile observed experimentally.
Figure 8.
Schematic illustration of the proposed mechanism for the release of doxorubicin from double-walled PDLLA(PLGA) microspheres. PLGA core and PDLLA shell layer are represented by light and dark brown respectively, while doxorubicin molecules are represented by green dots. (a) and (b) show the degradation process of formulations A and B microspheres respectively. Stage I: Initial microspheres before degradation. Stage II: Water penetration into the microspheres and pore formation on the PDLLA shell layer. Stage III: Increase in the number and size of pores on the PDLLA shell layer, and rapid erosion of the PLGA core. Stage IV: Release of doxorubicin into the aqueous medium through pores and/or cavities of the microspheres.
4. Conclusions
We report here a study of the drug release and degradation behavior of double-walled microspheres with a doxorubicin-loaded PLGA core surrounded by a PDLLA shell layer of different molecular weights. It was hypothesized that a higher molecular weight PDLLA shell layer could prevent water penetration into the inner drug-loaded core until a later time, and therefore control the drug release from the microspheres. The data presented herein show that different PDLLA molecular weights of the shell layer did not limit water penetration and formation of pores on the outer surface during the early stage of degradation. Water penetration into the microspheres resulted in the rapid erosion of the PLGA core inside the PDLLA shell within a time frame of about 40 days. Both microsphere formulations exhibited bulk erosion of PDLLA on a similar time scale despite different PDLLA molecular weights. The molecular weight of the shell layer did not influence the subsequent drug release from the microspheres as evidenced by the release profiles obtained experimentally. The rate of water penetration, onset of surface pore formation and degradation rate of polymer core may be the critical factors affecting the drug release and degradation behavior of double-walled microspheres.
Acknowledgments
The authors acknowledge the funding support from the National Institutes of Health (NIH, USA) and National Medical Research Council (NMRC, Singapore) under the grant numbers 1R01EB005181 and NMRC EDG11may084, respectively. Qingxing Xu acknowledges the scholarship support from Agency for Science, Technology and Research (A*STAR, Singapore) for NUS-UIUC Joint Ph.D. Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pekarek KJ, Jacob JS, Mathiowitz E. Double-walled polymer microspheres for controlled drug release. Nature. 1994;367:258–260. doi: 10.1038/367258a0. [DOI] [PubMed] [Google Scholar]
- 2.Pekarek KJ, Jacob JS, Mathiowitz E. One-step preparation of double-walled microspheres. Adv Mater. 1994;6:684–687. [Google Scholar]
- 3.Lee TH, Wang J, Wang CH. Double-walled microspheres for the sustained release of a highly water soluble drug: characterization and irradiation studies. J Control Release. 2002;83:437–452. doi: 10.1016/s0168-3659(02)00235-3. [DOI] [PubMed] [Google Scholar]
- 4.Rahman NA, Mathiowitz E. Localization of bovine serum albumin in double-walled microspheres. J Control Release. 2004;94:163–175. doi: 10.1016/j.jconrel.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Berkland C, Pollauf E, Pack DW, Kim K. Uniform double-walled polymer microspheres of controllable shell thickness. J Control Release. 2004;96:101–111. doi: 10.1016/j.jconrel.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Berkland C, Cox A, Kim K, Pack DW. Three-month, zero-order piroxicam release from monodispersed double-walled microspheres of controlled shell thickness. J Biomed Mater Res A. 2004;70:576–584. doi: 10.1002/jbm.a.30114. [DOI] [PubMed] [Google Scholar]
- 7.Pollauf EJ, Kim KK, Pack DW. Small-molecule release from poly(D,L-lactide)/poly(D,L-lactide- co-glycolide) composite microparticles. J Pharm Sci. 2005;94:2013–2022. doi: 10.1002/jps.20408. [DOI] [PubMed] [Google Scholar]
- 8.Tan EC, Lin R, Wang CH. Fabrication of double-walled microspheres for the sustained release of doxorubicin. J Colloid Interface Sci. 2005;291:135–143. doi: 10.1016/j.jcis.2005.04.089. [DOI] [PubMed] [Google Scholar]
- 9.Zheng W. A water-in-oil-in-oil-in-water (W/O/O/W) method for producing drug-releasing, double-walled microspheres. Int J Pharm. 2009;374:90–95. doi: 10.1016/j.ijpharm.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Choi DH, Park CH, Kim IH, Chun HJ, Park K, Han DK. Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. J Control Release. 2010;147:193–201. doi: 10.1016/j.jconrel.2010.07.103. [DOI] [PubMed] [Google Scholar]
- 11.Nie H, Dong Z, Arifin DY, Hu Y, Wang CH. Core/shell microspheres via coaxial electrohydrodynamic atomization for sequential and parallel release of drugs. J Biomed Mater Res A. 2010;95:709–716. doi: 10.1002/jbm.a.32867. [DOI] [PubMed] [Google Scholar]
- 12.Nie H, Fu Y, Wang CH. Paclitaxel and suramin-loaded core/shell microspheres in the treatment of brain tumors. Biomaterials. 2010;31:8732–8740. doi: 10.1016/j.biomaterials.2010.07.080. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad Z, Zhang HB, Farook U, Edirisinghe M, Stride E, Colombo P. Generation of multilayered structures for biomedical applications using a novel tri-needle coaxial device and electrohydrodynamic flow. J R Soc Interface. 2008;5:1255–1261. doi: 10.1098/rsif.2008.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YH, Bai MY, Chen DR. Multidrug encapsulation by coaxial tri-capillary electrospray. Colloids Surf B Biointerfaces. 2011;82:104–110. doi: 10.1016/j.colsurfb.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Kim W, Kim SS. Synthesis of biodegradable triple-layered capsules using a triaxial electrospray method. Polymer. 2011;52:3325–3336. [Google Scholar]
- 16.Lee WL, Hong M, Widjaja E, Loo SC. Formation and degradation of biodegradable triple-layered microparticles. Macromol Rapid Commun. 2010;31:1193–1200. doi: 10.1002/marc.200900811. [DOI] [PubMed] [Google Scholar]
- 17.Lee WL, Widjaja E, Loo SC. Designing drug-loaded multi-layered polymeric microparticles. J Mater Sci Mater Med. 2012;23:81–88. doi: 10.1007/s10856-011-4508-z. [DOI] [PubMed] [Google Scholar]
- 18.Lee WL, Yu PO, Hong M, Widjaja E, Loo SC. Designing multilayered particulate systems for tunable drug release profiles. Acta Biomater. 2012;8:2271–2278. doi: 10.1016/j.actbio.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji H, Mizuno A, Ikada Y. Properties and morphology of poly(L-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly(Llactide) film in phosphate-buffered solution. J Appl Polym Sci. 2000;77:1452–1464. [Google Scholar]
- 20.Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. J Control Release. 2007;122:338–344. doi: 10.1016/j.jconrel.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems - a review. Int J Pharm. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 22.Tamada JA, Langer R. Erosion kinetics of hydrolytically degradable polymers. Proc Natl Acad Sci USA. 1993;90:552–556. doi: 10.1073/pnas.90.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym Int. 2005;54:36–46. [Google Scholar]
- 24.Park TG. Degradation of poly(D,L-lactic acid) microspheres: effect of molecular weight. J Control Release. 1994;30:161–173. [Google Scholar]
- 25.Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- 26.Shieh L, Tamada J, Chen I, Pang J, Domb A, Langer R. Erosion of a new family of biodegradable polyanhydrides. J Biomed Mater Res. 1994;28:1465–1475. doi: 10.1002/jbm.820281212. [DOI] [PubMed] [Google Scholar]
- 27.Dang W, Saltzman WM. Controlled release of macromolecules from a degradable polyanhydride matrix. J Biomater Sci Polym Ed. 1994;6:297–311. doi: 10.1163/156856294x00374. [DOI] [PubMed] [Google Scholar]
- 28.Heller J, Barr J, Ng SY, Abdellauoi KS, Gurny R. Poly(ortho esters): synthesis, characterization, properties and uses. Adv Drug Deliv Rev. 2002;54:1015–1039. doi: 10.1016/s0169-409x(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 29.Pekarek KJ, Dyrud MJ, Ferrer K, Jong YS, Mathiowitz E. In vitro and in vivo degradation of double-walled polymer microspheres. J Control Release. 1996;40:169–178. [Google Scholar]
- 30.Leach KJ, Mathiowitz E. Degradation of double-walled polymer microspheres of PLLA and P(CPP:SA)20:80.I. In vitro degradation. Biomaterials. 1998;19:1973–1980. doi: 10.1016/s0142-9612(98)00108-2. [DOI] [PubMed] [Google Scholar]
- 31.Leach KJ, Takahashi S, Mathiowitz E. Degradation of double-walled polymer microspheres of PLLA and P(CPP:SA)20:80.II. In vivo degradation. Biomaterials. 1998;19:1981–1988. doi: 10.1016/s0142-9612(98)00109-4. [DOI] [PubMed] [Google Scholar]
- 32.Yang YY, Shi M, Goh SH, Moochhala SM, Ng S, Heller J. POE/PLGA composite microspheres: formation and in vitro behavior of double walled microspheres. J Control Release. 2003;88:201–213. doi: 10.1016/s0168-3659(02)00491-1. [DOI] [PubMed] [Google Scholar]
- 33.Shi M, Yang YY, Chaw CS, Goh SH, Moochhala SM, Ng S, et al. Double walled POE/PLGA microspheres: encapsulation of water-soluble and water-insoluble proteins and their release properties. J Control Release. 2003;89:167–177. doi: 10.1016/s0168-3659(02)00493-5. [DOI] [PubMed] [Google Scholar]
- 34.Pollauf EJ, Berkland C, Kim KK, Pack DW. In vitro degradation of polyanhydride/polyester core-shell double-wall microspheres. Int J Pharm. 2005;301:294–303. doi: 10.1016/j.ijpharm.2005.06.004. [DOI] [PubMed] [Google Scholar]