Abstract
Little is known about the risk factors associated with hepatitis B virus (HBV) intrauterine transmission among HBsAg positive mothers. We conducted a study in Taiyuan, China including 1,133 HBsAg positive mothers and their babies. A total of 101 neonates had HBsAg and/or HBV DNA positive with an intrauterine transmission rate of 8.9%. Maternal menstrual irregularity (OR=4.95, 95%CI: 1.71, 14.33) and severe nausea during the first trimester (OR=1.86, 95%CI: 1.11, 3.09) were associated with an increased risk of intrauterine transmission, while cesarean delivery (OR=0.32, 95%CI: 0.20, 0.51) was associated with a decreased risk after adjusting for potential confounders. Maternal HBeAg positive was a strong independent predictor for intrauterine transmission (OR=2.56, 95%CI: 1.54, 4.27). A positive association between maternal HBV DNA levels and intrauterine transmission was suggested. Maternal HBIG administration during pregnancy, family history of HBV infection, and premature rupture of membranes were not associated with the risk of intrauterine transmission. The study confirmed that maternal HBeAg positive was a risk factor and cesarean delivery was a protective factor for intrauterine transmission. The new findings associated with menstrual irregularity and severe nausea during the first trimester warrant further investigation.
Keywords: China, Epidemiology, HBV, HBV DNA, HBeAg, HBsAg, intrauterine transmission
Introduction
Approximately two billion individuals worldwide have been infected with hepatitis B virus (HBV) and about 350 million live with chronic HBV infection (1). With a hepatitis B surface antigen (HBsAg) positive rate of approximately 10%, China’s is one of the highest in Asia (2). Mother-to-child transmissions, including intrauterine, labor, breast-feeding, and daily contact, are major routes of HBV infection and account for approximately half of HBV carriers (3, 4). The rate of intrauterine transmission, which accounts for the majority of mother-to-child transmissions, among HBsAg positive pregnant women ranges from 5% to 40% in different areas of China (2, 5). Understanding the risk factors of HBV intrauterine transmission will help to effectively control the spread of HBV infection.
Several studies investigating various factors (i.e., mode of delivery, history of abortion, antepartum hemorrhage, maternal HBV DNA load) related to HBV intrauterine transmission reported inconsistent findings (6–8). It has been suggested that maternal administration of hepatitis B immunoglobulin (HBIG) may prevent intrauterine infection (9), though its efficacy is controversial (10, 11).
To better elucidate these poorly understood risk factors, we conducted a study in Taiyuan, China to examine various maternal characteristics and serum markers of HBV infection during pregnancy and the risk of HBV intrauterine transmission.
Materials and methods
Eligible study subjects were HBsAg positive pregnant women who gave birth in the Third People Hospital of Taiyuan City between August 1, 2003 and August 1, 2009. A total of 2,467 pregnant women were eligible for the study and 1,133 agreed to participate. The protocol was reviewed and approved by the Human Investigation Committee at the Shanxi Medical University. Informed written consent was obtained from all participants.
Information was collected by trained interviewers using a standardized, structured questionnaire through face-to-face interviews. Maternal information included demographics and characteristics before and during pregnancy. Mode of delivery, maternal complications, and newborns’ information were obtained from medical records. All participants donated peripheral blood before delivery and 5ml of femoral venous blood was collected from each infant less than 24 hours after birth and prior to inoculations of hepatitis B vaccine and HBIG. Blood samples were processed within 24 hours of being drawn. Serum samples were stored at −20°C.
HBsAg and hepatitis B e antigen (HBeAg), were measured using ELISA (Kehua Biotechnology, Shanghai, China). HBV DNA levels were tested by FQ-PCR (Da’an Gene Co., Ltd., Sun Yat-Sen University, Guangdong, China). All procedures were performed according to the manufacturers' instructions. If the levels of HBV DNA were ≤103 copies/mL according to the fluorescent signals set by the operation manual the sample was considered negative, otherwise it was positive. Intrauterine transmission was defined as finding HBsAg and/or HBV DNA positive in the peripheral blood of newborns within 24 hours of birth and before active or passive immune prophylaxis (11).
Newborns with intrauterine transmission of HBV were considered cases, those without were considered controls. In univariate analyses, Chi-squire tests were used for categorical data and student t-tests were used for continuous variables. Odds ratios (OR) and 95% confidence interval (CI) were estimated using unconditional logistical regression to measure the association between maternal characteristics and intrauterine transmission. All analyses were performed using SAS software Version 9.3 (SAS Institute).
Results
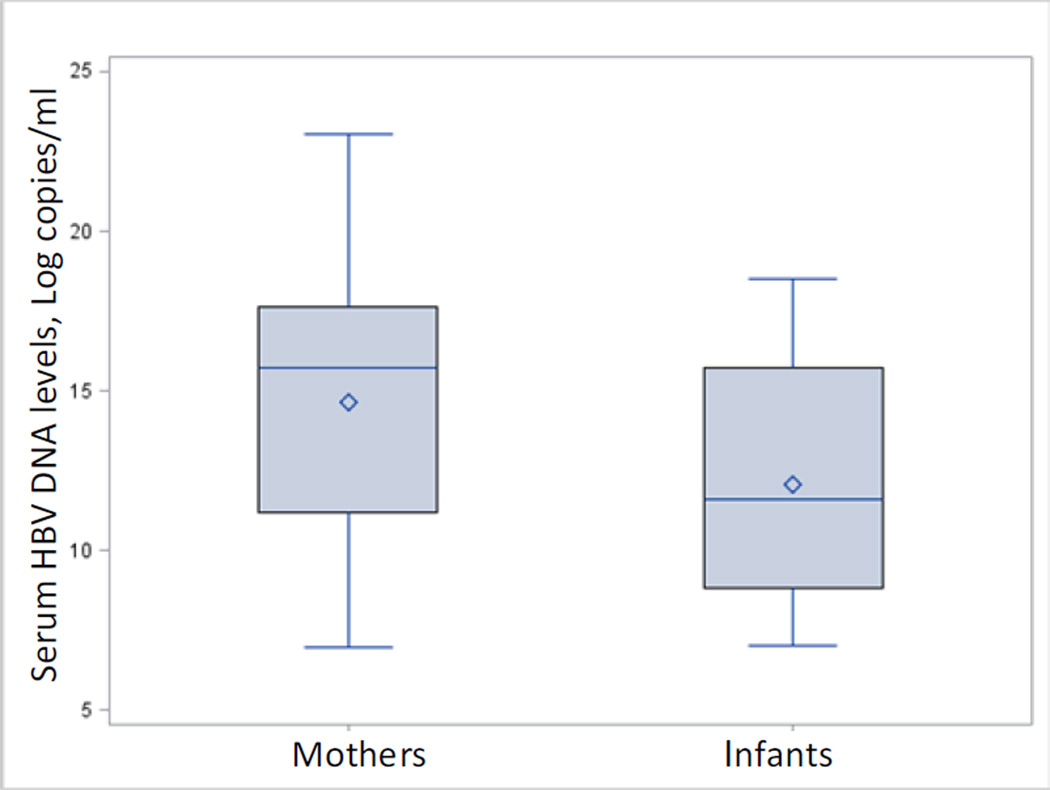
The study detected 101 newborns with serum HBsAg or HBV DNA positive (8.9%). The mean levels of HBV DNA were higher among mothers than infected infants (Figure 1). As shown in Table 1, distributions of maternal and newborn characteristics were similar across cases and controls.
Figure 1.
HBV DNA levels of mothers and infected infants. Mean levels of mothers and infected infants were 1.9 × 108 and 1.0 × 107 copies/ml, respectively.
Table 1.
Distributions of selected maternal and newborn characteristics among cases and controls
| Characteristics | Cases (N=101) Number (%) |
Controls (N=1032) Number (%) |
P |
|---|---|---|---|
| Maternal Characteristics | |||
| Age (years) | |||
| Mean (SD) | 27.27(5.05) | 26.53(4.54) | 0.16 |
| Highest educational levels | |||
| <High school | 53(52.5) | 564(54.7) | |
| ≥High School | 48(47.5) | 468(45.3) | 0.68 |
| Newborn Characteristics | |||
| Birth weight (g) | |||
| <2500 | 1(1.0) | 22(2.1) | |
| 2500–3999 | 92(91.1) | 908(88.0) | |
| ≥4000 | 8(7.9) | 102(9.9) | 0.59 |
| Gestational weeks | |||
| <37 | 1(1.0) | 43(4.2) | |
| 37–41 | 98(97.0) | 953(92.3) | |
| >41 | 2(2.0) | 36(3.5) | 0.20 |
| Gender | |||
| Girl | 48(47.5) | 470(45.5) | |
| Boy | 53(52.5) | 562(54.5) | 0.70 |
| Apgar1 score | |||
| 0–3 | 3(3.0) | 18(1.7) | |
| 4–7 | 31(30.7) | 283(27.4) | |
| 8–10 | 67(66.3) | 731(70.8) | 0.51 |
In univariate analyses (Table 2), menstrual regularity (P=0.0004), maternal HBeAg positive (P<0.0001), maternal HBV DNA positive (P=0.0004), pregnancy reaction (severe nausea/vomiting during the first trimester) (P=0.02), and cesarean section (P<0.0001) were significantly associated with HBV intrauterine transmission.
Table 2.
Univeriate analyses of associations between maternal characteristics and intrauterine HBV infection
| Characteristics | Cases | Controls | |||
|---|---|---|---|---|---|
| No Number (%) |
Yes Number (%) |
No Number (%) |
Yes Number (%) |
P-value | |
| Pregregnancy | |||||
| HBV vaccine injection | 93(93.0) | 7(7.0) | 924(91.1) | 90(8.9) | 0.53 |
| Menstrual regularity | 6(6.0) | 94(94.0) | 13(1.3) | 1016(98.7) | 0.0004 |
| History of abortion | 53(53.0) | 47(47.0) | 548(53.6) | 474(46.4) | 0.91 |
| Family history of HBV infection | 82(81.2) | 19(18.8) | 790(76.6) | 242(23.4) | 0.29 |
| Pregnancy | |||||
| Maternal HBeAg positive | 36(38.7) | 57(61.3) | 597(62.8) | 353(37.2) | <0.0001 |
| Maternal HBV DNA positive | 32(33.3) | 64(66.7) | 496(52.2) | 454(47.8) | 0.0004 |
| HBIG injection | 27(27.3) | 72(72.7) | 317(31.0) | 707(69.0) | 0.45 |
| HBV vaccine injection | 97(99.0) | 1(1.0) | 1008(99.2) | 8(0.8) | 0.56 |
| Medication use during pregnancy | 96(96.0) | 4(4.0) | 994(96.7) | 34(3.3) | 0.71 |
| Pregnancy reaction | 76(75.2) | 25(24.8) | 867(84.3) | 162(15.7) | 0.02 |
| Pregnancy-induced hypertension | 95(94.1) | 6(5.9) | 981(95.7) | 44(4.3) | 0.44 |
| Antepartum hemorrhage | 100(99.0) | 1(1.0) | 1008(97.7) | 24(2.3) | 0.38 |
| Delivery | |||||
| Cesarean section | 72(71.3) | 29(28.7) | 477(46.2) | 555(53.8) | <0.0001 |
| Premature rupture of membranes | 64(63.4) | 37(36.6) | 691(69.7) | 301(30.3) | 0.19 |
| Amniotic fluid pollution | 84(83.2) | 17(16.8) | 790(77.9) | 224(22.1) | 0.22 |
| Abnormality of umbilical cord | 66(65.3) | 35(34.7) | 701(67.9) | 331(32.1) | 0.60 |
| Praevia placenta | 101(100.0) | 0(0.0) | 1025(99.3) | 7(0.7) | 1.00 |
After adjusting for important covariates (Table 3), women who had irregular menstruation experienced increased risk of HBV intrauterine transmission (OR=4.95, 95%CI: 1.71–14.33). Pregnancy reaction was associated with 86% increased risk (95%CI: 1.11–3.09). Cesarean delivery was associated with a reduced risk compared to vaginal delivery (OR=0.32, 95%CI: 0.20–0.51). HBeAg positive women had more than twice the risk of intrauterine transmission compared to HBeAg negative women (OR=2.56, 95%CI: 1.54–4.27). Being HBV DNA positive was not significantly associated with intrauterine transmission (OR=1.44, 95%CI: 0.86–2.41), although risk of transmission increased with increasing levels of maternal HBV DNA (P for trend=0.032).
Table 3.
Multivariate analysis of associations between maternal characteristics and intrauterine HBV infection
| Characteristics | Cases | Controls | OR*(95%CI) |
|---|---|---|---|
| Pregregnancy menstrual regularity | |||
| Yes | 94 | 1016 | 1.00 |
| No | 6 | 13 | 4.95(1.71–14.33) |
| HBeAg positive | |||
| No | 36 | 597 | 1.00 |
| Yes | 57 | 353 | 2.56(1.54–4.27) |
| HBV DNA load | |||
| Negative | 32 | 496 | 1.00 |
| Positive | 64 | 454 | 1.44(0.86–2.41) |
| ≤106 copies/ml | 15 | 162 | 1.31(0.67–2.53) |
| >106–≤108 copies/ml | 41 | 255 | 1.48(0.82–2.67) |
| >108 copies/ml | 8 | 37 | 1.82(0.72–4.62) |
| P for trend | 0.032 | ||
| Pregnancy reaction | |||
| No | 76 | 867 | 1.00 |
| Yes | 25 | 162 | 1.86(1.11–3.09) |
| Cesarean section | |||
| No | 72 | 477 | 1.00 |
| Yes | 29 | 555 | 0.32(0.20–0.51) |
Adjusted for the variables listed in the table.
Discussion
We observed an increased risk of intrauterine transmission associated with HBeAg positive women and those with high HBV DNA levels, which was consistent with the majority of early studies (8, 12–14). Both HBeAg and HBV DNA positive indicate active viral replication. Dwivedi et al. (14) found that combination of HBeAg and HBV DNA was a more sensitive marker of intrauterine transmission than HBeAg alone; we observed no such association.
Maternal HBIG administration during pregnancy may neutralize HBV in the maternal body and subsequently reduce HBV viremia and risk of intrauterine transmission (9). A recent meta-analysis found that maternal HBIG injection significantly reduced the risk of intrauterine transmission (15), however heterogeneity between included studies makes the validity questionable. We found no association between maternal administration of HBIG and HBV intrauterine transmission. Recent Chinese chronic hepatitis B prevention guidelines did not advocate HBIG use for women in advanced stages of pregnancy to prevent intrauterine transmission (16). The World Health Organization, World Gastroenterology Organization, and the U.S. Centers for Disease Control and Prevention do not endorse any schedule of HBIG injections in pregnant HBV carriers (17–19).
Cesarean delivery has the least placental contraction and has been speculated to have the least maternal-fetal transfusion (20). It also limits direct contact of the fetus with infected secretions or blood in the maternal genital tract. The finding that cesarean delivery was protective for HBV intrauterine transmission from the current study was consistent with most earlier studies (14, 21–23) but not all (8).
The study found that menstrual irregularity and severe nausea/vomiting during the first trimester were associated with an increased risk of HBV intrauterine transmission, which had not been previously reported. Although the underlining mechanisms are unclear, diseased liver caused by HBV infection may lead to impaired metabolism, inactivation of estrogens, and accumulation of estrogens in the body (24). Abnormal estrogen levels could cause menstrual irregularity and severe nausea/vomiting during the first trimester. Although many women at this age are less likely to have severe liver disease, we found that women who were HBsAg positive before pregnancy were slightly more likely to experience menstrual irregularity and/or severe nausea during the first trimester compared with women who were HBsAg negative before pregnancy (result not shown). Future studies should examine of the effect of comorbid liver diseases in order to understand these associations.
A major strength of the study was that vein blood samples from newborns were used for serological markers of HBV infection; many previous studies used cord blood increasing the chance of false positive diagnosis of neonatal HBV infection. However, obtaining vein blood samples from newborns was difficult and invasive, resulting in lower participation (45.9%).
In conclusion, we confirmed that HBeAg positive and vaginal delivery were independent risk factors for intrauterine transmission. New findings of menstrual irregularity and severe nausea during the first trimester warrants further investigation.
Acknowledgements
This study was supported by grants 30070669 and 30671794 from the National Natural Science Foundation of China, grant 2008-50 from Returned Personnel Science Research Project of Shanxi Province, China, and grant HD70324-01 from the National Institutes of Health.
References
- 1.World Health Organization. Hepatitis B. WHO Fact Sheet 204. [Accessed May 2012]; (Revised August 2008) Available at: http://www.who.int/mediacentre/factsheets/fs204/en/
- 2.Zhang SL, Yue YF, Bai GQ, Shi L, Jiang H. Mechanism of intrauterine infection of hepatitis B virus. World J Gastroenterol. 2004;10(3):437–438. doi: 10.3748/wjg.v10.i3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamdani-Belghiti S, Bouazzaou NL. Mother-child transmission of hepatitis B virus. State of the problem and prevention. Arch Pediatr. 2000;7(8):879–882. doi: 10.1016/s0929-693x(00)80199-2. [DOI] [PubMed] [Google Scholar]
- 4.Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165(9):837–846. doi: 10.1001/archpediatrics.2011.72. [DOI] [PubMed] [Google Scholar]
- 5.Shao ZJ, Zhang L, Xu JQ, et al. Mother-to-infant transmission of hepatitis B virus: a Chinese experience. J Med Virol. 2011;83(5):791–795. doi: 10.1002/jmv.22043. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Zhu Q, Zhang X. Effect of delivery mode on maternal-infant transmission of hepatitis B virus by immunoprophylaxis. Chin Med J (Engl) 2002;115(10):1510–1512. [PubMed] [Google Scholar]
- 7.Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus--a systematic review. Virol J. 2008;5:100. doi: 10.1186/1743-422X-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67(1):20–26. doi: 10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Q, Yu G, Yu H, et al. A randomized control trial on interruption of HBV transmission in uterus. Chin Med J (Engl) 2003;116(5):685–687. [PubMed] [Google Scholar]
- 10.Li P, Liu XR, Cheng W, Wang T, Zhou XE. Clinical study on the effect of long period and high dosage HBV immunoglobulin in treatment of preventing HBV's intrauterine spreading. Practical J Clin Med. 2010;7(4):30–31. [Google Scholar]
- 11.Li XM, Shi MF, Yang YB, et al. Effect of hepatitis B immunoglobulin on interruption of HBV intrauterine infection. World J Gastroenterol. 2004;10(21):3215–3217. doi: 10.3748/wjg.v10.i21.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai H, Zhang L, Ma L, Dou XG, Feng GH, Zhao GZ. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J Gastroenterol. 2007;13(26):3625–3630. doi: 10.3748/wjg.v13.i26.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soderstrom A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis. 2003;35(11–12):814–819. doi: 10.1080/00365540310016547. [DOI] [PubMed] [Google Scholar]
- 14.Dwivedi M, Misra SP, Misra V, et al. Seroprevalence of hepatitis B infection during pregnancy and risk of perinatal transmission. Indian J Gastroenterol. 2011;30(2):66–71. doi: 10.1007/s12664-011-0083-y. [DOI] [PubMed] [Google Scholar]
- 15.Shi Z, Li X, Ma L, Yang Y. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother-to-child transmission-a meta-analysis. Int J Infect Dis. 2010;14(7):e622–e634. doi: 10.1016/j.ijid.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 16.The guidelines of prevention and treatment for chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2005;13(12):881–891. [PubMed] [Google Scholar]
- 17.World Health Organization. Hepatitis B. [accessed May 2012]; Available at: http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_2.pdf.
- 18.World Gastroenterology Organisation. WGO Practice Guideline-Hepatitia B. [Accessed May 2012]; Available at: http://www.worldgastroenterology.org/hepatitis-b.html.
- 19.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR-16):1–33. quiz CE1–4. [PubMed] [Google Scholar]
- 20.Wong VC, Lee AK, Ip HM. Transmission of hepatitis B antigens from symptom free carrier mothers to the fetus and the infant. Br J Obstet Gynaecol. 1980;87(11):958–965. doi: 10.1111/j.1471-0528.1980.tb04458.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin HH, Kao JH, Hsu HY, Mizokami M, Hirano K, Chen DS. Least microtransfusion from mother to fetus in elective cesarean delivery. Obstet Gynecol. 1996;87(2):244–248. doi: 10.1016/0029-7844(95)00385-1. [DOI] [PubMed] [Google Scholar]
- 22.Lee SD, Lo KJ, Tsai YT, et al. Role of caesarean section in prevention of mother-infant transmission of hepatitis B virus. Lancet. 1988;2(8615):833–834. doi: 10.1016/s0140-6736(88)92792-4. [DOI] [PubMed] [Google Scholar]
- 23.Sharma R, Malik A, Rattan A, Iraqi A, Maheshwari V, Dhawan R. Hepatitis B virus infection in pregnant women and its transmission to infants. J Trop Pediatr. 1996;42(6):352–354. doi: 10.1093/tropej/42.6.352. [DOI] [PubMed] [Google Scholar]
- 24.John TMS. Women and hepatitis: the estrogen connection. Originally published in Hepatitis Magazine, 2004. [Accessed June 2012];Reprinted with permission. Available at: http://www.hepcchallenge.org/pdf/women%20and%20hepatitis_hepmag_tms_reformat1006.pdf. [Google Scholar]