Abstract
The mechanism by which cancer mediates muscle atrophy has been delineated in the past 3 decades, and includes a prominent role of tumor-derived cytokines, such as IL-6, TNFα and IL-1. These cytokines interact with their cognate receptors on muscle to activate the downstream transcription factor NF-κB and induce sarcomere proteolysis. Experimentally, inhibiting NF-κB signaling largely prevents cancer-induced muscle wasting, indicating its prominent role in muscle atrophy. Resveratrol, a natural phytoalexin found in the skin of grapes, has recently been shown to inhibit NF-κB in cancer cells, which led us to hypothesize that it might have a protective role in cancer cachexia. Therefore, we investigated if daily oral resveratrol could protect against skeletal muscle loss and cardiac atrophy in an established mouse model. We demonstrate resveratrol inhibits skeletal muscle and cardiac atrophy induced by C26 adenocarcinoma tumors through its inhibition of NF-κB (p65) activity in the skeletal muscle and heart. These studies demonstrate for the first time the utility of oral resveratrol therapy to provide clinical benefit in cancer-induced atrophy through the inhibition of NF-κB in muscle. These findings may have application in the treatment of diseases with parallel pathophysiologies such as muscular dystrophy and heart failure.
Keywords: resveratrol, cancer cachexia, skeletal muscle, cardiac, atrophy, NF-κB inhibition
Introduction
Cancer cachexia is a wasting syndrome that affects as many as 80% of cancer patients with advanced disease 1–3. While cancer cachexia is estimated to be the cause of death in more than 20% of all cancer patients, the morbidity and collapse in the quality of life affects nearly all patients 2, 4, 5. Clinically, the manifestations of cancer cachexia are well established and include loss of skeletal muscle mass and adipose tissue, anorexia, asthenia, and anemia with considerable alterations in lipid, carbohydrate and protein metabolism 2, 5, 6. Patients remain in a hypermetabolic state as the tumor burden increases, due to the tumor production of catabolic pro-inflammatory cytokines 5
Cancer cachexia is a complex metabolic disorder which diminishes lean body mass in response to increases in tumor-released inflammatory cytokines, including TNFα, IL1, and IL-6 7, 8. These tumor derived cytokines interact with their receptors on striated muscle to activate the transcription factor NF-κB to enhance muscle atrophy, by enhancing the degradation of the sarcomere9–12. The accelerated protein degradation and proteolysis of the contractile apparatus is mediated by the ubiquitin proteasome system, including specific ubiquitin ligases in muscle such as muscle ring finger-1 (MuRF1)7, 13. Inflammatory cytokine-driven NF-κB activation is central in muscle atrophy found in a number of diseases including muscular dystrophy, heart failure, sepsis, and cancer 14–16. Pharmacologic and genetic inhibition of NF-κB prevents ubiquitin-dependent muscle wasting 10, 17 and can lead to retention of muscle mass, strength, and can promote muscle regeneration 18–20. Consequently, inhibition of NF-κB activity has enormous potential for the prevention and treatment of muscle atrophy.
Resveratrol, a natural phytoalexin found in the skin of grapes, has recently been shown to have beneficial effects on a variety of disease processes, including hepatocellular carcinoma21 and neurodegenerative disease 22. Resveratrol’s activity in these diseases has been due, in part, to its ability to inhibit NF-κB nuclear translocation and accumulation. It does this by primarily inhibiting IκB kinase (IKK), whose activation is necessary for NF-κB activity. In the present study, we test the hypothesis that resveratrol treatment inhibits cancer-induced cachexia by inhibiting NF-κB to protect against cancer-induced cardiac atrophy in vivo.
Materials and Methods
Cell Lines
The transplantable C-26 adenocarcinoma cells were maintained as previously described 23, 24
Animals and Tumor Implantation
Ninety-six female CD2F1 mice aged 45 to 70 days (weight 19 to 22 grams) were obtained from the Charles River Laboratories (Wilmington, MA) and randomly divided into four groups: 1) control mice, 2) control mice treated with resveratrol (100 mg/kg, 200 mg/kg, or 500 mg/kg), 3) tumor-bearing mice, and 4) tumor-bearing mice treated with resveratrol (100 mg/kg, 200 mg/kg, or 500 mg/kg). On day 0, the mice selected to go in the tumor-bearing groups were injected subcutaneously in the right flank with 150 µL (approximately 750,000 cells) of C-26 adenocarcinoma cells.
Treatment with Resveratrol
Trans-resveratrol (Cayman Chemical, Ann Arbor, MI, Catalog #70675) was solubulized in 1.5% methylcellulose./ 0.2% Tween-20 with vigorous vortexing. Beginning on day 6, mice received daily single oral treatment delivered intragastrically using a 20 g gavage needle. Resveratrol treated mice received daily single gavage treatment of 100 mg/kg, 200mg/kg, or 500mg/kg resveratrol, while controls received a comparable treatment of 250 µl of vehicle alone.
Body Composition Analysis
Prior to tumor injection, all animals underwent body composition analysis using quantitative magnetic resonance systems (QMR) echo MRI (EchoMRI-100, Echo Medical Systems, Houston TX).
Echocardiography
Echocardiography was performed on conscious mice using a Visual Sonics Vevo 770 ultrasound biomicroscopy system as previously described by our laboratory 25.
Total RNA isolation/Real Time PCR determination of mRNA Expression
Total RNA was isolated from flash frozen tissue using Trizol (Sigma Chemical, St. Louis, MO), cDNA made using High Capacity cDNA Archive kit (Applied Biosystems). Semi-quantitative RT-PCR was performed using an MJ Research Inc. Thermo Cycler (Waltham, MA) and New England Biolab (Ipswich, MA) reagents using the primers detailed in supplemental data.
NF-κB activity assay
Nuclear extracts were isolated from heart and gastrocnemius muscle immediately after harvest and NF-κB activity determined according to the manufacturer’s protocol (Cayman Chemical, Ann Arbor MI).
Additional materials and methods can be found in the supplemental data section.
Results
Resveratrol showed no evidence of drug induced toxicity at the levels used in this study
Pharmacological studies detailing the metabolism of resveratrol both in animal and humans have been carried out, and all indicate rapid absorption, distribution, metabolism and excretion of resveratrol 26, 27. After oral administration, wide tissue distribution in rats has been observed after 24 hours and significantly decreases after 72 hours.28 Toxicological studies have also been completed with tolerance of dosages as high as 700 mg/kg in rats 28 without toxic effects, and several Phase I clinical trials are underway at oral doses as high as 100 mg/kg/day in humans26, 28. We therefore dosed resveratrol orally at 100–500 mg/kg/day in the present study. We monitored toxicity by histology and lethality throughout the study. No events surrounding drug delivery were noted, including any obvious toxicity or lethality caused by daily oral gavage of resveratrol 100–500 mg/kg/day. Additionally, we did not observe any drug-induced hepatotoxicity or nephrotoxicity across all doses of resveratrol delivery, as confirmed by a blinded, independent pathologist on histological examination (Supplemental Figure 1).
Resveratrol protects against C26 adenocarcinoma-induced weight loss in mice
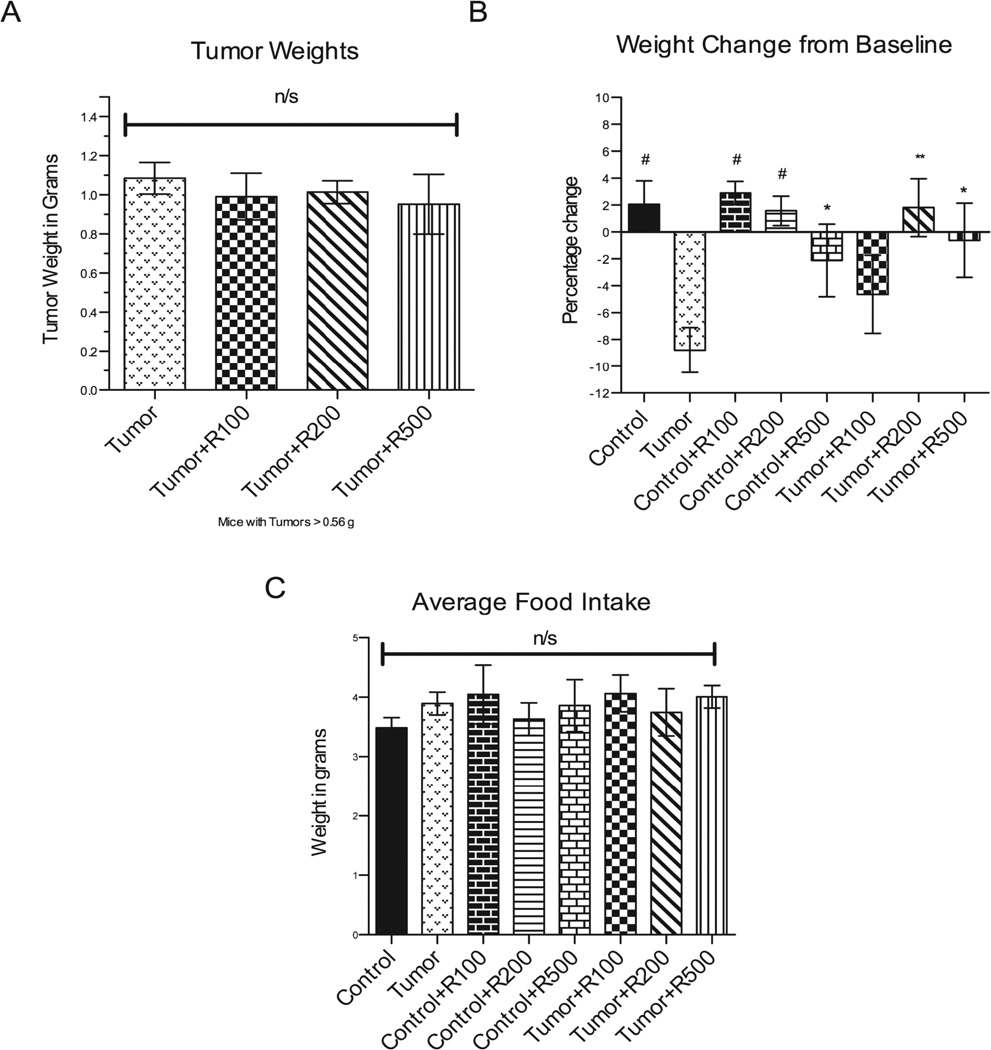
We assayed resveratrol’s ability to prevent cancer-induced skeletal and cardiac muscle atrophy in the C26-adenocarcinoma mouse model29 previously described by our laboratory 24. Cultured C26 adenocarcinoma cells were injected into the back flank where it develops into a tumor and induces muscle wasting in CD2F1 mice within 2 weeks. In the present study, tumors were palpable by day 6 after inoculation in the tumor bearing subset mice. On Day 17, at completion of the study, there were no significant differences in tumor weights among all tumor bearing mice (Figure 1A). Tumor bearing mice had a mean weight loss of 8.8±1.7% from their baseline weights, while control mice gained 2.1±1.8% over the experimental period (Figure 1B), despite all groups having an equivalent food intake (Figure 1C). In contrast, mice challenged with tumors and treated with 200 mg/kg of resveratrol gained weight similar to the control group. Mice treated with 500 mg/kg and challenged with tumor also maintained their weight compared to tumor bearing mice (Figure 1B). Mice with tumors treated with 100 mg/kg of resveratrol were not protected against the tumor-induced cachexia, losing 4.6 ± 2.9% of their body weight from baseline. These findings demonstrate that resveratrol at doses >/= 200 mg/kg protects against tumor-induced weight loss in this mouse model.
Figure 1. Final tumor weights and average food intake.
(A) There were no significant differences in tumor weights amongst tumor bearing groups at all tumor weights and amongst the subset of tumors larger than 0.56 g at time of harvest using unpaired t-tests. (B) Significant changes in carcass weight loss was seen in the untreated tumor bearing mice compared to control mice, control mice receiving resveratrol at all doses and tumor bearing mice receiving resveratrol at the 200 mg/kg and 500 mg/kg doses. Carcass weight was determined by subtracting the weight of the tumor from the body weight. Total carcass weights were analyzed and represented by the percentage change from baseline among all groups. Also no significant differences were noted in (C) average food intake per mouse per day. n/s = no significance detected ## = p <0.0001, # = p <0.001, ** = p < 0.01, * = p < 0.05. For (A) N=24 (Tumor), N=8 (Tumor +R100), N=7 (Tumor +R200), N=8 (Tumor +R500). For (B) and (C) N=24 (Tumor), N=8 (Tumor +R100), N=7 (Tumor +R200), N=8 (Tumor +R500).
To delineate if resveratrol had any independent affects on muscle mass, control mice without tumor were treated with 100–500 mg/kg in parallel with treated mice challenged with tumor. We found that non-tumor control mice treated with resveratrol alone exhibited body weights equal to control mice at lower doses (100 mg/kg, 200 mg/kg). At the highest dose tested (500 mg/kg), mice lost weight compared to age-matched controls (Figure 1B). These findings demonstrate that 500 mg/kg resveratrol may cause some weight loss in the absence of cachexia, while the lower levels (100–200 mg/kg) do not affect body weight. None of the resveratrol doses tested cause an increase in muscle mass, suggesting that the protective effects of resveratrol were not due to independent increases in muscle mass.
Resveratrol protects against cachexia-induced lean body and fat mass loss
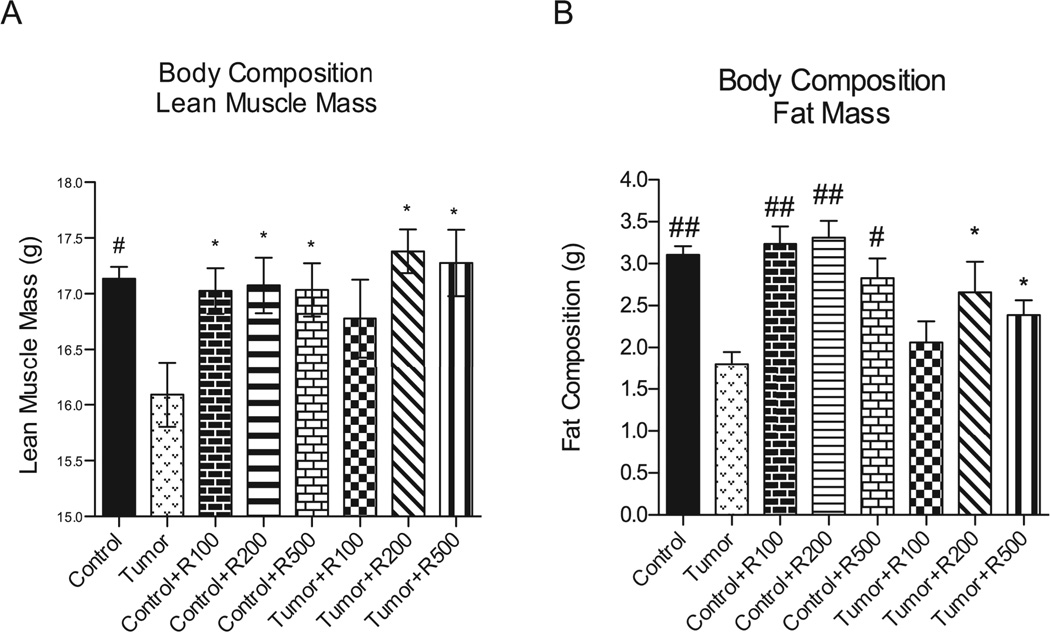
Cancer cachexia is characterized by the loss of both skeletal muscle mass and adipose tissue resulting from alterations in protein and lipid metabolism. 2, 5, 6 We next determined the body composition of mice challenged with tumors in the presence or absence of resveratrol by MRI body composition analysis to determine lean body mass (LBM) and fat mass (FM). We identified that 200 and 500 mg/kg of resveratrol significantly protected against tumor-induced loss of LBM and FM composition in tumor-bearing mice (Figure 2A and 2B). Compared to controls, untreated tumor bearing mice lost 5.3% LBM (16.1 ± 0.3 vs. 17.1 ± 0.1 g) in contrast to resveratrol-treated tumor bearing mice, which were completely protected (LBM (200 mg/kg)=17.4 ± 0.19 g ; LBM (500 mg/kg)=17.3 ± 0.3 g). Untreated tumor bearing mice lost 33.3% of their limited FM, compared to control mice (1.80 ± 0.15 vs. 3.1 ± 0.10 g). Tumor-challenged mice treated with 200 and 500 mg/kg were significantly protected (2.7 ± 0.36 g and 2.4 ± 0.17 g, respectively). Resveratrol treatment given to control mice without tumors did not affect LBM or FM. Since resveratrol did not cause increases in total body mass (including LBM and FM) in the absence of tumor, these studies indicate that resveratrol protection against tumor induced weight loss is not due to its ability to increase muscle weight, LBM, or FM by itself.
Figure 2. Final body composition among non-tumor bearing and tumor bearing mice.
Whole mouse body composition was determined and graphed to show final mass in grams among all groups. (A) Significant lean muscle mass loss was seen in the untreated tumor bearing mice compared to control mice, control mice receiving resveratrol at all dosages and tumor bearing mice receiving resveratrol at the 200 mg/kg and 500 mg/kg levels. (B) Fat mass compositions were compared among the same subsets with significant fat mass loss shown in the untreated tumor bearing group. The tumor bearing groups receiving resveratrol showed significant responses by maintaining more fat mass in the 200 mg/kg and 500 mg/kg groups. The untreated and treated control groups also were significantly higher in fat mass composition compared to untreated tumor bearing. ## = p <0.0001, # = p <0.001, ** = p < 0.01, * = p < 0.05. For (A) and (B) N=24 (Tumor), N=8 (Tumor +R100), N=8 (Tumor +R200), N=8 (Tumor +R500). For (C) N=17 (Tumor), N=6 (Tumor +R100), N=6 (Tumor +R200), N=7 (Tumor +R500).
Resveratrol inhibits tumor-induced gastrocnemius and cardiac atrophy
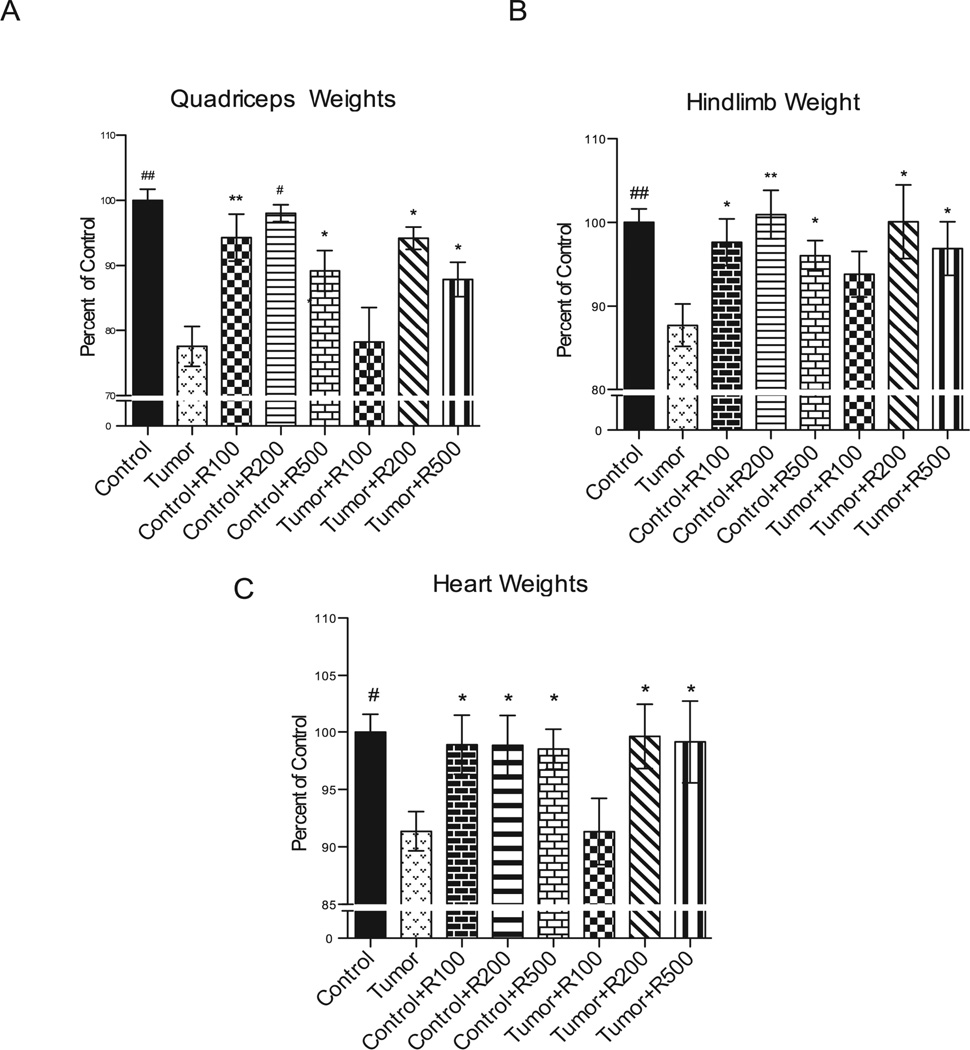
Isolated quadriceps and the heart were collected and weighed immediately after harvest. Tumor bearing mouse quadriceps weighted significantly less (~23%) than corresponding quadriceps muscle from control mice (Figure 3A). Isolated quadriceps muscle from control mice treated with 100 mg/kg, 200 mg /kg and 500 mg/kg resveratrol were not significantly different from control mice (Figure 3A). In contrast, quadriceps muscle from tumor-challenged mice treated with 200 mg /kg and 500 mg/kg resveratrol were significantly higher (16.6 ± 6.5%, 10.3 ± 4.9%, respectively) than untreated tumor-challenged mice (Figure 3A), suggesting that >= 200 mg/kg resveratrol protected against tumor-induced skeletal muscle atrophy. Tumor-bearing mice demonstrated significant hind limb atrophy as evidenced by a 12.2% loss of mass compared to non-tumor-bearing controls (Figure 3B). To determine a more general effect on skeletal muscle, we also weighed hind limb mass. Hind limb muscle mass was preserved in resveratrol treated (200 and 500 mg/kg) tumor-bearing animals as evidenced by significant differences in treated tumor-bearing mice having a 12.4 ± 5.7% and 9.2 ± 4.3% higher mass, respectively, than untreated tumor challenged mice. Resveratrol treatment in control mice did not significantly differ from control mice at all 3 doses tested.
Figure 3. Final weights among non-tumor bearing and tumor bearing mice.
(A) Quadriceps were individually analyzed and significant weight loss was shown in the untreated tumor bearing group compared to control, treated controls at all dosages, as well as the treated tumor bearing groups at the 200 and 500 mg/kg doses. The 100 mg/kg treated tumor bearing group was not significantly different in quadriceps weight from the non-treated tumor bearing group.(B) The tumor bearing groups receiving resveratrol showed significant responses by maintaining hindlimb weights comparable to control in the 200 mg/kg and 500 mg/kg groups. The untreated and treated control groups also were significantly higher in hindlimb weights compared to untreated tumor bearing mice. Hindlimb weights were compared among the same subsets with significant weight loss shown in the untreated tumor bearing group. (C) Significant decreases in heart weight were seen in the untreated tumor bearing mice compared to control mice, control mice receiving resveratrol at all doses and tumor bearing mice receiving resveratrol at the 200 mg/kg and 500 mg/kg doses. Change in heart weights among non-tumor bearing and tumor bearing mice. Individual hearts were weighed and are graphically represented here to show heart weight as a percentage of average control heart weight among all groups. ## = p <0.0001, # = p <0.001, ** = p < 0.01, * = p < 0.05. For (A)-(C) N=24 (Control), N=8 (Control +R100), N=8 (Control + R200), N=8 (Control +R500), N=17 (Tumor), N=6 (Tumor + R100), N=6 (Tumor + R200), N=7 (Tumor +R500).
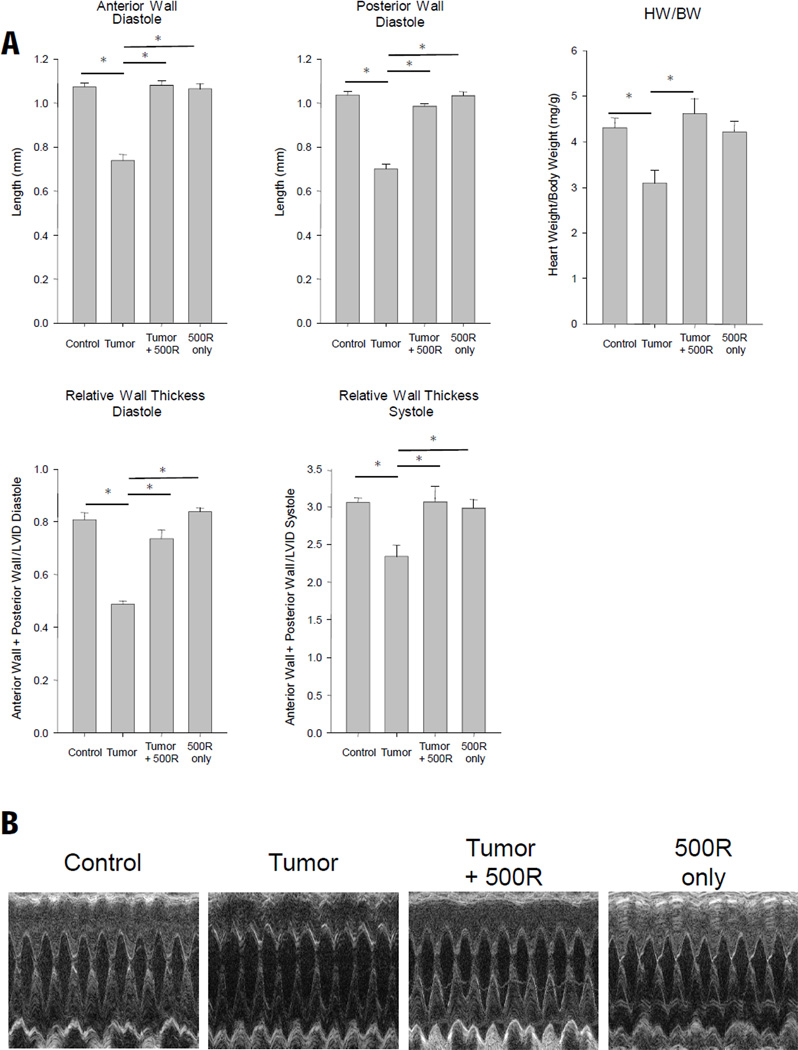
Tumor bearing mice exhibited a significant loss of total heart weight compared to control mice. Treatment with 200 and 500 mg/kg was protective against this 8.6% total heart weight loss identified after 17 days of tumor burden (Figure 3C). Treatment of control mice with 100 mg/kg, 200 mg/kg, 500 mg/kg of resveratrol did not affect total heart weight (Figure 3C). To further characterize the effects of resveratrol on the heart, we performed in vivo trans-thoracic echocardiography on conscious mice from control, tumor-challenged with and without 500 mg/kg resveratrol (Figure 4A, 4B, Table 1). We identified that 17 days of tumor burden induced a decrease in anterior and posterior wall of the left ventricle, reflecting the cardiac atrophy identified by a decrease in heart weight / body weight ratio and relative wall thickness (Figure 4). In our first set of studies, mice challenged with tumor and assayed by echocardiography lost approximately 8.8% of their body weight after 17 days of tumor (Figure 1B). However, in our echocardiography studies, they lost only 6.8% (=21.8–20.4/21.8) (tumor vs. control, Table 1). This may be due to the sample size and tumor size variation we seen in individual mice. Treatment of tumor challenged mice with 500 mg/kg resveratrol completely prevented the decrease in anterior and posterior wall thickness (Figure 4A, 4B, Table 1). Treatment of control mice with resveratrol did not affect baseline LV wall thickness or heart weight (Figure 4A, Table 1), suggesting that protection resveratrol afforded the heart was not drug induced increases in muscle mass, but more likely due to an inhibition of the signaling pathways mediating cardiac atrophy.
Figure 4. Resveratrol protects against tumor induced cardiac atrophy.
(A) Conscious echocardiography of the left ventricle indentified significant decreases in tumor-induced anterior and posterior wall thickness in diastole, heart weight to body weight ratio (HW/BW), and relative wall thickness, that were not identified when 500 mg/kg/day resveratrol was given. (B) Representative M-mode recordings from images used in the analysis of (A). A one-way ANOVA was performed to determine significance, followed by a Holm-Sidak pairwise comparison to significance between groups. *p<0.05. BW, body weight, HW, heart weight; RWT=LVPW;d/s+LVAntW; d/s / LVEDD or LVEDS; LV mass (index)=[1.055* ( (ExLVD;d)3- (LVEDD;d)3). N=4 per group.
Table 1. Transthoracic echocardiography on unanesthetized sham, tumor, or tumor plus resveratrol treatment in age-matched mice 16 days after tumor implantation (±SE).
A one-way ANOVA was performed to determine significance.
| Control N=8 |
Tumor N=4 |
Tumor + 500R N=4 |
Control + 500R N=4 |
|
|---|---|---|---|---|
| Body Weight (g) | 21.8 ± 0.4 | 20.4 ± 1.1 | 21.6 ± 0.8 | 20.6 ± 0.4 |
| HR (bpm) | 651 ± 9 | 617 ± 27 | 640 ± 13 | 659 ± 13 |
| LVEDD (mm) | 2.63 ± 0.09 | 2.95 ± 0.08* | 2.82 ± 0.13 | 2.50 ± 0.07 |
| LVESD (mm) | 1.11 ± 0.05 | 1.35 ± 0.07 | 1.19 ± 0.10 | 1.08 ± 0.06 |
| LV %EF | 88.97 ± 0.46 | 85.78 ± 2.78 | 88.64 ± 1.86 | 88.20 ± 0.94 |
| LV Vol; d (ml) | 25.9 ± 2.2 | 33.8 ± 2.2 | 30.6 ± 3.5 | 22.8 ± 1.5 |
| LV Vol; s (ml) | 2.9 ± 0.3 | 4.7 ± 0.7 | 3.5 ± 0.8 | 1.7 ± 1.5 |
BW, body weight; LV, left ventricle; HR, heart rate; LVEDD, LV end diastolic dimension; LVESD, LV end systolic dimension; LV EF%=[ (LV Vol;d-LV Vol;s/LV Vol;d )*100];LV Vol; d, LV volume in diastole; LV Vol;s, LV volume in systole; LV Post W.
p<0.05 vs Control, Tumor + 500R, and Control + 500R.
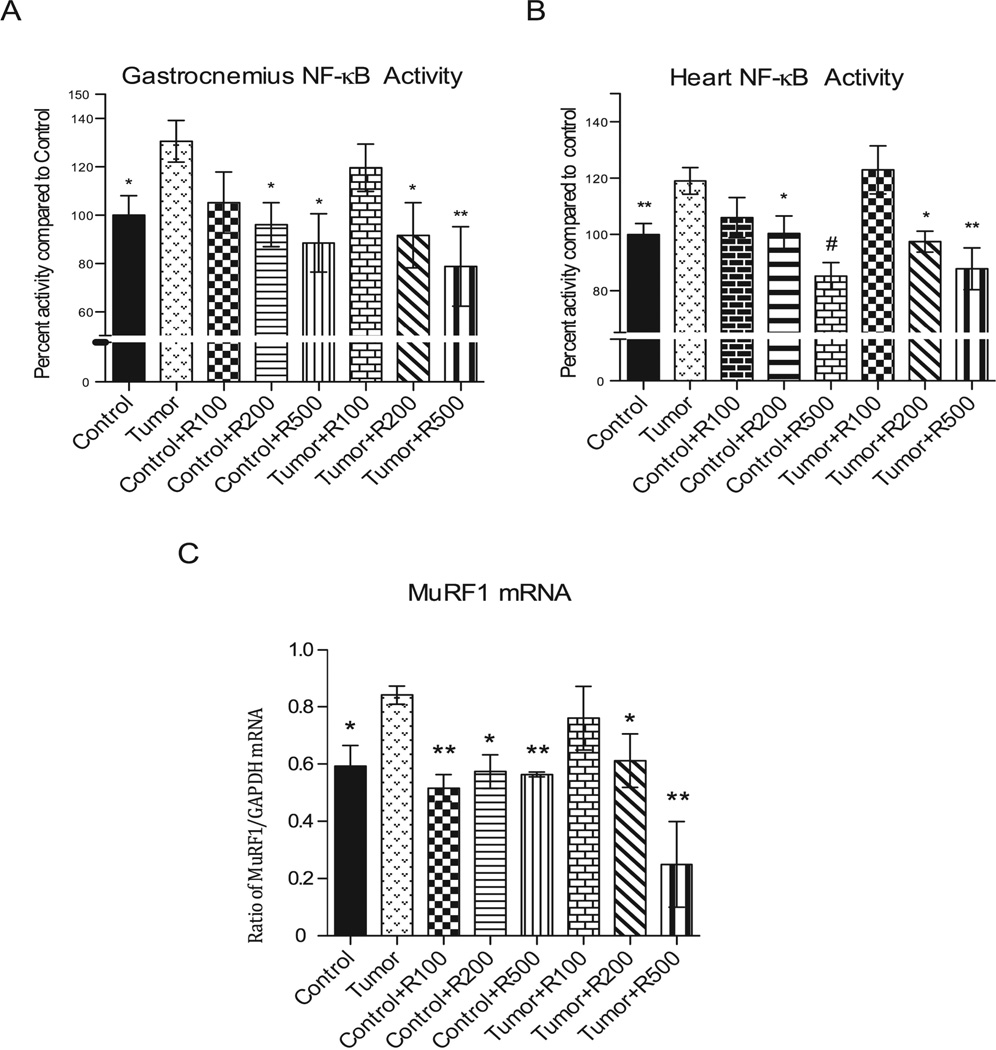
Resveratrol inhibits NF-κB transcription factor activity in tumor induced skeletal muscle and heart atrophy
The C-26 adenocarcinoma tumor induces cachexia through its release of TNFα, IL-1, IL-6, which interact with their cognate receptors on muscle to activate the downstream transcription factor NF-κB30–34, to induce cachexia and muscle atrophy. Recent studies have demonstrated experimentally the dominant role of NF-κB in muscle atrophy 18–20, 35, 36 and resveratrol’s ability to inhibit NF-κB in cancer cells 37. Therefore, we investigated if resveratrol’s inhibition of tumor-induced gastrocnemius and heart atrophy was due in part to its inhibition of NF-κB (Figure 5). We identified that nuclear bound NF-κB (p65 subunit) activity was significantly elevated in tumor-induced gastrocnemius and heart, and completely reversed in mice treated with the highest levels of resveratrol (200 mg/kg and 500 mg/kg) (Figure 5A, 5B). Gastrocnemius muscle from untreated tumor bearing mice had 30.5% higher mean NF-κB activity compared to control mice (Figure5A). A significant decrease in NF-κB (p65) activity was seen in tumor bearing mice given 200 and 500 mg/kg of resveratrol (decreased 39% and 51%, respectively) (Figure5A). Treated control mice also had a significant decrease in NF-κB (p65) activity after treatment with 200 and 500 mg/kg dosage (decreased 34%, and 41%, respectively) compared to untreated tumor challenged mice, indicating that resveratrol decreased endogenous NF-κB activity induced by tumor in skeletal muscle.
Figure 5. Nuclear factor-kappa B activity (p65).
(A) Nuclear extractions on gastrocnemius muscle were analyzed looking at p65 bound DNA activity. Significant increase in activity was noted in untreated tumor bearing mice compared to control. Tumor bearing mice treated with resveratrol showed a significant decrease in NF-κB activity at 200 mg/kg and 500 mg/kg dosages. Also of note in the treated non-tumor bearing group NF-κB activity was significantly decreased and comparable to control with administration of resveratrol at the 200 mg/kg and 500 mg/kg dosages. (B) The tumor bearing groups receiving 200 mg/kg and 500 mg/kg resveratrol showed significant inhibition of tumor-induced NF-κB activity. (C) Tumor bearing mice receiving 200 mg/kg and 500 mg/kg resveratrol had an inhibition of tumor-induced MuRF1 expression. mRNA expression was measured and depicted here as normalized to GAPDH controls. # = p <0.001, ** = p < 0.01, * = p < 0.05. For (A) and (B): N=12 (Control); N=4 (Control + R100), N=4 (Control + R200), N=4 (Control + R500), N=4 (Tumor), N=4 (Tumor +R200), N=4 (Tumor + R500). For (C): N=4 (Control), N=3 (Control + R100), N=3 (Control + R200), N=3 (Control + R500), N=4 (Tumor), N=3 (Tumor +R100), N=3 (Control + R200), N=3 (Control +R500).
Analysis of the heart revealed that tumor challenge induced a 19% increase in NF-κB activity compared to control hearts (Figure 5B). Treatment of tumor bearing mice with 200 and 500 mg/kg resveratrol significantly decreased NF-κB (p65) activity compared to tumor challenged mice (21.6%, and 31.2%, respectively) (Figure 5B). Treatment with 200 and 500 mg/kg resveratrol induced a significant reduction in NF-κB (p65) activity in control mice (18.7% and 33.8%, respectively) compared to untreated tumor challenged mice. Recent studies have identified that NF-κB induction of E3 ubiquitin ligases regulate atrophy is skeletal muscle10, 11. Furthermore, our laboratory has determined a critical role of the cardiac ubiquitin ligase muscle ring finger-1 (MuRF1) in cardiac atrophy 25. Specifically, we identified that mice lacking MuRF1 were essentially unable to atrophy in response to dexamethasone 25. This contrasts with skeletal muscle, where both MuRF1 and Atrogin-1 play a partial role in inhibiting skeletal muscle atrophy 13. We next focused our investigation on how resveratrol’s inhibition of NF-κB might affect cardiac MuRF1 expression in vivo.
Resveratrol inhibits cardiac MuRF1 mRNA expression
Tumor challenge induced MuRF1 mRNA expression in the heart significantly (~20%) after 17 days of tumor (Figure 5C). While resveratrol treatment did not affect MuRF1 expression in control animals, 200 and 500 mg/kg resveratrol significantly inhibited the tumor-induced MuRF1 compared to tumor challenge alone (~25% and ~75%, respectively), reaching MuRF1 mRNA levels significantly less than that found in control animals (Figure 5C). Since MuRF1 plays a pivotal role in cardiac atrophy in vivo 25, and MuRF1 is regulated in part by NF-κB 10, 11, these findings suggest one mechanism by which resveratrol inhibits tumor-induced cardiac atrophy by the inhibition of NF-κB-induced MuRF1 expression in vivo.
Discussion
The C26-adenocarcinoma model of cancer cachexia has been used over the last 3 decades for research on the natural history of carcinomas and anti-tumor therapy, as recently reviewed by Aulino, et al., 2010 29. Nearly 190 papers can be found by searching PubMed for C26 cancer, many focusing on C26-induced cachexia 29. The C26-adenocarcinoma model was established in 1975 in an effort to create models which could be used to test biological- and chemo-therapies 38. It can be established in nude and SCID mice, as well as wild type mice, suggesting that lymphocytes play little, if any, role in the pathophysiology of tumor progression 31. The predominant mechanism by which the C26-adenocarcinoma cell line induces cachexia is through its release of inflammatory cytokines 31, paralleling the mechanism by which cancer causes cachexia in humans 8.
Initial studies of the C26-adenocarcinoma mouse model, as used in the present study, identified a prominent role of IL-6 in the induction of muscle atrophy. In these studies, inhibiting IL-6 using cytokine specific reagents prevented muscle atrophy induced in C26-adenocarcinoma bearing mice. Additional studies identified that IL-6 was not the sole mechanism for the induction of muscle cachexia 34, 39. The C26-adenocarcinoma cells were also found to secrete TNFα, IL-1, and IL-6 in vivo, along with yet to be identified cachexia factors that induce skeletal muscle atrophy in vivo 31, 33. These pro-inflammatory cytokines bind to their cognate receptors found on skeletal muscle and heart, to activate the transcription factor NF-κB 40, which mediates their downstream effects. The activation of NF-κB is essential to the process of atrophy in striated muscle. This has been proven convincing by using dominant negative inhibitory NF-κB complexes (IKK), which effectively inhibits NF-κB. Over-expression of a dominant negative IKKα/β-EGFP fusion protein, which inhibits NF-κB markedly inhibited disuse induced atrophy up to 70% in vivo 17. Other studies have also identified that pharmacologic or genetic inhibition of IKK/NF-κB similarly inhibits ubiquitin dependent muscle wasting (proteolysis) 10. In these studies, NF-κB inhibition inhibited the expression of the ubiquitin ligase MuRF1, one of two ubiquitin ligases that are necessary for skeletal muscle atrophy to occur 10.
Proinflammatory mediators, such as TNFα, are potent inducers of the ubiquitin ligase MuRF1 13. Recent studies have shown that MuRF1 expression is regulated by the transcription factor NF-κB 10, 41, which is activated by a number of proinflammatory cytokines, including TNFα. Similarly, the C26-adenocarcinoma tumor in mice induces protein catabolism by inducing the ubiquitin ligase MuRF1 42. C26 burden induces specific loss of thick, but not thin filament sarcomere components 24 and alters myosin isoform expression 43, consistent with the actions of MuRF1 44. The dystrophin complex is decreased in C26 adenocarcinoma-bearing mice, a phenomenon essential for muscle degradation 42. This model has allowed the demonstration of the role of stem cells in skeletal muscle during cachexia45, 46.
While MuRF1 plays only a partial role in mediating skeletal muscle atrophy in vivo 13, our laboratory has recently identified that MuRF1 activity is necessary to induce ANY cardiac atrophy 25. Specifically, we identified that the significant dexamethasone-induced atrophy induced in wild type hearts is essentially absent in MuRF1 −/− mice 25. We therefore focused our present studies on the ubiquitin ligase MuRF1 expression in the heart. We hypothesized that resveratrol’s inhibition of cardiac NF-κB would reduce cardiac MuRF1 expression, representing one mechanism by which cardiac atrophy is inhibited in the C26-adenocarcinoma-induced cachexia mouse model. In fact, we did identify that resveratrol inhibited tumor-enhanced MuRF1 expression in vivo (Figure 5C). Therefore, our findings are consistent resveratrol’s prevention of cardiac atrophy to be due, in part, to the inhibition of MuRF1, possibly through its inhibition of NF-κB. However, MuRF1 is also regulated by other transcription factors such as FOXO transcription factors 47–49, so it is possible that resveratrol inhibits MuRF1 by mechanisms other than NF-κB in the heart. The findings in the present study demonstrate resveratrol protects against cancer induced cardiac atrophy by inhibiting NF-κB and MuRF1, suggesting one or more mechanisms by which it is cardioprotective.
Cachexia in human cancer patients presents as muscle loss, often with the loss of fat associated with anorexia, inflammation, and insulin resistance 50. Previous studies have demonstrated that C26-adenocarcinoma induced cachexia induces a loss of both lean muscle and fat, as we identified in the present study 51, 52. The details of how cachexia causes fat loss has not been completely delineated, but increased expression of hormone-sensitive lipase is a down-stream effect of factors secreted by tumors 50. While it might be possible inflammatory cytokines and NF-κB may mediate this in muscle, not enough about these complex signaling pathways in adipocytes is currently known to support this hypothesis 53.
One of the strengths of the current study is that resveratrol did not affect the C26-adenocarcinoma tumor size, allowing us to determine the resveratrol’s efficacy specifically at the level of skeletal muscle and heart. However, it is important to note that resveratrol has been reported to inhibit tumor growth, including multiple myeloma cells (IM-9 cell line), HELA cells (cervix carcinoma), and K-562 cells (chronic myeloid leukemia)37, 54, 55. In fact, the efficacy of NF-κB inhibition by resveratrol to protect against skeletal muscle loss induced by the MAC16 tumor (using 1 mg/kg/day) in a previous study by Wyke et al.56 could be attributed to its inhibition of tumor growth itself, making the present study unique from a mechanistic point of view. The ability of resveratrol to inhibit tumor growth itself may be of great benefit to patients with cachexia. Along with our present study, these findings indicate that resveratrol protects against cancer cachexia by 2 distinct mechanisms: 1) by inhibiting tumor growth directly, inducing cell death resulting in a reduction of total cytokine release; and 2) by inhibiting cytokine-driven NF-κB signaling at the level of the skeletal muscle and heart. Since resveratrol does not affect the C26-adenocarcinoma tumor in the present study, resveratrol may be even more potent then the results of the present study demonstrate.
The contribution of cardiac complications in human cancer cachexia has not been studied widely. However, many of the inflammatory mediators found cachexia are found in heart failure and directly cause cardiac dysfunction, including IL-6, IL-1, and TNFα. The best studied is TNFα, which itself can induce heart failure and has been implicated in the pathophysiology of a number of heart diseases 7. This suggests that cardiac involvement may be common in cardiac cachexia, particularly in severe disease. Experimentally, cachexia has been shown to induce heart failure, disrupt myocardial structure, and alter composition of the contractile proteins in mouse models 43, 57. We did not identify gross systolic dysfunction by echocardiography in the present study. However, larger tumors forming over longer periods may have led to cardiac dysfunction in our model.
In the present study, we demonstrate for the first time that resveratrol directly inhibits skeletal muscle and cardiac atrophy at oral doses of 200–500 mg/kg/day in a mouse model of tumor-induced cachexia and cardiac atrophy without any apparent toxicity to the mouse. A recent study performed by Busquets, et al. found that reseveratrol did not prevent cancer-induced skeletal muscle wasting, using a rat model with Yoshida AH-130 ascites hepatoma (treated with 1 mg/kg/day resveratrol intraperitoneally) or in mice bearing the Lewis lung carcinoma (at 5 and 25 mg/kg/day resveratrol intraperitoneally)58. Rats were also given oral resveratrol (3 mg/kg/day) in combination with fish oil intragastrically 58. Note that significantly lower doses were used in the intragastric model compared to our present study (100–500 mg/kg/day). The inability of resveratrol to protect against skeletal muscle mass or body weight loss in tumor-bearing rodents may therefore be due to the lower doses of resveratrol used in this earlier study. Consistent with this hypothesis, our use of 100 mg/kg/day by oral gavage did not have any effect on muscle loss, while a dose of at least 200 mg/kg/day was necessary to protect again tumor-induced cachexia. The effective doses in the current mouse study are roughly 3 times the dose tested in humans without toxicity. In s phase I clinical trial, a single dose of 5 g/day have been given to humans without obvious toxicity. 26 In an average 70 kg person, this dose would be approximately 71 mg/kg/day (5000 mg/70 kg), or approximately 2.8 times less than the minimum effective dose found in the current studies (200 mg/kg/day vs. 71 mg/kg/day).
The effects of long term resveratrol exposure in the context of treating cancer cachexia in the present model are not known. However, it should be pointed out that mice receiving 500 mg/kg/day (Figure 1B) lost weight in the presence or absence of tumor, whereas mice receiving lower doses did not. Since these small, but significant changes were seen when 500 mg/kg/day was given over a short period of time (11 days), future studies should be careful of the potential side effects with oral resveratrol therapy (i.e. weight loss) when given at doses of 500 mg/kg/day. This would be particularly important in longer term studies lasting greater than 11 days, which is likely how this drug would be used to treat cachexia in patients.
Current therapies for cachexia include single modality therapy or combinations of progestational agents, glucocorticoids, selective COX-2 inhibitors, or non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin or indomethacin. Newer treatments have been developed to inhibit cytokines or NF-κB to some degree. These include high dose progestins59, eicosapentaenoic acid (EPA) 60, β-Hydroxy-β-methylbutyrate (HMB) 61, thalidomide 62, and NSAID’s 63. However, these agents have adverse side effects and results have been variable and marginal in reversing cachexia clinically. Resveratrol is currently being used in a variety of human diseases, including hepatocellular carcinoma21 and neurodegenerative disease 22 with no marked toxicity 7. It may therefore have application to cachexia (including skeletal muscle and cardiac atrophy) and more broadly to heart failure in a variety of contexts in human disease, although this has currently not been tested directly.
Supplementary Material
Histological examination of liver and kidney tissues were undertaken at 20X magnification searching for signs of drug toxicity. (A) At the time of sacrifice, livers were harvested and histological specimens prepared in the standard fashion as described in the methods section. Normal liver parenchyma was noted with uniform cytoplasm between all groups as seen in the control sample. (B) No drug toxicity was detected in the liver from any resveratrol treated groups as seen in the 500 mg/kg tumor bearing resveratrol treated mouse histologically. (C,D) No evidence of hepatocyte loss, inflammation, or steatosis was detected in any groups at any dosages. Histological analysis of the kidney revealed normal glomeruli, tubules and interstitium with no evidence of acute tubular necrosis, glomerulosclerosis, or interstitial inflammation as demonstrated in a control mouse (C), as well as in a 500 mg/kg resveratrol treated tumor bearing mouse (D). N=4 mice per group were analyzed histologically (Control, Control + 100R, Control + 200R, Control + 500R, Tumor, Tumor + 100R, Tumor + 200 R, Tumor + 500 R). One representative mouse from control and Tumor + 500R is shown in the figure.
Acknowledgements
All work was performed at the University of North Carolina at Chapel Hill, USA
Grant support
S.S. was supported by the University of North Carolina Department of Otolaryngology/Head and Neck Surgery. This work was supported by University of North Carolina Program in Translational Science Grant (to M.C.), the American Heart Association Scientist Development Grant (to M.W.), and the National Heart, Lung, and Blood Institute grant R01HL104129 (to M.W.).
Non-standard abbreviations
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- IKK
IκB kinase
- MAC
murine adenocarcinoma
- LBM
lean body mass
- FM
fat mass
- MuRF1
muscle ring finger-1
- UPS
ubiquitin proteasome system
Footnotes
Conflict of interest
S.S., M.C., K.M., L.W., X.Y., J.R., D.G., M.W. declare no conflicts of interest.
References
- 1.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 2.Fearon KC, Moses AG. Cancer cachexia. Int J Cardiol. 2002;85:73–81. doi: 10.1016/s0167-5273(02)00235-8. [DOI] [PubMed] [Google Scholar]
- 3.Inagaki J, Rodriguez V, Bodey GP. Proceedings: Causes of death in cancer patients. Cancer. 1974;33:568–573. doi: 10.1002/1097-0142(197402)33:2<568::aid-cncr2820330236>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Warren S. The immediate causes of death in cancer. Am J Med Sci. 1935;184:610–616. [Google Scholar]
- 5.Argiles JM, Busquets S, Lopez-Soriano FJ. The pivotal role of cytokines in muscle wasting during cancer. Int J Biochem Cell Biol. 2005;37:2036–2046. doi: 10.1016/j.biocel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Costelli P, Baccino FM. Cancer cachexia: from experimental models to patient management. Curr Opin Clin Nutr Metab Care. 2000;3:177–181. doi: 10.1097/00075197-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Pajak B, Orzechowska S, Pijet B, Pijet M, Pogorzelska A, Gajkowska B, et al. Crossroads of cytokine signaling--the chase to stop muscle cachexia. J Physiol Pharmacol. 2008;59(Suppl 9):251–264. [PubMed] [Google Scholar]
- 8.Carson JA, Baltgalvis KA. Interleukin 6 as a Key Regulator of Muscle Mass during Cachexia. Exerc Sport Sci Rev. 2010;38:168–176. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 10.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Wyke SM, Tisdale MJ. NF-kappaB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin-proteasome system in skeletal muscle. Br J Cancer. 2005;92:711–721. doi: 10.1038/sj.bjc.6602402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drott C, Ekman L, Holm S, Waldenstrom A, Lundholm K. Effects of tumor-load and malnutrition on myocardial function in the isolated working rat heart. J Mol Cell Cardiol. 1986;18:1165–1176. doi: 10.1016/s0022-2828(86)80042-6. [DOI] [PubMed] [Google Scholar]
- 13.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med. 2008;86:1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tisdale MJ. Is there a common mechanism linking muscle wasting in various disease types? Curr Opin Support Palliat Care. 2007;1:287–292. doi: 10.1097/SPC.0b013e3282f35238. [DOI] [PubMed] [Google Scholar]
- 16.Tisdale MJ. Are tumoral factors responsible for host tissue wasting in cancer cachexia? Future Oncol. 2010;6:503–513. doi: 10.2217/fon.10.20. [DOI] [PubMed] [Google Scholar]
- 17.Van Gammeren D, Damrauer JS, Jackman RW, Kandarian SC. The IkappaB kinases IKKalpha and IKKbeta are necessary and sufficient for skeletal muscle atrophy. FASEB J. 2009;23:362–370. doi: 10.1096/fj.08-114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eley HL, Russell ST, Tisdale MJ. Attenuation of muscle atrophy in a murine model of cachexia by inhibition of the dsRNA-dependent protein kinase. Br J Cancer. 2007;96:1216–1222. doi: 10.1038/sj.bjc.6603704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, et al. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest. 2006;116:2945–2954. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010;36:43–53. doi: 10.1016/j.ctrv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Pallas M, Casadesus G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, et al. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6:70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell TM, Ardeshirpour F, Asher SA, Winnike JH, Yin X, George J, et al. Metabolomic analysis of cancer cachexia reveals distinct lipid and glucose alterations. Metabolomics. 2008;4:216–225. [Google Scholar]
- 24.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis MS, Rojas M, Li L, Selzman CH, Tang RH, Stansfield WE, et al. Muscle ring finger 1 mediates cardiac atrophy in vivo. Am J Physiol Heart Circ Physiol. 2009;296:H997–H1006. doi: 10.1152/ajpheart.00660.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 27.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 28.Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47:2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Aulino P, Berardi E, Cardillo VM, Rizzuto E, Perniconi B, Ramina C, et al. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer. 2010;10:363. doi: 10.1186/1471-2407-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturlan S, Beinhauer BG, Oberhuber G, Huang L, Aasen AO, Rogy MA. In vivo gene transfer of murine interleukin-4 inhibits colon-26-mediated cancer cachexia in mice. Anticancer Res. 2002;22:2547–2554. [PubMed] [Google Scholar]
- 31.Yasumoto K, Mukaida N, Harada A, Kuno K, Akiyama M, Nakashima E, et al. Molecular analysis of the cytokine network involved in cachexia in colon 26 adenocarcinoma-bearing mice. Cancer Res. 1995;55:921–927. [PubMed] [Google Scholar]
- 32.Zhou W, Jiang ZW, Jiang J, Li N, Li JS. [Role of NF-kappa B in cancer cachexia] Zhonghua Wai Ke Za Zhi. 2004;42:683–686. [PubMed] [Google Scholar]
- 33.Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li JS. Role of NF-kappaB and cytokine in experimental cancer cachexia. World J Gastroenterol. 2003;9:1567–1570. doi: 10.3748/wjg.v9.i7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soda K, Kawakami M, Kashii A, Miyata M. Manifestations of cancer cachexia induced by colon 26 adenocarcinoma are not fully ascribable to interleukin-6. Int J Cancer. 1995;62:332–336. doi: 10.1002/ijc.2910620317. [DOI] [PubMed] [Google Scholar]
- 35.Smith HJ, Wyke SM, Tisdale MJ. Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate. Cancer Res. 2004;64:8731–8735. doi: 10.1158/0008-5472.CAN-04-1760. [DOI] [PubMed] [Google Scholar]
- 36.Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes-McNary M, Baldwin AS., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 38.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35:2434–2439. [PubMed] [Google Scholar]
- 39.Soda K, Kawakami M, Kashii A, Miyata M. Characterization of mice bearing subclones of colon 26 adenocarcinoma disqualifies interleukin-6 as the sole inducer of cachexia. Jpn J Cancer Res. 1994;85:1124–1130. doi: 10.1111/j.1349-7006.1994.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 41.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421–432. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Diffee GM, Kalfas K, Al-Majid S, McCarthy DO. Altered expression of skeletal muscle myosin isoforms in cancer cachexia. Am J Physiol Cell Physiol. 2002;283:C1376–C1382. doi: 10.1152/ajpcell.00154.2002. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185:1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarzkopf M, Coletti D, Sassoon D, Marazzi G. Muscle cachexia is regulated by a p53-PW1/Peg3-dependent pathway. Genes Dev. 2006;20:3440–3452. doi: 10.1101/gad.412606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berardi E, Aulino P, Murfuni I, Toschi A, Padula F, Scicchitano BM, et al. Skeletal muscle is enriched in hematopoietic stem cells and not inflammatory cells in cachectic mice. Neurol Res. 2008;30:160–169. doi: 10.1179/174313208X281046. [DOI] [PubMed] [Google Scholar]
- 47.Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, et al. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab. 2008;295:E785–E797. doi: 10.1152/ajpendo.00646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 49.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004;287:E591–E601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- 50.Tisdale MJ. Cancer cachexia. Curr Opin Gastroenterol. 2010;26:146–151. doi: 10.1097/MOG.0b013e3283347e77. [DOI] [PubMed] [Google Scholar]
- 51.Weyermann P, Dallmann R, Magyar J, Anklin C, Hufschmid M, Dubach-Powell J, et al. Orally available selective melanocortin-4 receptor antagonists stimulate food intake and reduce cancer-induced cachexia in mice. PLoS One. 2009;4:e4774. doi: 10.1371/journal.pone.0004774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 55.Baatout S, Derradji H, Jacquet P, Ooms D, Michaux A, Mergeay M. Enhanced radiation-induced apoptosis of cancer cell lines after treatment with resveratrol. Int J Mol Med. 2004;13:895–902. [PubMed] [Google Scholar]
- 56.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br J Cancer. 2004;91:1742–1750. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer-induced cachexia in mice. Int J Oncol. 2010;37:347–353. doi: 10.3892/ijo_00000683. [DOI] [PubMed] [Google Scholar]
- 58.Busquets S, Fuster G, Ametller E, Olivan M, Figueras M, Costelli P, et al. Resveratrol does not ameliorate muscle wasting in different types of cancer cachexia models. Clin Nutr. 2007;26:239–244. doi: 10.1016/j.clnu.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Loprinzi CL, Schaid DJ, Dose AM, Burnham NL, Jensen MD. Body-composition changes in patients who gain weight while receiving megestrol acetate. J Clin Oncol. 1993;11:152–154. doi: 10.1200/JCO.1993.11.1.152. [DOI] [PubMed] [Google Scholar]
- 60.Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52:1479–1486. doi: 10.1136/gut.52.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.May PE, Barber A, D'Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479. doi: 10.1016/s0002-9610(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 62.Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, Goggin PM. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut. 2005;54:540–545. doi: 10.1136/gut.2004.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lundholm K, Gelin J, Hyltander A, Lonnroth C, Sandstrom R, Svaninger G, et al. Anti-inflammatory treatment may prolong survival in undernourished patients with metastatic solid tumors. Cancer Res. 1994;54:5602–5606. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histological examination of liver and kidney tissues were undertaken at 20X magnification searching for signs of drug toxicity. (A) At the time of sacrifice, livers were harvested and histological specimens prepared in the standard fashion as described in the methods section. Normal liver parenchyma was noted with uniform cytoplasm between all groups as seen in the control sample. (B) No drug toxicity was detected in the liver from any resveratrol treated groups as seen in the 500 mg/kg tumor bearing resveratrol treated mouse histologically. (C,D) No evidence of hepatocyte loss, inflammation, or steatosis was detected in any groups at any dosages. Histological analysis of the kidney revealed normal glomeruli, tubules and interstitium with no evidence of acute tubular necrosis, glomerulosclerosis, or interstitial inflammation as demonstrated in a control mouse (C), as well as in a 500 mg/kg resveratrol treated tumor bearing mouse (D). N=4 mice per group were analyzed histologically (Control, Control + 100R, Control + 200R, Control + 500R, Tumor, Tumor + 100R, Tumor + 200 R, Tumor + 500 R). One representative mouse from control and Tumor + 500R is shown in the figure.