Abstract
While acute or chronic inflammation is a common component of many clinical disorders, the underlying processes can be highly distinct. In recent years, the complement system has been associated with a growing number of immunological and inflammatory conditions that include degenerative diseases, cancer and transplant rejection. It becomes evident that excessive activation or insufficient control of complement activation on host cells can cause an immune imbalance that may fuel a vicious cycle between complement, inflammatory cells and tissue damage that exacerbates clinical complications. Although the exact involvement of complement needs to be carefully investigated for each disease, therapeutic modulation of complement activity emerges as attractive target for upstream inhibition of inflammatory processes. This review provides an update about the functional and collaborative capabilities of complement, highlights major disease areas with known complement contribution, and indicates the potential for complement as focal point in immunomodulatory strategies for treating inflammatory diseases.
Inflammation is a recognized hallmark of disease, yet the knowledge about underlying mechanism that shape the inflammatory response and its resolution has been largely extended in recent years. Given the classic perception of complement as defense system against microbial intruders, it may appear surprising that this ancient pillar of innate immunity was identified as a contributor in various inflammatory pathologies. On the other hand, it becomes evident that complement not only acts as sensor of pathogens but also recognizes diseased and damaged host cells, and closely collaborates with other immune and defense systems to eliminate potential danger (1, 2). This interplay serves as vital triage system that tailors the immune response according to the threat level. However, insufficient, excessive or poorly controlled complement activation can tip the balance between health and disease and lead to self-attack of host cells (1–3). In the worst case, a vicious cycle between tissue damage, complement activation and immune attack perpetually recreates inflammatory stimulators rather than resolving them. In view of this upstream position in inflammatory homeostasis, there is growing interest in understanding the role of complement in pathological processes and in exploiting complement targets for therapeutic modulation (3, 4). Fortunately, our knowledge about the functions of complement in health and disease has much improved, and new discoveries revealed a fascinating crosstalk network that ties complement closely into the immune-inflammatory network (1, 5). Here we provide an update on complement and its dialog with associated systems, discuss major disease areas and indicate opportunities for therapeutic intervention (see the accompanying review (6) for more).
Complement beyond microbial defense
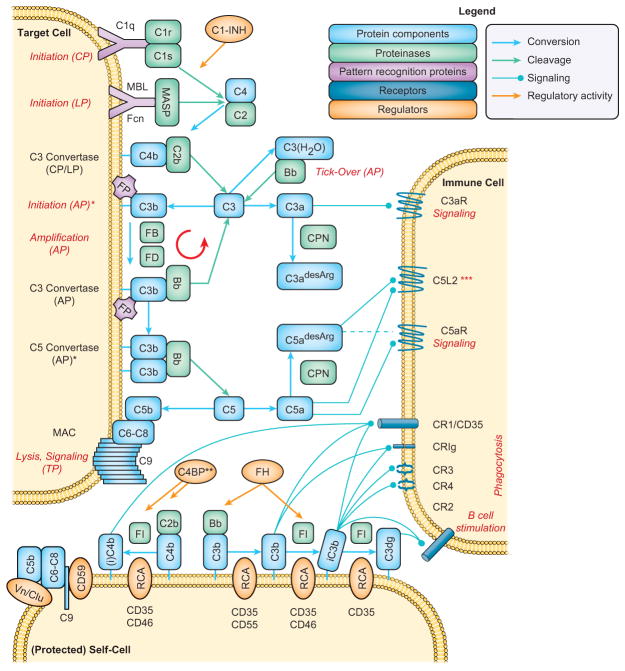
The past decade revealed a new perception of complement that reaches beyond the elimination of pathogens and includes key functions in immune surveillance, homeostasis, and mediation of inflammatory responses (1, 2). The hub-like organization of complement and its cell-surface-directed action (Fig. 1), involving some fifty constituents such as pattern-recognition molecules (PRM), protein components, proteases, regulators, and cell-surface receptors, is essential for adjusting the complement response to different triggers (Fig. 2A). When faced with foreign intruders, binding of PRM to molecular surface patterns can trigger distinct initiation pathways. In the classical pathway (CP), this is mainly mediated by binding of the C1 complex, consisting of the PRM C1q and the proteases C1r and C1s, to immunoglobulin patches on the pathogen. In the lectin pathway (LP), microbial carbohydrates are recognized by mannose-binding lectin (MBL) or ficolins in complex with MBL-associated serine proteases (MASP). Through activation of C2 and C4, both pathways lead to the assembly of C3 convertase complexes, which cleave the abundant plasma protein C3 into an anaphylatoxin fragment (C3a) and the opsonin C3b. The alternative pathway (AP), is induced by conversion of C3 to its hydrolyzed form C3(H2O), either spontaneously at a low rate in solution or accelerated by contact of C3 with various surfaces (tick-over (7)), which leads to the formation of initial AP C3 convertases. Once C3b is deposited on target surfaces, it promotes amplification of the response via the AP by forming additional C3 convertases via a tiered mechanism that involves binding of factor B (FB) and proteolytic activation by factor D (FD) to result in the C3bBb complex (8). Properdin (factor P; FP) further supports AP-mediated amplification by stabilizing the C3bBb convertase. Continuous deposition of C3b favors generation of C5 convertases that convert component C5 into C5b, which initiates formation of membrane attack complexes (MAC; C5b-9) that lyse susceptible cells (e.g., Gram-negative bacteria). Cleavage of C5 releases the chemokine C5a that, together with C3a, attracts immune cells to sites of activation via binding to the anaphylatoxin receptors C5aR (CD88) and C3aR, respectively. Carboxypeptidases rapidly convert C3a and C5a into their desarginated forms, resulting in a shift in their activity/specificity profiles. Phagocytic cells recognize C3b-opsonized surfaces via complement receptor 1 (CR1; CD35), which facilitates phagocytosis and mediates the degradation of C3b to iC3b, C3c and C3dg by factor I (FI). Whereas iC3b is the primary ligand for the integrin receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18), both iC3b and C3dg also interact with CR2 (CD21) that is part of the B cell co-receptor complex and reduces the threshold of B cell activation. Additional receptors for C3b/iC3b (i.e., CR of the immunoglobulin superfamily; CRIg) and for C1q (e.g., gC1qR) also participate in the recognition and elimination of opsonized cells. While host cells are probed by a constant low level of AP activation (referred to as “tick-over” mechanism), they express membrane-bound regulators of complement activation (RCA) that either destabilize convertases (CD35, CD55) or act as cofactors for the FI-mediated degradation of C3b to iC3b (CD35, CD46) and C3dg (CD35) and of C4b to iC4b (CD35, CD46) (1, 9). In addition, the soluble RCAs C4b-binding protein (C4BP) and factor H (FH) recognize host cell-surface patterns and contribute to the regulation of the CP/LP and AP convertases, respectively. Finally, the membrane regulator CD59 and soluble vitronectin and clusterin prevent MAC formation on host cells. Apoptotic cells induce yet another response that lies in between that observed for foreign and host cells; while the recognition of surface modifiers on apoptotic cells by PRMs induces opsonization, the presence of RCA prevents excessive amplification and, consequently, the generation of C5a or MAC. Thereby, complement facilitates the elimination of apoptotic cells, immune complexes and cellular debris without inducing inflammatory triggers (Fig. 2B) (1).
FIGURE 1.
Simplified scheme of the complement activation network. *Only part of the functional spectrum of properdin (FP) is visualized: FP may act as pattern recognition molecule and recruit C3b from plasma to the target surface (AP initiation); in addition, it stabilizes both the AP C3 and C5 convertases. Only the AP C5 convertase (C3bBb3b) is shown; a CP/LP C5 convertase (C4b2b3b) is also formed. **The regulation of the CP/LP C3 convertase is depicted a one-step process but follows a two-step mechanism similar to C3b, including decay acceleration (C4BP, CD35) and FI-mediated degradation to iC4b (C4BP, CD35, CD46). ***The function of C5L2 is not fully described and may be content-specific; C5a and C5a-desArg bind equally well to C5L2 whereas their binding and signaling profiles on C5aR is distinct. The binding of C3a-desArg to C5L2 remains controversial. Abbreviations: AP, alternative pathway; C1-INH, C1 inhibitor; C3aR, C3a receptor; C4BP, C4b-binding protein; C5aR, C5a receptor; C5L2, C5a receptor-like 2; Clu, clusterin; CP, classical pathway; CPN, carboxypeptidase-N; CR, complement receptor; FB, factor B; Fcn, ficolins; FD, factor D; FH, factor H; FI, factor I; LP, lectin pathway; MAC, membrane attack complex; MASP, MBL-associated protease; MBL, mannose-binding lectin; RCA, regulator of complement activation; Vn, vitronectin.
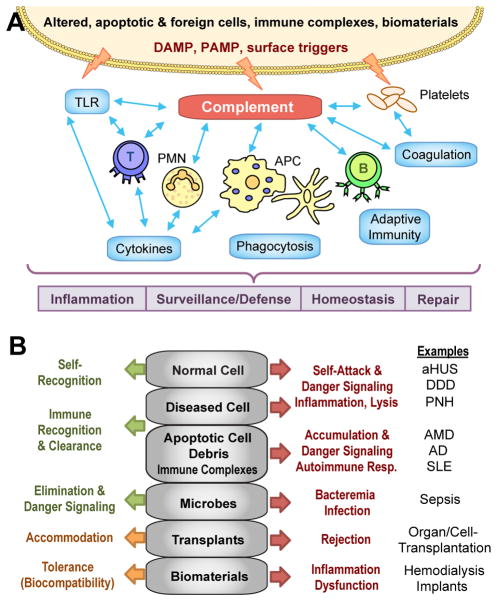
FIGURE 2.
(A) Triggered directly by foreign and altered surfaces, the complement network resides upstream of most defense and homeostatic systems, thereby acting as an important mediator in physiological and pathophysiological processes. Abbreviations: DAMP, damage-associated molecular patterns; PAMP, pathogen-associated molecular pattern; PMN, polymorphonuclear cells; TLR, Toll-like receptor. (B) While complement-mediate immune surveillance and mediation usually provides adequate physiological response (green arrow) to distinct surfaces (grey), any excessive trigger or inadequate regulation (e.g., due to deficiencies or polymorphisms) may lead to pathophysiological reactions (red arrow) that require therapeutic intervention. In the case of foreign surfaces (i.e., transplants, materials), complement-targeted modulation (orange arrow) may improve tolerance and compatibility. Abbreviations: AD, Alzheimer’s disease; aHUS, atypical hemolytic uremic syndrome; AMD, age-related macular degeneration; DDD, dense deposit disease; PNH, paroxysmal nocturnal hemoglobinuria; SLE, systemic lupus erythematosus.
While complement was first described decades ago, recent discoveries have challenged key concepts, revealed new players and redefined roles for established ones. For example, pentraxins were identified as mediators of complement activation (10), properdin was attributed PRM functionality (leading to AP initiation by recruiting C3b from the plasma pool to the surface) (11), and C5L2 (GPR77) was classified as an anaphylatoxin receptor, though its functional implication is still under investigation (12). The LP recently gained interest due to the discovery of new PRM (e.g., PTX3), regulators, and bypass routes that contributed to our understanding of this pathway and to the identification of therapeutic targets and templates (13, 14). In addition, LP components have been associated with a role in AP modulation, as MASP-1 was shown to mediate the maturation of FD from its inactive pro-form in mice and in vitro (15). Finally, the contribution of central (i.e., hepatic) versus peripheral complement production (e.g., by migratory and tissue-resident cells) in complement-driven pathologies is increasingly investigated, particularly in the context of transplantation (16).
Yet it is the discovery of the dialogue between complement and other physiological systems that has profoundly changed how we perceive complement as inflammatory mediator and therapeutic target (1). For example, it is evident that during injuries and certain disease conditions, complement and coagulation act in a concerted manner (17). While some coagulation enzymes directly cleave and activate C3 or C5 (18), thereby bypassing traditional initiation pathways, the products of such activation may themselves influence coagulation as shown for the stimulation of tissue factor expression by C5a (19). The LP may also play a linking role as MASP-2 can activate prothrombin and induce clot formation, while MBL and ficolins were described to bind fibrinogen and fibrin (20, 21). Furthermore, there are links between platelet activation and the activity of complement and contact systems through an interplay involving hydrolyzed C3, RCA, chondroitin sulfate, P-selectin, gC1qR and other components (22). Although complement and the Toll-like receptors (TLR) have both been described as ‘first line of defense’ systems, it is only recently that the extent of cooperation between these two pathways has been realized. The crosstalk between TLR, CD14 and MyD88 on the Toll side and complement components including C5aR, CR3, CD46, CD55 and gC1qR is important for antimicrobial defense but also contributes to inflammatory disorders (23). Despite its classification as a part of humoral innate immunity, complement strongly mediates adaptive and cellular immune responses. The role of complement in the stimulation and maturation of B cells via CD21 is well described and critically contributes to autoimmune and other diseases (24). Our knowledge about the crosstalk between complement and T cells, however, is still unfolding (25). Complement appears to modulate T cell immunity during induction, contraction and effector phases by acting directly on T cells or by affecting antigen-presenting cells (APC). The interaction between T cells and APC induces a concerted reaction (enhanced secretion of C3, FB, FD and C5 with downregulation of CD55) that favors complement activation on immune cells, local generation of C3a and C5a, enhanced T cell proliferation, and cytokine release on APC that provokes a shift towards Th1 immunity (25). Moreover, CD46 has been identified as a central element in the IL2-dependent transformation of Th1 cells into a regulatory state (25), and C5aR and CD46 signaling have been associated with the function of γδT-cells (26, 27). New insight was also achieved concerning the cooperation between complement and Fcγ receptors (FcγR) in the removal of immune complexes (IC). For example, a positive feedback loop has been reported, in which C5aR stimulation promotes the expression of activating FcγR; binding of autoantibodies induces the local secretion of C5 by activated cells, thereby increasing the generation of C5a and, consequently, C5aR signaling (28). More recently, a negative feedback mechanism has been discovered, in which high-galactose IC bridge inhibitory FcγRIIB and the lectin receptor dectin-1 to induce signaling that counteracts C5aR-mediated responses (29, 30). Finally, complement was shown to modulate the activities of other key players of cellular immunity such as NK and NKT cells (31), myeloid-derived suppressor cells (32), or mast cells (33). These observations underscore the importance of complement in physiological processes but also explain why dysregulation of the complement system may result in far-reaching clinical consequences.
A balancing act between health and disease
Though complement is considered a ‘master of sensing’ that discriminates between foreign, altered and healthy self surfaces, several triggers may lead to a dysfunctional triage of potential danger (Fig, 2B). Dysfunctions, deficiencies or polymorphisms of complement components are often factors that tip the balance (3), but tissue damage or confrontation with non-self surfaces (e.g., biomaterials, transplants) can also lead to excessive activation. Importantly, disruption of the complement balance with increased production of effector molecules may trickle down the immune system and contribute to autoimmune, inflammatory, degenerative, hematological, and ischemic disorders. Despite the variety of disease manifestations, the involvement of complement typically follows a common scheme that involves the recognition of potential (though not always “real”) danger patterns, an insufficiently controlled amplification loop, and the stimulation of downstream inflammatory responses. The activated immune system, in addition to complement attack itself, may exacerbate the problem by further disrupting tissue integrity and creating additional danger patterns, thereby fueling a vicious cycle between complement activation, immune stimulation and inflammation. What varies between individual disorders, however, are the triggers, dynamics, extent and localization of complement involvement.
In the case of age-related and degenerative diseases, it is the slow but steady accumulation of debris that can act as a promoter. While disease etiologies are often complex, the “fitness” of the complement system is considered a key determinant in how fast a symptomatic threshold is reached. Age-related macular degeneration (AMD), a degenerative eye disease and major cause of blindness in elderly people, has evolved into a focal point of complement drug discovery. Initiated by the discovery of complement proteins in the characteristic subretinal drusen deposits and strong disease correlations with polymorphisms in FH, research has meanwhile identified additional genetic associations (e.g., C3, FB) and established an imbalanced complement system as key contributor to the development of both geographic atrophy (dry AMD) and choroidal neovascularization (wet AMD) (34). While questions about disease triggers and the role of systemic versus locally produced complement remain controversial (35), a recent study suggested a contribution of the oxidative stress adduct malondialdehyde as initiator and provided a functional explanation about the disease correlation of the FH Y402H polymorphism (36). Although complement inhibition at various levels has shown promise in models of AMD, the translation into a clinical application has proven challenging. Another age-related disease that gained interest is Alzheimer’s disease (AD), especially after genome-wide association studies (GWAS) revealed disease correlations with CR1 and clusterin (37). Indeed, the importance of locally synthesized complement in the brain becomes increasingly evident and encompasses physiological functions such as synapse formation but also inflammatory roles in trauma, stroke and AD (38). For example, accumulation of amyloid proteins in AD was shown to trigger complement primarily via C1q-mediated CP activation; the role of the AP, and the contribution of individual pathways, may vary in different models of AD and requires further examination (38, 39). The C5a-C5aR axis seems to play an important role in this neuropathology by modulating inflammatory responses, and C5aR antagonists have shown beneficial effects in AD models (38, 40).
For reasons not fully resolved, the kidney appears to be particularly susceptible to complement attack, and several glomerular diseases show strong correlation with disturbed AP activity (41). In atypical hemolytic uremic syndrome (aHUS), a rare disease characterized by hemolytic anemia, thrombocytopenia and renal impairment, complement polymorphisms (e.g., FH, CD46, C3), deletions (e.g., FH-related proteins; FHR) or autoantibodies (e.g., against FH) can cause perpetual self-attack (41, 42). More recently, two other forms of thrombotic microangiopathies, i.e., hemolytic uremic syndrome caused by Shiga toxin-producing E. coli (STEC-HUS) and thrombotic thrombocytopenic purpura (TTP), have been more closely linked to AP activation via mechanisms involving P-selectin and platelet thrombi, respectively (43). While dysfunctional AP activity is a driving force of these diseases, activation of C5 appears to be fueling the cycle by causing endothelial cell damage. Indeed, C5-targeted inhibition has shown promising effects in these disorders, and Eculizumab has meanwhile been approved for the treatment of aHUS (44). Another series of kidney disorders characterized by dense renal deposits of C3 in the absence of CP markers have recently been classified under the name C3 glomerulopathy and include dense deposit disease (DDD) and CFHR5 nephropathy, among other forms (41).
Disruption or exhaustion of complement-mediated clearance of immune complexes and apoptotic cells, and of its bridging to adaptive immunity, are contributing factors of autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) or antiphospholipid antibody syndrome (APS) (45, 46). Bidirectional crosstalk between complement and FcγR appears to be of particular importance in this disease class as they cooperate in shaping B cell responses (47). The participation of complement pathways and components may differ considerably. In SLE, CP activation and complement consumption by autoimmune complexes can be influenced by autoantibodies against C1q or deficiencies in CP components, whereas AP involvement appears to be more prominent in the case of RA. Finally, certain forms of anti-neutrophil cytoplasmic antibody (ANCA)-induced vasculitis, inflammatory small-vessel disorders caused by autoantibodies against neutrophil constituents, were linked to complement: When affected vascular endothelial surfaces trigger complement activation, the generated C5a attracts and primes neutrophils, which in turn adhere to the endothelium. Primed neutrophils express antigens that are recognized by ANCAs, which leads to their activation and release of factors that induce cell damage, thereby fueling an amplification cycle that culminates in necrotizing inflammation (48). C5aR-directed therapy has shown success in a glomerular ANCA vasculitis model (49) and drugs blocking at the level of C5 or C5aR are currently evaluated.
Some autoimmune diseases also impose a higher risk for developing pregnancy-related complications, and important roles have been revealed for complement in both healthy and pathological pregnancy (50). Whereas complement is important for the protection of mother and fetus from pathogens, pregnancy also seems to be a state particularly vulnerable to excessive complement activity (50). For example, complement is suspected to be a major factor in both antibody-dependent (i.e., in women with APS) and –independent pregnancy loss, via mechanisms that likely involve C5a-mediated impairment of placental angiogenesis. Similar dysregulation of angiogenesis may also be involved in preeclampsia, a major pregnancy complication characterized by sudden onset of hypertension and proteinuria. In contrast to C5a signaling, which is considered a detrimental factor in the etiology of preeclampsia, C1q has recently been attributed a preventive role against abnormal placentation in a mouse model of the disease (51). Finally, complement activation was found significantly elevated in preterm birth, and complement activation products such as Bb or C3a have been described as predictive markers of preterm delivery; however, it is not yet clear whether and under which circumstances complement activation is a contributor to or a consequence of events leading to premature delivery (50). While complement therapeutics have shown encouraging effects in models of pregnancy disorders (52–54), the translation into the clinic is often challenging when involving pregnant patients.
Whereas the lytic power of complement on most eukaryotic cells is restricted, erythrocytes are comparatively susceptible to MAC attack, and several hemolytic disorders have ties to complement (55). In paroxysmal nocturnal hemoglobinuria (PNH), a somatic mutation disables the synthesis of glycosyl-phosphatidylinositol anchors and prevents the expression of CD55 and CD59 on mature blood cells. As erythrocytes naturally lack CD46, PNH erythrocytes show a very low capacity for complement regulation and are prone to intravascular lysis with severe hemolytic and thrombotic consequences. Whereas treatment of this orphan disease has long been limited to transfusion and allogeneic stem cell transplantation, the introduction of Eculizumab to the clinic has drastically changed disease management; nevertheless, complement inhibition at the level of C5 appears not to be sufficient for all patients and inhibitory strategies that act on the C3 level are currently considered (55). In addition to PNH, complement-mediated hemolysis is also observed in aHUS (see above), and in cold-agglutinin disease (CAD), in which IgM autoantibodies against certain erythrocyte antigens bind at low temperature (i.e., mainly in peripheral capillaries) and lead to CP activation and lysis (55).
Ischemic diseases constitute another widespread pathological area in which complement is integrally involved. Ischemia-reperfusion injury (IRI) occurs when blood flow to tissue is restored after a prolonged time of occlusion, and is relevant in clinical conditions ranging from stroke and myocardial infarction to trauma, sepsis, shock and cardiopulmonary bypass (CPB) surgery (56, 57). The pathological mechanisms behind IRI are multifactorial and complex, and current models suggest that the ischemic phase leads to cellular changes, exposition of neoepitoes, adhesion of polymorphonuclear cells, release of cytokines and production of reactive oxygen species that can trigger apoptosis and necrosis; reperfusion, on the other hand, is characterized by leukocyte adhesion and increased permeability. Complement is considered to be involved in aspects of both phases from the recognition of neoepitopes with subsequent opsonization and amplification to the C5a-mediated modulation of cellular responses and upregulation of adhesion molecules. While the exact contribution of individual complement pathways may vary depending on the model or disorder, recent studies have reemphasized the importance of the LP, and in particular the MBL:MASP-2 complex in complement-mediated effects of IRI (56, 57).
IRI is also a major and inevitable contributor to transplant-related complications, especially when organs are transplanted after circulatory arrest of the donor, which can lead to the induction of IRI as described above (16). In addition to the chemotactic and inflammatory effects of C5a, deposition of sublytic MAC has also been shown to induce direct cell activation with release of mediators such as IL-6 or TNF. Importantly, complement activation is a major culprit in allograft rejection, via direct tissue damage or by shaping the alloreactive T cell response. Peripheral synthesis of complement components by the donor organ has a strong impact in this context. Both the production (via B cell-costimulation) and effect of alloantibodies (via CP/LP activation) are complement-driven events in antibody-mediated rejection (AMR) (16). In the case of Langerhans islet transplantation in diabetic patients, the occurrence of a thromboinflammatory response known as ‘instant blood-mediated inflammatory reaction’ is caused by rapid complement activation and limits transplantation efficiency due to islet destruction (58). A particularly interesting, yet still incompletely understood, phenomenon in the context of transplantation is accommodation, in which transplant cells become ‘resistant’ to complement-mediated destruction; the promise of therapeutic complement inhibition for inducing accommodation of renal allografts was shown both for C5 inhibition (59) and after C3 depletion via cobra venom factor (60) in mice and non-human primates, respectively. Yet transplants are not the only non-self surfaces that trigger defense responses by complement and coagulation; products of modern medicine such as biomedical devices and implants, drug delivery vehicles, extracorporeal circuits and other artificial materials can all induce biomaterial-induced thromboinflammatory reactions (61). Such incompatibility responses are known to influence the outcome of CPB surgery, during which circuit materials, blood/air interfaces in the oxygenator, activated platelets, and protamine complexes (generated to neutralize soluble heparin at the end of the procedure) can activate complement and contribute to systemic inflammatory response syndrome (SIRS; see below) (61, 62). Despite moving out of the spotlight of complement-targeted therapy after years of clinical evaluation with soluble CR1 and anti-C5 antibodies (63, 64), CPB surgery remains a clinical problem and a promising indication for complement therapeutics (62). Another emerging area is hemodialysis; even modern dialyzer membranes activate complement significantly and contribute to perpetual inflammation in patients suffering from end-stage renal disease; therapeutic C3 inhibition was shown to prevent complement activation and reduced markers of immune cell activation, inflammation and coagulation (65, 66). Alongside soluble inhibitors, the coating of materials with passive (e.g., polyethyleneglycol) or active (e.g., FH-binding peptides) moieties is considered an attractive strategy (61, 67).
Other inflammatory diseases with complement contribution include allergic asthma and periodontitis. The ties between complement and asthma have long been recognized, yet the involvement appears to be complex. Under asthmatic conditions, complement is not only activated through the CP via allergen-antibody complexes but C3 and C5 might also be cleaved by proteases derived from certain allergens (e.g., house dust mites). The resulting C3a and C5a act synergistically in creating a proallergenic immune environment, yet C5a may also protect from maladaptive Th2 immunity during allergen sensitization (68). An important yet complex role in asthma has also been attributed to C5L2 (69). Whereas previous therapeutic attempts focused on C5aR, the scope has recently been expanded to include inhibitors at the levels of C5 and C3 (68). Relatedly, C5a has also been implicated in the exacerbation of chronic obstructive pulmonary disease (70). Though an involvement of complement in periodontal disease was proposed before (71), the intricate mechanisms behind the pathogenesis of periodontitis have only recently been revealed. In this biofilm-driven chronic inflammatory disease that leads to progressive bone loss of the teeth, an intense dialogue between complement effectors and receptors (C5aR, CR3), the TLR system (TLR4, CD14) and the oral microbiome with its keystone pathogen Porphyromonas gingivalis shapes the disorder (72). As C5a signaling is at the center of immune evasion and inflammatory activities, C5aR-directed therapies have been evaluated and shown encouraging results (73, 74). Finally, complement-mediated processes have been recognized critical for bone-related disorders and injury (e.g., via anaphylatoxin effects on osteoclast formation), thereby suggesting another potential indication area for complement therapeutics (75).
Perhaps the most severe effects of complement activation are seen in acute-phase conditions, often associated with SIRS (see above), in which the host is confronted with a dramatic increase of damage- and/or pathogen-associated molecular patterns (76). In trauma, for example, the initial traumatic impact combined with posttraumatic IRI can trigger a devastating cascade of immuno-inflammatory reactions with complement contribution, which may sustain SIRS (77). As a complication of trauma, or as an independent incident, massive infection may overwhelm the protective functions of complement and other innate immunity components (e.g., TLR) and provoke sepsis (78). The early pathogen-induced hyperinflammatory response with complement and immune cell activation, a cytokine storm and coagulopathy may result in SIRS and persist even after the pathogen is cleared; C5a-dependent signaling seems to be a major player in those devastating events. Independent of the trigger, SIRS can induce secondary tissue damage, multi-organ failure and, ultimately, death (76–78). Despite the prevalence and severity of sepsis, treatment of this condition has proven to be difficult, though complement therapies at the level of initiation (e.g., C1-INH), amplification (targeting C3) or signaling (blocking the C5a-C5aR axis) have shown promising results.
While complement plays a dual role in many diseases, the dilemma between beneficial and adverse effects is especially pronounced in cancer (79). On the one hand, complement may recognize altered surface pattern and attack cancer cells. Complement can also be therapeutically engaged for the killing of tumor cells via complement-dependent cytotoxicity (CDC); for example, antibodies directed against the surface antigen CD20 that is statically expressed on mature and malignant B cells, induce a CP-mediated complement attack and trigger FcγR activation and other mechanisms that lead to cell death, thereby making them valuable tools for the therapy of lymphoma or certain autoimmune disorders when applied in a well-adjusted dose regimen (80, 81). On the other hand, tumor cells may increase the expression of complement regulators as evasion mechanism, and strategies to increase the efficiency of CDC via regulator-specific inhibitors or knock down via siRNA have been investigated (82). Importantly, though, complement appears to be more intricately involved in tumor progression than originally anticipated, with several studies demonstrating that complement activation and release of C5a may actually create a more favorable environment for tumor growth by shaping immune cell populations and/or angiogenesis in certain cancer models (32, 83, 84).
Conclusions
The progression in GWAS, the availability of improved disease models, and the unprecedented insight into molecular details of humoral and cellular immunology have changed our perception of the role of complement in health and disease. While complement-mediated pathologies so far have often been looked at in an isolated manner, common pattern within a wide spectrum of disease forms begin to emerge. In this context, the importance of the intense crosstalk between complement and other physiological systems and the interplay between complement, infection, immunity, and inflammation has become particularly evident. In view of the upstream and mediating position of complement in inflammatory events, it is expected that the list of diseases with association to imbalanced complement will continue to grow in the years to come. Unquestionably, complement may not be the main driving force in some of these disorders, yet may still be a critical factor that can tip the balance between induction and resolution of inflammation. Even in knowledge of strong complement involvement and with identified risk genes, the translation into disease mechanisms or even therapeutic strategies may remain challenging, as the case of AMD has shown. Profound investigation of involved triggers and complement pathways in each disease, and a holistic interpretation in the context of inflammation and immunity will be required to rapidly achieve clinical benefit. Fortunately, an impressive body of research in recent years has created a broad arsenal of complement inhibitors that can be used to dissect molecular pathways but may also pave the way to complement-targeted immunomodulatory therapies (see accompanying review (6)).
Acknowledgments
We thank Dr. Robert A. DeAngelis and Dr. Edimara Reis for critically reading the manuscript and for their valuable discussion.
This work was supported by National Institutes of Health grants AI003040, AI068730, AI072106, AI097805, EY020633, GM097747 and DE021685.
Abbreviations used in this article
- AD
Alzheimer’s disease
- aHUS
atypical hemolytic uremic syndrome
- AMD
age-related macular degeneration
- ANCA
anti-neutrophil cytoplasmic antibodies
- AP
alternative pathway
- APC
antigen-presenting cells
- APS
antiphospholipid antibody syndrome
- C3aR
C3a receptor
- C4BP
C4b-binding protein
- C5aR
C5a receptor
- CAD
cold agglutinin disease
- CPB
cardiopulmonary bypass
- CDC
complement-dependent cytotoxicity
- CP
classical pathway
- CR
complement receptor
- CRIg
complement receptor of the immunoglobulin superfamily
- DDD
dense deposit disease
- FB
factor B
- FD
factor D
- FH
factor H
- FI
factor I
- FP
properdin
- GPI
glycosylphosphatidylinositol
- IC
immune complex
- IRI
ischemia/reperfusion injury
- LP
lectin pathway
- MAC
membrane attack complex
- MASP
MBL-associated serine proteases
- MBL
mannose-binding lectin
- PNH
paroxysmal nocturnal hemoglobinuria
- PRM
pattern-recognition molecule
- PTX
pentraxin
- RA
rheumatoid arthritis
- RCA
regulator of complement activation
- SIRS
systemic inflammatory response syndrome
- SLE
systemic lupus erythematosus
- STEC
shiga toxin-producing E. coli
- TLR
Toll-like receptor
Footnotes
Disclosures
D.R. and J.D.L. are the inventors of patents and/or patent applications that describe the use of complement inhibitors for therapeutic purposes. J.D.L. is the founder of Amyndas Biotherapeutics and Amyndas Pharmaceuticals, which are developing complement inhibitors for clinical applications.
References
- 1.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leslie M. Immunology. The new view of complement. Science. 2012;337:1034–1037. doi: 10.1126/science.337.6098.1034. [DOI] [PubMed] [Google Scholar]
- 3.de Cordoba SR, Tortajada A, Harris CL, Morgan BP. Complement dysregulation and disease: from genes and proteins to diagnostics and drugs. Immunobiology. 2012;217:1034–1046. doi: 10.1016/j.imbio.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Ricklin D, Lambris JD. Progress and trends in complement therapeutics. Adv Exp Med Biol. 2013;734:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013 doi: 10.4049/jimmunol.1203200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson B, Nilsson Ekdahl K. The tick-over theory revisited: is C3 a contact-activated protein? Immunobiology. 2012;217:1106–1110. doi: 10.1016/j.imbio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Forneris F, Ricklin D, Wu J, Tzekou A, Wallace RS, Lambris JD, Gros P. Structures of C3b in complex with factors B and D give insight into complement convertase formation. Science. 2010;330:1816–1820. doi: 10.1126/science.1195821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hourcade D, Liszewski MK, Krych-Goldberg M, Atkinson JP. Functional domains, structural variations and pathogen interactions of MCP, DAF and CR1. Immunopharmacology. 2000;49:103–116. doi: 10.1016/s0162-3109(00)80296-9. [DOI] [PubMed] [Google Scholar]
- 10.Doni A, Garlanda C, Bottazzi B, Meri S, Garred P, Mantovani A. Interactions of the humoral pattern recognition molecule PTX3 with the complement system. Immunobiology. 2012;217:1122–1128. doi: 10.1016/j.imbio.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Kemper C, Atkinson JP, Hourcade DE. Properdin: emerging roles of a pattern-recognition molecule. Annu Rev Immunol. 2010;28:131–155. doi: 10.1146/annurev-immunol-030409-101250. [DOI] [PubMed] [Google Scholar]
- 12.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degn SE, Jensenius JC, Bjerre M. The lectin pathway and its implications in coagulation, infections and auto-immunity. Curr Opin Organ Transplant. 2011;16:21–27. doi: 10.1097/MOT.0b013e32834253df. [DOI] [PubMed] [Google Scholar]
- 14.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, Dudler T, Parent B, Lhotta K, Wallis R, Farrar CA, Sacks S, Lee H, Zhang M, Iwaki D, Takahashi M, Fujita T, Tedford CE, Stover CM. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi M, Ishida Y, Iwaki D, Kanno K, Suzuki T, Endo Y, Homma Y, Fujita T. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207:29–37. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 17.Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement--their role in inflammation. Semin Immunopathol. 2012;34:151–165. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor crosstalk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 20.Krarup A, Wallis R, Presanis JS, Gal P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2:e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo Y, Nakazawa N, Iwaki D, Takahashi M, Matsushita M, Fujita T. Interactions of ficolin and mannose-binding lectin with fibrinogen/fibrin augment the lectin complement pathway. J Innate Immun. 2010;2:33–42. doi: 10.1159/000227805. [DOI] [PubMed] [Google Scholar]
- 22.Hamad OA, Back J, Nilsson PH, Nilsson B, Ekdahl KN. Platelets, complement, and contact activation: partners in inflammation and thrombosis. Adv Exp Med Biol. 2012;946:185–205. doi: 10.1007/978-1-4614-0106-3_11. [DOI] [PubMed] [Google Scholar]
- 23.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–224. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, Hayday A, Kemper C. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han G, Geng S, Li Y, Chen G, Wang R, Li X, Ma Y, Shen B. gammadeltaT-cell function in sepsis is modulated by C5a receptor signalling. Immunology. 2011;133:340–349. doi: 10.1111/j.1365-2567.2011.03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, Gessner JE. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, Winkler A, Hess C, Reid DM, Majoul IV, Strait RT, Harris NL, Kohl G, Wex E, Ludwig R, Zillikens D, Nimmerjahn F, Finkelman FD, Brown GD, Ehlers M, Kohl J. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18:1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricklin D, Reis ES, Lambris JD. A sweet spot to control complement-induced inflammation. Nat Med. 2012;18:1340–1341. doi: 10.1038/nm.2916. [DOI] [PubMed] [Google Scholar]
- 31.Fusakio ME, Mohammed JP, Laumonnier Y, Hoebe K, Kohl J, Mattner J. C5a regulates NKT and NK cell functions in sepsis. J Immunol. 2011;187:5805–5812. doi: 10.4049/jimmunol.1100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett. 2010;128:36–45. doi: 10.1016/j.imlet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karagianni N, Adamis AP. The case for complement and inflammation in AMD: open questions. Adv Exp Med Biol. 2010;703:1–7. doi: 10.1007/978-1-4419-5635-4_1. [DOI] [PubMed] [Google Scholar]
- 36.Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert JC, Amouyel P. Genetics of Alzheimer’s disease: new evidences for an old hypothesis? Curr Opin Genet Dev. 2011;21:295–301. doi: 10.1016/j.gde.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonseca MI, Chu SH, Berci AM, Benoit ME, Peters DG, Kimura Y, Tenner AJ. Contribution of complement activation pathways to neuropathology differs among mouse models of Alzheimer’s disease. J Neuroinflammation. 2011;8:4. doi: 10.1186/1742-2094-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, Taylor SM, Woodruff TM, Tenner AJ. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J Immunol. 2009;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickering M, Cook HT. Complement and glomerular disease: new insights. Curr Opin Nephrol Hypertens. 2011;20:271–277. doi: 10.1097/MNH.0b013e328345848b. [DOI] [PubMed] [Google Scholar]
- 42.Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 44.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Fremeaux-Bacchi V. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–657. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 45.Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276–286. doi: 10.1016/j.jaut.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 47.Karsten CM, Kohl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology. 2012;217:1067–1079. doi: 10.1016/j.imbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Chen M, Kallenberg CG. ANCA-associated vasculitides--advances in pathogenesis and treatment. Nat Rev Rheumatol. 2010;6:653–664. doi: 10.1038/nrrheum.2010.158. [DOI] [PubMed] [Google Scholar]
- 49.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denny KJ, Woodruff TM, Taylor SM, Callaway LK. Complement in pregnancy: a delicate balance. Am J Reprod Immunol. 2013;69:3–11. doi: 10.1111/aji.12000. [DOI] [PubMed] [Google Scholar]
- 51.Singh J, Ahmed A, Girardi G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension. 2011;58:716–724. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- 52.Qing X, Redecha PB, Burmeister MA, Tomlinson S, D’Agati VD, Davisson RL, Salmon JE. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011;79:331–339. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- 53.Lappas M, Woodruff TM, Taylor SM, Permezel M. Complement C5A regulates prolabor mediators in human placenta. Biol Reprod. 2012;86:190. doi: 10.1095/biolreprod.111.098475. [DOI] [PubMed] [Google Scholar]
- 54.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, Lambris JD, Holers VM, Salmon JE. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Risitano AM. Paroxysmal nocturnal hemoglobinuria and other complement-mediated hematological disorders. Immunobiology. 2012;217:1080–1087. doi: 10.1016/j.imbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 56.Banz Y, Rieben R. Role of complement and perspectives for intervention in ischemia-reperfusion damage. Ann Med. 2012;44:205–217. doi: 10.3109/07853890.2010.535556. [DOI] [PubMed] [Google Scholar]
- 57.Gorsuch WB, Chrysanthou E, Schwaeble WJ, Stahl GL. The complement system in ischemia-reperfusion injuries. Immunobiology. 2012;217:1026–1033. doi: 10.1016/j.imbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant. 2011;16:620–626. doi: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- 59.Rother RP, Arp J, Jiang J, Ge W, Faas SJ, Liu W, Gies DR, Jevnikar AM, Garcia B, Wang H. C5 blockade with conventional immunosuppression induces long-term graft survival in presensitized recipients. Am J Transplant. 2008;8:1129–1142. doi: 10.1111/j.1600-6143.2008.02222.x. [DOI] [PubMed] [Google Scholar]
- 60.Chen Song S, Zhong S, Xiang Y, Li JH, Guo H, Wang WY, Xiong YL, Li XC, Chen Shi S, Chen XP, Chen G. Complement inhibition enables renal allograft accommodation and long-term engraftment in presensitized nonhuman primates. Am J Transplant. 2011;11:2057–2066. doi: 10.1111/j.1600-6143.2011.03646.x. [DOI] [PubMed] [Google Scholar]
- 61.Ekdahl KN, Lambris JD, Elwing H, Ricklin D, Nilsson PH, Teramura Y, Nicholls IA, Nilsson B. Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Adv Drug Deliv Rev. 2011;63:1042–1050. doi: 10.1016/j.addr.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stahl GL, Shernan SK, Smith PK, Levy JH. Complement activation and cardiac surgery: a novel target for improving outcomes. Anesth Analg. 2012;115:759–771. doi: 10.1213/ANE.0b013e3182652b7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lazar HL, Keilani T, Fitzgerald CA, Shapira OM, Hunter CT, Shemin RJ, Marsh HC, Jr, Ryan US T. P. C. S. S. Group. Beneficial effects of complement inhibition with soluble complement receptor 1 (TP10) during cardiac surgery: is there a gender difference? Circulation. 2007;116:I83–88. doi: 10.1161/CIRCULATIONAHA.106.677914. [DOI] [PubMed] [Google Scholar]
- 64.Smith PK, Shernan SK, Chen JC, Carrier M, Verrier ED, Adams PX, Todaro TG, Muhlbaier LH, Levy JH PRIMO–CABG II Investigators. Effects of C5 complement inhibitor pexelizumab on outcome in high-risk coronary artery bypass grafting: combined results from the PRIMO-CABG I and II trials. J Thorac Cardiovasc Surg. 2011;142:89–98. doi: 10.1016/j.jtcvs.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 65.DeAngelis RA, Reis ES, Ricklin D, Lambris JD. Targeted complement inhibition as a promising strategy for preventing inflammatory complications in hemodialysis. Immunobiology. 2012;217:1097–1105. doi: 10.1016/j.imbio.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kourtzelis I, Markiewski MM, Doumas M, Rafail S, Kambas K, Mitroulis I, Panagoutsos S, Passadakis P, Vargemezis V, Magotti P, Qu H, Mollnes TE, Ritis K, Lambris JD. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu YQ, Qu H, Sfyroera G, Tzekou A, Kay BK, Nilsson B, Nilsson Ekdahl K, Ricklin D, Lambris JD. Protection of nonself surfaces from complement attack by factor H-binding peptides: implications for therapeutic medicine. J Immunol. 2011;186:4269–4277. doi: 10.4049/jimmunol.1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Kohl J. A complex role for complement in allergic asthma. Expert Rev Clin Immunol. 2010;6:269–277. doi: 10.1586/eci.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J. A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J Immunol. 2010;185:6741–6752. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]
- 70.Marc MM, Kristan SS, Rozman A, Kern I, Flezar M, Kosnik M, Korosec P. Complement factor C5a in acute exacerbation of Chronic Obstructive Pulmonary Disease. Scand J Immunol. 2010;71:386–391. doi: 10.1111/j.1365-3083.2010.02385.x. [DOI] [PubMed] [Google Scholar]
- 71.Allison AC, Schorlemmer HU. Activation of complement by the alternative pathway as a factor in the pathogenesis of periodontal disease. Lancet. 1976;2:1001–1004. doi: 10.1016/s0140-6736(76)90837-0. [DOI] [PubMed] [Google Scholar]
- 72.Hajishengallis G, Lambris JD. Complement and dysbiosis in periodontal disease. Immunobiology. 2012;217:1111–1116. doi: 10.1016/j.imbio.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abe T, Hosur KB, Hajishengallis E, Reis ES, Ricklin D, Lambris JD, Hajishengallis G. Local Complement-Targeted Intervention in Periodontitis: Proof-of-Concept Using a C5a Receptor (CD88) Antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breivik T, Gundersen Y, Gjermo P, Taylor SM, Woodruff TM, Opstad PK. Oral treatment with complement factor C5a receptor (CD88) antagonists inhibits experimental periodontitis in rats. J Periodontal Res. 2011;46:643–647. doi: 10.1111/j.1600-0765.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 75.Schoengraf P, Lambris JD, Recknagel S, Kreja L, Liedert A, Brenner RE, Huber-Lang M, Ignatius A. Does complement play a role in bone development and regeneration? Immunobiology. 2013;218:1–9. doi: 10.1016/j.imbio.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 76.Barratt-Due A, Pischke SE, Brekke OL, Thorgersen EB, Nielsen EW, Espevik T, Huber-Lang M, Mollnes TE. Bride and groom in systemic inflammation--the bells ring for complement and Toll in cooperation. Immunobiology. 2012;217:1047–1056. doi: 10.1016/j.imbio.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 77.Neher MD, Weckbach S, Flierl MA, Huber-Lang MS, Stahel PF. Molecular mechanisms of inflammation and tissue injury after major trauma--is complement the “bad guy”? J Biomed Sci. 2011;18:90. doi: 10.1186/1423-0127-18-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2012 doi: 10.1016/j.it.2012.09.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Markiewski MM, Lambris JD. Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends Immunol. 2009;30:286–292. doi: 10.1016/j.it.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 81.Beurskens FJ, Lindorfer MA, Farooqui M, Beum PV, Engelberts P, Mackus WJ, Parren PW, Wiestner A, Taylor RP. Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients. J Immunol. 2012;188:3532–3541. doi: 10.4049/jimmunol.1103693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kolev M, Towner L, Donev R. Complement in cancer and cancer immunotherapy. Arch Immunol Ther Exp (Warsz) 2011;59:407–419. doi: 10.1007/s00005-011-0146-x. [DOI] [PubMed] [Google Scholar]
- 83.Corrales L, Ajona D, Rafail S, Lasarte JJ, Riezu-Boj JI, Lambris JD, Rouzaut A, Pajares MJ, Montuenga LM, Pio R. Anaphylatoxin c5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189:4674–4683. doi: 10.4049/jimmunol.1201654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nunez-Cruz S, Gimotty PA, Guerra MW, Connolly DC, Wu YQ, DeAngelis RA, Lambris JD, Coukos G, Scholler N. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 2012;14:994–1004. doi: 10.1593/neo.121262. [DOI] [PMC free article] [PubMed] [Google Scholar]