Abstract
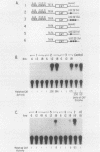
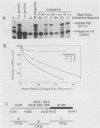
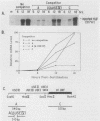
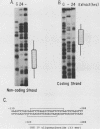
Normal development requires that individual genes be expressed in their correct temporal patterns, but the mechanisms regulating this process during early embryogenesis are poorly understood. We have studied the early and late sea urchin histone genes during embryogenesis to address the molecular mechanisms controlling temporal gene expression. By measuring the changes in expression of cloned H1-beta DNA constructs after microinjection into fertilized one-cell zygotes, we demonstrated that a highly conserved 30-base-pair segment of DNA between positions -288 and -317 (USE IV) is responsible for the transcriptional activation of this late histone gene at the late blastula stage. In this report, we demonstrate that an oligonucleotide corresponding to USE IV acts as an embryonic enhancer element capable of activating the simian virus 40 early promoter in a stage-specific manner. Using an in vivo competition assay and in vitro DNase I footprinting and mobility shift assays, we also identified a protein(s) that interacts with this enhancer. Results of the competition assay suggested that this factor acts to stimulate transcription of the H1-beta gene. The factor was found to be stored in mature eggs as well as in all embryonic stages examined. The mobility of the factor found in eggs, however, differed from that of the embryonic form, which suggested that posttranslational modification occurs after fertilization.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Thézé N., Thiebaud P., Hill R. L., Britten R. J., Davidson E. H. Developmental appearance of factors that bind specifically to cis-regulatory sequences of a gene expressed in the sea urchin embryo. Genes Dev. 1988 Sep;2(9):1074–1088. doi: 10.1101/gad.2.9.1074. [DOI] [PubMed] [Google Scholar]
- Carey M. F., Gerrard S. P., Cozzarelli N. R. Analysis of RNA polymerase III transcription complexes by gel filtration. J Biol Chem. 1986 Mar 25;261(9):4309–4317. [PubMed] [Google Scholar]
- Childs G., Maxson R., Kedes L. H. Histone gene expression during sea urchin embryogenesis: isolation and characterization of early and late messenger RNAs of Strongylocentrotus purpuratus by gene-specific hybridization and template activity. Dev Biol. 1979 Nov;73(1):153–173. doi: 10.1016/0012-1606(79)90144-1. [DOI] [PubMed] [Google Scholar]
- Childs G., Nocente-McGrath C., Lieber T., Holt C., Knowles J. A. Sea urchin (lytechinus pictus) late-stage histone H3 and H4 genes: characterization and mapping of a clustered but nontandemly linked multigene family. Cell. 1982 Dec;31(2 Pt 1):383–393. doi: 10.1016/0092-8674(82)90132-5. [DOI] [PubMed] [Google Scholar]
- Colin A. M., Catlin T. L., Kidson S. H., Maxson R. Closely linked early and late histone H2B genes are differentially expressed after microinjection into sea urchin zygotes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):507–510. doi: 10.1073/pnas.85.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin A. M. Rapid repetitive microinjection. Methods Cell Biol. 1986;27:395–406. [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Flytzanis C. N., Lee J. J., Robinson J. J., Rose S. J., 3rd, Sucov H. M. Lineage-specific gene expression in the sea urchin embryo. Cold Spring Harb Symp Quant Biol. 1985;50:321–328. doi: 10.1101/sqb.1985.050.01.041. [DOI] [PubMed] [Google Scholar]
- Flytzanis C. N., Brandhorst B. P., Britten R. J., Davidson E. H. Developmental patterns of cytoplasmic transcript prevalence in sea urchin embryos. Dev Biol. 1982 May;91(1):27–35. doi: 10.1016/0012-1606(82)90004-5. [DOI] [PubMed] [Google Scholar]
- Flytzanis C. N., Britten R. J., Davidson E. H. Ontogenic activation of a fusion gene introduced into sea urchin eggs. Proc Natl Acad Sci U S A. 1987 Jan;84(1):151–155. doi: 10.1073/pnas.84.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flytzanis C. N., McMahon A. P., Hough-Evans B. R., Katula K. S., Britten R. J., Davidson E. H. Persistence and integration of cloned DNA in postembryonic sea urchins. Dev Biol. 1985 Apr;108(2):431–442. doi: 10.1016/0012-1606(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein U., Tjian R. Temporal pattern of alcohol dehydrogenase gene transcription reproduced by Drosophila stage-specific embryonic extracts. Nature. 1988 Feb 4;331(6155):410–415. doi: 10.1038/331410a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Hendricks M. B., Hemminki K., Weinberg E. S. Histone gene switch in the sea urchin embryo. Identification of late embryonic histone messenger ribonucleic acids and the control of their synthesis. Biochemistry. 1979 Jun 26;18(13):2707–2716. doi: 10.1021/bi00580a004. [DOI] [PubMed] [Google Scholar]
- Hough-Evans B. R., Franks R. R., Cameron R. A., Britten R. J., Davidson E. H. Correct cell-type-specific expression of a fusion gene injected into sea urchin eggs. Dev Biol. 1987 Jun;121(2):576–579. doi: 10.1016/0012-1606(87)90193-x. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Hart R. P., Nevins J. R. An enhancer-like element in the adenovirus E2 promoter contains sequences essential for uninduced and E1A-induced transcription. Proc Natl Acad Sci U S A. 1985 Jan;82(2):381–385. doi: 10.1073/pnas.82.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Bell J., Lyons G., Maxson R. Synthesis and turnover of late H2B histone mRNA in developing embryos of the sea urchin, Strongylocentrotus purpuratus. Dev Biol. 1988 Sep;129(1):147–158. doi: 10.1016/0012-1606(88)90169-8. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Katula K. S., Hough-Evans B. R., Britten R. J., Davidson E. H. Ontogenic expression of a CyI actin fusion gene injected into sea urchin eggs. Development. 1987 Nov;101(3):437–447. doi: 10.1242/dev.101.3.437. [DOI] [PubMed] [Google Scholar]
- Kemler I., Busslinger M. Characterization of two nonallelic pairs of late histone H2A and H2B genes of the sea urchin: differential regulation in the embryo and tissue-specific expression in the adult. Mol Cell Biol. 1986 Nov;6(11):3746–3754. doi: 10.1128/mcb.6.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Knowles J. A., Childs G. J. Comparison of the late H1 histone genes of the sea urchins Lytechinus pictus and Strongelocentrotus purpuratus. Nucleic Acids Res. 1986 Oct 24;14(20):8121–8133. doi: 10.1093/nar/14.20.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. A., Childs G. J. Temporal expression of late histone messenger RNA in the sea urchin Lytechinus pictus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2411–2415. doi: 10.1073/pnas.81.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. A., Lai Z. C., Childs G. J. Isolation, characterization, and expression of the gene encoding the late histone subtype H1-gamma of the sea urchin Strongylocentrotus purpuratus. Mol Cell Biol. 1987 Jan;7(1):478–485. doi: 10.1128/mcb.7.1.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. An enhancer responsible for activating transcription at the midblastula transition in Xenopus development. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2331–2335. doi: 10.1073/pnas.84.8.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z. C., Childs G. Characterization of the structure and transcriptional patterns of the gene encoding the late histone subtype H1-beta of the sea urchin Strongylocentrotus purpuratus. Mol Cell Biol. 1988 Apr;8(4):1842–1844. doi: 10.1128/mcb.8.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z. C., Maxson R., Childs G. Both basal and ontogenic promoter elements affect the timing and level of expression of a sea urchin H1 gene during early embryogenesis. Genes Dev. 1988 Feb;2(2):173–183. doi: 10.1101/gad.2.2.173. [DOI] [PubMed] [Google Scholar]
- Lieber T., Weisser K., Childs G. Analysis of histone gene expression in adult tissues of the sea urchins Strongylocentrotus purpuratus and Lytechinus pictus: tissue-specific expression of sperm histone genes. Mol Cell Biol. 1986 Jul;6(7):2602–2612. doi: 10.1128/mcb.6.7.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livant D. L., Cutting A. E., Britten R. J., Davidson E. H. An in vivo titration of regulatory factors required for expression of a fusion gene in transgenic sea urchin embryos. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7607–7611. doi: 10.1073/pnas.85.20.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T. Expression and organization of histone genes. Annu Rev Genet. 1983;17:239–277. doi: 10.1146/annurev.ge.17.120183.001323. [DOI] [PubMed] [Google Scholar]
- Maxson R., Mohun T., Gormezano G., Childs G., Kedes L. Distinct organizations and patterns of expression of early and late histone gene sets in the sea urchin. Nature. 1983 Jan 13;301(5896):120–125. doi: 10.1038/301120a0. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Flytzanis C. N., Hough-Evans B. R., Katula K. S., Britten R. J., Davidson E. H. Introduction of cloned DNA into sea urchin egg cytoplasm: replication and persistence during embryogenesis. Dev Biol. 1985 Apr;108(2):420–430. doi: 10.1016/0012-1606(85)90045-4. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Novak T. J., Britten R. J., Davidson E. H. Inducible expression of a cloned heat shock fusion gene in sea urchin embryos. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7490–7494. doi: 10.1073/pnas.81.23.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci F., Stein E. M. Oscillatory singular integrals and harmonic analysis on nilpotent groups. Proc Natl Acad Sci U S A. 1986 Jan;83(1):1–3. doi: 10.1073/pnas.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner G., Schirm S., Müller-Baden B., Weber F., Schaffner W. Redundancy of information in enhancers as a principle of mammalian transcription control. J Mol Biol. 1988 May 5;201(1):81–90. doi: 10.1016/0022-2836(88)90440-8. [DOI] [PubMed] [Google Scholar]
- Schirm S., Jiricny J., Schaffner W. The SV40 enhancer can be dissected into multiple segments, each with a different cell type specificity. Genes Dev. 1987 Mar;1(1):65–74. doi: 10.1101/gad.1.1.65. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Vitelli L., Kemler I., Lauber B., Birnstiel M. L., Busslinger M. Developmental regulation of micro-injected histone genes in sea urchin embryos. Dev Biol. 1988 May;127(1):54–63. doi: 10.1016/0012-1606(88)90188-1. [DOI] [PubMed] [Google Scholar]
- Wilson C., Cross G. S., Woodland H. R. Tissue-specific expression of actin genes injected into Xenopus embryos. Cell. 1986 Nov 21;47(4):589–599. doi: 10.1016/0092-8674(86)90623-9. [DOI] [PubMed] [Google Scholar]
- Wirth T., Staudt L., Baltimore D. An octamer oligonucleotide upstream of a TATA motif is sufficient for lymphoid-specific promoter activity. Nature. 1987 Sep 10;329(6135):174–178. doi: 10.1038/329174a0. [DOI] [PubMed] [Google Scholar]