Abstract
Background and Purpose
Bimodal dose–response relationships have been demonstrated in animals and humans following morphine administration. We examined if systemic administration of morphine, in extremely low (μg) and high (mg, analgesic) doses, changed the learning process.
Experimental Approach
In the social learning test, an adult rat investigates a juvenile. The juvenile is submitted to a second encounter after a few days and investigation by the adult should be reduced. Morphine was administered before the first encounter between rats, and the critical test was performed 24, 72 or 168 h later, when animals were re-exposed to each other, in the absence of morphine.
Key Results
Low doses of morphine, comparable with endogenous brain concentrations, enhanced long-term memory recognition; while high doses did the reverse, indicating the adult failed to recognize the juvenile. Recognition of a familiar rat appeared to be mediated within the brain accessory olfactory bulb (AOB) by an opioid system intrinsic to the olfactory system through μ-opioid receptors (MORs). At this supraspinal site, the PLC/PKC signalling pathway was activated by extremely low morphine doses.
Conclusions and Implications
Morphine treatment administration may either disrupt or facilitate social memory, depending on the dose, extending to memory formation the bimodal effects of morphine previously shown in pain. Social memory formation elicited by extremely low morphine doses, was mediated within the AOB by an opioid system, intrinsic to the olfactory system through MORs.
Keywords: memory, social learning, morphine, behavioural animal model, opioid receptor
Introduction
Biphasic dose–response relationships have been demonstrated in animals and humans following administration of opioids (Jacquet and Lajtha, 1973). Beyond the analgesic effect exerted by morphine at analgesic doses, acute thermal hyperalgesia has been demonstrated by different authors after morphine administration to rodents at extremely low doses (Kayser et al., 1987; Crain and Shen, 2001). Memory and the process of learning in mammals are affected by opioid agonists, which interfere with these processes and produce amnesia. Particularly, systemic administration of exogenous morphine impaired memory processes in mice and rats submitted to the passive avoidance test (Castellano, 1975). Several studies have reported facilitation of memory retention by opiate antagonists, primarily naloxone, in normal animals in a variety of tasks, such as passive avoidance (Izquierdo, 1980).
Different levels of ability to distinguish and recognize individuals exist in a wide range of species and have been shown essential for establishing social relationships as including membership in a group, dominance hierarchies, territorial networks and numerous other aspects of social behaviour association (Brennan and Keverne, 1997). Thus, social recognition reflects the ability of one animal to learn and remember the identity of another and may be considered as an important component of survival in animal groups. Animals recognize each other on the basis of multimodal sensory characteristics, conjunctively encoded (Thor and Holloway, 1982; Gao et al., 2009). Under laboratory conditions, social learning and memory in animals can be studied by exposing rodents to each other for an initial meeting then testing their recognition as a function of time. During the first investigation of the juvenile rat, the adult rat is using olfactory investigation to collect and store information regarding the novel rat. The olfactory investigation time will be reduced during a second exposure to the juvenile rat. Namely, a social memory was stored during the initial encounter and retrieved during the second encounter. In this study, we examined the effects of morphine administration in rats on social memory formation, over a range of doses and times. We particularly investigated if the direction of change in social memory capacity in response to morphine administration could reflect either enhancement or impairment, as related to morphine dose. Several publications demonstrate the role played by different brain regions on morphine induced memory impairment. Therefore, in these experiments, we sought to investigate a central site of action of systemically administered morphine on social memory enhancement.
Methods
Animals
All animal care and experimental procedures complied with the European community guidelines for animal care (DL 116/92, Application of the European Communities Council Directive of 24 November 1986, 86/609/EEC) and the ethical policy of Florence University, which follows the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH publication no.85-23, revised 1996; University of Florence assurance number: A5278-01). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 340 animals were used in these experiments. Adult (12–15 weeks) and juvenile (5–6 weeks) male rats were purchased from Morini (Morini, Italy). Animals were housed two to three per cage in clear plastic enclosures (30 × 45 × 19), were given food and water ad libitum and were maintained under a reversed light cycle (10:00 h light off, 22:00 h light on). The experiments were always conducted between 10:00 and 18:00 h during the nocturnal portion of the reversed light cycle. Juvenile rats were used as the stimulus animals to minimize aggression. New sets of animals were used for each experiment; no rat was tested at more than one evaluation time or drug dose.
Social memory assessment
Social memory test is based on the propensity of an adult rodent to inspect an unknown juvenile rodent. In this paradigm (Dantzer et al., 1987), two rats (one adult and one juvenile) are exposed to each other for a short time (2 min), and the time that the adult explores the juvenile is measured. Then rats are re-exposed 24, 72 or 168 h after (second exposure). Re-exposing the rat also to an unfamiliar juvenile was used as control. The decrement in investigation time between the first and second exposure is used as an indication of social memory. On the day of the first exposure, group-housed adult and juvenile rats were brought to the observation room to acclimate to the environment. An adult rat was then placed into an empty plastic cage under weak light and allowed to habituate to the test environment for 15 min. Following the 15 min acclimation period, a juvenile rat was placed into the cage with the adult rat for 2 min. The duration of the social investigatory behaviour within the 2 min period was recorded on videotape under white illumination. The tapes were later scored by a highly trained observer, unaware of the treatments. The social investigatory behaviour that was quantified included direct contact or sniffing, following, nosing, grooming, pawing or generally inspecting any body surface of the novel juvenile rat within 1 cm2. Social interaction was quantified as the sum duration of sniffing, grooming, pawing or inspecting elicited by the adult rat towards the juvenile. Non-social exploration comprised walking and sniffing around the cage, self-grooming and rearing (upright posture). The re-exposure at a fixed interval between the same adult and juvenile pair was conducted exactly like the first exposure. A minimum initial investigation time of 24 s (Kogan et al., 2000) was required to qualify for an exposure test.
Surgery to deliver drugs to the accessory olfactory bulb (AOB)
Anaesthetized rats were stereotaxically implanted with 7 mm stainless steel guide cannula (26-gauge) into the AOB at position: A +6.2, L −1.5, V −4.5 according to the atlas of Paxinos and Watson (1998) with the bregma as zero. A dummy cannula (33-gauge stainless steel wire) was inserted into each guide cannula immediately after surgery to reduce the incidence of occlusion. Five to seven days after surgical recovery, solutions were injected into the AOB by microinjection unit, which extended 1.0 mm beyond the tips of the guide cannula. Each microinjection unit was attached to a 5 μL Hamilton microsyringe via polyethylene tubing, and administration was controlled by an infusion pump.
Olfaction test
Foraging test
Food was witheld from individually housed rats for 16–24 h before testing. Successively, small pieces of chocolate were made available to rats for 12 h. Chow was given to rats when chocolate was fully consumed. Two days later, food was again kept away for 16–24 h. Then the rat was transferred from his home cage to a holding cage. Then a small piece of chocolate was set on the bedding of the cage, and the rat came back to his home cage. The latency to detect the piece of chocolate was measured. The procedure was repeated three more times, locating small pieces of chocolate in different positions beneath 2–3 cm of levelled bedding (Mencio-Wszalek et al., 1992).
Olfactory habituation test
A small perforate tube was filled with cotton, added of a 10 μL drop of lemon natural extract and placed in the cage of each rat for 1 min. The procedure was repeated four times at 10 min intervals. In a 10 min later trial, 10 μL drops of vanilla natural extract were added to lemon in the tube, which was set in the rat cage. Olfactory investigation (nasal contact with the tube) was recorded (Paolini and McKenzie, 1993).
Histology
At the conclusion of the experiments, 1% Evans blue dye was administered to rats according to the microinjection procedure described above for intra-AOB administration. A postmortem histological control of the injection site was performed on cryostat sections of unfixed brains. If the cannula tip was outside the AOB or if the region had sustained extensive damage, the data of that rat were excluded from statistical analysis.
Data analysis
The effect on social memory retrieval of morphine at different doses was tested by a two-factor repeated-measures analysis using a mixed linear model. The considered factors are morphine dose (between group factor), trial (repeated factor, initial versus trial) or familiar versus unfamiliar juvenile (trial versus trial) and the interaction between these two factors. The same model was used to test the interval and trial effect on social memory with and without morphine administration except that, in this case, the considered factor are the exposure interval (the between group factor) and trial (initial versus trial) or familiar versus unfamiliar juvenile (trial versus trial). A three-factor repeated measures model was used in presence of morphine co-administration with different substances. Multiple comparisons were done via least square mean contrasts. Fisher's protected least significant difference procedure for post hoc comparison was used to verify significance between two means of behavioural results. Data were analysed with the StatView software for the Macintosh (Abacus Concept, 2011). A significance level (α) less than 0.01 or 0.05 was considered significant.
Materials
The following drugs were used: morphine HCl; the non-selective opioid receptor antagonist naloxone; the μ-opioid receptor (MOR) antagonist, d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP); the δ-opioid receptor (DOR) antagonist, 7-benzylidenenaltrexone (BNTX) and the κ- opioid receptor (KOR) antagonist, norbinaltorphimine (nor-BNI); PLC inhibitor U73122 and corresponding inactive analogue U73343; PKC inhibitor calphostin C (Sigma Chemicals, St Louis, MO, USA); PKA inhibitor H-89 (Calbiochem, San Diego, CA, USA). Drugs were administered in a volume of 3.0 μL per rat by intracranial injection, and 5 mL kg−1 by i.p. injection. All drugs were dissolved in isotonic (NaCl 0.9%) saline solution immediately before use. Morphine was administered i.p. 5 min before starting of the test (first exposure of juvenile to adult rat); naloxone, CTOP, BMTX, nor-BNI, U73122, U73343, calphostin C and H-89 were administered to the AOB immediately before morphine administration. Naloxone was administered both i.p. or intra-AOB. Receptor nomenclature follows Alexander et al., (2011).
Results
Effect of morphine on social recognition behaviour in rat
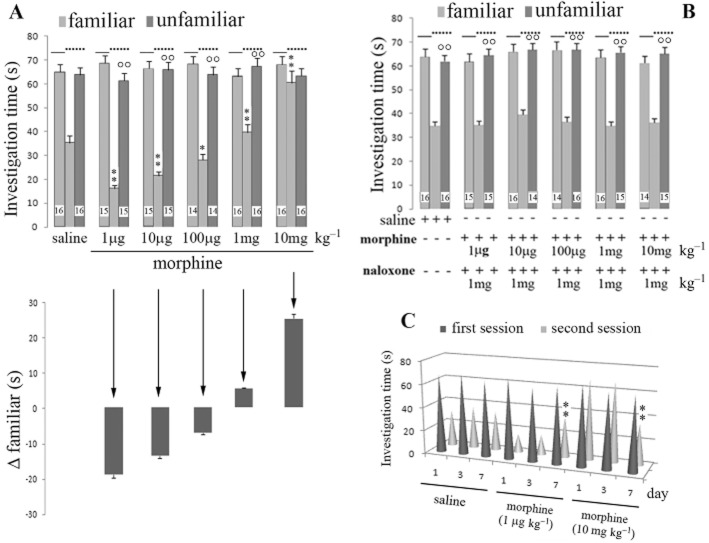
The investigation time, 24 h after the initial exposure of the adult to the juvenile rat, was measured after saline (control) and morphine injection over an range of doses (1 μg kg−1– 10 mg kg−1) given systemically (i.p.) 5 min before the initial exposure. Such treatment with morphine at extremely low doses (1 or 10 μg kg−1) 5 min before first exposure of juvenile to adult rat, induced a significant reduction in investigation time, 24 h after the initial exposure (Figure 1A). Higher morphine doses (1 or 10 mg kg−1) increased the time spent in exploration of the juvenile rat by the adult rat. At the highest dose (10 mg kg−1), the time spent by the adult rat in investigating a familiar juvenile rat was not significantly different from the time spent investigating an unfamiliar rat (Figure 1A). When morphine at the higher doses was co-administered with naloxone (1 mg kg−1, i.p.), social recognition times returned to control values (Figure 1B). Naloxone also prevented the reduction in investigation time when was co-administered with morphine at the lowest dose (1 μg kg−1). A dose–response curve to morphine in the rat social learning test, similar to that after 24 h (1 day), was obtained by re-exposing the adult to the juvenile rat after 72 h (3 days) (data not shown). Thus the change in investigation time of a familiar juvenile after morphine at 1 μg kg−1 or 10 mg kg−1 was the same after a re-exposure interval of 3 days as it was after a re-exposure interval of 1 day. However, after 7 days re-exposure interval, both the lower and higher morphine dose failed to alter the investigation time (Figure 1C).
Figure 1.
Dose–effect curve of morphine in the social learning test. (A) Investigation times of juveniles by adult rats is shown during the first exposure and on re-exposure after 24 h. Dat are shown for familiar and unfamiliar rats after morphine administration at different doses (1 μg–10 mg kg−1) 5 min before the first exposure to the social learning test. (B) The same experiment was repeated in the presence of naloxone (1 mg kg−1). C: Effect of different intervals (1, 3 or 7 days) between exposures on the investigation time versus familiar rat for systemic saline and morphine administration. Vertical bars represent SEM; **α < 0.01 or *α < 0.05, significantly different from saline treatment (A, B) or day interval investigation (C); °°α < 0.01, familiar versus unfamiliar juvenile (A, B). The numbers shown in the Figure represent the number of animals used in each experiment (A, B). At least 10 animals were used in panel C.
Effect on social memory of intra-AOB co-administration of opioid antagonist with systemic morphine
Rats were injected with systemic morphine (1 μg kg−1) and intra-AOB naloxone or CTOP (MOR) or BMTX (DOR) or nor-BNI (KOR) antagonists 5 min before the first session of the social learning test. BMTX and nor-BNI did not modify the effect on social memory acquisition, induced by morphine at the low dose (1 μg kg−1) (Figure 2). Co-treatment with CTOP given intra-AOB completely reversed the effect of low dose morphine, to control values (after saline) (Figure 2).
Figure 2.
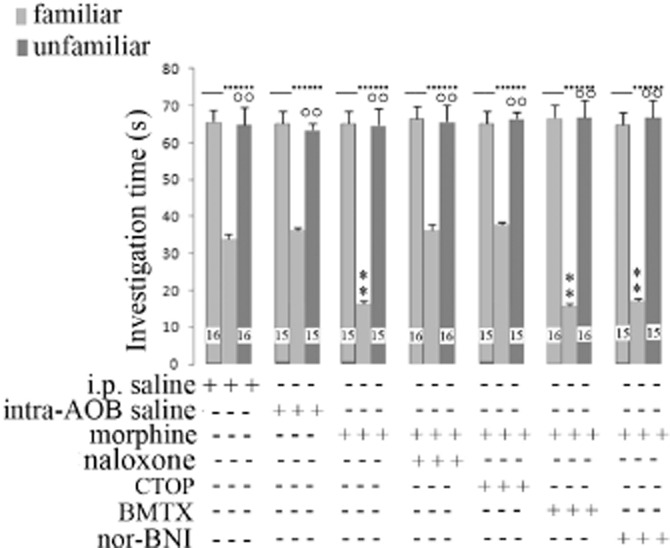
Social learning test in rats treated systemically with morphine and opioid antagonists given intra-AOB. Investigation times are shown for rats treated with 1 μg kg−1 morphine and the MOR antagonist CTOP (30 ng kg−1), the DOR antagonist BMTX (80 ng kg−1) or the KOR antagonist nor-BNI (10 ng kg−1) given intra-AOB, 5 min before starting the social learning test. During the second session, both the juvenile rat previously investigated and a completely unknown juvenile rat were exposed to investigation by the adult rat. Vertical bars represent SEM; **α < 0.01, significantly different from saline treatment or °°α < 0.01 familiar versus unfamiliar juvenile. The numbers shown in the Figure represent the number of animals used in each experiment.
Effect of co-administration of morphine with PLC, PKC and PKA inhibitors on morphine social learning test
The PLC inhibitor U73312 and the PKC inhibitor calphostin C were injected intra-AOB, concurrently with 1 μg kg−1 morphine i.p., 5 min before the first session of social learning test. These inhibitors completely reversed the effects of morphine and the investigation times after 24 h interval re-exposure returned to values observed after saline (Figure 3A,B). In contrast, co-administration of the PKA inhibitor H-89 intra-AOB did not affect the change in investigation time after 24 h induced by 1 μg kg−1 morphine i.p. (Figure 3C).
Figure 3.
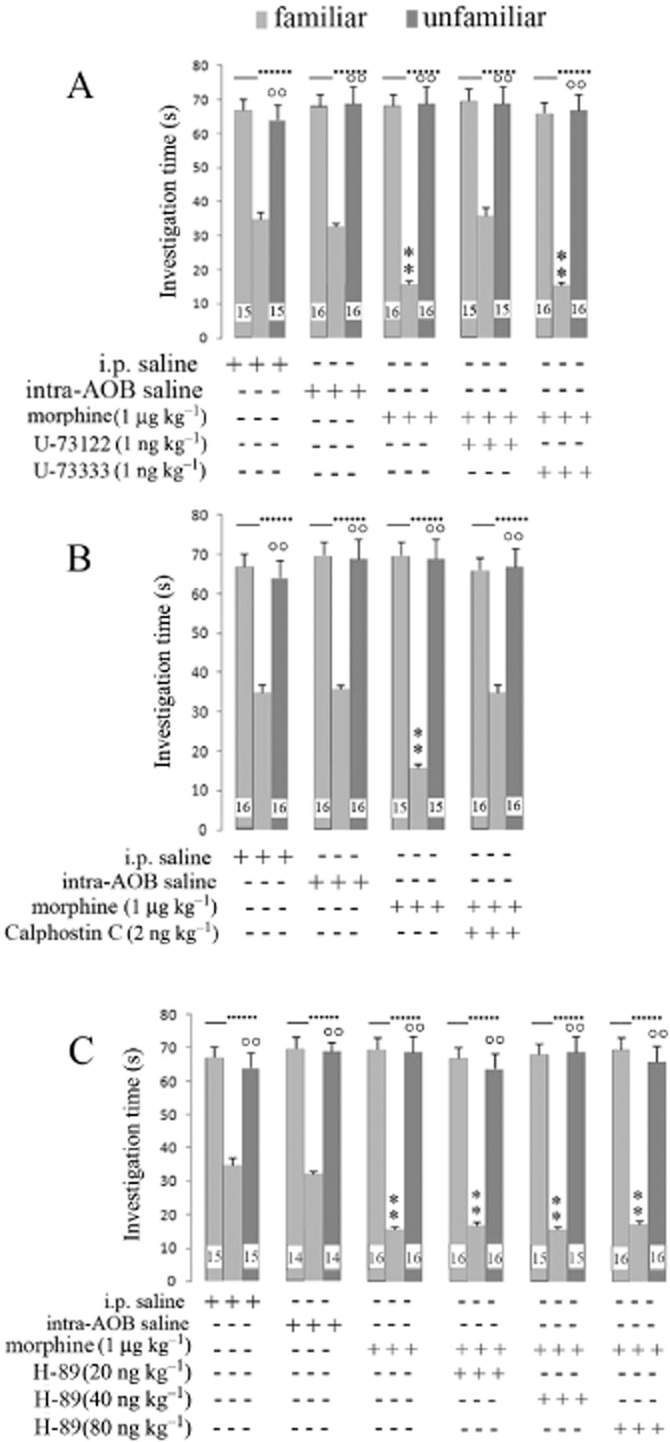
Social learning test in rats given morphine in presence of different signalling inhibitors. (A) Investigation times after systemic morphine (1 μg kg−1) together with intra-AOB injection of the PLC inhibitor U73122 or its inactive analogue U-73333. (B) Investigation times after systemic morphine (1 μg kg−1) with intra-AOB injection of calphostin C (PKC inhibitor). (C) Investigation times after systemic morphine (1 μg kg−1) with intra-AOB injection of the PKA inhibitor H-89. Vertical bars represent SEM; **α < 0.01, significantly different from saline treatment or °°α < 0.01 familiar versus unfamiliar juvenile. The numbers shown in the Figure represent the number of animals used in each experiment.
Effects of treatments alone on social learning test
When naloxone, CTOP, BMTX, nor-BNI, U73122, U73343, calphostin C and H-89 were administered alone at the highest doses used in our experiments following the same schedule of administration, they did not modify the investigation times during the first and second session of the social learning test, relative to the values in saline treated rats (data not shown).
Olfactory tests
The time spent in locating a preferred food placed on the bedding surface or buried in bedding by rats treated with 1 μg kg−1 or 10 mg kg−1 morphine (i.p.) or previously submitted to surgery was not different from that spent by untreated/unoperated animals (controls) (Figure 4A). Similarly, these doses of morphine or intracranial surgery alone did not modify the amount of time spent investigating a lemon-scented cylinder during repeated 1 min presentations, relative to values in control rats (Figure 4B). The animals showed dishabituation when vanilla was added to lemon inside cylinder (Figure 4B).
Figure 4.
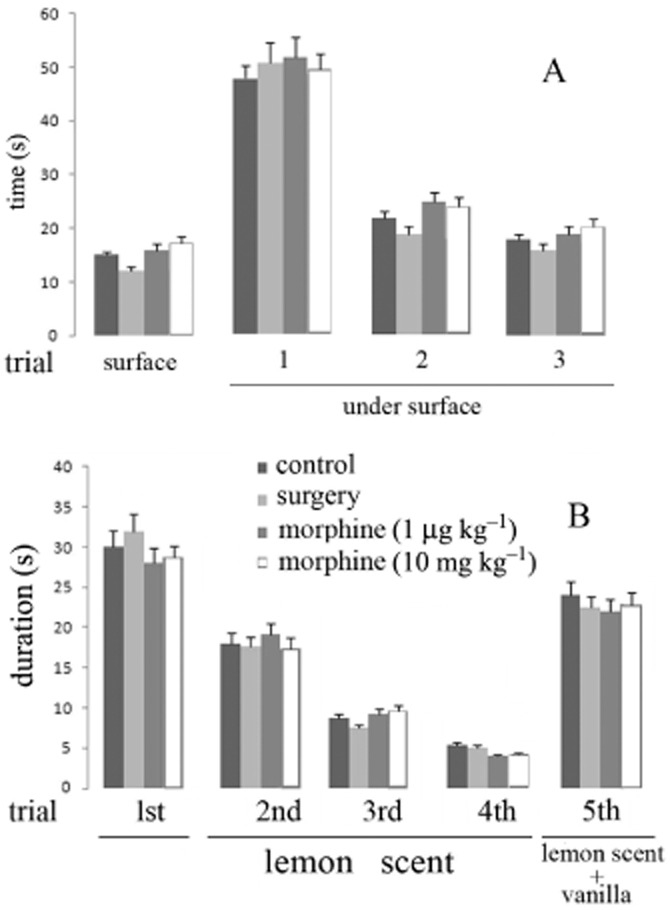
Olfactory guided behaviour in rat. (A) Time required by rats to find small pieces of chocolate, on the surface or beneath smoothed wood-chip bedding, in three successive trials. (B) Amount of time spent investigating a lemon-scented cylinder during repeated 1 min presentation training time in the presence of lemon alone or lemon and vanilla combination. At least 10 rats were used in each experiment. Vertical bars represent SEM.
Histology
Histology confirmed that 92% of the rats used in the experiments had cannula placement within the AOB (Figure 5).
Figure 5.
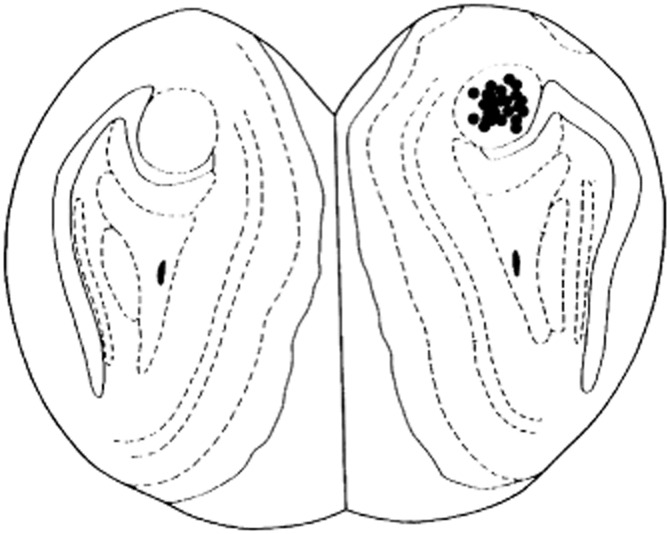
Schematic representation of the distribution of microinfusion sites within the AOB area of rat brain. The numbers of the filled circles is less than the total number of rats because of overlaps.
Discussion
The results of this study confirmed that rats can effectively form long-term social memories of another rat, as previously reported by Young, (2002). Juvenile recognition was present during the second exposure if the adult spent significantly less time investigating the previously exposed juvenile. In our experiments, the adult rat recognized a familiar juvenile when tested at 1–3 days (long term) after a single 2 min interaction, but lost familiarity with the individual when tested at 7 days after the initial interaction. The familiarity gained by an adult rat when exposed to an unfamiliar one was reliable and spontaneous. Our results are consistent with reports of learning and recognition in group-housed rodents from other laboratories (Kogan et al., 2000), which showed that the adult rat forms social memories that last for several days after a single exposure. When adult rats were given morphine at analgesic doses (mg kg-1) before the first trial with juvenile rat, investigation time dramatically increased during re-exposure of the two animals on a subsequent occasion. An increase between the time spent investigating the juvenile during the first and second exposures indicates that the adult failed to recognize the familiar juvenile. Therefore, social memory could be disrupted with morphine administration at analgesic doses, as previously shown in rats tested in social behaviour situations (Panksepp et al., 1980). In contrast, when adult rats were treated with morphine at extremely low doses (μg kg-1) before the first encounter with the juvenile, long-term memory was dramatically strengthened as re-exposure of the two animals at a subsequent occasion was characterized by a shorter investigation time by adult rat, compared with values obtained after saline administration. At both doses of morphine, investigation times returned to control values when systemic naloxone was co-administered with morphine. These findings show that social memory acquisition in rats can be modulated bidirectionally by different doses of morphine, according to a paradigm previously established as a social learning test (Dantzer et al., 1987). We were able to exclude possible effects of morphine on olfactory function, as measured in olfactory habituation and olfactory foraging tasks. In both these tests, rats given morphine at either low or high doses, learned to locate hidden food as rapidly as saline-treated rats and spent the same times investigating lemon-scented cotton, showing no difference with controls.
Different brain regions such as the amygdala, hippocampus and cortex, have been demonstrated as the site of action of morphine in memory impairment after administration to rodents at analgesic doses (Westbrook et al., 1997; Bianchi et al., 2010; Lu et al., 2010). The social investigatory behaviour used in our experiments included direct contact or sniffing, following, nosing or generally inspecting any body surface of the novel juvenile rat. There is evidence that olfactory system plays a main role in processing olfactory signals relevant to social discrimination (Tobin et al., 2010). The AOB, which is located in the dorsal–posterior region of the main olfactory bulb, receives axonal input from the vomeronasal organ forming synapses with mitral cells projecting to a number of areas in the brain, including cortex, amygdala and hypothalamus and deficits in memory have been observed following bilateral olfactory bulbectomy (Douma et al., 2011). This suggested us that a site of action of systemically administered morphine on social memory might be located in the AOB, a site where opioid receptors are present although these are relatively few in number (Mansour et al., 1994; Ding et al., 1996). Therefore, we used microinjection of selective opioid receptor antagonists to assess the role of one brain region, the AOB, as the central site of action of morphine at low (μg kg-1) doses on memory formation. We showed that that the surgical procedure did not interfere with social recognition, as rats submitted to habituation and olfactory foraging tasks showed a behaviour similar to that of un-operated controls. Our experiments showed that co-administration of systemic morphine with intra-AOB injection of naloxone restored investigation times to control, saline-induced values. Exposure of the AOB to DOR and KOR antagonists did not modify the effects induced by morphine on social memory acquisition. Particularly, exposure of rat AOB to the MOR antagonist CTOP completely reversed the effect on social memory formation of the low morphine dose, showing that recognition of a familiar rat appeared to be mediated within the AOB by an opioid system intrinsic to the olfactory system through the MOR.
Activation of opioid receptors may trigger many signalling mechanisms and the cAMP/PKA pathway is the major biochemical pathway for opioid signalling. Our data showed that co-administration of extremely low doses of morphine with the highest concentration of PKA inhibitor, did not modify recognition time, suggesting that different signalling pathways might underlie the effects of such doses of morphine on social memory formation. The application of PLC or PKC blockers to the AOB, the low systemic dose of morphine did restore to control values with the time spent by the adult rat investigating the juvenile, suggesting that the PLC/PKC inositol–lipid signalling pathway was involved in this memory formation. Further experiments are needed to confirm this assumption.
The opioids interact with a large family of GPCRs and the molecular mechanisms of ligand recognition and signal transduction in GPCRs are usually interpreted assuming that these receptors act as monomeric proteins. However, this assumption is now challenged by a number of results suggesting that GPCRs can function as oligomeric proteins. Thus, the phenomenon of homo- and hetero-oligomerization between the GPCRs is now a new paradigm with which all the key functions of these receptors, including the mechanisms of signal transduction, can be reinterpreted. Thus, the bimodal effects of morphine may result from an added complexity in signalling because of receptor oligomerization. In cell culture, a switch from G-protein-coupled to G-protein-independent signalling was dependent on agonist concentration (Sun et al., 2007) and based on a differential asymmetric/symmetric activation of a dimeric receptor (Rovira et al., 2010). From studies regarding the identification of specific signalling pathways for opioid anti-nociception, tolerance addiction and dependence, opioids producing these different effects have been linked to different intracellular cascades, preferentially acting on more than one receptor type (Morgan and Christie, 2011, Dang and Christie, 2012).
In conclusion, memory and the process of learning are affected by opioid agonists, which interfere with these processes and produce amnesia. Several studies have reported facilitation of memory retention by opioid antagonists such as naloxone, whereas the systemic administration of morphine at analgesic doses impairs memory processes (Izquierdo, 1980). Our experiments showed that social memory formation appears to be impaired in presence of morphine at analgesic doses (mg kg-1). In contrast, we showed that an opposite effect was induced on memory formation by morphine at low (μg kg-1) doses. The mechanism underlying the enhancement of recognition response is mediated by the activation of opioid receptors by morphine given systemically in low doses (1-10 μg kg-1). According to Glare and Walsh (1991), about 2% of an i.p. dose of 1μg kg-1 morphine enters the brain, yielding concentrations in the CNS comparable to those of the endogenous opioids, estimated to be at nanomolar levels (Hackler et al., 1997). This is compatible with the assumption that endogenous opioids sustain essential survival ability in non-human animals as memorization of attack place (Siegfried and Frischknecht, 1989) or mechanisms of attention (Rodgers, 1982) or stranger presence inside the environment, a process in which we have showed that olfactory system plays a major role. Impairment in social interaction is the characteristic feature of autism and rodent behavior classed as social learning has been described as a possible model of the social aspects of autism (Moy et al., 2008). Deficits in olfactory identification, but not detection, have been found in patients affected by autistic spectrum disorder (Suzuki et al., 2003; Bennetto et al., 2007). The possible clinical significance of the endogenous opioid system at olfactory brain regions in human diseases exhibiting abnormal social interaction remains to be explored.
Acknowledgments
This work was supported by MIUR grants (DR149-2009).
Glossary
- AOB
accessory olfactory bulb
- MOR
μ-opioid receptor
Conflict of interest
The authors have no conflict of interest to report.
References
- Abacus Concept. StatView. Berkeley, CA: Abacus Concept; 1992. [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetto L, Kuschner ES, Hyman SL. Olfaction and taste processing in autism. Biol Psychiatry. 2007;62:1015–1021. doi: 10.1016/j.biopsych.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Lehmann D, Vivoli E, Norcini M, Ghelardini C. Involvement of PLC-beta3 in the effect of morphine on memory retrieval in passive avoidance task. J Psychopharmacol. 2010;24:891–896. doi: 10.1177/0269881108102013. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Castellano C. Effects of morphine and heroin on discrimination learning and consolidation in mice. Psychopharmacologia. 1975;42:235–242. doi: 10.1007/BF00421262. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Acute thermal hyperalgesia elicited by low-dose morphine in normal mice is blocked by ultra-low-dose naltrexone, unmasking potent opioid analgesia. Brain Res. 2001;888:75–82. doi: 10.1016/s0006-8993(00)03010-9. [DOI] [PubMed] [Google Scholar]
- Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol. 2012;165:1704–1716. doi: 10.1111/j.1476-5381.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Douma TN, Borre Y, Hendriksen H, Olivier B, Oosting RS. Simvastatin improves learning and memory in control but not in olfactory bulbectomized rats. Psychopharmacology (Berl) 2011;216:537–544. doi: 10.1007/s00213-011-2245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XM, Elmer GI, Adams-Huet B, Tamminga CA. Social memory in mice: disruption with an NMDA antagonist and attenuation with antipsychotic drugs. Pharmacol Biochem Behav. 2009;2:236–242. doi: 10.1016/j.pbb.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glare PA, Walsh TD. Clinical pharmacokinetics of morphine. Ther Drug Monit. 1991;13:1–23. doi: 10.1097/00007691-199101000-00001. [DOI] [PubMed] [Google Scholar]
- Hackler L, Zadina JE, Ge LJ, Kastin AJ. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 1997;18:1635–1639. doi: 10.1016/s0196-9781(97)00259-3. [DOI] [PubMed] [Google Scholar]
- Izquierdo I. Effects of a low and a high dose of beta-endorphin on acquisition and retention in the rat. Behav Neural Biol. 1980;30:460–464. doi: 10.1016/s0163-1047(80)91292-3. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. Morphine action at central nervous system sites in rat: analgesia or hyperalgesia depending on site and dose. Science. 1973;182:490–492. doi: 10.1126/science.182.4111.490. [DOI] [PubMed] [Google Scholar]
- Kayser V, Besson JM, Guilbaud G. Paradoxical hyperalgesic effect of exceedingly low doses of systemic morphine in an animal model of persistent pain (Freund's adjuvant-induced arthritic rats) Brain Res. 1987;414:155–157. doi: 10.1016/0006-8993(87)91338-2. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lu G, Zhou QX, Kang S, Li QL, Zhao LC, Chen JD, et al. Chronic morphine treatment impaired hippocampal long-term potentiation and spatial memory via accumulation of extracellular adenosine acting on adenosine A1 receptors. J Neurosci. 2010;30:5058–5070. doi: 10.1523/JNEUROSCI.0148-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencio-Wszalek T, Ramirez VD, Dluzen DE. Age-dependent changes in olfactory-mediated behavioral investigations in the male rat. Behav Neural Biol. 1992;57:205–212. doi: 10.1016/0163-1047(92)90164-y. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164:1322–1334. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, et al. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Paolini AG, McKenzie JS. Effects of lesions in the horizontal diagonal band nucleus on olfactory habituation in the rat. Neuroscience. 1993;57:717–724. doi: 10.1016/0306-4522(93)90017-a. [DOI] [PubMed] [Google Scholar]
- Paxinos and Watson. The Rat Brain in Stereotaxic Coordinates. 4th edn. San Diego: Academic Press; 1998. [Google Scholar]
- Rodgers RJ. Delayed effects of naloxone on responsiveness to environmental novelty in rats. Psychopharmacology (Berl) 1982;78:230–233. doi: 10.1007/BF00428156. [DOI] [PubMed] [Google Scholar]
- Rovira X, Pin JP, Giraldo J. The asymmetric/symmetric activation of GPCR dimers as a possible mechanistic rationale for multiple signalling pathways. Trends Pharmacol Sci. 2010;31:15–21. doi: 10.1016/j.tips.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Siegfried B, Frischknecht HR. Place avoidance learning and stress-induced analgesia in the attacked mouse: role of endogenous opioids. Behav Neural Biol. 1989;52:95–107. doi: 10.1016/s0163-1047(89)90206-9. [DOI] [PubMed] [Google Scholar]
- Sun Y, Huang J, Xiang Y, Bastepe M, Jüppner H, Kobilka BK, et al. Dosage-dependent switch from G protein-coupled to G protein-independent signaling by a GPCR. EMBO J. 2007;26:53–64. doi: 10.1038/sj.emboj.7601502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Critchley HD, Rowe A, Howlin P, Murphy DG. Impaired olfactory identification in Asperger's syndrome. J Neuropsychiatry Clin Neurosci. 2003;15:105–107. doi: 10.1176/jnp.15.1.105. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Social memory of the male laboratory rat. J Comp Physiol Psychol. 1982;96:1000–1006. [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464:413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook RF, Good AJ, Kiernan MJ. Microinjection of morphine into the nucleus accumbens impairs contextual learning in rats. Behav Neurosci. 1997;111:996–1013. doi: 10.1037//0735-7044.111.5.996. [DOI] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]