Abstract
Background and Purpose
Activation of GABAB receptors in the dentate gyrus (DG) enhances granule cell (GC) activity by reducing synaptic inhibition imposed by hilar interneurons. This disinhibitory action facilitates signal transfer from the perforant path to the hippocampus. However, as the two main molecular subtypes, GABAB(1a,2) and GABAB(1b,2) receptors, prefer axonal terminal and dendritic compartments, respectively, they may modulate the hilar pathways at different synaptic localizations. We examined their relative expression and functions in the DG.
Experimental Approach
The localization of GABAB subtypes was revealed immunohistochemically using subunit-selective antibodies in GABAB1a–/– and GABAB1b–/– mice. Effects of subtype activation by the GABAB receptor agonist, baclofen, were examined on the perforant path-stimulated GC population activities in brain slices.
Key Results
GABAB(1a,2) receptors were concentrated in the inner molecular layer, the neuropil of the hilus and hilar neurons at the border zone; while GABAB(1b,2) receptors dominated the outer molecular layer and hilar neurons in the deep layer, showing their differential localization on GC dendrite and in the hilus. Baclofen enhanced the GC population spike to a larger extent in the GABAB1b–/– mice, demonstrating exclusively disinhibitory roles of the GABAB(1a,2) receptors. Conversely, in the GABAB1a–/– mice baclofen not only enhanced but also inhibited the population spike during GABAA blockade, revealing both disinhibitory and inhibitory effects of GABAB(1b,2) receptors.
Conclusions and Implications
The GABAB(1a,2) and GABAB(1b,2) receptor subtypes differentially modulate GC outputs via selective axonal terminal and dendritic locations in the hilar pathways. The GABAB(1a,2) receptors exclusively mediate disinhibition, thereby playing a greater role in gating signal transfer for hippocampal spatial and pattern learning.
Keywords: disinhibition, GABAB receptors, baclofen, GABAB1a, GABAB1b, multi-electrode, dentate gyrus
Introduction
GABAB receptors are GPCRs for the main inhibitory neurotransmitter, GABA. They participate in many brain functions including cognition, reward and anxiety via modulation of synaptic transmission (Bowery et al., 2002; Bettler et al., 2004). Activation of pre-synaptic GABAB receptors decreases neurotransmitter release by inhibiting voltage-gated Ca2+ channels and vesicular release; activation of somatodendritic GABAB receptors modulates Ca2+ channels and G-protein-coupled inward-rectifying K+ channels, resulting in postsynaptic inhibition. However, presynaptic GABAB receptors at excitatory and inhibitory synapses in neuronal networks induce inhibitory and disinhibitory effects respectively. GABAB receptor activation in the hippocampal CA1 and CA3 synaptic circuits is predominantly inhibitory because of the inhibition of glutamate release via presynaptic heteroreceptors (Nicoll, 2004; Chen et al., 2006). Conversely, in the dentate gyrus (DG), GABAB receptors primarily exert disinhibition on granule cells (GCs) as GABA release from hilar interneurons is reduced (Burgard and Sarvey, 1991; Mott and Lewis, 1991; Mott et al., 1993).
The disinhibitory role of GABAB receptors is important for the gate control of inputs from the entorhinal cortex to the hippocampus for spatial and pattern learning (Gilbert et al., 2001; Leutgeb et al., 2007; Moser et al., 2008). The perforant path (PP) forms excitatory synapses on dendrites of GCs, and spikes generated in GCs propagate into the hippocampus. However, the GCs are strongly inhibited by a divergent population of local interneurons in the hilus and the molecular layer (Freund and Buzsaki, 1996; Scharfman and Witter, 2007), which release GABA to activate ionotropic GABAA and metabotropic GABAB receptors on GCs and induce fast and slow inhibitory currents (Otis and Mody, 1992; Otis et al., 1993). Hilar interneurons in the border zone are particularly important for this role because they project to perisomatic and proximal dendritic regions (Freund and Buzsaki, 1996; Amaral et al., 2007), exerting synaptic inhibition that shunts action potential generation (Ben-Ari et al., 2005). GABAB receptors are densely expressed in hilar interneurons (Bischoff et al., 1999; Kulik et al., 2003), and activation of these somatodendritic receptors decreases firing of hilar border neurons (Mott et al., 1999), reduces GABA release on GCs and enhances spike discharge (Burgard and Sarvey, 1991; Mott and Lewis, 1991; Mott et al., 1993). However, presynaptic GABAB receptors may also reduce hilar inhibition by decreasing excitatory inputs to the hilus and inhibiting GABA release from hilar axonal terminals on to GCs.
Pre- and postsynaptic GABAB receptors show molecular diversity (Pinard et al., 2010; Ulrich and Bettler, 2007). GABAB(1a,2) and GABAB(1b,2) receptors are the two main molecular subtypes assembled by the obligatory heterodimerization between the GABAB1 isoforms, 1a and 1b, and the GABAB2 subunit. They show no significant pharmacological or signalling differences, but their expression is independently regulated in neuronal populations and differs between synaptic compartments (Bischoff et al., 1999; Fritschy et al., 1999; Margeta-Mitrovic et al., 1999; Vigot et al., 2006). At glutamatergic synapses, the GABAB(1a,2) receptors are preferentially expressed in presynaptic compartments, directed exclusively by the N-terminal ‘sushi’ domains on the 1a subunit; while the GABAB(1b,2) receptors, lacking the ‘sushi’ domains, are primarily confined at the default dendritic location (Vigot et al., 2006; Tiao et al., 2008; Biermann et al., 2010). The GABAB(1a,2) receptors are also the exclusive presynaptic autoreceptors inhibiting GABA release between layer 1 and 5 cortical neurons, with the GABAB(1b,2) receptors at the dendrite (Perez-Garci et al., 2006). However, the GABAB(1b,2) receptors can also inhibit GABA release in the CA1 (Vigot et al., 2006) and the thalamus (Ulrich et al., 2007), probably via somatodendritic inhibition of interneurons.
Both 1a and 1b transcripts are expressed in hilar neurons and GCs in the DG, but high density receptor expression is shown in the molecular layer and the hilus with the relative expression of two receptor subtypes unclear (Bischoff et al., 1999). Although somatodendritic GABAB receptors on the hilar border neurons, presumably of the GABAB(1b,2) subtype, mediate disinhibition (Mott et al., 1999), genetic deletion of the 1a, but not 1b isoform, impairs synaptic plasticity in the hippocampus and novel object recognition performance of the GABAB1a–/– mice (Vigot et al., 2006; Jacobson et al., 2007), implicating a critical role for the GABAB(1a,2) receptors in pattern learning. We tested the hypothesis that GABAB(1a,2) receptors exert greater disinhibition on GCs by presynaptic heteroreceptor inhibition of excitatory inputs to the hilus and autoreceptor inhibition of GABA release on GC dendrites in the molecular layer. We, therefore, examined the anatomical localization of the GABAB subtypes in the DG of the GABAB1a–/– and GABAB1b–/– mice, and investigated their individual roles in modulating GC output.
Methods
All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). The use of animals was in accordance with the Animals in Scientific Procedures Act (1986) UK. GABAB1a knockout mice (1a–/–), GABAB1b knockout mice (1b–/–) and their wild-type (WT) littermates were bred from heterozygous pairs and maintained on a Balb/c background (Vigot et al., 2006). The transgenic mice were back-crossed with WT Balb/c mice (B & K Universal, Hull, Yorkshire, UK) every three generations. Animals were group-housed with food and water available ad libitum in a room with temperature control (23 ± 0.5°C) and a 12/12 h light/dark cycle. The genotypes of the mice were determined at approximately 5 weeks of age by PCR on DNA extracts from tail biopsies. Sequences of the primers used in genotyping were as described previously (Vigot et al., 2006). Male mice (aged 5–12 weeks) exhibited no spontaneous seizures or other basal behavioural abnormalities (Jacobson et al., 2006), in contrast to the GABAB1–/– knockout mice that displayed several forms of complex seizures (Schuler et al., 2001). The use of drug and molecular target nomenclature conforms to the Guide to Receptors and Channels (Alexander et al., 2011).
Electrophysiological recordings of synaptic potentials in dentate GCs using a multi-electrode array
Sagittal sections of both brain hemispheres were cut at a thickness of 300 μm using a vibratome (Series 1000, Vibratome, St. Louis, MO, or a Leica VT1200, Leica Microsystems GmbH, Wetzlar, Germany). Transverse sections of the DG and the hippocampus were harvested and transferred to oxygenated (5% CO2/95% O2) artificial cerebrospinal fluid, which contained (in mM) NaCl (123), Na2CO3 (25), glucose (10), KCl (3.7), CaCl2 (2.5), NaH2PO4 (1.4) and MgSO4 (1.2), and maintained at 31°C. One slice was transferred to a multi-electrode probe (MED-P210A; MED64, Alpha MED Sciences, Osaka, Japan), which consists of 64 indium tin oxide and platinum black electrodes arranged in an 8 by 8 grid with an inter-electrode distance of 100 μm. Slices were orientated with a row of electrodes in line with the GC layer of the inner, enclosed blade of the DG (Figure 3A) and held in place using a nylon mesh and a platinum ring. Bath perfusion was at a rate of 1–2 mL·min−1, and the artificial cerebrospinal fluid was continuously oxygenated (5% CO2/95% O2), and heated to 31 ± 0.5°C using an in-line heater (Warner Instruments, Hamden, CT).
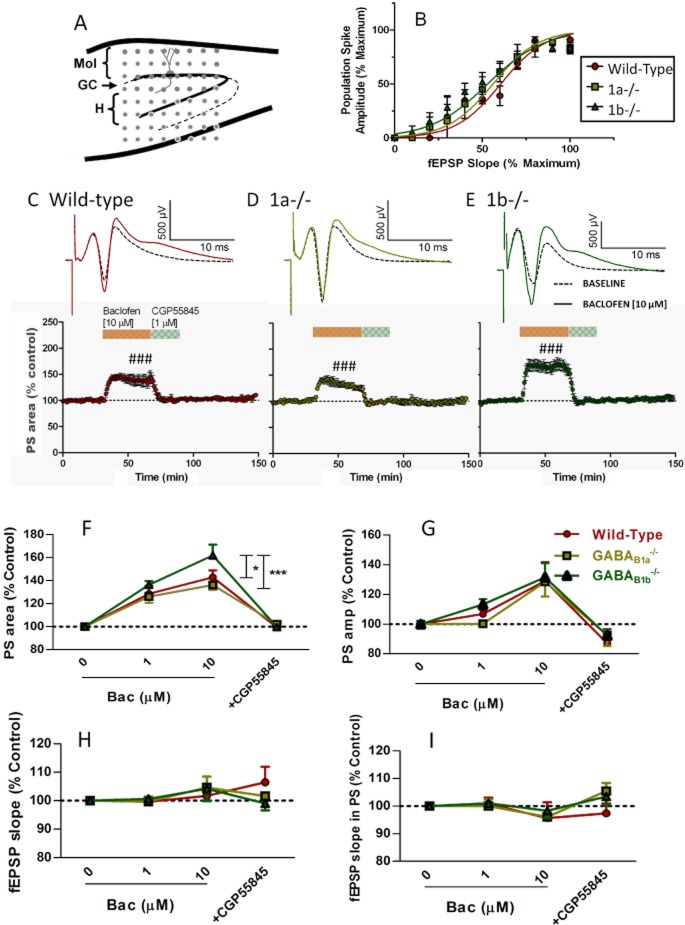
Figure 3.
The GABAB receptor agonist, baclofen (Bac), enhances the PS in the DG. (A) A schematic of the multi-electrode positions in the DG for simultaneous recordings of the fEPSPs and the PSs. Electrical stimulation was applied to an electrode in the outer two-thirds of the molecular layer (Mol) and evoked fEPSPs were recorded in the outer molecular layer and PSs adjacent to the GC layer. (B) fEPSPs and PSs were simultaneously recorded at stimulation intensities ranging from 10 to 110 μA with a 10 μA incremental step. The PS amplitude and the fEPSP slope were normalized and plotted at all stimulation intensities. The relationships between the PS amplitude and the fEPSP slope are similar between the wild-type and the 1a–/– and 1b–/– mice (F[2224] = 0.917, P > 0.05), indicating unaltered coupling between the excitatory synaptic transmission and the GC excitability. (C–E) Bath application of 10 μM Bac significantly increased the PS areas in all genotypes (###P < 0.001, compared to baseline). The effects were rapidly reduced by CGP55845, showing GABAB receptor activation. F. Bac concentration-dependently increased the PS area in all mice (***F[3,71] = 84.6). At 10 μM, Bac induced a significantly larger effect in the 1b–/– mice compared with the wild-type (*) and 1a–/– mice (***). Bac also concentration-dependently increased the PS amplitude in all genotypes (G, ***F[3,71] = 17.6). (H and I) The fEPSP slopes were not altered by the application of Bac or CGP55845 in all genotypes (G, F[1,15] = 0.3, P > 0.05; I, F[3,71] = 2.1, P > 0.05). Repeated-measure two-way anova followed by Bonferroni post test was used for comparison (*P < 0.05, **P < 0.01 and ***P < 0.001).
Electrical pulses (0.2 ms in duration) were delivered every 60 s to stimulate the PP via one of the electrodes in the array positioned in the outer two-thirds of the molecular layer. Stimulation, recording and the analysis of extracellular potentials was performed using the Mobius software (version 0.3.7; Alpha MED Sciences). The negatively deflected field excitatory postsynaptic potentials (fEPSPs) were recorded in the dendrite field of GCs in the outer molecular layer. Signals with amplitude greater than 200 μV were captured and used for off-line analysis. Due to submerged perfusion method and the low impedance of the surface electrodes (Chen et al., 2006), the amplitude of the field potentials recorded by multi-electrodes are small, but the cut-off amplitude was more than three times the peak-to-peak noise level. The fEPSP slope was measured using the 10–90% slope function, and the absolute value (mV·ms−1) was used to assess the excitatory synaptic strength.
The population spike (PS) was recorded from the soma of the GCs where the field potential waveform displayed positively deflected fEPSP superimposed by a large negatively deflected spike. The relative amplitudes of the fEPSP and the spike differ between recording sites. Recordings towards the hilar region displayed a relatively larger fEPSP as the site gets closer to the current source. Therefore, all PSs with amplitudes larger than 500 μV were analysed. The amplitude of the PS (mV) was measured from the negative peak to the line connecting the positive peaks for the summation of synchronized action potentials from the GCs. The initial slope of the positively deflected fEPSP was also measured by the 10–90% slope function (mV·ms−1), which mirrors the negative fEPSP slope of the same neuronal population as an evaluation of excitatory synaptic strength. The area under the curve (mV × ms, PS area) was calculated including any additional spikes at longer latencies induced by drug treatment to evaluate the ensemble effect of all events. The input–output relationship was examined in all slices using escalating stimulation intensities (10 to 110 μA in steps of 10 μA), and approximately 80% of the maximum responses was used for further pharmacological studies.
Effects of GABAB receptor agonist and antagonist were examined by bath application following a 30 min baseline recording (control). Recordings from 3 to 10 electrodes were analysed independently, and the mean drug effect was obtained for each slice. This exercise averages out variability between different recording sites and hence enhances the reproducibility of pharmacological effects between experiments (Chen et al., 2006). In addition, the effects of GABAB receptor agonist and antagonist were similar on fEPSPs and PSs elicited by different stimulating electrodes in the molecular layer to recruit the lateral or medial perforant path, respectively, so that the data sets were pooled. There was also no apparent age (5–12 weeks)-dependent effect.
Immunohistochemical staining and analysis
The specificity of the anti-GABAB2 antibody (AB5394; Millipore Ltd, Watford, Hertfordshire, UK) has been confirmed using the GABAB2–/– mice (Gassmann et al., 2004). The anti-GABAB1 antibody (sc-14006; Santa Cruz Biotechnology Inc., Insight Biotechnology Ltd, Wembley, Middlesex, UK) was raised against the immunogen sequence specific for GABAB1 subunit (NCBI mouse protein library), and the immunostaining produced the same pattern of subunit distribution in the hippocampus and the DG as shown in previous studies (Kulik et al., 2003; Vigot et al., 2006). Notably, GABAB1 staining in the stratum lucidum of the CA3 area was significantly reduced in the 1a–/– mice (Vigot et al., 2006).
The brain block containing the DG was fixed in ice-cold paraformaldehyde (4%) for 4 h immediately following decapitation. Antigen retrieval was carried out by heating the tissue in sodium citrate buffer for 130 s in a 750W microwave oven, as described by Fritschy et al. (1999). Coronal sections of 30 μm thick were cut in a cryostat (Microtome, UK) at −21°C and incubated overnight (approximately 15 h) at 4°C with primary antibodies raised against either the GABAB1 or the GABAB2 subunit. The bound primary antibodies were labelled with biotinylated secondary antibodies (Jackson Immuno Research Laboratories Inc, West Grove, PA). The tissue-bound biotin molecules were then labelled with a peroxidase–avidin complex (Vectastain ABC Kit, Vector Laboratories Ltd, Peterborough, Cambridgeshire, UK) in PBS with 0.5% Triton X-100 (v/v) for 30 min. Sections were then stained using DAB (3,3′-diaminobenzidine, ImmPACT DAB Substrate; Vector Laboratories Ltd) under visual guidance. Negative control sections were incubated without the primary antibodies to evaluate any unspecific binding by the secondary antibody.
Brain tissues from each of the three genotypes (WT, 1a–/– and 1b–/–) were processed simultaneously to minimize variability in staining intensity. The Nissl stain was also performed to examine the architectonic structure of the tissue in the knockout mice. Images were captured using a Nikon light microscope with a fitted digital camera (DS-2Mvm, Nikon Instruments Europe, Kingston, UK). The number of stained hilar neurons was counted in each section. The hilar border neurons were those located in a single cell layer immediately adjacent to the GC layer. The deep layer neurons were those in between the upper and lower border layers, excluding the CA4 area. The relative staining intensities of the neuropil of the molecular layers were scored at high (3), medium (2), low (1) and very low (0) levels within the same batch of staining. The mean for each DG was obtained from 8–20 sections across the septotemporal axis and used for comparison between genotypes. Note that the relative staining levels do not necessarily conform to a linear scale. Nonparametric tests were, therefore, used for comparison between genotypes.
Pharmacological agents
GABAB receptor agonist (±)-baclofen (referred to hence forward as baclofen) was purchased from Sigma-Aldrich Company Ltd. (Poole, Dorset, UK). GABAA receptor antagonist (–)-bicuculline methochloride (referred to hence forward as bicuculline) and GABAB receptor antagonist CGP55845 was purchased from Tocris Bioscience (Bristol, UK). All other reagents used were of analytical grade and purchased from either Sigma-Aldrich or Fisher Scientific UK Ltd. (Loughborough, Leicestershire, UK).
Statistical analysis
Data are expressed as mean ± SEM, and the n represents the number of different animals (see Results). Treatment effects over the time course were compared using paired t-test or repeated-measures one-way anova followed by Tukey's multiple comparisons, where appropriate. Treatment effects among the three genotypes were analysed using anova followed by Bonferroni's post hoc test or nonparametric tests, where appropriate. Statistical significance was taken as P < 0.05.
Results
Localization of GABAB receptor subtypes in the molecular layer
Previous studies show that transcripts for both 1a and 1b subunits are expressed in GCs and hilar neurons (Bischoff et al., 1999), but radioligand binding and immunohistochemical labelling of heteromeric GABAB receptors are mainly found in the hilus and the molecular layer, indicating predominant receptor expression on neuronal processes (Kulik et al., 2003). The mossy fibre terminals in the CA3 are immunopositive for the 1a subunit (Vigot et al., 2006; Guetg et al., 2009), showing selective trafficking of this subtype to GC axonal terminals. Conversely, GABAB(1b,2) receptors are probably expressed on dendrites of GCs in the molecular layer (Kulik et al., 2003) at their default dendritic localization (Biermann et al., 2010). Here, we examined the anatomical localization of the GABAB receptor subtypes in the DG in the 1a–/– and 1b–/– mice. Both anti-GABAB1 (B1) and anti-GABAB2 (B2) antibodies were used because co-localization of B1 and B2 subunits is essential for the assembly of heteromeric functional GABAB receptors (Kulik et al., 2003; Gassmann et al., 2004; Vigot et al., 2006). B1 expression in the soma alone without B2 is indicative of B1 retention in the endoplasmic reticulum, as the binding of the B2 subunit enables surface expression of the heteromeric assembly (Margeta-Mitrovic et al., 2000; Gassmann et al., 2004).
The cellular architecture of the DG in the knockout brains were examined using the Nissl stain (Figure 1A-C), and no apparent alteration from the WT was observed. Intense B1 immunostaining was found in the soma and proximal dendrites of hilar neurons and the neuropil of the molecular layer and GC layer in WT mice (Figure 1D-F); B2 staining was mainly found in the neuropil of the hilus and the molecular layer (Figure 1G), indicating that the B1 and B2 subunits are co-localized in the neuropil of the hilus and the molecular layer for heteromeric receptor assembly (Bischoff et al., 1999; Kulik et al., 2003). Few neuronal cell bodies were stained in the molecular layer, in comparison to the number labelled by the Nissl stain (Figure 1A-C), consistent with a low level of GABAB receptors in interneurons of the molecular layer (Kulik et al., 2003). We, therefore, further analysed the subtype expression patterns in the molecular layer and the hilus.
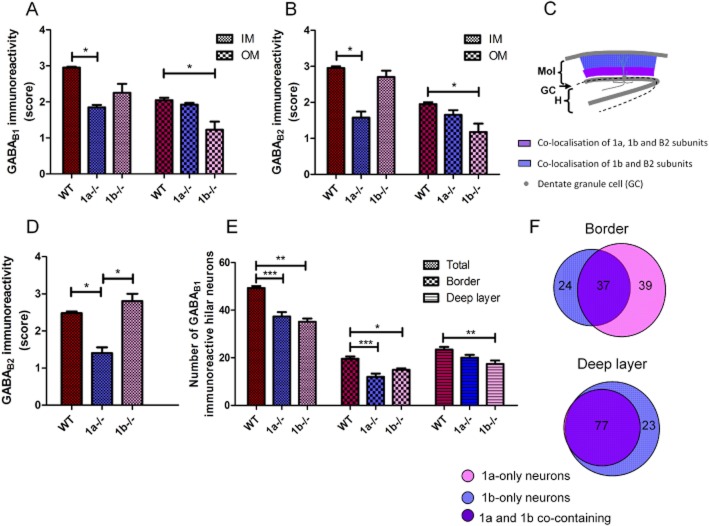
Figure 1.
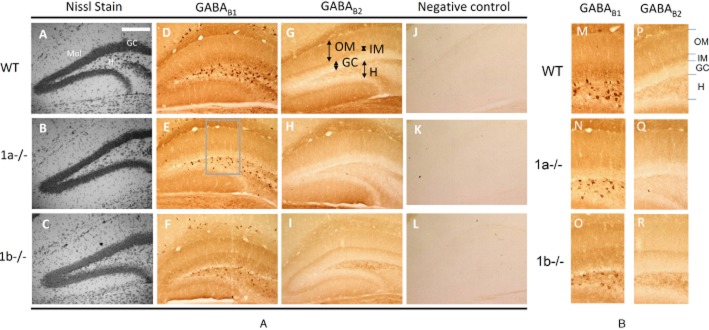
Distribution of GABAB receptor subunits in the DG of 1a–/– and 1b–/– mice. (A–C) The Nissl stain in the DG shows no significant anatomical differences for cell populations in the GC layer, molecular layer (Mol) and the hilus (H) between the wild-type (WT), 1a–/– and 1b–/– mice. (D–F) GABAB1 immunolabelling in the DG using an immunoperoxidase method reveals high intensity staining in the cell bodies and proximal dendrites of hilar neurons, and in the neuropil of the inner molecular layer (IM, see panel G). The number of immunopositive hilar neurons is lowered in the 1a–/– (E) and 1b–/– mice (F). The neuropil staining is reduced in the IM in the 1a–/– mice and the outer molecular layer (OM, see panel G) in the 1b–/– mice. (G–I) GABAB2 immunoperoxidase labelling in the DG show that the relative neuropil staining intensities in the IM and the OM were reduced in the 1a–/– (H) and the 1b–/– mice (I) respectively. (J–L) Examples of negative control sections processed without the addition of GABAB1 or GABAB2 antibodies show low levels of background staining. The scale bar for all sections (200 μm) is shown in panel A. (M–R) Enlarged sections from panels D–I (see the frame in panel E) showing the immunostaining patterns in the molecular layer and hilar neurons.
In the molecular layer of the WT mice, immunoreactivity for both B1 and B2 was more intense in a narrow band immediately adjacent to the GC layer in the inner molecular layer (IM) than the outer molecular layer (OM) (Figure 1D, G and Figure 2A, B). In the 1a–/– mice, B1 and B2 staining was both reduced in the IM, but not the OM (Figure 1E and H, and Figure 2A and B, P < 0.05, Kruskal–Wallis test and Dunn's post tests, n = 4 brains of each genotype), showing restricted co-localization of the 1a and B2 subunits in the IM, which form the GABAB(1a,2) receptors. Correspondingly, in the 1b–/– mice, B1 and B2 staining (Figure 1F, I) was both significantly reduced in the OM (Figure 2A and 2B, P < 0.05), but not the IM, showing predominance of the GABAB(1b,2) receptors in the OM. A schematic that depicts the localization of the subunits in the molecular layer is shown in Figure 2C, where the GABAB(1a,2) receptors are concentrated in the IM, and the GABAB(1b,2) subtype dominates the OM. Enlarged sections (see the frame in Figure 1E) that include the hilus and the enclosed blade of the molecular layer are shown in Figure 1M–R to illustrate the differential expression of GABAB(1a,2) and GABAB(1b,2) receptors. The IM localization of the axonal terminal-preferring GABAB(1a,2) receptors may indicate the presence of presynaptic autoreceptors at hilar pathways innervating the proximal dendrite of GCs only. The GABAB(1a,2) receptors may then inhibit hilar output, exerting a disinhibitory effect, whereas the GABAB(1b,2) receptors may be on GC dendrites in the OM (Kulik et al., 2003), thereby mediating an inhibitory effect on GC output.
Figure 2.
Comparison of GABAB receptor subtype expression in the molecular layer (A–C) and hilar neurons (D–F). (A) The relative intensity scores of B1 immunostaining were significantly reduced in the IM in the 1a–/– mice (*) and in the OM only in the 1b–/– mice (*) compared with the WT. (B) The relative intensity scores of B2 immunostaining were significantly reduced in the IM in the 1a–/– (*) and the OM in the 1b–/– mice (*). The scores range from 0 (no staining) to 3 (the highest intensity) and were used for all sections the brain processed simultaneously. Each score was the mean from 8–20 sections. Nonparametric Kruskal–Wallis test and Dunn's post tests were performed. (C) A schematic of the relative co-localization of 1a, 1b and B2 subunits in the molecular layer. (D) The relative intensity scores of B2 immunostaining were significantly reduced in the hilus in the 1a–/– mice (*P < 0.05, nonparametric Kruskal–Wallis test and Dunn's post tests). (E) The total number of immunopositive hilar neurons (Total) was significantly decreased in the 1a–/– (***) and 1b–/– mice (***). The number of neurons at the hilus–GC border zone (Border) also significantly decreased in the 1a–/– (***) and 1b–/– mice (*). However, the number of hilar neurons in deep layers was only significantly lower in the 1b–/– mice (*). The data were mean counts from 8–20 sections per brain. Two-way anova and Bonferroni's multiple comparison tests were used (*P < 0.05, **P < 0.01, ***P < 0.001). N = 4 brains of each genotype. (F) Venn diagrams illustrate the relative percentages of hilar neurons containing 1a, 1b or both in the border zone and the deep layer respectively.
Localization of the GABAB receptor subtypes in the hilus
Hilar neurons include a diverse population of GABAergic interneurons, which receive excitatory inputs from GC axons and project to the molecular layer to exert feedback inhibition on GC dendrites (Amaral et al., 2007). Previous studies show that GABAB receptors are expressed on GABAergic interneurons (Bischoff et al., 1999; Kulik et al., 2003), but not the mossy cell membrane (Mott et al., 1999; Nahir et al., 2007). Hilar neurons were strongly labelled by the B1 antibody, but B2 staining was distributed in the neuropil, highlighting a non-somatic localization of heteromeric assemblies.
The neuropil staining of B2 in the hilus (Figure 1P-R) was significantly reduced in the 1a–/– (Figure 2D, P < 0.05, Kruskal–Wallis test and Dunn's post tests, n = 4 brains of each genotype), showing significant presence of the GABAB(1a,2) receptors on neuronal processes. Given their preferred localization on axonal terminals, the GABAB(1a,2) receptors may include presynaptic heteroreceptors on GC axonal terminals innervating the hilus, similar to those expressed on mossy fibre terminals in the CA3 (Guetg et al., 2009). Activation of the GABAB(1a,2) heteroreceptors can inhibit excitatory inputs from GCs to reduce hilar neuron activity and induce disinhibition on GCs. Presumably, presynaptic GABAB(1a,2) heteroreceptors can also reduce excitatory inputs on the excitatory mossy cells (Nahir et al., 2007), resulting in further decreased excitation in the hilus.
Hilar neurons were intensely stained by the B1 antibody, showing somatic expression of the subunit. Hilar neurons at the border zone and the deep layer also differentially innervate the somatodendritic regions of GCs via proximal and distal pathways in the IM and OM, respectively (Freund and Buzsaki, 1996; Amaral et al., 2007). Given the co-localization of 1a and B2 subunits in the IM, the GABAB(1a,2) receptors may be expressed on the axonal terminals of hilar border neurons. In correlation to the terminal expression, the 1a subunit may be abundantly expressed in the soma of these neurons due to retention in the endoplasmic reticulum, although its absence in the soma does not rule out the possibility of exclusive axonal terminal localization as in the case of GCs. We found that the total number of B1-positive hilar neurons was reduced by 24.2 ± 6.6% in the 1a–/– mice (P < 0.001, n = 4 mice, Figures 1E and 2E) and by 29.8 ± 3.1% (P < 0.001, n = 4 mice, Figures 1F and 2E) in the 1b–/– mice, compared with the WT mice (a total of 49.3 ± 0.7 labelled neurons per section, n = 4 mice), showing that ∼24% of the hilar neurons contain the 1a subunit only and ∼30% the 1b only, while the rest (∼46%) comprise both subunits. In addition, the number of neurons located in the border zone immediately adjacent to the GC layer was reduced by a greater extent in the 1a–/– mice (39.0 ± 9.8%, P < 0.001, n = 4, Figure 2E), while the rest of hilar neurons in the deep layer was only significantly reduced in the 1b–/– mice (by 23.3 ± 7.5%, P < 0.01, n = 4, Figure 2E), demonstrating prevalence of 1a-only neurons in the border zone and 1b-only ones in the deep layer. The relative distributions of the two isoforms are depicted in the Venn diagrams in Figure 2F, where a relatively higher percentage of hilar border neurons express the 1a subunit only, and all deep-layer neurons express the 1b subunit.
Despite the relatively different somatic localization of 1a and 1b subunit, most hilar neurons contain the 1a. The 1a-containing hilar border neurons included fusiform cells and pyramidal-shaped cells with prominent apical dendritic tuft extending to the GC layer, resembling the basket cells (Freund and Buzsaki, 1996; Amaral et al., 2007). These cells potentially innervate both perisomatic and proximal dendritic regions of GCs. However, as the 1a and B2 subunits were mainly co-localized in the IM, the GABAB(1a,2) receptors may then be only expressed at axonal terminals innervating the proximal dendrite. In addition, the 1a subunit was expressed in ∼77% deep-layer neurons, but its expression level was low in the OM, indicating a deficiency in axonal terminal expression. Therefore, despite the abundance of somatic expression, the hilar neurons show differentially axonal terminal expression of the 1a subunit in the IM.
Activation of GABAB(1a,2) receptors enhances GC population spikes to a greater extent
The differential localization of the GABAB receptor subtypes in the DG indicates their potentials to differentially modulate GC output at axonal terminal and dendritic sites of hilar pathways. We, therefore, studied the PP-GC synaptic transmission in the DG of the WT, 1a–/– and 1b–/– mice. Electrical stimulation in the outer two-thirds of the molecular layer activates the PP and evokes synaptic potentials in the GC dendrite and action potential in the cell body (Ault and Nadler, 1982; Burgard and Sarvey, 1991; Mott and Lewis, 1991; Mott et al., 1993). We recorded fEPSPs and PSs from the outer molecular layer and the GC layer, respectively, using a multi-electrode array ( Figure 3A). The evoked potentials were blocked by the ionotropic glutamate receptor antagonist, CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) (20 μM; data not shown), confirming the glutamatergic nature of the PP-GC synapses. Both the fEPSP slope and the PS amplitude increased with the stimulation intensity (10–110 μA with 10 μA step) in all WT (n = 7), 1a–/– (n = 8) and 1b–/– (n = 7) mice. The input–output relationships did not differ between the genotypes (F[2350] = 0.60, P > 0.05 for fEPSP slopes; F[2240] = 0.89, P > 0.05 for PS amplitudes), nor did the relationship between the PS amplitude and the fEPSP slope (F[2224] = 0.917, P > 0.05, Figure 3B), demonstrating similar coupling between the excitatory inputs and GC firing in WT and GABAB1 isoform knockout mice in the single-pulse stimulation paradigm.
Baclofen concentration-dependently (1 and 10 μM) augmented the PS in all mice (Figure 3C–E upper traces). An additional spike was induced following the initial spike in the PS (Figure 3C–E, upper panels), indicating increased excitability of GCs due to reduced late-onset synaptic inhibition. The amplitude of the first spike was also enhanced at 10 μM baclofen (Figure 3G) by 129 ± 12 % (P < 0.05, n = 7) for WT; 128 ± 10% (P < 0.05, n = 8) for 1a–/– and 131 ± 10% (P < 0.05, n = 7) for 1b–/– mice. To include both spikes in the analysis, the area under the curve (PS area) was measured and baclofen significantly increased the PS area in all genotypes (Figure 3C–E lower panels, ###P < 0.001 compared with its own baseline). Furthermore, the increase of the PS area was significantly greater in the 1b–/– mice than in the WT (P < 0.05) and 1a–/– mice (P < 0.001, Figure 3F), showing a larger effect mediated by the GABAB(1a,2) receptors. As the two receptor subtypes do not differ pharmacologically (Vigot et al., 2006), the greater effect by the GABAB(1a,2) receptors may reflect their relative localization in the hilar inhibitory pathways (Figures 1 and 2).
Despite the enhancement in PS amplitude and PS area, the initial slope of the fEPSPs recorded in the OM was not enhanced by baclofen (Figure 3I, F[1,19] = 2.3, P > 0.05, 7 WT, 10 1a–/– and 7 1b–/– mice), nor was the initial slope of the positive fEPSP in the PS (Figure 3H, F[3,71] = 2.1, P > 0.05), demonstrating a lack of GABAB heteroreceptors on the excitatory axonal terminals of the PP in the mouse DG. This is in agreement with the lack of presynaptic GABAB receptors on PP terminals in the stratum lacunosum moleculare of the mouse CA1 (Price et al., 2008). The GABAB receptor-selective antagonist, CGP55845 (1 μM), also failed to affect the fEPSP slope (Figure 3I, F[1,16] = 2.6, P > 0.05, WT, n = 7; 1a–/–, n = 10 and 1b–/–, n = 7 mice), showing a lack of endogenous GABAB receptor activation upon single-pulse PP stimulation. The enhanced PS, therefore, demonstrates disinhibitory effects of the GABAB receptors. The additional spike in the PS further indicates reduced feedback inhibition, consistent with the intense expression of GABAB receptors in the hilus.
GABAA receptor blockade prevents baclofen-induced disinhibition and reveals an inhibition by GABAB(1b,2) receptors only
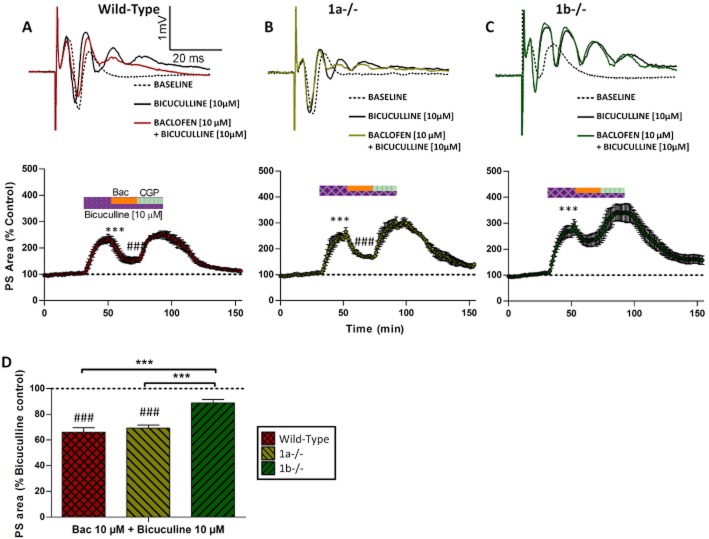
To confirm that the potentiation of the PS is due to disinhibition, we examined effects of baclofen in the presence of GABAA receptor antagonist, bicuculline (10 μM), because the GABAA receptor blockade is expected to abolish feedback synaptic inhibition and hence baclofen-induced disinhibition via reduced GABA release. Bicuculline induced multiple spiking in the PSs in all slices, showing facilitated action potential generation in GCs resulting in a spike train. As a result, the PS area was significantly increased (Figure 4A–C, PS area, P < 0.001 compared to baseline). In the presence of bicuculline, baclofen (10 μM) failed to enhance the PS in any genotype (P > 0.05), confirming that the PS enhancement was due to GABAA receptor-dependent disinhibition.
Figure 4.
The baclofen-induced enhancement of the PS is GABAA receptor-dependent. (A–C) The GABAA receptor antagonist, bicuculline (10 μM), induced multiple spikes in the PSs and significantly (***P < 0.001, compared with the baseline) increased the PS area, showing increased GC excitability. In the presence of bicuculline, baclofen (Bac) failed to increase the PS area because of the blockade of GABAA receptors. However, Bac significantly decreased the PS area in wild-type and 1a–/– mice (###P < 0.001, compared with bicuculline treatment), but not in 1b–/– mice, showing an inhibitory effect mediated by GABAB(1b,2) receptors. CGP55845 reduced the effect of Bac, confirming GABAB receptor activation. A comparison of Bac-induced inhibition between genotypes is shown in panel D (***P < 0.001, one-way anova followed by Tukey's test).
However, in the presence of GABAA blockade, baclofen reduced the PS area in WT and 1a–/– mice (Figure 4A–D, P < 0.001 compared with baseline, n = 7 for WT, n = 6 for 1a–/–), but not 1b–/– mice (P > 0.05, n = 6). The amplitude of the first spike in the PS was also reduced but not the initial slopes of the fEPSP, indicating a direct postsynaptic inhibition on GCs, but not a pre-terminal inhibition of the PP-GC transmission. The GABAB(1b,2) receptors, therefore, also mediate a postsynaptic inhibition at GC dendrites (Vigot et al., 2006; Tiao et al., 2008; Biermann et al., 2010). This additional inhibitory effect is in contrast to the exclusive disinhibitory effects of GABAB(1a,2) receptors, reflecting their differences in anatomical localization.
Discussion
We show for the first time that GABAB receptor subtypes differentially modulate synaptic inhibition on GCs at different localizations of the hilar pathways in the DG. The axonal terminal-preferring GABAB(1a,2) receptors are predominantly located in the neuropil of the inner molecular layer and the hilus, and in hilar border neurons. Activation of this subtype exclusively decreases GABAA receptor-mediated synaptic inhibition, thereby indicating presynaptic reduction of excitatory inputs to the hilus and also inhibition of hilar outputs. The dendrite-preferring GABAB(1b,2) receptors dominate the outer molecular layer and deep-layer hilar neurons. This subtype may, therefore, mediate the disinhibitory and inhibitory effects on GCs via dendritic inhibition of hilar neurons and GCs respectively. The GABAB(1a,2) receptors are also more effective at augmenting GC output, highlighting a greater role of these presynaptic receptors in gating spike transmission from the DG to the hippocampus for spatial and pattern learning.
The use of 1a–/– and 1b–/– mice isolates GABAB(1a,2) and GABAB(1b,2) receptor expression without altering the cellular architecture of the DG (Figure 1). Previous studies using these mice have revealed differential roles of the GABAB receptor subtypes in synaptic circuitry (Ulrich and Bettler, 2007; Pinard et al., 2010). Most notably, the GABAB(1a,2) receptors are the exclusive presynaptic receptors on glutamatergic and GABAergic synapses, trafficked by the ‘sushi’ domains on the 1a subunit (Tiao et al., 2008; Biermann et al., 2010), while the GABAB(1b,2) predominant dendritic receptors (Vigot et al., 2006; Ulrich et al., 2007; Guetg et al., 2009). The differential localization of the subtypes in synaptic compartments enables them to differently modulate synaptic functions and cognitive behaviours. Here, we evaluated the subtypes in modulating synaptic inhibition in the DG.
Disinhibitory and inhibitory roles of GABAB(1b,2) receptors in the DG
Baclofen-induced enhancement of GC activity has been attributed to decreased inhibitory actions from hilar pathways (Burgard and Sarvey, 1991; Mott and Lewis, 1991; Mott et al., 1993). GABAB receptors are highly expressed on hilar interneurons (Bischoff et al., 1999; Mott et al., 1999; Kulik et al., 2003). Activation of these receptors hyperpolarizes the membrane potential and decreases hilar neuronal excitability (Mott et al., 1999), which, in turn, reduces GABA release and synaptic inhibition on GCs. We demonstrate that selective activation of the GABAB(1b,2) receptor subtype in the 1a–/– mice enhances GC PSs via disinhibition (Figures 3 and 4), consistent with activation of dendritic GABAB(1b,2) receptors on hilar neurons (Figures 1 and 2). Furthermore, we show that GABAB(1b,2) receptors also mediate a direct inhibition on GCs during GABAA receptor blockade, agreeing with a hyperpolarizing action at the extrasynaptic compartments of GC dendrites in the outer molecular layer (Kulik et al., 2003). Therefore, dendritic GABAB(1b,2) receptors on hilar neurons and GCs mediate disinhibition and inhibition of GC output respectively.
Exclusively disinhibitory roles of GABAB(1a,2) receptors in the DG
More importantly, this study reveals that the axonal terminal-preferring GABAB(1a,2) receptors collectively mediate disinhibition of the GCs without inhibition of the PP-GC transmission. In the molecular layer where GC dendrites are located, the GABAB(1a,2) receptors are densely expressed in a narrow band of the inner molecular layer immediately adjacent to the GCs. Given their preferential presynaptic localization and the exclusive disinhibitory effects, the GABAB(1a,2) receptors are envisaged to be on GABAergic axonal terminals acting as autoreceptors. Activation of these autoreceptors can effectively decrease GABAergic inhibition on proximal dendrites that shunts dendritic potentials (Ben-Ari et al., 2005), consistent with the enhanced GC output (Figure 3). In addition, disinhibition via presynaptic autoreceptors may be stronger than postsynaptic hyperpolarization, which can be easily overcome by strong depolarization on hilar neurons (Mott et al., 1999), further supporting a greater disinhibitory effect of GABAB(1a,2) receptors.
Hilar interneurons are activated by GC axons, inducing feedback inhibition onto GC dendrites in the molecular layer (Freund and Buzsaki, 1996; Amaral et al., 2007). Given their exclusive disinhibitory roles, the GABAB(1a,2) receptors expressed in the neuropil of the hilus (Figure 1 and 2) are potentially presynaptic heteroreceptors on GC axonal terminals innervating hilar neurons, similar to those expressed at mossy fibre terminals in the CA3 (Vigot et al., 2006; Guetg et al., 2009). Activation of these receptors reduces excitatory inputs to hilar neurons, decreases hilar neuron activity and, subsequently, feedback inhibition on GC dendrite, manifesting a disinhibitory action that can synergize with the autoreceptors in the inner molecular layer. If the GABAB(1a,2) receptors were autoreceptors at inhibitory terminals in the hilus, their activation would increase hilar neuron activity and enhance feedback inhibition on GCs, opposite to what was observed experimentally. Therefore, GABAB(1a,2) receptors in the neuropil of the hilus are potentially heteroreceptors on GC axonal terminals, mediating disinhibition in synergy with the autoreceptors.
Given that the majority of hilar neurons express the 1a subunit, it is potentially possible that the GABAB(1a,2) autoreceptors in the inner molecular layer are on axonal terminals of hilar neurons. The 1a-containing hilar neurons in the border zone include basket cells and fusiform cells, and they preferentially project to perisomatic and proximal dendritic regions of the GCs (Freund and Buzsaki, 1996; Amaral et al., 2007), thereby having the potential to traffic GABAB(1a,2) receptors to axonal terminals in the GC and the inner molecular layer. However, the restricted localization of GABAB(1a,2) receptors in the inner molecular layer suggests that only a subpopulation of the axons that project to the proximal dendrite of GCs may express the autoreceptors.
Heterogeneity of 1a expression between the soma and the terminal is more pronounced in deep-layer neurons, which, including hilar-perforant-path-related cells (Han et al., 1993), preferentially terminate on distal dendrites of GCs in the outer molecular layer. Despite that the majority of deep-layer hilar neurons contain both 1a and 1b subunits, a low level of GABAB(1a,2) expression is found in the outer molecular layer. This indicates that the deep-layer cells do not efficiently traffic the subunit to terminals. Potentially, axonal trafficking can be limited by the availability of intracellular binding proteins for ‘sushi’ domains (Vigot et al., 2006; Tiao et al., 2008; Biermann et al., 2010). Therefore, hilar neurons may regulate the spatial expression of autoreceptors on axon terminals across the molecular layer to selectively modulate inhibition on proximal dendrite of GCs.
Endogenous activation of GABAB receptor subtypes
While the blockade of GABAA receptors by the antagonist, bicuculline, markedly enhanced the PS (Figure 4), showing significant endogenous GABA release, GABAB receptors were not activated by the single-pulse PP stimulation. The selective antagonist, CGP55845, did not affect the PS or the fEPSP, probably because GABAB receptors are predominantly located extrasynaptically in the DG (Kulik et al., 2003), only activated by ‘spillover’ of GABA under synchronized and/or high-frequency activation (Scanziani, 2000). The GABAB(1a,2) autoreceptors concentrated in the inner molecular layer can potentially be activated by local release of GABA, producing activity-regulated gating of GC output.
Dendritic GABAB receptors in the outer molecular layer are, however, activated by GABA released from en passant axonal varicosities of neurogliaform cells (Armstrong et al., 2011), a subtype of GABAergic interneurons displaying extensive axonal arborizations, which form non-synaptic contacts with other cells (Tamas et al., 2003; Olah et al., 2009). Neurogliaform cells are present in the outer two-thirds of the molecular layer and PP stimulation activates these cells and induces GABAA and GABAB inhibitory postsynaptic currents on GCs (Armstrong et al., 2011). Although the GABAB antagonist had no effect on the PS, local receptor activation may modulate dendritic NMDA receptor activity and calcium signals (Chalifoux and Carter, 2010) and regulate synaptic plasticity. The two GABAB subtypes in the molecular layer are, therefore, potentially activated by different forms of endogenous GABA release.
Disinhibitory and inhibitory roles of GABAB(1a,2) receptors in the hippocampal tri-synaptic circuit
We also reveal a lack of presynaptic GABAB heteroreceptors on PP terminals in the mouse DG. GABAB(1a,2) receptors in the DG are, therefore, particularly important for disinhibition, which enhances GC output and spike transmission to the hippocampus.
Conversely, GABAB(1a,2) receptors in CA3 and CA1 areas are mainly presynaptic heteroreceptors inhibiting glutamate release and CA3 and CA1 outputs (Vigot et al., 2006; Guetg et al., 2009). The lack of GABAB(1a,2) receptors, therefore, alters the dynamic range of signal transfer in the tri-synaptic circuits between the PP and the CA1, resulting in decreased proportion of silent synapses, impaired long-term potentiation in the CA1 (Vigot et al., 2006; Guetg et al., 2009) and deficits in novel object recognition (Vigot et al., 2006; Jacobson et al., 2007). Furthermore, rapid changes in the expression of presynaptic GABAB receptors at hippocampal mossy fibre (Chandler et al., 2003) and CA1 inhibitory terminals (Wu and Leung, 1997) have been demonstrated following kindling, indicating axonal trafficking of the 1a subunit as an important regulatory mechanism in epileptogenesis.
In conclusion, the axon terminal-preferring GABAB(1a,2) and dendrite-preferring GABAB(1b,2) receptor subtypes are distinctly expressed in hilar inhibitory pathways and GCs and differentially modulate GC output. By regulating subunit composition and expression in neuronal circuits, GABAB receptor subtypes modulate a variety of behavioural states.
Acknowledgments
The work was supported by a BBSRC grant to YC (BB/E010296/1).
Glossary
- 1a–/–
GABAB1a knockout
- 1b–/–
GABAB1b knockout
- CGP55845
(2S)-3-[[(1S)-1-(3,4-Dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DG
dentate gyrus
- fEPSP
field excitatory postsynaptic potential
- GABA
gamma-amino butyric acid
- GC
granule cell
- IM
inner molecular layer
- OM
outer molecular layer
- PP
perforant path
- PS
population spike
- WT
wild-type
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164:1–2. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C, Szabadics J, Gabor T, Soltesz I. Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward gamma-aminobutyric acidergic modulators of entorhinal-hippocampal interplay. J Comp Neurol. 2011;519:1476–1491. doi: 10.1002/cne.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B, Nadler JV. Baclofen selectively inhibits transmission at synapses made by axons of Ca3 pyramidal cells in the hippocampal slice. J Pharmacol Exp Ther. 1982;223:291–297. [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R, Bernard C. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Biermann B, Ivankova-Susankova K, Bradaia A, Abdel Aziz S, Besseyrias V, Kapfhammer JP, et al. The Sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci. 2010;30:1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, et al. Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol. 1999;412:1–16. [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, et al. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Burgard EC, Sarvey JM. Long-lasting potentiation and epileptiform activity produced by GABAB receptor activation in the dentate gyrus of rat hippocampal slice. J Neurosci. 1991;11:1198–1209. doi: 10.1523/JNEUROSCI.11-05-01198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler KE, Princivalle AP, Fabian-Fine R, Bowery NG, Kullmann DM, Walker MC. Plasticity of GABA(B) receptor-mediated heterosynaptic interactions at mossy fibers after status epilepticus. J Neurosci. 2003;23:11382–11391. doi: 10.1523/JNEUROSCI.23-36-11382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Menendez-Roche N, Sher E. Differential modulation by the GABAB receptor allosteric potentiator 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)-phenol (CGP7930) of synaptic transmission in the rat hippocampal CA1 area. J Pharmacol Exp Ther. 2006;317:1170–1177. doi: 10.1124/jpet.105.099176. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Shaban H, Vigot R, Sansig G, Haller C, Barbieri S, et al. Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci. 2004;24:6086–6097. doi: 10.1523/JNEUROSCI.5635-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: a double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Guetg N, Seddik R, Vigot R, Turecek R, Gassmann M, Vogt KE, et al. The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J Neurosci. 2009;29:1414–1423. doi: 10.1523/JNEUROSCI.3697-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZS, Buhl EH, Lorinczi Z, Somogyi P. A High-Degree of Spatial Selectivity in the Axonal and Dendritic Domains of Physiologically Identified Local-Circuit Neurons in the Dentate Gyrus of the Rat Hippocampus. Eur J Neurosci. 1993;5:395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Bettler B, Kaupmann K, Cryan JF. GABAB1 receptor subunit isoforms exert a differential influence on baseline but not GABAB receptor agonist-induced changes in mice. J Pharmacol Exp Ther. 2006;319:1317–1326. doi: 10.1124/jpet.106.111971. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. Specific roles of GABA(B(1)) receptor isoforms in cognition. Behav Brain Res. 2007;181:158–162. doi: 10.1016/j.bbr.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, Haas CA, Lopez-Bendito G, Shigemoto R, et al. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV. Facilitation of the induction of long-term potentiation by GABAB receptors. Science. 1991;252:1718–1720. doi: 10.1126/science.1675489. [DOI] [PubMed] [Google Scholar]
- Mott DD, Xie CW, Wilson WA, Swartzwelder HS, Lewis DV. GABAB autoreceptors mediate activity-dependent disinhibition and enhance signal transmission in the dentate gyrus. J Neurophysiol. 1993;69:674–691. doi: 10.1152/jn.1993.69.3.674. [DOI] [PubMed] [Google Scholar]
- Mott DD, Li Q, Okazaki MM, Turner DA, Lewis DV. GABAB-Receptor-mediated currents in interneurons of the dentate-hilus border. J Neurophysiol. 1999;82:1438–1450. doi: 10.1152/jn.1999.82.3.1438. [DOI] [PubMed] [Google Scholar]
- Nahir B, Bhatia C, Frazier CJ. Presynaptic inhibition of excitatory afferents to hilar mossy cells. J Neurophysiol. 2007;97:4036–4047. doi: 10.1152/jn.00069.2007. [DOI] [PubMed] [Google Scholar]
- Nicoll RA. My close encounter with GABA(B) receptors. Biochem Pharmacol. 2004;68:1667–1674. doi: 10.1016/j.bcp.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Olah S, Miklos F, Gergely K, Csaba V, Rita B, Pal B, et al. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–U1113. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Mody I. Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J Neurophysiol. 1992;67:227–235. doi: 10.1152/jn.1992.67.1.227. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J Physiol. 1993;463:391–407. doi: 10.1113/jphysiol.1993.sp019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Pinard A, Seddik R, Bettler B. GABAB receptors: physiological functions and mechanisms of diversity. Adv Pharmacol. 2010;58:231–255. doi: 10.1016/S1054-3589(10)58010-4. [DOI] [PubMed] [Google Scholar]
- Price CJ, Scott R, Rusakov DA, Capogna M. GABA(B) receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J Neurosci. 2008;28:6974–6982. doi: 10.1523/JNEUROSCI.4673-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Witter M. The dentate gyrus – A comprehensive guide to structure, function, and clinical implications – Preface. Prog Brain Res. 2007;163:Ix–Xiii. [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- Tiao JY, Bradaia A, Biermann B, Kaupmann K, Metz M, Haller C, et al. The sushi domains of secreted GABA(B1) isoforms selectively impair GABA(B) heteroreceptor function. J Biol Chem. 2008;283:31005–31011. doi: 10.1074/jbc.M804464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Bettler B. GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol. 2007;17:298–303. doi: 10.1016/j.conb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Besseyrias V, Bettler B. Functional mapping of GABA(B)-receptor subtypes in the thalamus. J Neurophysiol. 2007;98:3791–3795. doi: 10.1152/jn.00756.2007. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Leung LS. Partial hippocampal kindling decreases efficacy of presynaptic GABAB autoreceptors in CA1. J Neurosci. 1997;17:9261–9269. doi: 10.1523/JNEUROSCI.17-23-09261.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]